Abstract
Due to the degradation of osteoarthritic (OA) cartilage in post-traumatic OA (PTOA), these tissues are challenging to study and manipulate in vitro. In this study, chondrocytes isolated from either PTOA (meniscal-release (MR) model) or normal (contralateral limb) cartilage of canine knee joints were used to form micropellets to assess the maintenance of the OA chondrocyte phenotype in vitro. Media samples from the micropellet cultures were used to measure matrix metalloproteinase (MMP), chemokine, and cytokine concentrations. Significant differences in matrix synthesis were observed as a function of disease with OA chondrocytes generally synthesizing more extracellular matrix with increasing time in culture. No donor dependent differences were detected. Luminex multiplex analysis of pellet culture media showed disease and time-dependent differences in interleukin (IL)-8, keratinocyte chemoattractant (KC)-like protein, MMP-1, MMP-2, and MMP-3, which are differentially expressed in OA. This memory of their diseased phenotype persists for the first 2 weeks of culture. These results demonstrate the potential to use chondrocytes from an animal model of OA to study phenotype alterations during the progression and treatment of OA.
Keywords: osteoarthritis, tissue engineering, cartilage, chondrocyte, animal model
Post-traumatic osteoarthritis (PTOA) often develops after injury to diarthrodial joints, such as the knee. To gain greater insights to the etiology and progression of cartilage damage in PTOA, researchers have adopted a number of in vivo and in vitro models that can successfully mimic aspects of the disease. Animal models of PTOA, including surgical destabilization of the knee joint, allow the researcher to address the full spectrum of pathologic changes observed in clinical PTOA.1–3 Specifically, in the meniscal-release (MR) model, development of osteoarthritic-like conditions is accelerated, and can be reproducibly generated over the period of a few months rather than years (as in humans). Further, a canine animal model provides for assessment in a clinically relevant species in which the anatomy, physiology, and biomechanics of the knee joint, as well as medical, surgical, and post-operative management strategies are similar to humans.4–6
In vitro culture provides opportunities for studying the biologic behavior of OA tissue and cells under more controlled conditions than in vivo. In this context, tissue explants offer ideal culture models as they maintain chondrocytes in their native extracellular matrix. The feasibility of culturing human OA cartilage explants, however, depends on the severity of OA, where possibly only irregular and fragile fragments and slivers of tissue may be harvestable. Therefore, the ability to isolate and expand chondrocytes from limited tissue quantities and subsequently culture them under conditions that maintain their OA phenotype is desirable.
Tissue engineering culture models can provide a platform to study diseased chondrocytes. Traditional tissue engineering approaches for disease modeling include mechanical overload7,8 or cytokine insult9,10 of healthy chondrocytes in 2D or 3D culture. Patient-specific culture models have been developed using cells derived from an individual’s cartilage (healthy or OA) or stem cells differentiated toward the chondrocyte phenotype. Mesenchymal stem cells may be derived from healthy or OA sources including bone marrow, adipose tissue, or synovium, as well as from iPSCs to produce disease-specific models of OA.11–15 However, these methods require the differentiation of cells to a chondrogenic phenotype. Studies utilizing OA chondrocytes may have an advantage as they are derived directly from the pathologic joint.
In the current study, our model system outlines the derivation of cells from pathologic cartilage with a well-defined history of joint injury, canine knee joints that have undergone MR surgery and subsequent development of PTOA, and use of these cells in a 3D tissue engineering culture model. Pellet culture has long been used to optimize culture conditions prior to the use of 3D hydrogels and provides analogous results.16 While previous research has characterized the behavior of human OA chondrocytes under numerous culture conditions, many of these studies do not provide a normal chondrocyte comparison,17 are limited by statistical matching power (age and gender)15,18 or use cells with age-related loss of chondrogenic capacity.18,19 Due to these differences, the reported synthesis capabilities of OA chondrocytes compared to healthy chondrocytes has been varied, with some groups reporting reduced20,21 and others reporting comparable or increased levels of matrix synthesis15,18 in both 2D and 3D culture.
This study aims to characterize the persistence of the disease phenotype in long-term culture (up to 4 weeks) using a well-established micropellet culture system. The maintenance of the OA phenotype is determined by micropellet production of de novo proteoglycan and collagen production as well as via micropellet release into the media of OA chemokines and cytokines observed previously to be elevated in the synovial fluid of dogs post-MR. As studies of OA cells in the literature are often limited to gene expression, this study, using an established 3D tissue engineering culture model with protein analysis and immunoanalysis, addresses a knowledge gap in our understanding of OA phenotype stability in culture. Moreover, insights gained from this study may help to establish a more biomimetic research platform for the study of OA through the incorporation of OA cells.
MATERIALS AND METHODS
Induction of Canine OA
MR surgery was performed on the knees of six adult research bred hound dogs (5 males, 1 female, 1-year-old (13–16 m), >20 kg) as described previously22 (Fig. 1). The procedure was approved by the University of Missouri’s Institutional Animal Care and Use Committee (IACUC #7332). This PTOA model is created using arthroscopic guidance and instrumentation so as to avoid the known joint health effects of open arthrotomy.23 Dogs were pre-medicated intramuscularly (IM) with xylazine (0.5 mg/kg), morphine (0.5 mg/kg), and glyco-pyrrolate (0.005 mg/kg); induced intravenously (IV) with propofol (6 mg/kg); intubated and maintained with isoflurane (1–2.5%) for the duration of the surgery based on veterinary medicine standard of care. After surgery, heart rate (HR), respiratory rate (RR), and rectal temperature were monitored until the dog was considered stable, alert, and sternal. Pain was assessed by monitoring HR, RR, the patient’s attitude and vocalizations, appetite, reaction to palpation of the surgical site and recorded for appropriate decision making and pain-relieving measures.
Figure 1.
Gross images of six donor canine knees 12 weeks post-surgery and prior to cartilage sample harvesting.
All dogs were assessed at least twice daily (morning and afternoon/evening) for the first 72 hours post-operatively. Dogs received two post-operative doses of morphine (0.5 mg/ kg IM or SQ). Each dose of morphine was given within 6 hours of the preceding dose (including the pre-operative dose). Tramadol (2–4 mg/kg bid) was started the evening of surgery, no longer than 6 h following the second postoperative dose of morphine. Two doses in total were given with approximately 12 hours between the first dose and second dose of Tramadol. Assessment of pain was performed and recorded immediately prior to administration of each dose of the analgesic agents. The dogs were housed in an AAALAC approved facility in 18–25 square foot cages (64–72°F) with one dog per cage. The dogs were given a 12-hours light/12-hours dark cycle, unlimited water from spout, dry dog food once a day and Nylabone Chews and Kong Dog Toys in the cage for enrichment.
After 12 weeks, the dogs were euthanized and the cartilage was grossly examined for OA changes (Fig. 1). No adverse events were noted. As in our previous work with the canine MR model, insult to the joint via radial transection of the medial meniscus resulted in primarily unicompartmental (medial) disease. This was characterized by cartilage erosion and degradation due to the changes in femoral and tibial articular cartilage contact pressures and areas by 12 weeks. Associated synovitis, effusion, and clinical signs of slowly progressive lameness have also been noted.22
Tissue Harvest and Isolation of Chondrocytes
Cartilage slivers were obtained from the limbs of dogs (Dog 219, 220, 221, 223, 224, 249) 12 weeks post-MR surgery for induction of OA with contralateral limbs serving as normal controls (Fig. 1). Tissues were enzymatically digested using collagenase type IV (Worthington Biochemical Corporation, Carlsbad, CA) for 8 h with stirring at 37°C after which cell suspensions were filtered through a 70 μm porous mesh and sedimented by centrifugation.5 Viable cells were counted and plated at 20 × 103 cells/cm2.
Creation and Culture of Micropellets
After two passages in Dulbecco’s Modified Eagle’s Media (DMEM, Life Technologies, Carlsbad, CA) containing 10% fetal bovine serum (FBS, Atlanta Biologicals, Norcross, GA), 1% antibiotic–antimycotic (Invitrogen Corporation, Carlsbad, CA), and a growth factor expansion cocktail (1 ng/ml TGF-β1, 5 ng/ml bFGF-2, and 10 ng/ml PDGF-ββ) (Invitrogen Corporation),24,25 confluent chondrocytes were trypsinized, counted and re-suspended.5 Chondrocytes from dogs 219, 223, and 249 were pooled by disease condition, normal (non-operated) or OA (MR surgery) (similar to previously utilized bovine cell culture protocols5,17,26), while chondrocytes from 220, 221, and 224 remained separated by dog and disease state to study effects of donor variability. Briefly, 1 ml of a 500,000 cells/ml suspension was aliquoted into 1.5 ml sterile screw-top tubes and pelleted by centrifugation. Individual micropellets were cultured for 28 days with media changes every 2–3 days in 1 ml of chondrogenic medium comprised of DMEM containing 50 μg/ml l-proline (Sigma–Aldrich, St. Louis, MO), 100 μg/ml sodium pyruvate (Sigma–Aldrich), 1% ITS +premix (BD Biosciences, San Jose, CA), 100 nM dexamethasone (Sigma–Aldrich), 1% antibiotic–antimycotic, (Invitrogen), 50 μg/ml ascorbic acid (Sigma–Aldrich), and continuously supplemented with 10 ng/ml transforming growth factor-beta-3 (TGF-β3, R&D Systems, Minneapolis, MN).5 Media samples were collected at each feeding (n =5). Micropellets were harvested, weighed and frozen at −20°C at days 0, 14, and 28 for biochemical analysis (n =5), or fixed in acid formalin ethanol for histology and immunohistochemistry (n =2).
Biochemical Analysis
Frozen samples were lyophilized, and the dry samples were digested in 0.5 mg/ml of proteinase K (MP Biomedicals, Santa Ana, CA) in 50 mM Tris buffered saline containing 1 mM EDTA, 1 mM iodoacetamide, and 10 μg/ml pepstatin A for 16 h at 56°C.27 Aliquots were analyzed for DNA, glycos-aminoglycan (GAG), and collagen (COL) using the picogreen (Invitrogen), 1,9 dimethylmethylene (DMMB) (Sigma–Aldrich) dye-binding,28 and orthohydroxyproline (OHP) assays. In the OHP assay, the proteinase K digested sample was hydrolyzed with 12 N HCl at 110°C overnight, dried, and re-suspended in assay buffer. OHP content was determined using a colorimetric assay in which chloramine T was reacted with dimethylaminobenzaldehyde and quantified using a 1:7.64 OHP-to-collagen mass ratio.29 Micropellet culture media were also assayed with the DMMB blue dye-binding assay to determine GAG lost to the media. Total GAG was determined as the sum of micropellet GAG at each time point and accumulated media GAG. GAG, total GAG, and collagen contents were each normalized to micropellet DNA.
Histological and Immunohistochemical Analysis
Acid-formalin ethanol fixed samples were embedded in paraffin wax (Fisher Scientific, Waltham, MA) and sectioned to a thickness of 8 μm. Sections were subsequently stained with alcian blue (pH =1.0, Sigma–Aldrich) and picrosirius red (Sigma–Aldrich) to determine GAG and collagen distribution, respectively. Immunohistochemistry was performed to determine the distribution of collagen Type II (rabbit polyclonal anti-collagen type II, EMD Millipore, Billerica, MA.30 Distribution of the staining intensity through the center (horizontal diameter) of each sample was analyzed in ImageJ (NIH). Grayscale values were normalized to the maximum grayscale value across the cross-section.
Media Chemokine and Cytokine Analysis
A 25 μl aliquot was taken from each of the media samples from pooled donor pellet groups at days 0, 14, and 28 for chemokine and cytokine analysis (n =5). Each aliquot was analyzed in duplicate using a Luminex multiplex canine cytokine and chemokine immunoassay (Millipore Corporation, St. Louis, MO) on the xMAP platform (Qiagen Inc, Valencia, CA) for interleukin (IL)-6, IL-8, keratinocyte derived chemoattractant (KC)-like protein, and Monocyte Chemoattractant Protein-1 (MCP-1).31 A multiplex human MMP immuoassay (R&D Systems) based on the xMAP assay platform which has been previously shown to react with canine samples,32 was used to analyze the media samples for five matrix metalloproteinases (MMP): MMP-1, MMP-2, MMP-3, MMP-9, and MMP-13 as described in ref.31 For the xMAP assays, media samples were mixed with antichemokine, anticytokine, or anti-MMP monoclonal antibody-charged 5.6 μm polystyrene microspheres. Streptavidin-phycoerythrin and a biotinylated polyclonal secondary antibody were added following overnight incubation at 4°C with the polystyrene microspheres.31,32 The median fluorescence intensity was used to determine the concentration (pg/ml) of each chemokine and cytokine.
Statistics
A two-way ANOVA with repeated measures with Fisher’s Least Significance Difference post hoc tests (α =0.05) was performed with main factors set as time and disease condition, and donor as the repeated measure. Biochemical properties served as the dependent variable. A separate set of two-way ANOVA tests with main factors set as time and disease state were used for chemokine and cytokine data. Data are reported as the mean ± standard deviation of 4–5 samples per time point and group. All statistical tests were performed in Statistica (Tulsa, OK).
RESULTS
Effects of Disease State and Donor on ECM Synthesis
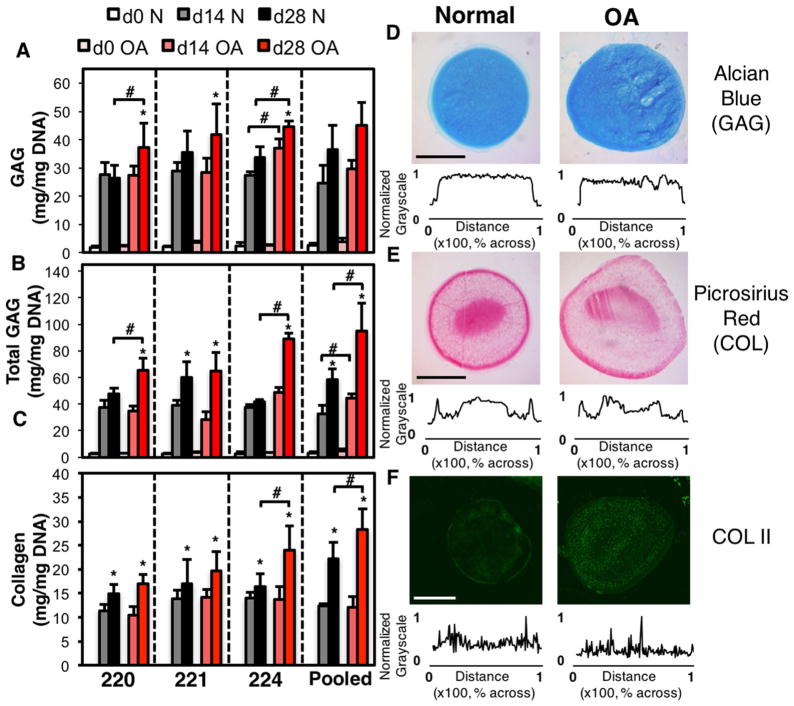
The average cell yield was 172 × 103±85×103 cells/gram of tissue. Two passages led to a ~200-fold increase in cell number during expansion. There were no observed differences in the DNA content of the chondrocyte pellets, regardless of time, donor, or disease state (not shown, p =0.46). Accordingly, pellet extracellular matrix synthesis was normalized by DNA content to identify biosynthesis changes. Across all biochemical analyses, there were no statistical differences between donors within the same disease state. Pellet GAG was significantly different with respect to disease (p =0.004), time (p =0.0001), and as a function of time and disease (p =0.009). No significant differences were detected as a function of donor. More specifically, by day 28, pellet GAG was significantly greater in OA pellets from dog 220 (p =0.01), and 224 (p =0.001) and with trending increases in pellets synthesized by dog 221 (p =0.15) and pooled dogs (p =0.23) compared to normal chondrocyte pellets (Fig. 2A). Total pellet GAG was significantly different between disease states (p =0.005), time (0.0001), time and disease (p =0.0001) and donor and disease (p =0.032) at day 28. Thus, while there are no intra-disease differences between donors alone there exist disease-dependent differences in total GAG synthesis. At day 28, total GAG synthesis was significantly greater in OA pellets from dog 220 (p =0.003), dog 224 (p =0.0002), and pooled dogs (p =0.03) (Fig. 2B). Overall, pellet collagen was only significant with respect to disease (p =0.03) and time (p =0.005). By day 28, collagen content was significantly higher in OA chondrocyte pellets synthesized by dog 224 (p =0.03) and pooled groups (p =0.04) and trending toward increases in pellets synthesized by dog 220 (p =0.14) compared to normal controls for each dog (Fig. 2C).
Figure 2.
Pellet GAG (A), total GAG (sum of pellet GAG and media GAG) and pellet collagen (C) normalized by DNA content as a function of donor and culture time. All groups significant to day 0. Representative histological and immunohistological staining for pooled donor chondrocyte pellets. Alcian blue (GAG) (D), picrosirius red (collagen) (E) and collagen type II (F) staining of pellets formed from normal and osteoarthritis chondrocytes and cultured for 28 days. Graphs show grayscale intensity across the diameter normalized to the maximum intensity of the histological section. *p <0.05 versus day 14, #p <0.05 versus normal. All groups significant to day 0. n =4–6 Scale bar =1 mm.
The results of the biochemical data were supported by histology and immunohistochemistry. After 28 days of culture, alcian blue staining of pellets for GAG was relatively uniform across all dog donors and disease states (Fig. 2D). Picrosirius red pellets had a dense ring of collagen staining around the edges with locally intense staining in the center of pellets prepared from the pooled dogs (Fig. 2E). Collagen II staining was observed throughout pellets synthesized by normal and OA chondrocytes. Brighter immunostaining of collagen II was observed for OA pellets from pooled donors (Fig. 2F).
Chemokine and Cytokine Expression Profiles Vary With Culture Duration and Disease State
MMP, chemokine, and cytokine expression levels were evaluated in media from pooled donor pellets at days 0, 14, and 28. Concentration of MMP-1 and MMP-3 increased with time in culture for both normal (pMMP1 <0.00001, pMMP3 < 0.00001) and OA chondrocyte pellets (pMMP1 = 0.01, pMMP3 < 0.00001) (Fig. 3A and C). Conversely, MMP-2 concentrations decreased with time in culture (pN = 0.002, pOA < 0.00001) (Fig. 1B). MMP-9 levels remained constant during pellet culture for normal chondrocytes, but decreased with culture time for OA pellets (p <0.001) (Fig. 3D). Media concentration of MMP-13 remained constant (Fig. 3E). While trends were mostly similar between the two groups, expression of MMP1 (p0 = 0.02, p14 < 1 × 10−5, p28 = 0.01) (Fig. 3A) and MMP-2 (p0 < 1 × 10−5, p14 = 1 × 10−5, p28 = 0.01) (Fig. 3B) were significantly elevated in OA pellet media at all time points. MMP-9 media expression was significantly decreased in OA pellets only at day 28 (p =0.01) (Fig. 1D). MMP-3 concentrations were slightly elevated in OA pellets compared to their normal counterparts at days 14 and 28 (p14 = 0.17, p28 = 0.21) (Fig. 3C). No differences were observed between disease states for MMP-13 pellets (p =0.64, Fig. 3E).
Figure 3.

MMP concentrations from culture media of pooled donor pellets synthesized from normal or osteoarthritic chondrocytes. *p <0.05 versus Day 0, **p <0.05 versus Day 14, #p <0.05 versus normal. n =5.
No significant differences were observed as a function of culture time or disease state in the media concentration of IL-6 (p =0.57, Fig. 4A). Concentrations of IL-8 (Fig. 4B), KC (Fig. 4C) and MCP-1 (Fig. 4D) (pIL8-N = 2 × 10−6, pIL8-OA = 1 × 10−6 pKC-N < 1 × 1035, pKC-OA < 1 × 10−5, pMCP1-N = 0.0004, pMCP1-OA = 0.009) all significantly decreased with culture time. Meanwhile, the concentration of KC was significantly elevated in normal pellet groups at days 0 and 14 (p0=2 ×10−6, p14 < 1 × 10−6), but was significantly lower in OA pellet groups by day 28 (p <1 ×10−5) (Fig. 4C). Finally, MCP-1 levels were slightly elevated in OA groups at day 14 (p14 = 0.10) (Fig. 4D).
Figure 4.
Chemokine and cytokine concentrations from culture media of pooled donor pellets synthesized from normal or osteoarthritic chondrocytes. *p <0.05 versus Day 0, **p <0.05 versus Day 14, #p <0.05 versus normal. n =5.
DISCUSSION
Biochemical analysis of the cartilage micropellets revealed no differences among donors for pellets fabricated from either normal or OA chondrocytes. Overall, pellet GAG, total GAG, and pellet collagen was significantly increased with time in culture and more elevated in OA chondrocyte pellets (Fig. 2). Specifically, the biochemical composition between pellets from normal and OA chondrocytes became significantly different at day 28, suggesting that an underlying difference between the chondrocytes derived from healthy and pathologic tissue exists in our 3D culture system with time. Interestingly, we observed a greater ECM content for the pooled pellet group relative to the ECM content of each individual donor that was pooled. This finding may suggest an interactive effect between cells of different donors. We have previously noted a similar effect of pooling for canine chondrocyte-seeded agarose constructs.5 These results support previous reports that OA chondrocytes can have an increased metabolic activity due to cellular attempts to balance of catabolic and anabolic pathways, even though the disease is characterized by a loss of ECM.33,34
The differences between normal and OA chondrocytes are also captured by the time-dependent changes of key chemokines, cytokines, and matrix metalloproteinases in the micropellet culture media. Previously, through analysis of the synovial fluid of naturally occurring canine OA and induced canine OA, we have identified IL-8 as highly sensitive and MCP-1 as highly specific biomarkers in canine OA. Analysis of canine synovial fluid 12 weeks post-MR surgery showed a trending increase in MCP-1 expression and a significant increase in IL-8 expression compared to baseline and non-operated contralateral controls.31 A similar evaluation of dogs found that in naturally occurring (not surgically induced) canine OA, MCP-1 was significantly higher pre-surgery (lavage and stabilization of the cruciate ligament) compared to post-surgery and normal dogs. Similarly, IL-8 and KC were significantly higher pre-surgery compared to normal dogs and decreased with surgery (although not significantly). While MMP expression was below the resolution of the assay in the induced canine OA synovial fluid aspirate, evaluation of dogs with naturally occurring OA had no changes in MMP-2 and MMP-3 synovial fluid expression before and after surgery and compared to healthy dogs.31
In this study, culture media profiles from OA chondrocyte micropellets paralleled a subset of the synovial fluid markers measured in spontaneously occurring OA and MR dogs.31 Significantly increased expression of MMP-1 and MMP-2 was observed in OA micropellet culture media at all time points. Elevated MMP-3 concentrations were detected at days 14 and 28. OA research has included investigation into a number of different MMPs, including MMP-1, MMP-2, and MMP-3 which target collagen, gelatin fragments, and proteoglycans, respectively.15,35 These MMPs are typically up-regulated in early stage OA and post-traumatic OA.34,36–38 Additionally, these markers are highly expressed by OA and cytokine stimulated healthy chondrocytes in 2D and 3D cell culture.15,39,40 Observed increases in MMP-3 expression are closely linked to elevated expression of MCP-1 and IL-8.41,42 In this study, increased MMP-1, -2, and -3 expression resulted in increased loss of GAG to the media. The observed deviations from the MR animal model may be exacerbated by in vitro culture conditions. There were no observed differences in MMP-13 expression detected in the micropellet media, suggesting that both normal and OA chondrocytes did not undergo hypertrophic differentiation during time in culture.43 This may also be the result of a loss of MMP-13 expression during cell expansion in 2D as this has been observed by others.44,45 In previous canine studies, no measurable amounts of MMP-9 or MMP-13 were detected in synovial fluid samples.
Similar to the results of previous canine OA bio-marker studies, the results of this study identified time-dependent differences in IL-8, KC, and MCP-1 media concentrations. IL-8, a neutrophil chemoattractant, is elevated in rheumatoid arthritis and constitutively expressed by OA synoviocytes.46,47 Expression of IL-8 in OA chondrocytes can be regulated by cytokines in vitro and induce chondrocyte hypertrophy, which is commonly seen in OA.48 Similarly, MCP-1 attracts mononuclear cells such as monocytes and memory T cells and is frequently associated with rheumatoid arthritis. Studies have shown that MCP-1 expression is elevated by OA chondrocytes and can be regulated by IL-1β in vitro.41,46 More recently, these markers, in addition to MMP-1 and IL-6, were confirmed as biomarkers for OA in human patients undergoing knee arthroplasty.37 Likewise, our in vitro model exhibits higher media concentrations of both IL-8 at day 0 and significantly greater concentrations of IL-8 at day 14 with elevated levels of MCP-1.31 The delayed increase in MCP-1 expression may be related to the clinically observed positive correlation between MCP-1 and IL-8 expression.37 At day 28, no differences in media concentrations of either IL-8 or MCP-1 are detected between normal and OA micropellet cultures.
Like IL-8, KC is a murine protein and a member of the CXC cytokines family and is a neutrophil chemoattractant. In the dog, mouse antibodies against KC are capable of detecting a KC-like proteins in fluids such as synovial fluid.31 Up-regulation of KC may also encourage chondrocyte hypertrophy.48 Clinical results have demonstrated that variations in KC expression may be useful for identifying differences between cruciate disease and other types of OA, as changes were only observed in dogs suffering from OA after ACL transection.31 Here, we observed decreased expression of KC in OA chondrocyte pellets at day 0, but significantly elevated levels at day 14 with no differences by day 28. This result suggests that in our in vitro model, KC expression is regulated by related factors such as IL-8, as they show similar expression trends.
Using chondrocytes isolated from pathologic cartilage harvested from an animal model of OA, as done in this study, decouples donor age and gender from disease specific results. Unlike human studies, the canine animal model allows for a donor matched healthy tissue control, the contralateral limb. Further, by using chondrocytes isolated from an animal model of OA, we are able to reduce the effects of donor to donor variability that are frequently observed in OA chondrocytes obtained from human clinical samples. The ability to control these variables allows us to design studies that look at cellular alterations in OA relatively free of contaminating variables. Using cells derived from animal models also allows for the flexibility to study OA pathology as a function of age as OA can be induced in juvenile or adult animals. Although animal models may not fully capture the naturally occurring disease states, these options may provide insights on early intervention strategies for mitigating the effects of OA.
Taken together, our OA chondrocyte pellet data indicate that for at least a period of 2 weeks in 3D cell culture, OA chondrocytes exhibit a memory of their disease phenotype. These findings are encouraging and consistent with the literature findings showing increased and correlated synovial fluid expression of MMP-1, IL-8, KC, and MCP-1 in human and canine OA.31,37 These results may further suggest that epigenetic changes in the diseased joint promote memory of the OA phenotype for at least 2 weeks, even after cells have been isolated from the OA tissue. Accordingly, as this OA phenotype wanes with increasing time in culture, there may be techniques available to preserve the disease phenotype, including growth factor treatment for the minimization of de-differentiation,17,49 stimulation with mechanical loading50,51 or oxygen tension,44,52 administration of pro-inflammatory cytokines,15,39 and co-culture with synovium53,54 or immune cells.34 This preliminary work provides a baseline from which more optimal protocols may be developed to preserve the OA phenotype in culture, for studies of cellular changes associated with PTOA, or perhaps to convert the cells to a more “normal” phenotype as a cell source for therapeutic applications.18,55
Acknowledgments
Research reported in this publication was supported by NIAMS, NCRR, and NIBIB of the National Institutes of Health under Award Numbers AR060361, AR059038, 1S10RR027943, and 5P41EB002520. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
AUTHORS’ CONTRIBUTIONS
All authors contributed significantly to the work reported in this manuscript. AMS and CTH designed the experiments.
AMS and AMS carried out the experiments and collected the data. AMS, GAA, JCB, JLC, and CTH supervised and advised the work. AMS, AMS, GAA, JCB, JLC, and CTH wrote the paper. All authors have read and approved the final submitted manuscript.
References
- 1.Gregory MH, Capito N, Kuroki K, et al. A review of translational animal models for knee osteoarthritis. Arthritis. 2012;2012:764621. doi: 10.1155/2012/764621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marshall K, Chan A. Arthroscopic anterior cruciate ligament transection induces canine osteoarthritis. J Rheum. 1996;23:338–343. [PubMed] [Google Scholar]
- 3.Kuroki K, Cook CR, Cook JL. Subchondral bone changes in three different canine models of osteoarthritis. Osteoarthritis Cartilage. 2011;19:1142–1149. doi: 10.1016/j.joca.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Altman RD, Tenenbaum J, Latta L, et al. Biomechanical and biochemical properties of dog cartilage in experimentally induced osteoarthritis. Ann Rheum Dis. 1984;43:83–90. doi: 10.1136/ard.43.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng KW, Lima EG, Bian L, et al. Passaged adult chondrocytes can form engineered cartilage with functional mechanical properties: a canine model. Tissue Eng Part A. 2010;16:1041–1051. doi: 10.1089/ten.tea.2009.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pond MJ, Nuki G. Experimentally-induced osteoarthritis in the dog. Ann Rheum Dis. 1973;32:387–388. doi: 10.1136/ard.32.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan AR, Dong EY, Ateshian GA, et al. Response of engineered cartilage to mechanical insult depends on construct maturity. Osteoarthritis Cartilage. 2010;18:1577–1585. doi: 10.1016/j.joca.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patwari P, Fay J, Cook MN, et al. In vitro models for investigation of the effects of acute mechanical injury on cartilage. Clin Orthop Relat Res. 2001;391(Suppl):S61–S71. doi: 10.1097/00003086-200110001-00007. [DOI] [PubMed] [Google Scholar]
- 9.Kuroki K, Stoker AM, Cook JL. Effects of proinflammatory cytokines on canine articular chondrocytes in a three-dimensional culture. Am J Vet Res. 2005;66:1187–1196. doi: 10.2460/ajvr.2005.66.1187. [DOI] [PubMed] [Google Scholar]
- 10.Goldring MB, Berenbaum F. The regulation of chondrocyte function by proinflammatory mediators: prostaglandins and nitric oxide. Clin Orthop Relat Res. 2004;427(Suppl):S37–S46. doi: 10.1097/01.blo.0000144484.69656.e4. [DOI] [PubMed] [Google Scholar]
- 11.Willard VP, Diekman BO, Sanchez-Adams J, et al. Use of cartilage derived from murine induced pluripotent stem cells for osteoarthritis drug screening. Arthritis Rheum. 2014;66:3062–3072. doi: 10.1002/art.38780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sampat SR, O’Connell G, Fong JV, et al. Growth factor priming of synovium derived stem cells for cartilage tissue engineering. Tissue Eng Part A. 2011;17:2259–2265. doi: 10.1089/ten.tea.2011.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diekman BO, Rowland CR, Lennon DP, et al. Chondrogenesis of adult stem cells from adipose tissue and bone marrow: induction by growth factors and cartilage-derived matrix. Tissue Eng Part A. 2010;16:523–533. doi: 10.1089/ten.tea.2009.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy JM, Dixon K, Beck S, et al. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002;46:704–713. doi: 10.1002/art.10118. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Davison N, Moroni L, et al. Evaluating osteoarthritic chondrocytes through a novel 3-dimensional in vitro system for cartilage tissue engineering and regeneration. Cartilage. 2012;3:128–128. doi: 10.1177/1947603511429698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Connell GD, Tan AR, Cui V, et al. Human chondrocyte migration behaviour to guide the development of engineered cartilage. J Tissue Eng Regen Med. 2015 doi: 10.1002/term.1988. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsieh-Bonassera ND, Wu I, Lin JK, et al. Expansion and redifferentiation of chondrocytes from osteoarthritic cartilage: cells for human cartilage tissue engineering. Tissue Eng Part A. 2009;15:3513–3523. doi: 10.1089/ten.tea.2008.0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dehne T, Karlsson C, Ringe J, et al. Chondrogenic differentiation potential of osteoarthritic chondrocytes and their possible use in matrix-associated autologous chondrocyte transplantation. Arthritis Res Ther. 2009;11:R133. doi: 10.1186/ar2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barbero A, Grogan S, Schafer D, et al. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthritis Cartilage. 2004;12:476–484. doi: 10.1016/j.joca.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Dorotka R, Bindreiter U, Vavken P, et al. Behavior of human articular chondrocytes derived from nonarthritic and osteoarthritic cartilage in a collagen matrix. Tissue Eng. 2005;11:877–886. doi: 10.1089/ten.2005.11.877. [DOI] [PubMed] [Google Scholar]
- 21.Tallheden T, Bengtsson C, Brantsing C, et al. Proliferation and differentiation potential of chondrocytes from osteoarthritic patients. Arthritis Res Ther. 2005;7:R560–R568. doi: 10.1186/ar1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luther JK, Cook CR, Cook JL. Meniscal release in cruciate ligament intact stifles causes lameness and medial compartment cartilage pathology in dogs 12 weeks postoperatively. Vet Surg. 2009;38:520–529. doi: 10.1111/j.1532-950X.2009.00520.x. [DOI] [PubMed] [Google Scholar]
- 23.Farr J, Mathew LM, Stoker AM, et al. Effects on exposed articular cartilage during open surgical procedures: a comparison of various fluids in an animal model. Arthroscopy. 2015;31:113–117. doi: 10.1016/j.arthro.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 24.Francioli SE, Martin I, Sie CP, et al. Growth factors for clinical-scale expansion of human articular chondrocytes: relevance for automated bioreactor systems. Tissue Eng. 2007;13:1227–1234. doi: 10.1089/ten.2006.0342. [DOI] [PubMed] [Google Scholar]
- 25.Alegre-Aguaron E, Sampat SR, Xiong JC, et al. Growth factor priming differentially modulates components of the extracellular matrix proteome in chondrocytes and synovium-derived stem cells. PLoS ONE. 2014;9:e88053. doi: 10.1371/journal.pone.0088053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mauck RL, Soltz MA, Wang CC-B, et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252–260. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 27.Riesle J, Hollander AP, Langer R, et al. Collagen in tissue-engineered cartilage: types, structure, and crosslinks. J Cell Biochem. 1998;71:313–327. doi: 10.1002/(sici)1097-4644(19981201)71:3<313::aid-jcb1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 28.Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 29.Hollander AP, Heathfield TF, Webber C, et al. Increased damage to type II collagen in osteoarthritic articular cartilage detected by a new immunoassay. J Clin Invest. 1994;93:1722–1732. doi: 10.1172/JCI117156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly TA, Wang CC, Mauck RL, et al. Role of cell-associated matrix in the development of free-swelling and dynamically loaded chondrocyte-seeded agarose gels. Biorheology. 2004;41:223–237. [PubMed] [Google Scholar]
- 31.Garner BC, Stoker AM, Kuroki K, et al. Using animal models in osteoarthritis biomarker research. J Knee Surg. 2011;24:251–264. doi: 10.1055/s-0031-1297361. [DOI] [PubMed] [Google Scholar]
- 32.Breshears LA, Cook JL, Stoker AM, et al. Detection and evaluation of matrix metalloproteinases involved in cruciate ligament disease in dogs using multiplex bead technology. Vet Surg. 2010;39:306–314. doi: 10.1111/j.1532-950X.2010.00675.x. [DOI] [PubMed] [Google Scholar]
- 33.Goldring MB. The role of the chondrocyte in osteoarthritis. Arthritis Rheum. 2000;43:1916–1926. doi: 10.1002/1529-0131(200009)43:9<1916::AID-ANR2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 34.Sun L, Wang X, Kaplan DL. A 3D cartilage—inflammatory cell culture system for the modeling of human osteoarthritis. Biomaterials. 2011;32:5581–5581. doi: 10.1016/j.biomaterials.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshihara Y, Nakamura H, Obata K, et al. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann Rheum Dis. 2000;59:455–461. doi: 10.1136/ard.59.6.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alam MR, Ji JR, Kim MS, et al. Biomarkers for identifying the early phases of osteoarthritis secondary to medial patellar luxation in dogs. J Vet Sci. 2011;12:273–280. doi: 10.4142/jvs.2011.12.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monibi F, Roller BL, Stoker A, et al. Identification of synovial fluid biomarkers for knee osteoarthritis and correlation with radiographic assessment. J Knee Surg. 2015;29:242–247. doi: 10.1055/s-0035-1549022. [DOI] [PubMed] [Google Scholar]
- 38.Catterall JB, Stabler TV, Flannery CR, et al. Changes in serum and synovial fluid biomarkers after acute injury ( NCT00332254) Arthritis Res Ther. 2010;12:R229. doi: 10.1186/ar3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan Z, Bau B, Yang H, et al. Freshly isolated osteoarthritic chondrocytes are catabolically more active than normal chondrocytes, but less responsive to catabolic stimulation with interleukin-1? Arthritis Rheum. 2005;52:136–143. doi: 10.1002/art.20725. [DOI] [PubMed] [Google Scholar]
- 40.Flannery CR, Little CB, Caterson B, et al. Effects of culture conditions and exposure to catabolic stimulators (IL-1 and retinoic acid) on the expression of matrix metalloproteinases (MMPs) and disintegrin metalloproteinases (ADAMs) by articular cartilage chondrocytes. Matrix Biol. 1999;18:225–237. doi: 10.1016/s0945-053x(99)00024-4. [DOI] [PubMed] [Google Scholar]
- 41.Yuan GH, Masuko-Hongo K, Sakata M, et al. The role of C-C chemokines and their receptors in osteoarthritis. Arthritis Rheum. 2001;44:1056–1070. doi: 10.1002/1529-0131(200105)44:5<1056::AID-ANR186>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 42.de Bruin T, de Rooster H, van Bree H, et al. Interleukin-8 mRNA expression in synovial fluid of canine stifle joints with osteoarthritis. Vet Immunol Immunopathol. 2005;108:387–397. doi: 10.1016/j.vetimm.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 43.Goldring MB, Otero M, Plumb DA, et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur Cell Mater. 2011;21:202–220. doi: 10.22203/ecm.v021a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Markway BD, Cho H, Johnstone B. Hypoxia promotes redifferentiation and suppresses markers of hypertrophy and degeneration in both healthy and osteoarthritic chondrocytes. Arthritis Res Ther. 2013;15:R92. doi: 10.1186/ar4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diao HJ, Yeung CW, Yan CH, et al. Bidirectional and mutually beneficial interactions between human mesenchymal stem cells and osteoarthritic chondrocytes in micromass co-cultures. Regen Med. 2013;8:257–269. doi: 10.2217/rme.13.22. [DOI] [PubMed] [Google Scholar]
- 46.Seitz M, Loetscher P, Dewald B, et al. Production of interleukin-1 receptor antagonist, inflammatory chemotactic proteins, and prostaglandin E by rheumatoid and osteoarthritic synoviocytes-regulation by IFN-gamma and IL-4. J Immunol. 1994;152:2060–2065. [PubMed] [Google Scholar]
- 47.Remick DG, DeForge LE, Sullivan JF, et al. Profile of cytokines in synovial fluid specimens from patients with arthritis. Interleukin 8 (IL-8) and IL-6 correlate with inflammatory arthritides. Immunol Invest. 1992;21:321–327. doi: 10.3109/08820139209069371. [DOI] [PubMed] [Google Scholar]
- 48.Merz D, Liu R, Johnson K, et al. IL-8/CXCL8 and growth-related oncogene alpha/CXCL1 induce chondrocyte hypertrophic differentiation. J Immunol. 2003;171:4406–4415. doi: 10.4049/jimmunol.171.8.4406. [DOI] [PubMed] [Google Scholar]
- 49.Loeser RF, Pacione CA, Chubinskaya S. The combination of insulin-like growth factor 1 and osteogenic protein 1 promotes increased survival of and matrix synthesis by normal and osteoarthritic human articular chondrocytes. Arthritis Rheum. 2003;48:2188–2196. doi: 10.1002/art.11209. [DOI] [PubMed] [Google Scholar]
- 50.Lima EG, Tan AR, Tai T, et al. Physiologic deformational loading does not counteract the catabolic effects of interleukin-1 in long-term culture of chondrocyte-seeded agarose constructs. J Biomech. 2008;41:3253–3259. doi: 10.1016/j.jbiomech.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jeon JE, Schrobback K, Hutmacher DW, et al. Dynamic compression improves biosynthesis of human zonal chondrocytes from osteoarthritis patients. Osteoarthritis Cartilage. 2012;20:906–915. doi: 10.1016/j.joca.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 52.Schrobback K, Klein TJ, Crawford R, et al. Effects of oxygen and culture system on in vitro propagation and redifferentiation of osteoarthritic human articular chondrocytes. Cell Tissue Res. 2011;347:649–663. doi: 10.1007/s00441-011-1193-7. [DOI] [PubMed] [Google Scholar]
- 53.Cook JL, Kuorki K, Stoker AM, et al. Review of in vitro models and development and initial validation of a novel co-culture model for the study of osteoarthritis. Curr Rheum Rev. 2007;3:172–182. [Google Scholar]
- 54.Patwari P, Lin SN, Kurz B, et al. Potent inhibition of cartilage biosynthesis by coincubation with joint capsule through an IL-1-independent pathway. Scand J Med Sci Sports. 2009;19:528–535. doi: 10.1111/j.1600-0838.2009.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cavallo C, Desando G, Facchini A, et al. Chondrocytes from patients with osteoarthritis express typical extracellular matrix molecules once grown onto a three-dimensional hyaluronan-based scaffold. J Biomed Mater Res. 2009;93:86–95. doi: 10.1002/jbm.a.32547. [DOI] [PubMed] [Google Scholar]