Abstract
Objective
Executive Function, a set of cognitive skills important to social and academic outcomes, is a specific area of cognitive weakness in children with congenital heart disease (CHD). We evaluated the prevalence and profile of executive dysfunction in a heterogeneous sample of school aged children with CHD, examined whether children with executive dysfunction are receiving school services and support, and identified risk factors for executive dysfunction at school age.
Design
91 school aged patients completed questionnaires, including the Behavior Rating Inventory of Executive Function (BRIEF) and a medical history questionnaire. An age and gender matched control sample was drawn from a normativedatabase.
Results
CHD patients had a higher rate of parent reported executive dysfunction (OR=4.37, p<0.0001), especially for working memory (OR=8.22, p<0.0001) and flexibility (OR=8.05, p<0.0001). Those with executive dysfunction were not more likely to be receiving school services (p>0.05). Gender, premature birth (≤37 weeks), and CHD with aortic obstruction were predictive of executive dysfunction, especially for behavior regulation skills.
Conclusions
School aged children with CHD have an increased prevalence of executive dysfunction, especially problems with working memory and flexibility, and are underserved by the school system. The increased risk for executive dysfunction in those with CHD and prematurity or CHD with aortic obstruction suggests an etiology of delayed brain development in the fetal and neonatal periods, while male gender may increase susceptibility to brain injury. This study highlights the need for regular neurodevelopmental follow up in children with CHD, and a need to better understand mechanisms that contribute to adverse neurodevelopmental outcomes.
Keywords: congenital heart disease, neurodevelopment, outcomes, executive function
Introduction
As survivorship has improved for children with complex congenital heart disease (CHD), emerging research shows that children with CHD are at high risk for neurodevelopmental problems (1–2). There are abnormalities in brain maturation and brain injury that are present in infancy, even prior to surgical intervention (3–6). Neurodevelopmental problems in CHD are thought to be related to disrupted fetal and neonatal brain development and subsequent increased susceptibility to brain injury (7). MRI abnormalities have been found in older children and adolescents with CHD (8), and are associated with cognitive impairments (9). Population based studies suggest children with CHD access special education services at a higher rate than those without CHD (10). In addition, the rate of children needing educational assistance increases over the course of development, and though most children have normal intellectual functioning, there are problems with attention, executive skills, memory, visual-spatial skills, and social/pragmatic skills (8, 11–13).
Previous studies document a range of outcomes of children with CHD, but there are limitations in what they address. First, many examine only specific cardiac diagnoses, or divide children into smaller groups by cardiac diagnosis. This limits the ability to evaluate specific disease factors as they relate to outcome. For example, single ventricle defects and CHD with aortic obstruction likely alter the fetal circulation, affecting fetal brain development (14), and subsequently may impact cognitive outcomes. Second, many studies primarily address outcomes in infants or toddlers (15–17). Some studies extend into school age and adolescence (8, 12–13, 18-21), but few take into account the aspects of a child’s medical or demographicprofile that may predict a specific cognitive outcome beyond surgical factors and IQ. There is some suggestion that the severity of the defect as indicated by postnatal cyanosis, need for single ventriclepalliation, or CHD with aortic obstruction, along with other medical complications such as seizures, stroke, abnormal neurological examination, abnormalities on MRI, premature birth, extended hospital stay, or use of mechanical support, incur higher risk for neurodevelopmental consequences (2). This study addresses gaps in the extant literature by examining a heterogeneous sample of children with CHD requiring surgical repair in the first year of life, and by examining specific aspects of a child’s disease as predictors, rather than dividing children into diagnostic subgroups a priori.
Executive functioning (EF) has been identified as a specific area of cognitive impairment in CHD that is critical to social development and academic learning (8, 12, 22–25). EF describes a set of behaviors responsible for purposeful, goal directed activity (26). It is used to organize and direct cognitive activity, emotional responses, and overt behavior. Developmentally, these skills emerge in toddler/preschool years and develop substantially through childhood, adolescence and early adulthood, mirroring increasing environmental demands. Given this, difficulties in EF become more apparent over time. Children with executive dysfunction are often overlooked by general practitioners and schools, as intellectual development can be unrelated to executive skills problems, or problems with executive skills may be masked by stronger intellectual skills on some testing (27–28). Despite this, executive dysfunction is strongly related to a child’s development, learning, behavior, and academic success, and it has been suggested that EF is a better predictor of classroom performance and academic achievement than intellectual or early academic skills (29–30). In this way, it is possible that school age children with CHD who would potentially qualify for accommodations and/or services are being under identified.
The aims of this study are to 1) evaluate the prevalence and profile of executive dysfunction in a heterogeneous sample of school aged children with CHD, 2) to examine whether school aged children with executive dysfunction are receiving school services and supports, and 3) to identify which indicators of medical severity represent risk factors for executive dysfunction. We hypothesize that children with CHD are at high risk for executive dysfunction at school age, and given previous reports we expect a high prevalence of impairment in this area. In addition, we hypothesize that those patients whose cardiac defect is more likely to alter the fetal and neonatal brain circulation or which predisposes to hypoxic injury (e.g., single ventricle defects, CHD with aortic obstruction, or cyanosis), and those with more complicating medical factors (such as neurological events or prematurity) will have increased prevalence of executive dysfunction.
Methods
Participants and Procedures
Children with CHD were recruited via social media (Facebook posts in CHD specific groups), in-hospital advertisements, and at cardiology or neuropsychology clinic visits. Patients were included if they had CHD requiring open heart surgery within the first year of life. Patients were excluded if they were diagnosed with a genetic syndrome that would better explain their cognitive and behavioral profile, or if they had a substantial, identified genetic finding that was presumed to have a large influence across organ systems. Study procedures were approved by the Institutional Review Board at Children’s National Health System. Parents gave written informed consent, children between 7-10 years of age provided verbal assent to participate, and children over 11 years of age provided written assent to participate. As part of the informed consent process, participants gave permission to contact the child’s cardiologist for a recent clinic note, which was used to confirm cardiac diagnosis.
Parents completed a set of questionnaires that were delivered and returned by mail or in person. Study data were collected and managed using REDCap (31). This included a demographic and medical history questionnaire, where parents reported any prior neurological findings (including presence of MRI abnormalities if MRI was available, abnormal EEG, non-febrile seizures, or stroke), pregnancy/birth history, and other information on their child’s medical and educational history. The Behavior Rating Inventory of Executive Function (BRIEF) is a standardized questionnaire completed by the primary caregiver or parent that has been widely used in research and clinical settings to assess the presence and severity of executive dysfunction in day to day situations (32). It is composed of three broad indices (General Executive Composite, the Metacognitive Index, and the Behavior Regulation Index) and eight subscales.. The metacognitive index is comprised of five subscales; initiate (how well an individual independently initiates tasks), working memory (holding information in mind, manipulating information in mind), planning/organization (using systematic, well planned approaches to tasks), organization of materials, and monitor (monitoring one’s behavior, or task approach). The Behavior Regulation Index is comprised of three subscales, including inhibit (an index of impulsive behavior or acting before thinking), shift (the ability to maintain a flexible approach to problem solving or behavior), and emotional control (the ability to manage and regulate emotional responses). Age-based T-scores are computed for each subscale and index, and a score of 65 or higher is considered a clinically significant problem. To examine the prevalence of parent reported executive dysfunction, we classified each subject’s scores on subscales as clinically elevated (T≥65) or not elevated. In addition, an age and gender matched control sample was drawn from the normative database for the BRIEF for statistical comparisons as described in detail below. In order to reduce the number of statistical tests performed, while retaining detailed information about executive skills profiles, only subscale scores were entered into analyses, as the indices are directly derived from the subscales and would provide overlapping information.
Each patient was assigned to one of four previously described diagnostic classes (33): Class I – two ventricle CHD without aortic obstruction, Class II – two ventricle CHD with aortic obstruction, Class III – single ventricle CHD without aortic obstruction, or Class IV – single ventricle CHD with aortic obstruction. Table 1 presents the specific diagnoses in each cardiac class. CHD Class was determined by the study cardiologist (MTD) based on the information given in the history form and/or the medical records. Any CHD diagnosis which included aortic valve stenosis or coarctation, hypoplasia, or interruption of the aortic arch was considered to have aortic obstruction. Single ventricle palliation vs. two ventricle repair was determined by the type of surgical repair undertaken. Cyanosis was coded based on specific diagnosis and anticipated postnatal clinical presentation. Given that the number of patients in each class was too small for multivariate analyses of individual diagnostic classes, subjects were instead compared based on important physiological components of their cardiac diagnosis, including single ventricle (Class III and IV) vs. two ventricle (Class I and II) repair, CHD with aortic obstruction (Class II and IV) vs. no aortic obstruction (Class I and III), and lesions with postnatal cyanosis vs. acyanotic. Based on parent report and available records, classifications were also made for medical risk variables, including prematurity (≤37 weeks gestation), and the presence of neurological abnormality (the presence of any of the following by parent report/records: stroke, seizures, MRI or EEG abnormality).
Table 1.
Diagnoses and Classifications of the obtained sample
| Classification | Frequency by diagnosis, N=91 |
|---|---|
| Class 1 2V, no aortic obstruction 37 Total |
6 dextro-Transposition of the great arteries and intact ventricular septum 3 dextro-Transposition of the great arteries and ventricular septal defect 6 Tetralogy of Fallot 5 Tetralogy of Fallot/Pulmonary atresia 4 Truncus arteriosus 4 Ventricular septal defect 3 Atrioventricular canal defects 6 Other 2V defects |
| Class 2 2V, aortic obstruction 15 Total |
1 Truncus arteriosus (with Interupted aortic arch) 2 Ventricular septal defect with Coarctation 3 Coarctation/Arch hypoplasia 9 Other 2V defects with aortic obstruction |
| Class 3 SV, no aortic obstruction 19 Total |
4 Pulmonary atresia with intact ventricular septum 15 Other functional SV defects |
| Class 4 SV, aortic obstruction 20 Total |
13 Hypoplastic left heart syndrome 7 Other functional SV defects with aortic obstruction |
2V = Two Ventricle, SV = Single Ventricle
Statistical Analyses
Fisher’s exact tests were used to determine whether parents of children with CHD were more likely to report elevations across subscales of the BRIEF relative to controls drawn from an archival database. Fisher’s exact tests were also used to evaluate whether children with elevated BRIEF subscale scores were more likely to be receiving special education services or supports. Multivariate logistic regression models were then implemented to examine the odds of elevation in each of the BRIEF subscales in relation to available medical and demographic risk variables (single/two ventricle, cyanosis, aortic obstruction, presence of any neurological abnormality, prematurity (≤37 weeks/full term), and gender). An effect was considered statistically significant if α level in a 2-tailed test was less than 0.05. Cyanosis was not included in the models as our preliminary analysis suggested it did not significantly contribute to the models.
Results
Prevalence of executive dysfunction
Ninety-one children with CHD (mean age 9.08 years, SD = 2.71, range 6-17; 53 male) participated in the study. The sample was a combination of clinically referred patients that volunteered to participate in the study (n = 26), a local sample that volunteered for a research appointment (n = 15), and volunteers from around the country who completed questionnaires by mail (n = 50). Ninety-one age and gender matched controls with data on the BRIEF questionnaire were drawn from the normative database from the BRIEF. Descriptive data for each group are presented in Table 2.
Table 2.
Descriptive Statistics for CHD and Controls on the BRIEF
| Indices/subscales | Controls | All CHD | Class I | Class II | Class III | Class IV |
|---|---|---|---|---|---|---|
| Metacognitive Index | 51.98 (9.84) | 59.43 (12.23) | 58.30 (12.11) | 63.07 (14.00) | 58.95 (13.36) | 59.25 (15.11) |
| Initiate | 50.05 (9.31) | 57.84 (11.95) | 56.08 (12.13) | 61.60 (12.87) | 57.32 (13.68) | 58.75 (8.95) |
| Working Memory | 51.77 (9.82) | 60.88 (11.95) | 59.97 (11.42) | 63.33 (11.90) | 60.32 (14.52) | 61.25 (10.90) |
| Planning/Organization | 51.21 (10.03) | 58.70 (12.69) | 57.81 (12.30) | 60.47 (15.44) | 59.00 (13.87) | 58.75 (10.71) |
| Organization of Materials | 49.46 (9.48) | 55.41 (11.37) | 55.51 (10.91) | 59.07 (14.27) | 52.32 (10.05) | 55.40 (11.00) |
| Monitor | 53.92 (10.72) | 56.91 (12.38) | 55.57 (13.49) | 59.40 (12.67) | 58.21 (12.15) | 56.30 (10.59) |
| Behavior Regulation Index | 50.54 (9.43) | 57.55 (14.67) | 55.84 (14.75) | 61.53 (16.37) | 55.89 (12.00) | 59.30 (15.78) |
| Inhibit | 52.22 (9.90) | 54.95 (14.22) | 54.95 (15.10) | 57.40 (13.97) | 52.68 (12.28) | 55.25 (15.11) |
| Shift | 48.89 (9.07) | 58.13 (14.74) | 54.76 (12.52) | 60.07 (18.00) | 60.95 (13.07) | 60.25 (17.14) |
| Emotional Control | 50.44 (9.68) | 57.19 (14.23) | 55.38 (14.20) | 62.80 (16.65) | 53.95 (12.24) | 59.40 (13.00) |
Results are mean T-Scores for each scale, mean = 50, standard deviation = 10. A T-Score ≥ 65 is considered a significant elevation.
BRIEF = Behavior Rating Inventory of Executive Function, CHD = Congenital Heart Disease
There was a high prevalence of parent reported executive dysfunction in our sample, with 64.8% of parents reporting at least one elevation on the BRIEF, compared to 29.7% of controls (Odds Ratio = 4.37; 95% CI: 2.35, 8.14, p < 0.0001). Figure 1 and Table 3 show the percentage of the sample with clinically significant elevations by subscale. Parents of children with CHD were more likely to endorse clinically significant elevations across BRIEF subscales (all p < 0.05, Table 3), except for Inhibit (p = 0.07, Table 3). Working Memory and Shift were most frequently endorsed as problematic, with parents of children with CHD being over eight times more likely to endorse a problem than parents of healthy controls.
Fig 1.
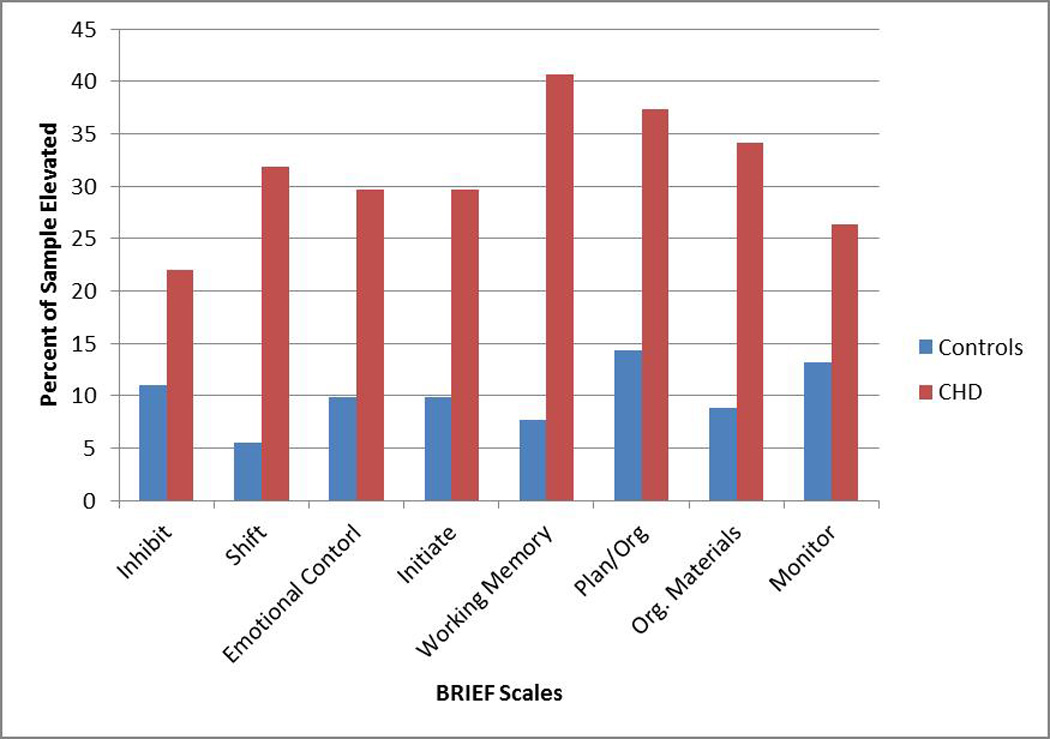
Prevalence of Executive Dysfunction: Percent of the sample reporting clinically significant elevations (T score ≥ 65) on the BRIEF
Table 3.
Comparisons of Clinically Elevated BRIEF Scores in Children with CHD vs. Controls
| Indices/subscales | % Elevated Scores |
Odds Ratio |
95% CI | P-value* | |
|---|---|---|---|---|---|
| Controls N (%) |
CHD N (%) |
||||
| Metacognitive Index | |||||
| Initiate | 9 (9.9%) | 27 (29.7%) | 3.84 | 1.69, 8.75 | 0.0013 |
| Working Memory | 7 (7.7%) | 37 (40.7%) | 8.22 | 3.42, 19.77 | <0.0001 |
| Planning/Organization | 13 (14.3%) | 34 (37.4%) | 3.58 | 1.73, 7.39 | 0.0006 |
| Organization of Materials | 8 (8.8%) | 31 (34.1%) | 5.36 | 2.30, 12.48 | <0.0001 |
| Monitor | 12 (13.2%) | 24 (26.4%) | 2.36 | 1.10, 5.07 | 0.0397 |
| Behavior Regulation Index | |||||
| Inhibit | 10 (11%) | 20 (22%) | 2.28 | 1.00, 5.20 | 0.07 |
| Shift | 5 (5.5%) | 29 (31.9%) | 8.05 | 2.95, 21.95 | <0.0001 |
| Emotional Control | 9 (9.9%) | 27 (29.7%) | 3.84 | 1.69, 8.75 | 0.0013 |
Note:
Fisher’s exact test
BRIEF = Behavior Rating Inventory of Executive Function, CHD = Congenital Heart Disease
Access to services
Thirty-three percent of children with CHD were receiving some form of support in the school setting (such as an IEP, 504, or similar student support plan if in a private school setting). Those children with CHD who had at least one area of executive dysfunction were not more likely to be receiving services in the school setting when compared with those who did not endorse any problems (χ2 (1, N=91) = 0.044, p = 0.833). Similarly, the odds of receiving services or supports in the school setting were not significantly higher for children with CHD endorsing problems in any specific area of EF (Table 4), though there was a trend towards children with inhibitory control problems being more likely to receive services.
Table 4.
Odds of Children with CHD and Clinically Elevated BRIEF Scores Receiving Special Education Services
| Indices/subscales | N (%) Receiving Services |
Odds Ratio | 95% CI | P-value* |
|---|---|---|---|---|
| Metacognitive Index | ||||
| Initiate | 11 (40.7%) | 1.63 | 0.64, 4.15 | 0.34 |
| Working Memory | 13 (35.1%) | 1.18 | 0.49, 2.86 | 0.82 |
| Planning/Organization | 14 (41.2%) | 1.80 | 0.73–4.39 | 0.25 |
| Organization of Materials | 9 (29%) | 0.76 | 0.30–1.94 | 0.64 |
| Monitor | 10 (41.7%) | 1.68 | 0.64–4.41 | 0.32 |
| Behavior Regulation Index | ||||
| Inhibit | 10 (50%) | 2.55 | 0.92, 7.06 | 0.10 |
| Shift | 12 (41.4%) | 1.73 | 0.69, 4.33 | 0.34 |
| Emotional Control | 11 (40.7%) | 1.63 | 0.64, 4.15 | 0.34 |
Note:
Fisher’s exact test
BRIEF = Behavior Rating Inventory of Executive Function, CHD = Congenital Heart Disease
Relationship between medical/demographic risk factors and executive dysfunction
Results of the multivariate logistic models are presented in Table 5in which the p-values of Hosmer and Lemeshow Goodness-of-Fit Tests are all statistically insignificant (p > 0.05), indicating that all logistic regression models fit the data very well. Prematurity showed the strongest impact on executive dysfunction in children with CHD, especially with respect to behavioral regulation, with significantly increased risk for elevated scores on the Inhibit and Emotional Control subscales, and increased risk on Initiate and Working Memory scales relative to full-term children with CHD. The presence of CHD with aortic obstruction significantly increased risk for elevated scores on the Emotional Control and Organization of Materials scales. Male gender was associated with increased risk for elevated scores on the Inhibit, Shift, Monitor, and Planning/Organization scales. Contrary to our hypothesis, children with single ventricle defects were not more likely to experience executive dysfunction. In fact, children with two ventricle CHD were more likely to have problems with Emotional Control (OR=3.53, 95% CI: 1.02, 12.15) and Organization of Materials (OR=3.75, 95% CI: 1.23, 11.45).. The presence of neurological abnormalities was not associated with executive dysfunction for children with CHD.
Table 5.
Results of Logistic Regression Models by BRIEF Subscale
| Variable | Indices/Subscale |
|||||||
|---|---|---|---|---|---|---|---|---|
| Metacognitive Index |
Behavior Regulation Index |
|||||||
| Initiate |
Working Memory |
Planning/ Organization |
Organization of Materials |
Monitor | Inhibit | Shift |
Emotional Control |
|
| OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
|
| Single Ventricle | 0.61 (0.22, 1.71) |
0.72 (0.28, 1.88) |
0.58 (0.22, 1.56) |
0.27 (0.09, 0.81)† |
0.48 (0.16, 1.47) |
0.30 (0.08, 1.12) |
1.48 (0.54, 4.07) |
0.28 (0.08, 0.98)† |
| Aortic Obstruction | 1.86 (0.69, 5.07) |
2.19 (0.84, 5.69) |
1.39 (0.52, 3.68) |
4.58 (1.58, 13.24)‡ |
1.44 (0.48, 4.31) |
2.04 (0.57, 7.32) |
1.87 (0.66, 5.25) |
5.95 (1.74, 20.37)‡ |
| Prematurity |
3.75 (1.20, 11.75)† |
3.31 (1.05, 10.41)† |
2.97 (0.95, 9.26) |
2.72 (0.82, 9.09) |
2.82 (0.85, 9.31) |
11.12 (2.62, 47.12)‡ |
3.11 (0.95, 10.15) |
15.12 (3.56, 64.17)‡ |
| Neurological Abnormality | 0.70 (0.21, 2.30) |
0.54 (0.17, 1.67) |
0.75 (0.24, 2.34) |
0.61 (0.18, 2.07) |
2.35 (0.71, 7.76) |
0.58 (0.13, 2.63) |
0.52 (0.15, 1.85) |
0.49 (0.12, 1.99) |
| Male Gender | 1.06 (0.41, 2.78) |
2.04 (0.81, 5.13) |
2.88 (1.11, 7.51)† |
1.43 (0.53, 3.84) |
4.71 (1.46, 15.19) ‡ |
6.74 (1.62, 28.11)‡ |
3.97 (1.34, 11.73)† |
3.23 (0.996, 10.46) |
| Hosmer & Lemeshow Goodness-of-fit |
0.319 | 0.282 | 0.206 | 0.358 | 0.261 | 0.902 | 0.209 | 0.604 |
Notes:
significant at p ≤ 0.05
significant at p ≤ 0.01
BRIEF = Behavior Rating Inventory of Executive Function
Discussion
This study reveals a high prevalence of executive dysfunction in a sample ofschool age children with CHD requiring surgery in the first year of life. In the group as a whole, problems with working memory (mental maintenance and manipulation of information) and flexibility (rigid behavior and patterns of thinking) were most commonly reported. This pattern and prevalence of parent reported executive dysfunction is similar to previous reports (23). Several aspects of a child’s medical history and gender were associated with increased risk for executive dysfunction, especially for behavioral dysregulation.
Overall, prematurity was the strongest predictor of negative outcomes for both metacognitive skills and behavior regulation, followed by male gender and CHD with aortic obstruction. We propose that these medical and demographic risk factors may increase the risk for executive dysfunction by impacting brain development in the fetal and neonatal period. Neurodevelopmental problems in CHD are thought to be related to delayed fetal and neonatal brain development and subsequent susceptibility to brain injury (7). At term, neonates with CHD have brain MRI findings similar to that of premature infants born at 35 weeks gestation (4). Risk factors identified in this study impact fetal circulation, which is thought to be a primary mechanism of these maturational changes and subsequent injury. That is, the aortic obstruction may contribute to decreased antegrade flow in the ascending aorta in fetal life, thus likely contributing to delayed brain maturation in-utero (34). Prematurity also impacts brain maturation and susceptibility to injury. In fact, executive dysfunction has been identified as a specific area of concern in premature children (35–37). Given this, our data suggest that prematurity further impacts brain maturation in children with CHD, and therefore subsequent susceptibility to injury. Comparison to children with prematurity and no CHD would provide greater insights into the contribution of each risk factor and their cumulative effects. Finally, males may be more vulnerable to problems associated with brain immaturity, specifically to neonatal hypoxic-ischemic injury (38). Males and females are known to respond differently to neonatal hypoxic-ischemic injury, which may explain gender differences in prevalence for central nervous system disorders such as cerebral palsy (39). In addition, there may be different neuronal pathways for cell death in males and females (40), and estrogen may be neuroprotective (41).
Interestingly, children with single ventricle CHD were not more likely to have executive dysfunction at school age than children with two ventricle repair. In fact, children who underwent two ventricle repair in this study group were at increased risk for problems with emotional control and organization. This result suggests that all children with CHD, including those who have a two ventricular repair should be considered at risk for neurodevelopmental abnormalities. Since surgical and medical management of single ventricle patients is generally more complex, and since they may have more physiological complications (42–43), practitioners and researchers often assume that they will have worse neurocognitive outcomes across domains. Indeed, while some studies show worse performance on global outcomes, such as IQ (44), these findings only approach statistical significance when other patient specific factors are taken into account. That is, it may be that examination of these patient specific factors (e.g, specific physiological complications, specific disease-related factors such as aortic obstruction or other associated complications such as prematurity) may eventually explain more of the variance, and provide clues regarding mechanisms of action. Additionally, studies do not often look beyond coarse outcomes such as IQ to specific cognitive skill areas or profiles, or at times they focus exclusively on single ventricle patients (15, 45) or other specific diagnostic groups, such as transposition of the great arteries (12). Instead, examination of patient specific factors across diagnostic subgroups may be helpful in pinpointing potentially modifiable risk factors or mechanisms of action.
When viewed alongside previous work, this study highlights three important points. First, all children requiring surgical repair in the first year of life are at high risk for executive dysfunction at school age, regardless of cardiac diagnosis. Second, individual factors (such as prematurity, gender, and CHD with aortic obstruction) that potentially influence brain maturation and subsequent susceptibility to injury may be more predictive of neurodevelopmental outcomes than the broader distinction of single ventricle versus two ventricle CHD. Third, it is important to look beyond global measures like IQ in evaluating outcomes in children with CHD.
This study also suggests that many children who may qualify for services and supports in the school setting are not receiving them. Our finding that only 33% of children with CHD are receiving services is consistent with previous studies in infants and toddlers suggesting that only a small number of infants with CHD participating in a neurodevelopmental follow-up program that qualified to early intervention services were receiving therapies (46). There was a trend towards being more likely to receive services if a child with CHD had problems related to impulsivity; this makes intuitive sense, since impulsive children can be disruptive in a classroom setting. Despite this, our data suggest that impulsivity is not one of the more commonly reported problems in this group; only 22% of children with CHD had problems in this area. In other words, the majority of children in this sample will not likely be identified for services in the school setting. Taken together, these findings suggest neurodevelopmental assessment across childhood and adolescence is needed as identification of the problem is a critical first step to ensuring appropriate access to therapies and supports, and to continue to identify those children with more subtle difficulties in school age.
There are limitations to the current study which will be addressed in future work. A primary deficiency was reliance on parent report on questionnaire for identification of concomitant neurological abnormalities. This may have resulted in under-reporting of neurological abnormalities. Furthermore, there are likely differing standards for neurologic assessment over time and in different hospitals (e.g., not all hospitals routinely provide MRI or neurological examinations for cardiac patients). As such, our classification may represent only severe neurological abnormalities such as overt stroke or seizures. In the future, precise measures of neurological maturity and injury, even in the absence of overt symptoms, will likely prove more fruitful. Additionally, data regarding key medical variables were not always consistently available. This includes use of mechanical circulatory support, or specific surgical data including duration of cardiopulmonary bypass and/or hypothermic circulatory arrest. Identification of those with a prenatal vs. postnatal diagnosis and data relating to degree of hemodynamic compromise at presentation were not available and may be useful in future studies as predictors of outcome. Careful measurement of socioeconomic status was also not readily available across the sample. Given previous research, this will likely have a large impact on outcomes and will be included in future data collection and analysis. Though the use of the BRIEF, a self-report measure, allowed a broad sample of children across the country to participate, this limited the type of data available for analysis. While there are limitations to using solely parent report data, the BRIEF has been shown to be a powerful tool in assessment of EF unique from traditional paper-pencil measures (47), and even accounts for variance in neuroimaging findings of typically developing and patient populations (48–50).
This study has important implications for clinical practice. The incidence of executive dysfunction in patients with CHD is very high, and may significantly impact a child’s ability to succeed in multiple settings. Though neurodevelopmental follow up in children with CHD has been set as a practice guideline by the AHA (2), there are no guidelines for specific areas that need to be assessed at particular time points in development. While neurodevelopmental programs often prioritize patients with single ventricle CHD, or prioritize seeing very young children, there is evidence that all children with CHD requiring early surgical intervention require regular assessment through school age and adolescence, which can help ensure appropriate access to and continuity of services and therefore improve outcomes. This work also suggests that follow up should include a detailed evaluation of specific cognitive skills like EF. The goal of future research will be to identify which measures are the most sensitive and specific predictors of neurodevelopmental problems in children with CHD, and to work towards selection of effective screening tools (such as the BRIEF) that can be used routinely in cardiology clinics. Future research will also be directed towards evaluation of the prevalence of neurodevelopmental problems in children with decreased heart function or defects such as aortic stenosis undergoing catheter intervention, as they may share similar risk factors with respect to brain maturation. Evaluation of patient specific medical and demographic factors that may confer increased risk for executive dysfunction in later childhood will help create tiered levels of risk for assessment, and ‘flag’ those patients in need of closer monitoring and follow up. Finally, this work suggests a need for specific interventions at school age to improve EF, especially with respect to working memory and flexibility.
With increasing survivorship, there is a strong impetus to better understand those factors that impact a child’s quality of life. A better understanding of the complex medical and demographic factors that predict specific neurodevelopmental and psychosocial outcomes will help determine the mechanisms behind these outcomes. This will aid in the development of better standards of care and interventions to improve outcomes for children with CHD and their families.
Acknowledgments
Funding Sources: This work was funded by an award from the American Heart Association (13CRP14530003) and supported by the National Institute of Child Health and Human Development (NICHD) Intellectual and Developmental Disabilities Research Center (IDDRC) at Children’s National Health System (P30HD040677).
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest to disclose.
Author Contributions:
Jacqueline Sanz contributed to the concept/design of the study, securing funding, data collection, data analysis and interpretation, drafting and revising the article.
Madison Berl contributed to the concept/design of the study, securing funding, and critical revision of the article.
Anna Chelsea Armour contributed to the concept/design of the study, data collection, data analysis, and critical revision of the article.
Yao Cheng contributed to statistical analysis and interpretation, drafting and critical revision of the article.
Jichuan Wang contributed to the design of the study, statistical analysis and interpretation, and drafting and critical revision of the article.
Mary Donofrio contributed to the concept/design of the study, securing funding, data analysis and interpretation, drafting and revising the article.
References
- 1.Marino BS. New concepts in predicting, evaluating, and managing neurodevelopmental outcomes in children with congenital heart disease. Curr Opin Pediatr. 2013;25:574–584. doi: 10.1097/MOP.0b013e328365342e. [DOI] [PubMed] [Google Scholar]
- 2.Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor WJ, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management. Circulation. 2012;126:1143–1172. doi: 10.1161/CIR.0b013e318265ee8a. [DOI] [PubMed] [Google Scholar]
- 3.Andropoulos DB, Hunter JV, Nelson DP, Stayer S, Stark AR, McKenzie ED, et al. Brain immaturity is associated with MRI brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. J Thorac Cardiovasc Surg. 2010;139:543–556. doi: 10.1016/j.jtcvs.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Licht DJ, Shera DM, Clancy RR, Wernovsky G, Montenegro LM, Nicolson SC, et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:529–537. doi: 10.1016/j.jtcvs.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahle WT, Tavani F, Zimmerman RA, Nicolson SC, Galli KK, Gaynor WJ, et al. An MRI study of Neurological Injury before and after congenital heart surgery. Circulation. 2002;106:I–109–I–114. [PubMed] [Google Scholar]
- 6.Miller SP, McQuillen PS, Hamrick SEG, Xu D, Gidden DV, Charlton N, et al. Abmormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–1938. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 7.Volpe JJ. Encephalopathy of congenital heart disease--destructive and developmental effects intertwined. J Pediatr. 2014;164:962–965. doi: 10.1016/j.jpeds.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Bellinger DC, Wypij D, Rivkin MJ, DeMaso DR, Robertson RL, Dunbar-Masterson C, et al. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: Neuropsychological assessment and structural brain imaging. Circulation. 2011;124:1361–1369. doi: 10.1161/CIRCULATIONAHA.111.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rollins C, Watson CG, Asaro LA, Wypij D, Vajapeyam S, Bellinger DC, et al. White Matter Microstructure and Cognition in Adolescents with Congenital Heart Disease. J Pediatr. 2014;165(5):936–944. doi: 10.1016/j.jpeds.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riehle-Colarusso T, Autry A, Razzaghi H, Boyle CA, Mahle WT, Van Naarden Braun K, et al. Congenital Heart Defects and Receipt of Special Education Services. Pediatrics. 2015;136(3):496–504. doi: 10.1542/peds.2015-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellinger DC, Wypij D, Kuban KCK, Rappaport LA, Hickey PR, Wernovsky G, et al. Developmental and neurological status of children at 4 years of age after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. Circulation. 1999;100:526–532. doi: 10.1161/01.cir.100.5.526. [DOI] [PubMed] [Google Scholar]
- 12.Bellinger DC, Wypij D, duPlessis AJ, Rappaport LA, Jonas RA, Wernovsky G, Newburger JW. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: The Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1385–1396. doi: 10.1016/s0022-5223(03)00711-6. [DOI] [PubMed] [Google Scholar]
- 13.Miatton M, DeWolf D, Francois K, Thiery E, Vingerhoets G. Neuropsychological performance in school-aged children with surgically corrected congenital heart disease. J Pediatr. 2007;151:73–78. doi: 10.1016/j.jpeds.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 14.Donofrio MT, DuPlessis AJ, Limperopoulos C. Impact of Congenital Heart Disease on Fetal Brain Development. Curr Opin Pediatr. 2011;23:502–511. doi: 10.1097/MOP.0b013e32834aa583. [DOI] [PubMed] [Google Scholar]
- 15.Newburger JW, Sleeper LA, Bellinger DC, Goldberg CS, Tabbutt S, Lu M, et al. Early Developmental Outcome in Children With Hypoplastic Left Heart Syndrome and Related Anomalies. Circulation. 2012;125:2081–2091. doi: 10.1161/CIRCULATIONAHA.111.064113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mussatto KA, Hoffmann RG, Hoffman GM, Tweddell JS, Bear L, Cao Y, Brosig C. Risk and prevalence of developmental delay in young children with congenital heart disease. Pediatrics. 2014;133:e570–e577. doi: 10.1542/peds.2013-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellinger DC, Wernovsky G, Rappaport LA, Mayer JE, Castaneda AR, Farrell DM, et al. Cognitive development of children following early repair of transposition of the great arteries using deep hypothermic circulatory arrest. Pediatrics. 1991;87:701–707. [PubMed] [Google Scholar]
- 18.Karsdrop PA, Everaerd W, Kindt M, Mulder BJM. Psychological and cognitive functioning in children and adolescents with congenital heart disease: A meta-analysis. J Pediatr Psychol. 2007;32:527–541. doi: 10.1093/jpepsy/jsl047. [DOI] [PubMed] [Google Scholar]
- 19.Schaefer C, von Rhein M, Knirsch W, Huber R, Natalucci G, Caflisch J, et al. Neurodevelopmental outcome, psychological adjustment, and quality of life in adolescents with congenital heart disease. Dev Med Child Neurol. 2013;55:1143–1149. doi: 10.1111/dmcn.12242. [DOI] [PubMed] [Google Scholar]
- 20.Mulkey SB, Swearingen CJ, Melguizo MS, Reeves R, Rowell JA, Gibson N, et al. Academic Proficiency in Children After Early Congenital Heart Disease Surgery. Pediatr Cardiol. 2014;35:344–352. doi: 10.1007/s00246-013-0781-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahle WT, Clancy RR, Moss EM, Gerdes M, Jobes DR, Wernovsky G. Neurodevelopmental outcome and lifestyle assessment in school-aged and adolescent children with hypoplastic left heart syndrome. Pediatrics. 2000;105:1082–1089. doi: 10.1542/peds.105.5.1082. [DOI] [PubMed] [Google Scholar]
- 22.Calderon J, Bonnet D, Courtin C, Concordet S, Plumet M-H, Angeard N. Executive function and theory of mind in school-aged children after neonatal corrective cardiac surgery for transposition of the great arteries. Dev Med Child Neurol. 2010;52:1139–1144. doi: 10.1111/j.1469-8749.2010.03735.x. [DOI] [PubMed] [Google Scholar]
- 23.Cassidy AR, White MT, DeMaso DR, Newburger JW, Bellinger DC. Executive Function in Children and Adolescents with Critical Cyonotic Congenital Heart Disease. J Int Neuropsychol Soc. 2015;20:34–49. doi: 10.1017/S1355617714001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerstle M, Beebe DW, Drotar D, Cassedy A, Marino BS. Executive Functioning and School Performance among Pediatric Survivors of Complex Congenital Heart Disease. J Pediatr. 2016 doi: 10.1016/j.jpeds.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calderon J. Executive Function in Patients with Congenital Heart Disease: Only the Tip of the Iceberg? The Journal of Pediatrics. 2016 doi: 10.1016/j.jpeds.2016.02.066. [DOI] [PubMed] [Google Scholar]
- 26.Gioia GA, Isquith PK, Guy SC. Assessment of executive functions in children with neurological impairment. In: Simeonsson RJ, Rosenthal SL, editors. Psychological and Developmental Assessment: Children with Disabilities and Chronic Conditions. New York: Guilford Press; 2001. pp. 317–356. [Google Scholar]
- 27.Mahone EM, Hagelthorn KM, Cutting LE, Schuerholz LJ, Pelletier SF, Rawlins C, et al. Effects of IQ on Executive Function Measures in Children with ADHD. Child Neuropsychol. 2002;8:52–65. doi: 10.1076/chin.8.1.52.8719. [DOI] [PubMed] [Google Scholar]
- 28.Affra S. The relationship of intelligence to executive function and non-executive function measures in a sample of average, above average, and gifted youth. Arch Clin Neuropsychol. 2007;22:969–978. doi: 10.1016/j.acn.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Alloway TP, Alloway RG. Investigating the predictive roles of working memory and IQ in academic attainment. J Exp Child Psychol. 2010;106:20–29. doi: 10.1016/j.jecp.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Diamond A. Executive functions. Annu Rev Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavioral Rating Inventory of Executive Function (BRIEF) Florida: Psychological Assessment Resources; 2000. [Google Scholar]
- 33.Clancy RR, McGaurn SA, Wernovsky G, Spray TL, Norwood WI, Jacobs ML, et al. Preoperative risk-of-death prediction model in heart surgery with deep hypothermic circulatory arrest in the neonate. J Thorac Cardiovasc Surg. 2000;119:347–357. doi: 10.1016/S0022-5223(00)70191-7. [DOI] [PubMed] [Google Scholar]
- 34.Limperopoulos C, Tworetzky W, McElhinney DB, Newburger JW, Brown DW, Robertson RL, et al. Brain volume and metabolism in fetuses with congenital heart disease: Evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121:26–33. doi: 10.1161/CIRCULATIONAHA.109.865568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aarnoudse-Moens CSH, Smidts DP, Oosterlaan J, Duivenvoorden HJ, Weisglas-Kuperus N. Executive Function in Very Preterm Children at Early School Age. J Abnorm Child Psychol. 2009;37:981–993. doi: 10.1007/s10802-009-9327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edgin JO, Inder TE, Anderson PJ, Clark CA, Woodward LJ. Executive functioning in preschool children born very preterm: Relationship with early white matter pathology. J Int Neuropsychol Soc. 2008;14:90–101. doi: 10.1017/S1355617708080053. [DOI] [PubMed] [Google Scholar]
- 37.Mulder H, Pitchford NJ, Hagger MS, Marlow N. Development of Executive Function and Attention in Preterm Children: A Systematic Review. Dev Neuropsychol. 2009;34:393–421. doi: 10.1080/87565640902964524. [DOI] [PubMed] [Google Scholar]
- 38.Majnemer A, Limperopoulos C, Shevell M, Rohlicek C, Rosenblatt B, Tchervenkov C. Gender differences in the developmental outcomes of children with congenital cardiac defects. Cardiol Young. 2012;22:514–519. doi: 10.1017/S1047951111002071. [DOI] [PubMed] [Google Scholar]
- 39.Johnston MV, Hagberg H. Sex and the pathogenesis of cerebral palsy. Dev Med Child Neurol. 2007;49:74–78. doi: 10.1017/s0012162207000199.x. [DOI] [PubMed] [Google Scholar]
- 40.Du L, Bayir H, Lai Y, Zhang X, Kochanek PM, Watkins SC, et al. Innate Gender-based Proclivity in Response to Cytotoxicity and Programmed Cell Death Pathway. J Biol Chem. 2004;279:38563–38570. doi: 10.1074/jbc.M405461200. [DOI] [PubMed] [Google Scholar]
- 41.Hilton GD, Ndubuizu AN, McCarthy MM. Neuroprotective effects of estradiol in newborn female rat hippocampus. Dev Brain Res. 2004;150:191–198. doi: 10.1016/j.devbrainres.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Anderson P, Sleeper L, Mahony L, Colan SD, Atz AM, Breitbart RE, et al. Contemporary outcomes after the Fontan procedure: a Pediatric Heart Network multicenter study. J Am Coll Cardiol. 2008;52:85–98. doi: 10.1016/j.jacc.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atz A, Zak V, Mahony L, Uzark K, Shrader P, Gallaher D, et al. Survival data and predictors of functional outcome an average of 15 years after the fontan procedure: the pediatric heart network fontan cohort. Congenit Heart Dis. 2015;10:E30–E42. doi: 10.1111/chd.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forbess JM, Visconti KJ, Hancock-Friesen C, Howe RC, Bellinger DC, Jonas RA. Neurodevelopmental outcome after congenital heart surgery: Results from an institutional registry. Circulation. 2002;106:I–95–I–102. [PubMed] [Google Scholar]
- 45.Wernovsky G, Stiles KM, Gauvreau K, Gentles TL, DuPlessis AJ, Bellinger DC, et al. Cognitive development after the fontan operation. Circulation. 2000;102:883–889. doi: 10.1161/01.cir.102.8.883. [DOI] [PubMed] [Google Scholar]
- 46.Brosig Soto C, Olude O, Hoffmann RG, Bear L, Chin A, Dasgupta M, Mussatto K. Implementation of a Routine Developmental Follow-up Program for Children with Congenital Heart Disease: Early Results. Congenit Heart Dis. 2011;6:451–460. doi: 10.1111/j.1747-0803.2011.00546.x. [DOI] [PubMed] [Google Scholar]
- 47.Toplak ME, West RF, Stanovich KE. Practitioner Review: Do performance-based measures and ratings of executive function assess the same construct?: Performance-based and rating measures of EF. J Child Psychol Psychiat. 2013;54:131–143. doi: 10.1111/jcpp.12001. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Horst KK, Kronenberger WG, Hummer TA, Mosier KM, Kalnin AJ, et al. White matter abnormalities associated with disruptive behavior disorder in adolescents with and without attention-deficit/hyperactivity disorder. Psychiatry Res: Neuroimaging. 2012;202:245–251. doi: 10.1016/j.pscychresns.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Warren SL, Crocker LD, Spielberg JM, Engels AS, Banich MT, Sutton BP, et al. Cortical organization of inhibition-related functions and modulation by psychopathology. Front Hum Neurosci. 2013;7:271. doi: 10.3389/fnhum.2013.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ziegler G, Dahnke R, Winkler AD, Gaser C. Partial least squares correlation of multivariate cognitive abilities and local brain structure in children and adolescents. Neuroimage. 2013;82:284–294. doi: 10.1016/j.neuroimage.2013.05.088. [DOI] [PubMed] [Google Scholar]


