Abstract
Recently, there has been a surge of early phase trials of molecularly targeted agents (MTAs) and immunotherapies. These new therapies have different toxicity profiles compared to cytotoxic therapies. MTAs can benefit from new trial designs that allow inclusion of low-grade toxicities, late-onset toxicities, addition of an efficacy endpoint, and flexibility in the specification of a target toxicity probability. To study the degree of adoption of these methods, we conducted a Web of Science search of articles published between 2008 and 2014 that describe phase 1 oncology trials. Trials were categorized based on the dose-finding design used and the type of drug studied. Out of 1,712 dose-finding trials that met our criteria, 1,591 (92.9%) utilized a rule-based design, and 92 (5.4%; range 2.3% in 2009 to 9.7% in 2014) utilized a model-based or novel design. Over half of the trials tested an MTA or immunotherapy. Among the MTA and immunotherapy trials, 5.8% used model-based methods, compared to 3.9% and 8.3% of the chemotherapy or radiotherapy trials, respectively. While the percentage of trials using novel dose-finding designs has tripled since 2007, only 7.1% of trials use novel designs.
Keywords: phase 1 designs, dose-finding methods, targeted therapy, immunotherapy, optimal dose, maximum tolerated dose
1. Introduction
Dose-finding trials test new drugs or combinations of drugs with the goal of identifying a tolerable dose to be used in subsequent trials. In oncology, study designs for these trials were initially developed for testing cytotoxic agents, with the goal of determining the maximum tolerated dose (MTD). The primary outcome of these trials is usually binary, defined as the presence or absence of a dose limiting toxicity (DLT) in the first cycle of treatment based on the Common Terminology Criteria for Adverse Events (CTCAE) (NIH, 2009; NCI, 2014). The underlying assumption in phase 1 cancer trials is that the probability of toxicity monotonically increases with dose, and that doses with higher probabilities of toxicity yield higher probabilities of response (Green et al., 2012). Dose-finding methods for phase 1cancer trials can be broadly categorized into rule-based/algorithmic designs, such as the ‘3+3’ design (Storer, 1989), and model-based designs. The latter category includes the Continual Reassessment Method (CRM) (O’Quigley et al., 1990), its variations such as Time-to-Event CRM (TITE-CRM) (Cheung and Chappell, 2000) and modified CRM (Goodman et al., 1995; Piantadosi et al., 1998; Yuan et al., 2007), and Escalation with Overdose Control (EWOC) design (Babb et al., 1998). Compared to rule-based designs, model-based methods require a smaller sample size to achieve the same precision in estimation of the MTD and assign a higher proportion of patients to levels closer to the true MTD. Moreover, they allow more flexibility in the specification of a target probability of DLT. Despite these advantages, the adoption of model-based designs has been slow. Rogatko et al. (2007) reported that 98.4% of phase 1 dose-finding cancer trials published from 1991 to 2006 utilized a rule-based design. Le Tourneau et al. (2009) performed a similar review of articles published between 2007 and 2008 and concluded that 96.7% of dose-finding trials used rule-based algorithms.
In recent years, the landscape in cancer drug development has changed with more molecularly targeted agents (MTAs) and immunotherapies being tested in clinical trials. Starting in 2008, the number of Food and Drug Administration (FDA) approved MTAs has increased rapidly, with more than 60 MTAs available today for cancer treatment (NCI, 2014). These new MTAs and immunotherapies have distinct molecular pathways, which lead to different toxicity profiles compared to cytotoxic agents. A recent study reported that 25% of early phase MTA clinical trials defined grade 2 toxicities as a DLT (Le Tourneau et al., 2011). Moreover, the extended periods over which these therapies are administered raise concerns regarding late-onset and cumulative toxicities (e.g., rashes, fatigue, diarrhea and neuropathy), which affect patients’ quality of life (Verweij et al., 2010; Wigertz et al., 2012, Blay and Rutkowski, 2014). Furthermore, the dose-efficacy and dose-toxicity relationships may differ from those of cytotoxic agents, raising the possibility that efficacious doses may be found below the MTD (Korn, 2001; Parulekar and Eisenhauer, 2004; Cannistra, 2008; Moreno Garcia et al., 2014).
Given the aforementioned differences between cytotoxic agents and MTA / immunotherapies, the definitions of DLT and MTD, as well as the methods used to estimate the optimal dose need to be re-evaluated (Soria, 2011). Recent methods have been proposed to:
Account for late-onset toxicity without interrupting recruitment (Cheung and Chappell, 2000; Ivanova et al., 2016)
Allow for the specification of multiple toxicity thresholds, for example, thresholds for low and moderate grade toxicity (Lee et al., 2011; Cheng and Lee, 2015)
Estimate the MTD for combination therapies (Mandrekar et al., 2007; Mandrekar, 2014; Wages and Conaway, 2014)
Take into account toxicity and efficacy simultaneously (Braun, 2002; Ivanova, 2003; Thall and Cook, 2004; Zhang et al., 2006; Dragalin and Fedorov, 2006).
The acute need of implementing new methods for testing novel anticancer agents, led us to evaluate the use of statistical designs from 2008 to 2014. Not only the literature of dose-finding reviews needed an update (last ones covered 1991 – 2008: Rogatko et al., 2007; Le Tourneau et al., 2009), but the number of novel agents has increased substantially in these seven years. Our review complements the recent article on drug-combinations published by Riviere et al. (2015) and the article by Iasonos and O’Quigley (2014), with additional findings on single agents use, classification by therapy type (chemotherapy, radiotherapy, MTA and immunotherapy), and an updated list of trials implementing model-based designs. In addition, we redesign two trials that used the ‘3+3’ design to see how they would have benefitted from using a model-based approach instead.
2. Methods and Classification
A Web of Science (WOS) search was conducted on November 2, 2015 to identify phase 1 oncology trial articles published between 2008 and 2014. Only trials aimed at estimating the MTD were included, regardless of the number of investigational agents being tested. The database and the search criteria were selected based on the previous literature review performed by Rogatko et al. (2007) to provide continuity and comparability to the last major review evaluating dose-finding methods. The exact search criteria can be found in Appendix 1. Studies were grouped into four categories:
Animal studies (studies solely in animals)
Statistical/methodological studies (papers that proposed new designs or reviewed, compared and addressed other methodological considerations without having an explicit dose-finding trial published during our time frame of interest)
Dose-finding studies (articles in which the goal was to find the MTD)
Other (articles that did not fall under the aforementioned categories, such as articles discussing phase 2 studies, providing an overview of a drug’s PK/PD, or reviewing the entire drug development process for a particular agent).
Further classification of the dose-finding studies was based on the type of statistical design employed, as follows:
- Dose-Escalation (model-based or novel designs)
- Dose-Escalation (CRM), studies using the Continual Reassessment Method or its variations,
- Dose-Escalation (EWOC), studies using the EWOC design or its variations,
- Dose-Escalation (Novel designs), studies using a statistical methodology specifically tailored for the published study or other novel designs such as the ‘Cumulative Cohort Design’ by Ivanova and Chung (2007).
Dose-Escalation (‘A+B’), studies using rule-based algorithms, such as the ‘3+3’ design, the rolling six design (Skolnik et al., 2008) or the accelerated titration design (Simon et al., 1997).
Dose-Escalation (Other), studies using none of the above methods. These were primarily dose-finding studies that used intra-patient dose-escalation or studies that did not provide enough detail regarding the method used to assign patients to doses and estimate the MTD.
Two readers reviewed each article independently and assigned a study category. If discrepancies were found, a third reader was asked to review the inconsistent articles. Furthermore, the literature search was supplemented by a review of all articles published on Google Scholar between 2008 and 2014 that cited the original CRM, the modified CRM, the TITE-CRM, or the EWOC. Given that these were the most often used designs, the supplementation was performed to provide an updated list of trials using model-based designs, and to provide a conservative estimate of the uptake of these methods. Dose-finding studies from the reference search were matched against the original list of model-based and novel designs from the WOS search. Studies that were unique to the reference search were reviewed, and included only if the design used was among one of the four categories mentioned above. For model-based and novel designs, the pre-specified dose information used in the design phase of the trial, the sample size, the number of evaluable patients, the type of entity that sponsored the study, and the specific statistical design were recorded.
The types of investigational agents were recorded and categorized for all dose-finding studies. The classification was based on the escalating agent, as chemotherapy, radiotherapy, MTA, immunotherapy, or other. The chemotherapy category represented classical antimetabolites and included novel chemotherapeutic agents such as TAS-106 and Vosaroxin. The radiotherapy category included radiation therapy, radiation chemotherapy, radioimmunotherapy, radioembolization, and radiosensitizers. MTAs were defined as agents that specifically inhibited or manipulated a specific actionable target (other than antimetabolites that were categorized as chemotherapy). Trials that escalated both the chemotherapy agent and the MTA were classified as MTA. The immunotherapy category included check-point blockers, cytokines (other than pegfilgastrim), vaccines, viral agents, NGR-hTNF, and bromohydrin pyrophosphate (IPH 1101). When both chemotherapy and immunotherapy were escalated, the trial was classified as immunotherapy. ‘Other’ category included agents that were unable to be represented by the aforementioned categories, such as Tariquidar, lenalidomide, hypericin, denibulin, hylaronic acid, PHY-906, steroids, and boorotene. One study used as an illustrative example and published in a statistical journal was also classified in the ‘Other’ category.
All results are presented in tables as frequency counts and percentages. The number of studies using model-based or novel designs was calculated given the type of agent and year of publication. The time trend for each agent type was also tabulated separately. A separate section was included in the paper to illustrate two case examples of trials that would have benefitted from the use of a model-based design, but used the ‘3+3’ design instead.
3. Results
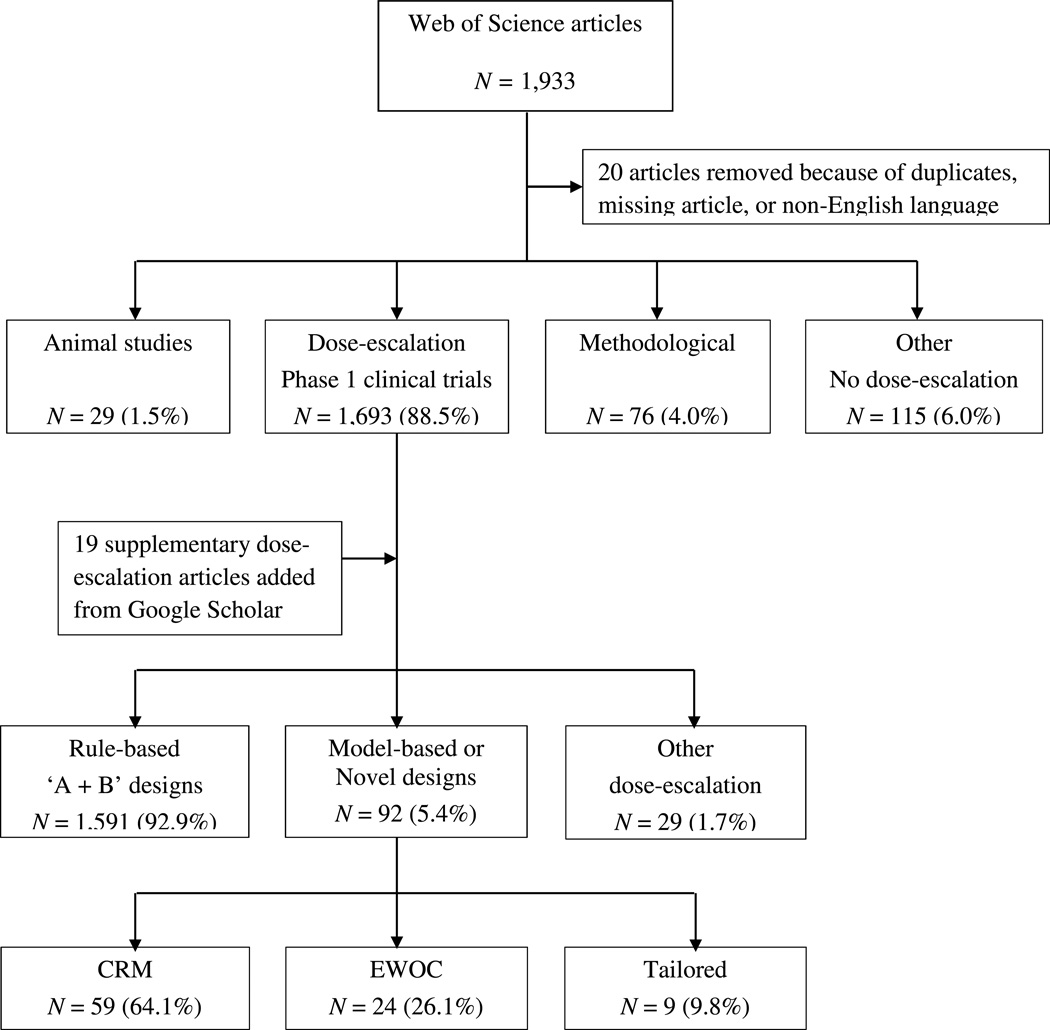
Of the 1,933 records identified on Web of Science, 20 were excluded because of duplicates, non-existing article links, published in a language other than English or published in print after 2014. Of the remaining 1,913 studies, 1,693 discussed phase 1 dose-escalation oncology trials, 29 were animal studies, 76 were statistical or methodological articles, and 115 were in the other category. The supplementary Google Scholar search produced 19 additional articles that utilized the CRM, variations of the CRM, or the EWOC.
Among the 1,712 articles that described dose-escalation trials, rule-based ‘A+B’ designs were used in 1,591 (92.9%) studies. Model-based or novel designs were used in 92 (5.4%) studies, of which 59 (64.1%) used the CRM or its variations, 24 (26.1%) used the EWOC or its variations, and the rest used other trial specific designs (see Figure 1). The percentage of studies utilizing model-based or novel designs varied throughout the years, ranging from 2.3% in 2009 to 9.7% in 2014 (Table 1). The percentage of studies using the CRM or EWOC ranged from 0.9% in 2009 to 6.1% in 2011, and from 0% in 2008 to 4.9% in 2014, respectively.
Figure 1. Classification of the reviewed dose-finding oncology studies published between 2008–2014.
CRM: Continual Reassessment Method; EWOC: Escalation with Overdose Control
Table 1.
Summary % (n) of dose-finding oncology trials that implemented a model-based or novel design by agent type and year of publication
| Year | MTA | Immuno Therapy |
Chemo Therapy |
Radiation Therapy |
Other Therapy† |
All Therapies |
|---|---|---|---|---|---|---|
| 2008 | 3.4% (3/87)* |
0% (0/13) |
5.5% (6/109) |
14.3% (1/7) |
9.1% (1/11) |
4.8% (11/227) |
| 2009 | 3.1% (2/65) |
22.2% (2/9) |
0% (0/116) |
9.1% (1/11) |
0% (0/11) |
2.4% (5/212) |
| 2010 | 3.5% (4/115) |
0% (0/16) |
2.1% (2/95) |
0% (0/9) |
0% (0/14) |
2.4% (6/249) |
| 2011 | 7.5% (8/107) |
8.3% (1/12) |
4.2% (4/95) |
0% (0/8) |
8.7% (2/23) |
6.1% (15/245) |
| 2012 | 6.5% (11/170) |
9.1% (1/11) |
1.8% (3/81) |
18.2% (2/11) |
17.6% (3/17) |
6.9% (20/290) |
| 2013 | 4.8% (8/166) |
5.6% (1/18) |
1.8% (1/55) |
11.1% (1/9) |
13.3% (2/15) |
4.9% (13/263) |
| 2014 | 9.8% (14/143) |
0% (0/6) |
13.8% (8/58) |
0% (0/5) |
0% (0/14) |
9.7% (22/226) |
In parentheses for a given year, the numerator represents the number of trials that used a model-based or novel design; the denominator represents the total number of trials that investigated a particular agent
One article with unspecified agent was included in the ‘Other Therapy’ category
Classification by type of therapy tested in phase 1 trials identified 853 (49.8%) trials of MTAs, 85 (5.0%) of immunotherapies, 609 (35.6%) of chemotherapies, 60 (3.5%) of radiotherapies, and 105 (6.1%) of other agents. As shown in Table 2, the percentage of trials investigating MTAs has increased sharply. In 2013 and 2014, 63% of all published dose-escalation trials tested a targeted agent. Across all years, 50 out of the 853 (5.9%) trials testing MTAs used a model-based or novel design, ranging from 3.1% in 2009 to 9.8% in 2014. For chemotherapy, 24 (3.9%) used a model-based or novel design, ranging from 0% in 2009 to 13.8% in 2014. For immunotherapy, radiotherapy and ‘other’ category, the number of trials that used a model-based or novel design was 5 out of 85 (5.9%), 5 out of 60 (8.3%) and 8 out of 105 (7.6%), respectively.
Table 2.
Summary % (n) of investigational agents tested in dose-finding oncology trials by year of publication
| Year | N* | MTA | Immuno Therapy |
Chemo Therapy |
Radiation Therapy |
Other Therapy† |
|---|---|---|---|---|---|---|
| 2008 | N = 227 | 38.3% (87) |
5.7% (13) |
48.0% (109) |
3.1% (7) |
4.9% (11) |
| 2009 | N = 212 | 30.7% (65) |
4.2% (9) |
54.7% (116) |
5.2% (11) |
5.2% (11) |
| 2010 | N = 249 | 46.2% (115) |
6.4% (16) |
38.2% (95) |
3.6% (9) |
5.6% (14) |
| 2011 | N = 245 | 43.7% (107) |
4.9% (12) |
38.8% (95) |
3.3% (8) |
9.4% (23) |
| 2012 | N = 290 | 58.6% (170) |
3.8% (11) |
27.9% (81) |
3.8% (11) |
5.9% (17) |
| 2013 | N = 263 | 63.1% (166) |
6.8% (18) |
20.9% (55) |
3.4% (9) |
5.7% (15) |
| 2014 | N = 226 | 63.3% (143) |
2.7% (6) |
25.7% (58) |
2.2% (5) |
6.2% (14) |
N represents the total number of dose-escalation oncology trials by year
One article with unspecified agent was included in the ‘Other Therapy’ category
A more detailed classification of the 92 dose-escalation studies that employed a model-based or novel design is shown in the Appendix 2. Of these 92 studies, more than 25% were designed using a continuous dose range or did not explicitly state the discrete set of dose levels used in the design. For studies employing discrete dose levels, the range varied from 2 to 16 with total sample sizes ranging from 9 to over 100. Academic institutions (48%) and industry-sponsored trials (41%) were the major contributors of these studies. The majority of the 92 trials (62%) were multicenter.
4. Redesigned Examples
Case Study 1
We redesigned two studies that used the ‘3+3’ design, but could have benefitted from the use of a model-based design. The study in Ghamande et al. (2014) was a phase 1 trial to determine the MTD of a novel camptothecin in patients with advanced solid tumors. A total of 54 patients were enrolled at 11 dose levels using the ‘3+3’ design. However, one of the doses was not specified a priori, but added during the trial as an intermediate level, after seeing 2 DLTs out of 6 patients at dose number 8. This ad-hoc approach not only contravened with the trial conduct, but it also affected the choice of the final MTD. If the original set of doses had been used, the trial would have stopped earlier (after enrolling 32 patients), and a lower dose level (dose level 8) would have been declared as the MTD. The protocol amendment increased the total sample size to 54 patients and declared dose level 10 as the MTD. With such a large number of dose levels, using the ‘3+3’ design can be inefficient and lead to a large sample size, like in this example. The study would have benefitted from using the CRM with assigning patients in cohorts of size 1 for faster escalation. Alternatively, rather than selecting a dose from a pre-specified set of 11 dose levels, since the drug was administered intravenously, a dose could be selected from a continuum of doses. We performed simulations to compare the ‘3+3’ design to the CRM with dose level 1 as the starting dose and a target DLT probability of 0.25 (see Table 3). We chose the target DLT probability of 0.25 since it has been shown that on average the ‘3+3’ design targets DLT probabilities between 0.16 and 0.27. (Ivanova, 2006). The CRM with an empirical dose-toxicity model and a vague normal prior distribution for the model parameter, β ~ N(0,1.34), was used (O’Quigley and Shen, 1996). A total of 2,000 simulations were run for the CRM and the ‘3+3’ using the statistical software R (2014). Using the CRM with cohorts of size 1 and a total sample size of 54 patients, the probability of selecting dose level 10 as the MTD was 63%, with an average of 23 patients treated at the MTD. In comparison, the ‘3+3’ design selected dose level 10 (with the true probability of DLT of 0.25) as the MTD 29% of the time, and selected dose level 9 (with the true probability of DLT of 0.15) 33% of the time. This indicates that the ‘3+3’ design is more likely to select a dose with DLT probability of 0.20 or lower rather than higher probabilities such as 0.25 or 0.30. Since the mean sample size for the ‘3+3’ design was 34 patients, and the total sample size of the trial before the protocol amendment was 32 patients, we also simulated the CRM with a sample size of 32 patients. The CRM selected the correct MTD 54% of the time with an average of 11 patients being assigned to this dose level. Thus, the CRM is more efficient in estimating the dose with the DLT probability of 0.25 compared to the ‘3+3’ design. It also assigns more patients to the true MTD on average.
Table 3.
Case Study 1: Probability of selecting each dose as the maximum tolerated dose (MTD). Results for the true MTD are in bold.
| Doses 1 – 7 |
Dose 8 |
Dose 9 |
Dose* 10 |
Dose 11 |
|
|---|---|---|---|---|---|
| Assumed DLT probabilities used in simulations |
0 | 0 | 0.15 | 0.25 | 0.35 |
| ‘3+3’ design (N = 34)** | 0 | 0.19 | 0.33 | 0.29 | 0.19 |
| CRM (N = 54) | 0 | 0 | 0.19 | 0.63 | 0.18 |
| CRM (N = 32) | 0 | 0 | 0.22 | 0.54 | 0.24 |
MTD declared by the original trial.
Average sample size obtained from 2,000 simulated trials.
CRM: Continual Reassessment Method
Case Study 2
Chen et al. (2014) reported the results of a phase 1 trial of maintenance sorafenib after stem cell transplantation in patients with acute myeloid lymphoma. The ‘3+3’ design with an expansion cohort was used to identify the MTD out of three dose levels. A DLT was defined as a grade 3 or higher treatment-related toxicity in the first 28 days that did not resolve within 14 days after suspending sorafenib. Twelve patients were used to identify the MTD, which was the highest dose level, and 10 additional patients were treated at that dose level. While only 1 DLT out of 6 was observed at the highest dose during the initial DLT window (28 day cycle), later on, 5 (31%) additional patients had to discontinue treatment due to toxicities possibly related to treatment. In this case late-onset toxicities may be contributing to a poorly tolerated dose. Thus, defining the MTD based on a longer time interval and using a design like the TITE-CRM, which accounts for late-onset toxicities, can help identify a better-tolerated dose. The TITE-CRM utilizes data in real time including data from patients still in follow-up, allows for enrollment of patients before previously enrolled patients complete the DLT evaluation window, and thus can significantly reduce the length of the trial. Table 4 compares the operating characteristics based on 2,000 simulations of the TITE-CRM versus the ‘3+3’ algorithm using the DLT probabilities observed during a longer time interval. For the TITE-CRM, we extended the DLT evaluation window to two cycles, and used sample sizes with (N = 22) and without the expansion cohort (N = 12). The target DLT probability was set at 0.20 with dose level 1 as the starting dose level and cohorts of size 1. The TITE-CRM used an empirical dose-toxicity model with a vague normal prior distribution as in the first case study.
Table 4.
Case Study 2: Probability of selecting each dose as the maximum tolerated dose (MTD). Results for the true MTD are in bold.
| Dose 1 |
Dose 2 |
Dose* 3 |
|
|---|---|---|---|
| Assumed DLT probabilities used in simulations |
0 | 0.20 | 0.40 |
| ‘3+3’ design (N = 11)** | 0.29 | 0.48 | 0.23 |
| TITE-CRM (N = 22) | 0.07 | 0.80 | 0.13 |
| TITE-CRM (N = 12) | 0.14 | 0.70 | 0.16 |
MTD declared by the end of the original trial was later deemed unsafe due to late onset toxicities.
Average sample size obtained from 2,000 simulated trials.
TITE-CRM: Time-o-event Continual Reassessment Method.
Extending the DLT evaluation window to capture delayed toxicities might lead to a different MTD. When accounting for delayed toxicities, the TITE-CRM identified dose level 2 as the MTD 80% and 70% of the time, for N = 22 and N = 12, respectively, compared to 48% for the ‘3+3’ design with an average sample size of 11 patients. In addition, under the TITE-CRM, the average number of patients treated at the MTD was 13 and 6 for sample sizes of 22 and 12, respectively.
5. Discussion
This study reviews dose-finding oncology trials published between 2008 and 2014 with the goal of providing updated statistics on trial design methodology. The review was motivated by the increased number of MTAs and immunotherapies, which bring new challenges in identifying the dose for subsequent phase 2 trials. The percentage of dose-finding studies that used a model-based or novel design between 2008 and 2014 has more than tripled since the last major review of the literature in 2007. However the actual fraction remains low (1.6% for the period of 1991–2006 versus 5.4% for the period of 2008 to 2014). While the percentage of trials using novel designs fluctuates from year to year, there seems to be a slight increase in the implementation of model-based or novel designs over time. Our findings indicate that from 2011 to 2014, the percentage of trials that employed model based or novel designs for testing MTAs approached 7%, double compared to previous years. However, this remains far below the desirable rate at which these new methods should be adopted. The recent post-marketing dose-optimization studies performed on several MTAs have made investigators reconsider the use of conventional dose-finding designs in the context of the new anticancer treatments (Minasian et al., 2014). In recent meetings convened by the FDA and the American Association of Cancer Research (AACR) in May of 2015 and 2016, a call was made in favor of using model-based methods, as they are more flexible in considering different toxicity profiles.
In order to provide a more complete and updated list of trials using CRM and EWOC, we supplemented our original search with model-based designs using citations from Google Scholar. Thus, the estimate of 5.4% may even be an overestimate of the true proportion of trials using model-based or novel designs. It should also be noted that this review was limited to published studies and our search criteria included “maximum tolerated dose”. As a result, we are only capturing information on published dose-finding studies aimed at estimating the MTD, and studies identifying biologically optimal dose or minimum effective dose may not be included. A search on www.clinicaltrials.gov was attempted to identify unpublished studies, but very limited information was available on the dose-finding methodology.
Reluctance to adopt new designs can be due to many factors. It is possible that not all clinical investigators or biostatisticians are familiar with or even aware of these methods (Jaki, 2013). Also, model-based and novel dose-escalation designs require close collaboration with statisticians and access to software and computational resources (Gonen, 2009; Jaki, 2013). These obstacles can be addressed through greater educational initiatives around model-based designs for statisticians in academia, pharmaceutical industry, or consortiums, and additional software development to facilitate the execution of model-based designs. In 2015, the AACR offered a biostatistics workshop focused on clinical trial designs for targeted agents for junior statisticians working in oncology. Short courses on phase 1 designs have been offered frequently at major statistical conferences such as the Society for Clinical Trials (SCT) Meeting, the International Biometric Society Eastern North American Region (ENAR) Meeting, and the American Statistical Association Joint Statistical Meeting (JSM). Another major obstacle is the misconception that model-based designs lead to observing more toxicities on average compared to the ‘3+3’ design. To the contrary, the average proportion of toxicities in these studies is acceptable and does not exceed the conventional DLT ceiling of 0.25–0.33. In a recent article, Iasonos and O'Quigley (2014) showed that in trials implementing model-based design such as CRM, TITE-CRM or EWOC, the average DLT probability was 0.18. An ultimate barrier is motivation and time (Gonen, 2009). At many institutions, statisticians have limited time to explore and implement novel methods. Even for statisticians who are aware and familiar with these designs, applying them requires more effort than the conventional ‘3+3’ designs.
With the different toxicity profiles displayed by MTAs and immunotherapy, it is becoming necessary and important to consider novel designs for dose-finding trials of new anticancer agents. Model-based designs can offer increased flexibility and efficiency compared to rule-based designs, as illustrated in the case examples presented above. In these examples, the novel methods would have improved the trial by decreasing the sample size and even identifying a different recommended dose level to be carried forward given the inclusion of late-onset or cumulative toxicities. While the '3+3' design is simple to implement, model-based designs are superior in quality of estimation of the MTD, in reducing trial duration, and in flexibility of defining the optimal dose.
Acknowledgments
Dr. Lee received research support for this study from the American Cancer Society (grant number MRSG-13-146-01-CPHPS). Dr. Ivanova’s work was supported in part by the NIH grant P01 CA142538. The authors would like to thank Daniel Kang MS, Yao Ma MS and Tianyi Sun MS for helping with categorizing the methods used in the trial, and Fatima Sarwar, MBBS and Masooma Sarwar, MBBS from Punjab Medical College, Faisalabad, Pakistan for helping with categorizing the agents used in the trial.
Appendix 1
Search term:
TOPIC: ("phase I" OR "phase 1" OR "phase one") AND TOPIC: (study OR studies OR trial)
AND TOPIC: (maximum tolerated dose)
Refined by: DOCUMENT TYPES=(ARTICLE) AND RESEARCH AREAS=(ONCOLOGY)
Timespan=2008–2014
Search language=Auto
Appendix 2
Table of the characteristics of dose-finding trials that used model-based or novel designs
| Year | First Author | Design | N* | Dose Levels | MTD drug type† | Entity | Center Type |
|---|---|---|---|---|---|---|---|
| 2008 | Adkison | Novel | 47 | Various | Radiotherapy | Academic | One Center |
| 2008 | De Bono | CRM | 39 | 7 | Targeted therapy | Industry | Two Centers |
| 2008 | Grossman | CRM | 12 18 |
Continuous | Chemotherapy | Consortium | Multicenter |
| 2008 | Guillot | CRM | 31 | 4 | Chemotherapy | Academic | Multicenter |
| 2008 | Gururangan | CRM | 16 19 |
Continuous | Chemotherapy | Consortium | Multicenter |
| 2008 | Jimeno | CRM | 21 | Continuous | Targeted therapy | Academic | One Center |
| 2008 | MacDonald | CRM | 31 | Continuous | Targeted therapy | Consortium | Multicenter |
| 2008 | Neuenschwander | CRM | 24 | 15 | Other therapy | Industry | Multicenter |
| 2008 | Rao | CRM | 17 | 5 | Chemotherapy | Academic | One Center |
| 2008 | Saji | CRM | 17 | 5 | Chemotherapy | Academic | Multicenter |
| 2008 | Truemper | CRM | 41 78 |
4 4 |
Chemotherapy | Academic | Multicenter |
| 2009 | Bailey | Novel | 50 | 5 | Targeted therapy | Industry | Multicenter |
| 2009 | Borghaei | EWOC | 39 13 |
Continuous | Immunotherapy | Academic | Multicenter |
| 2009 | Demetri | EWOC | 53 | 3 | Targeted therapy | Industry | Multicenter |
| 2009 | Li | CRM | 27 | Continuous | Immunotherapy | Academic | One Center |
| 2009 | Loeb | CRM | 13 | Continuous | Radiotherapy | Academic | Two Centers |
| 2010 | Andre | TITE- CRM |
23 10 |
3 4 |
Targeted therapy | Industry | Multicenter |
| 2010 | Fouladi | CRM | 29 21 |
Continuous | Targeted therapy | Consortium | Multicenter |
| 2010 | Furman | TITE- CRM |
18 | 3 | Targeted therapy | Academic | Multicenter |
| 2010 | O'Donnell | CRM | 14 15 |
4 | Chemotherapy | Academic | One Center |
| 2010 | Peereboom | CRM | 16 21 |
Continuous | Chemotherapy | Consortium | Multicenter |
| 2010 | Rathkopf | EWOC | 8 8 |
Continuous | Targeted therapy | Industry | Multicenter |
| 2011 | Fouladi | CRM | 9 | 2 | Targeted therapy | Consortium | Multicenter |
| 2011 | Gandhi | CRM | 35 12 |
Continuous | Targeted therapy | Industry | Multicenter |
| 2011 | Geoerger | CRM | 29 21 |
4 | Targeted therapy | Consortium | Multicenter |
| 2011 | Jakubowiak | TITE- CRM |
30 20 |
3 4 |
Other therapy | Industry | Multicenter |
| 2011 | Jerusalem | TITE- CRM |
40 | 4 | Targeted therapy | Industry | Multicenter |
| 2011 | Kim | CRM | 27 | 4 | Targeted therapy | Industry | Multicenter |
| 2011 | Koolen | CRM | 32 10 |
5 2 |
Chemotherapy | Industry | Multicenter |
| 2011 | Magenau | TITE- CRM |
46 | 3 | Chemotherapy | Academic | One Center |
| 2011 | Mehnert | CRM | 16 | Continuous | Targeted therapy | Academic | One Center |
| 2011 | Satoh | CRM | 20 21 |
3 4 |
Chemotherapy | Academic | Multicenter |
| 2011 | Smith | CRM | 32 | Continuous | Targeted therapy | Academic | One Center |
| 2011 | Terakura | CRM | 17 | Continuous | Chemotherapy | Academic | Multicenter |
| 2011 | Vansteenkist | TITE- CRM |
24 19 |
3 3 |
Targeted therapy | Industry | Multicenter |
| 2011 | Warren | CRM | 38 | Continuous | Other therapy | Consortium | Multicenter |
| 2011 | Di Stasi | CRM | 5 | 3 | Immunotherapy | Academic | One Center |
| 2012 | Ben-Josef | TITE- CRM |
50 | 6 | Radiotherapy | Academic | Two Centers |
| 2012 | Bendell | EWOC | 30 | Continuous | Targeted therapy | Industry | Multi center |
| 2012 | Crew | TITE- CRM |
30 | 3 | Other therapy | Academic | Multicenter |
| 2012 | Farid | CRM | 18 | 6 | Chemotherapy | Government | One Center |
| 2012 | Feng | TITE- CRM |
23 | 11 | Other therapy | Academic | One Center |
| 2012 | Geletneky | CRM | 18 | 3 | Immunotherapy | Academic | One Center |
| 2012 | Jakubowiak | TITE- CRM |
35 | 3 | Targeted therapy | Academic | Multicenter |
| 2012 | Jones | Novel | 21 | 12 | Targeted therapy | Academic | One Center |
| 2012 | Kawahara | CRM | 18 | 3 | Chemotherapy | Consortium | Multicenter |
| 2012 | Lu | CRM | 31 | Continuous | Targeted therapy | Academic | One Center |
| 2012 | Markman | EWOC | 57 | Continuous | Targeted therapy | Industry | Multicenter |
| 2012 | Mazard | CRM | 17 11 |
5 7 |
Chemotherapy | Academic | One Center |
| 2012 | Moulder | CRM | 15 | 4 | Targeted therapy | Academic | One Center |
| 2012 | Reardon | EWOC | 16 | Continuous | Targeted therapy | Industry | Multicenter |
| 2012 | Roberts | CRM | 15 14 |
Continuous | Targeted therapy | Industry | Multicenter |
| 2012 | Schneider | TITE- CRM |
12 | 7 | Targeted therapy | Academic | Multicenter |
| 2012 | Sinha | EWOC | 19 | 6 | Targeted therapy | Academic | One Center |
| 2012 | Tevaarwerk | TITE- CRM |
24 | Continuous | Other therapy | Academic | One Center |
| 2012 | Tsien | TITE- CRM |
38 | 5 | Radiotherapy | Academic | One Center |
| 2012 | Foster | Novel | 20 | 5 | Targeted therapy | Academic | Multicenter |
| 2013 | Angevin | EWOC | 19 | Continuous | Targeted therapy | Industry | Multicenter |
| 2013 | Cannon | Novel | 79 | Various | Radiotherapy | Academic | One Center |
| 2013 | Chiappella | CRM | 21 | 4 | Other therapy | Academic | Multicenter |
| 2013 | DeAngelo | EWOC | 39 23 28 20 |
Continuous | Targeted therapy | Industry | Multicenter |
| 2013 | Finn | EWOC | 25 | Continuous | Targeted therapy | Industry | Multicenter |
| 2013 | Harvey | EWOC | 30 | Continuous | Targeted therapy | Academic | One Center |
| 2013 | Larocca | CRM | 24 | 4 | Other therapy | Academic | Multicenter |
| 2013 | Mangiacavalli | CRM | 24 | 2 | Chemotherapy | Academic | One center |
| 2013 | Schott | TITE- CRM |
30 | 4 | Targeted therapy | Academic | Multicenter |
| 2013 | Sessa | EWOC | 101 | Continuous | Targeted therapy | Industry | Multicenter |
| 2013 | Sharma | EWOC | 22 6 19 8 |
5 5 3 2 |
Targeted therapy | Industry | Multicenter |
| 2013 | Thornton | CRM | 15 | 5 | Targeted therapy | Industry | One Center |
| 2013 | Cruz | CRM | 8 | 3 | Immunotherapy | Academic | One Center |
| 2014 | Besse | TITE- CRM |
22 18 |
4 4 |
Targeted therapy | Industry | Multicenter |
| 2014 | Das | CRM | 18 | 4 | Chemotherapy | Academic | One Center |
| 2014 | Fanale | EWOC | 111 | Continuous | Targeted therapy | Industry | Multicenter |
| 2014 | Gandhi | Novel | 60 | 16 | Targeted therapy | Industry | Multicenter |
| 2014 | Brennan | EWOC | 16 | 7 | Chemotherapy | Industry | One Center |
| 2014 | Isakoff | Novel | 32 | 9 | Targeted therapy | Industry | Multicenter |
| 2014 | Brana | Novel | 47 | 4 2 |
Targeted therapy | Industry | Multicenter |
| 2014 | Iyer | EWOC | 26 | 4 | Targeted therapy | Industry | Multicenter |
| 2014 | Tsimberidou | Novel | 27 | 3 | Chemotherapy | Industry | One Center |
| 2014 | Infante | EWOC | 53 | Continuous | Targeted therapy | Industry | Multicenter |
| 2014 | Doi | EWOC | 31 | 7 | Targeted therapy | Industry | Multicenter |
| 2014 | Rodon | EWOC | 83 | 7 | Targeted therapy | Industry | Multicenter |
| 2014 | Boulin | CRM | 21 | 5 | Chemotherapy | Academic | One Center |
| 2014 | Faderl | CRM | 30 20 |
4 | Chemotherapy | Academic | One Center |
| 2014 | Rodon | EWOC | 73 30 |
7 3 |
Targeted therapy | Academic | Multicenter |
| 2014 | Saura | EWOC | 17 | 2 | Targeted therapy | Industry | Multicenter |
| 2014 | Shaw | EWOC | 130 | 6 | Targeted therapy | Industry | Multicenter |
| 2014 | Ando | EWOC | 15 | 3 | Targeted therapy | Industry | One Center |
| 2014 | Khouri | CRM | 56 | 4 | Chemotherapy | Academic | One Center |
| 2014 | Kobayashi | CRM | 18 15 |
6 4 |
Chemotherapy | Industry | Multicenter |
| 2014 | Popovtzer | CRM | 25 | 5 | Chemotherapy | Academic | One Center |
| 2014 | Sharma | EWOC | 40 33 |
7 6 |
Targeted | Industry | Multicenter |
Abbreviation: CRM, Continual Reassessment Method; EWOC; Escalation with Overdose Control
Evaluable N, Multiple rows indicate trials with multiple arms
MTD drug type ‘Other’ category includes one unspecified agent
References
- Babb J, Rogatko A, Zacks S. Cancer phase I clinical trials: efficient dose escalation with overdose control. Statistics in Medicine. 1998;17:1103–1120. doi: 10.1002/(sici)1097-0258(19980530)17:10<1103::aid-sim793>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Blay JY, Rutkowski P. Adherence to imatinib therapy in patients with gastrointestinal stromal tumors. Cancer Treatmeant Reviews. 2014;40:242–247. doi: 10.1016/j.ctrv.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Braun TM. The bivariate continual reassessment method. extending the CRM to phase I trials of two competing outcomes. Control Clinical Trials. 2002;23:240–256. doi: 10.1016/s0197-2456(01)00205-7. [DOI] [PubMed] [Google Scholar]
- Cannistra SA. Challenges and pitfalls of combining targeted agents in phase I studies. Journal of Clinical Oncology. 2008;26:3665–3667. doi: 10.1200/JCO.2008.17.2676. [DOI] [PubMed] [Google Scholar]
- Chen YB, Li S, Lane AA, Connolly C, Del Rio C, Valles B, Curtis M, Ballen K, Cutler C, Dey BR, El-Jawahri A, Fathi AT, Ho VT, Joyce A, McAfee S, Rudek M, Rajkhowa T, Verselis S, Antin JH, Spitzer TR, Levis M, Soiffer R. Phase I trial of maintenance sorafenib after allogeneic hematopoietic stem cell transplantation for fms-like tyrosine kinase 3 internal tandem duplication acute myeloid leukemia. Biology of Blood and Marrow Transplant. 2014;20:2042–2048. doi: 10.1016/j.bbmt.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B, Lee SM. On the consistency of the continual reassessment method with multiple toxicity constraints. Journal of Statistical Planning and Inference. 2015;164:1–9. [Google Scholar]
- Cheung YK, Chappell R. Sequential designs for phase I clinical trials with late-onset toxicities. Biometrics. 2000;56:1177–1182. doi: 10.1111/j.0006-341x.2000.01177.x. [DOI] [PubMed] [Google Scholar]
- Dragalin V, Fedorov V. Adaptive designs for dose-finding based on efficacy–toxicity response. Journal of Statistical Planning and Inference. 2006;136:1800–1823. [Google Scholar]
- Ghamande S, Lin CC, Cho DC, Shapiro GI, Kwak EL, Silverman MH, Tseng Y, Kuo MW, Mach WB, Hsu SC, Coleman T, Yang JC, Cheng AL, Ghalib MH, Chuadhary I, Goel S. A phase 1 open-label, sequential dose-escalation study investigating the safety, tolerability, and pharmacokinetics of intravenous TLC388 administered to patients with advanced solid tumors. Investigational New Drugs. 2014;32:445–451. doi: 10.1007/s10637-013-0044-7. [DOI] [PubMed] [Google Scholar]
- Gonen M. Bayesian clinical trials: no more excuses. Clinical Trials. 2009;6(3):203–204. doi: 10.1177/1740774509105374. [DOI] [PubMed] [Google Scholar]
- Goodman SN, Zahurak ML, Piantadosi S. Some practical improvements in the continual reassessment method for phase I studies. Statistics in Medicine. 1995;14:1149–1161. doi: 10.1002/sim.4780141102. [DOI] [PubMed] [Google Scholar]
- Green S, Benedetti J, Smith A, Crowley J. Clinical Trials in Oncology. 3rd. Chapman and Hall/ CRC Press; 2012. [Google Scholar]
- Iasonos A, O'Quigley J. Adaptive dose-finding studies: a review of model-guided phase I clinical trials. Journal of Clinical Oncology. 2014;32:2505–2511. doi: 10.1200/JCO.2013.54.6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova A. A new dose-finding design for bivariate outcomes. Biometrics. 2003;59:1003–1009. doi: 10.1111/j.0006-341x.2003.00115.x. [DOI] [PubMed] [Google Scholar]
- Ivanova A. Escalation, group and A+B designs for dose-finding trials. Statistics in Medicine. 2006;25(21):3668–3678. doi: 10.1002/sim.2470. [DOI] [PubMed] [Google Scholar]
- Ivanova A, Chung Y. Cumulative cohort design for dose-finding. Journal of Statistical Planning and Inference. 2007;137:2316–2327. [Google Scholar]
- Ivanova A, Wang A, Foster M. The rapid enrollment design for Phase I clinical trials. Statistics in Medicine. 2016;35(15):2516–2524. doi: 10.1002/sim.6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaki T. Uptake of novel statistical methods for early-phase clinical studies in the UK public sector. Clinical Trials. 2013;10(2):344–346. doi: 10.1177/1740774512474375. [DOI] [PubMed] [Google Scholar]
- Korn EL, Arbuck SG, Pluda JM, Simon R, Kaplan RS, Christian MC. Clinical trial designs for cytostatic agents: are new approaches needed? Journal Clinical Oncology. 2001;19:265–272. doi: 10.1200/JCO.2001.19.1.265. [DOI] [PubMed] [Google Scholar]
- Lee SM, Cheng B, Cheung YK. Continual reassessment method with multiple toxicity constraints. Biostatistics. 2011;12:386–398. doi: 10.1093/biostatistics/kxq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Tourneau C, Lee JJ, Siu LL. Dose escalation methods in phase I cancer clinical trials. Journal National Cancer Institute. 2009;101:708–720. doi: 10.1093/jnci/djp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Tourneau C, Razak AR, Gan HK, Pop S, Dieras V, Tresca P, Paoletti X. Heterogeneity in the definition of dose-limiting toxicity in phase I cancer clinical trials of molecularly targeted agents: a review of the literature. European Journal of Cancer. 2011;47:1468–1475. doi: 10.1016/j.ejca.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Mandrekar SJ, Cui Y, Sargent DJ. An adaptive phase I design for identifying a biologically optimal dose for dual agent drug combinations. Statistics in Medicine. 2007;26:2317–2330. doi: 10.1002/sim.2707. [DOI] [PubMed] [Google Scholar]
- Mandrekar SJ. Dose-finding trial designs for combination therapies in oncology. Journal of Clinical Oncology. 2014;32:65–67. doi: 10.1200/JCO.2013.52.9198. [DOI] [PubMed] [Google Scholar]
- Minasian L, Rosen O, Auclair D, Rahman A, Pazdur R, Schilsky RL. Optimizing dosing of oncology drugs. Clinical Pharmacology Therapeutics. 2014;96:572–579. doi: 10.1038/clpt.2014.153. [DOI] [PubMed] [Google Scholar]
- Moreno Garcia V, Olmos D, Gomez-Roca C, Cassier PA, Morales-Barrera R, Del Conte G, Gallerani E, Brunetto AT, Schöffski P, Marsoni S, Schellens JH, Penel N, Voest E, Evans J, Plummer R, Wilson RH, Soria JC, Tabernero J, Verweij J, Kaye SB. Dose-response relationship in phase i clinical trials: a European Drug Development Network (EDDN) Collaboration Study. Clinical Cancer Research. 2014;20:5663–5671. doi: 10.1158/1078-0432.CCR-14-0719. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute: NIH. Common Terminology Criteria for Adverse Events (CTCAE). v4.0. CTEP. 2009 [Google Scholar]
- National Cancer Institute NCI. Fact Sheet - Targeted Cancer Therapies. 2014 Web: https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/targeted-therapies-fact-sheet.
- Storer BE. Design and analysis of phase I clinical trials. Biometrics. 1989;45:925–937. [PubMed] [Google Scholar]
- O'Quigley J, Pepe M, Fisher L. Continual reassessment method: a practical design for phase 1 clinical trials in cancer. Biometrics. 1990;46:33–48. [PubMed] [Google Scholar]
- O’Quigley J, Shen L. Continual reassessment method: A likelihood approach. Biometrics. 1996;52:673–684. [PubMed] [Google Scholar]
- Parulekar WR, Eisenhauer EA. Phase I trial design for solid tumor studies of targeted, non-cytotoxic agents: theory and practice. Journal National Cancer Institute. 2004;96:990–997. doi: 10.1093/jnci/djh182. [DOI] [PubMed] [Google Scholar]
- Piantadosi S, Fisher JD, Grossman S. Practical implementation of a modified continual reassessment method for dose-finding trials. Cancer Chemotherapy Pharmacology. 1998;41:429–436. doi: 10.1007/s002800050763. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. Available from: http://www.r-project.org/ [Google Scholar]
- Riviere MK, Le Tourneau C, Paoletti X, Dubois F, Zohar S. Designs of drug-combination phase I trials in oncology: a systematic review of the literature. Annals of Oncology. 2015;26:669–674. doi: 10.1093/annonc/mdu516. [DOI] [PubMed] [Google Scholar]
- Rogatko A, Schoeneck D, Jonas W, Tighiouart M, Khuri FR, Porter A. Translation of innovative designs into phase I trials. Journal Clinical Oncology. 2007;25:4982–4986. doi: 10.1200/JCO.2007.12.1012. [DOI] [PubMed] [Google Scholar]
- Simon R, Freidlin B, Rubinstein L, Arbuck SG, Collins J, Christian MC. Accelerated titration designs for phase I clinical trials in oncology. Journal of the National Cancer Institute. 1997;89:1138–1147. doi: 10.1093/jnci/89.15.1138. [DOI] [PubMed] [Google Scholar]
- Skolnik JM, Barrett JS, Jayaraman B, Patel D, Adamson PC. Shortening the timeline of pediatric phase I trials: the rolling six design. Journal of Clinical Oncology. 2008;26:190–195. doi: 10.1200/JCO.2007.12.7712. [DOI] [PubMed] [Google Scholar]
- Soria JC. Phase 1 trials of molecular targeted therapies: are we evaluating toxicities properly? Eur Journal Cancer. 2011;47:1443–1445. doi: 10.1016/j.ejca.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Thall PF, Cook JD. Dose-finding based on efficacy-toxicity trade-offs. Biometrics. 2004;60:684–693. doi: 10.1111/j.0006-341X.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- Verweij J, Disis ML, Cannistra SA. Phase I studies of drug combinations. Journal of Clinical Oncology. 2010;28:4545–4546. doi: 10.1200/JCO.2010.30.6282. [DOI] [PubMed] [Google Scholar]
- Wages NA, Conaway MR. Phase I/II adaptive design for drug combination oncology trials. Statistics in Medicine. 2014;33:1990–2003. doi: 10.1002/sim.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigertz A, Ahlgren J, Holmqvist M, Fornander T, Adolfsson J, Lindman H, Bergkvist L, Lambe M. Adherence and discontinuation of adjuvant hormonal therapy in breast cancer patients: a population-based study. Breast Cancer Research Treatments. 2012;133:367–373. doi: 10.1007/s10549-012-1961-4. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Chappell R, Bailey H. The Continual Reassessment Method for Multiple Toxicity Grades: A Bayesian Quasi-Likelihood Approach. Biometrics. 2007;63:173–179. doi: 10.1111/j.1541-0420.2006.00666.x. [DOI] [PubMed] [Google Scholar]
- Zhang W, Sargent DJ, Mandrekar S. An adaptive dose-finding design incorporating both toxicity and efficacy. Statistics in Medicine. 2006;25:2365–2383. doi: 10.1002/sim.2325. [DOI] [PubMed] [Google Scholar]