Abstract
Aim: Abnormal daily sleep duration and quality have been linked to hypertension, diabetes, stroke, and overall cardiovascular disease (CVD) morbidity & mortality. However, the relationship between daily sleep duration and quality with subclinical measures of CVD remain less well studied. This systematic review evaluated how daily sleep duration and quality affect burden of subclinical CVD in subjects free of symptomatic CVD.
Methods: Literature search was done via MEDLINE, EMBASE, Web of Science until June 2016 and 32 studies met the inclusion criteria. Sleep duration and quality were measured either via subjective methods, as self-reported questionnaires or Pittsburg Sleep Quality Index (PSQI) or via objective methods, as actigraphy or polysomnography or by both. Among subclinical CVD measures, coronary artery calcium (CAC) was measured by electron beam computed tomography, Carotid intima-media thickness (CIMT) measured by high-resolution B-mode ultrasound on carotid arteries, endothelial/microvascular function measured by flow mediated dilation (FMD) or peripheral arterial tone (PAT) or iontophoresis or nailfold capillaroscopy, and arterial stiffness measured by pulse wave velocity (PWV) or ankle brachial index (ABI).
Results: Subjective short sleep duration was associated with CAC and CIMT, but variably associated with endothelial dysfunction (ED) and arterial stiffness; however, subjective long sleep duration was associated with CAC, CIMT and arterial stiffness, but variably associated with ED. Objective short sleep duration was positively associated with CIMT and variably with CAC but not associated with ED. Objective long sleep duration was variably associated with CAC and CIMT but not associated with ED. Poor subjective sleep quality was significantly associated with ED and arterial stiffness but variably associated with CAC and CIMT. Poor objective sleep quality was significantly associated with CIMT, and ED but variably associated with CAC.
Conclusions: Overall, our review provided mixed results, which is generally in line with published literature, with most of the studies showing a significant relationship with subclinical CVD, but only some studies failed to demonstrate such an association. Although such mechanistic relationship needs further evaluation in order to determine appropriate screening strategies in vulnerable populations, this review strongly suggested the existence of a relationship between abnormal sleep duration and quality with increased subclinical CVD burden.
Keywords: Sleep duration, Sleep quality, Subclinical cardiovascular disease, Coronary Artery Calcium (CAC), Carotid intima media thickness (CIMT), Endothelial function, Flow Mediated Dilation (FMD), Arterial stiffness, Pulse Wave Velocity (PWV)
Introduction
A good hygienic sleep is an essential part of everyday life. It is estimated that humans spend one third of their lifetime sleeping. An estimated 50–70 million Americans suffer from sleep disorders, yet only 20% report it to their physicians1). Poor sleep may be a risk factor for cardiovascular disease (CVD) and has serious biological consequences2). A growing body of literature suggests a relationship of sleep parameters (sleep duration and sleep quality) with CVD, stroke, and all-cause morbidity & mortality3). A recent review of 23 studies determined that both short and long sleep is related to increased risk for all-cause mortality among both men and women, compared to individuals who report a medium amount of daily sleep (7–8 hours)4), however, some studies have shown that the sleep disturbances may be associated with CVD mortality with weaker evidence for women5). Additionally, acute and chronic sleep deprivation can lead to weight gain, impaired glucose tolerance, activate pro-inflammatory pathways, increased sympathetic activity and higher cortisol levels in healthy subjects6).
The epidemiological data suggests strong association of subjectively and/or objectively measured sleep parameters with classical CVD risk factors like hypertension, insulin resistance, hypercholesterolemia, type 2 diabetes and increase in the inflammatory markers leading to subclinical and clinical CVD7–10). The traditional risk stratification has relied mainly on scoring systems such as the Framingham risk score to determine the CVD risks11). However, newer non-invasive techniques that assess coronary artery calcium (CAC), carotid intima-media thickness (CIMT), flow-mediated dilation (FMD), Ankle Brachial Index (ABI) and pulse wave velocity (PWV) provide refined assessment of early atherosclerosis process, endothelial dysfunction and arterial stiffness potentially leading to clinical CVD events. The extent of CAC and CIMT correlates with plaque burden and atherosclerosis, decreased FMD correlates with endothelial dysfunction, decreased ABI but increased PWV measure increasing arterial stiffness12–15). There are some studies that show relationship of these non-invasive subclinical CVD markers with disturbed sleep parameters16, 17) and signify the importance of regular sleep habits but such relationships between the sleep parameters and subclinical CVD markers need to be studied in more detail. As early recognition of subclinical CVD in people with sleep disturbances may impact risk stratification and early treatment of subclinical CVD before the development of clinical CVD events.
The purpose of this systematic review is to evaluate the following: First the relationship between self-reported or/and objectively assessed sleep duration with markers of subclinical CVD. Second, the relationship between self-reported or/and objectively assessed sleep quality with markers of subclinical CVD. Third, the effect of acute and chronic sleep deprivation on subclinical CVD risks. Such association of sleep parameters with subclinical CVD would provide the importance of regular sleep habits and would provide evidence to the stakeholders to consider sleep disturbances as important risk factors for determination of future risk of CVD events. This systematic review may also highlight the role of noninvasive cardiovascular diagnostic procedures in those with sleep disturbances.
Methods
An electronic systematic literature search was performed using the EMBASE (via EMTREE) and Medline database (National Library of Medicine, Bethesda, MD via PubMed) for relevant literature up to March 2016 (Fig. 1). We used both MeSH and Emtree terms and relevant free text terms. The following search terms (synonyms and combinations) were used: “sleep duration” and/or “sleep quality”. These terms were combined with “sub clinical cardiovascular disease risks”, “coronary artery calcium (CAC)”, “carotid intima-media thickness (CIMT)”, “endothelial function and/or microvascular function via flow mediated dilation (FMD) and/or peripheral arterial tone (PAT) and/or iontophoresis and/or nailfold capillaroscopy”, and “arterial stiffness via pulse wave velocity (PWV)”, and/or ankle brachial index (ABI)”.
Fig. 1.
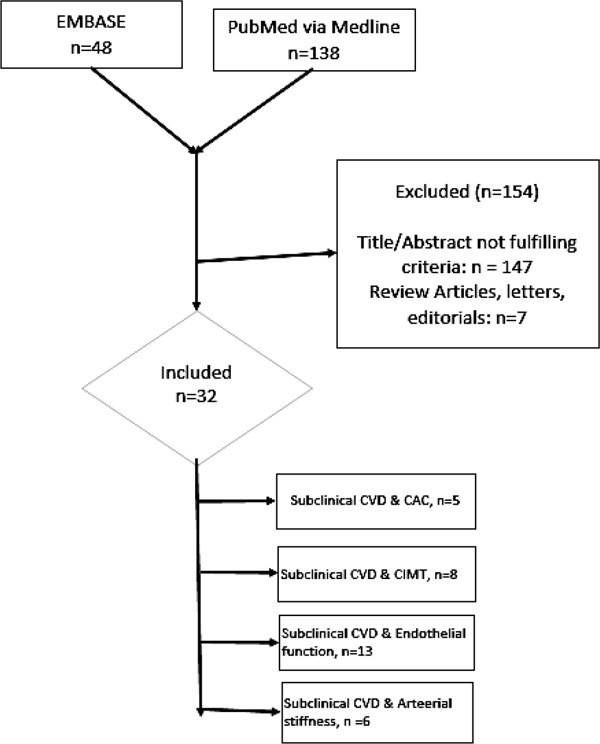
Literature Search Strategy
CVD: Cardiovascular disease, CAC: Coronary artery calcium, CIMT: Carotid intimamedia thickness.
The search results were manually scanned for relevant articles by three independent reviewers. References of obtained articles were used for additional articles. The search was limited to original research studies on human subjects published in English language. We included (1) original research articles, AND (2) studies that reported subjective and/or objective sleep duration AND/OR (3) studies that reported subjective and/or objective sleep quality, AND (4) studies that reported PSQI good vs PSQI bad sleep quality and/or insomnia vs. non-insomnia and/or shift vs. non-shift work and/or arousal index and/or sleep efficiency (%) and/or sleep Fragmentation index and/or REM/Non-REM sleep, AND (5) studies that reported an assessment of Non-Invasive Subclinical CVD assessment tools as Coronary Artery Calcium (CAC) and/or Carotid Intima Media Thickness (CIMT) and/or Endothelia function and/or Arterial stiffness. The aim of this review was to summarize the evidence of regular daily sleep duration and sleep quality with subclinical CVD, so we excluded any study that discussed the association of sleep disorders and clinical cardiovascular and cerebrovascular diseases. Many research studies have shown that sleep disorders like obstructive sleep apnea has been previously shown to be strongly associated with subclinical CVD18). In our exclusion criteria, we excluded (1) the letters, editorials (2) the studies on subjects with sleep disorders [obstructive sleep apnea (OSA), parasomnias, and narcolepsy] (3) the studies that discussed clinical CVDs (angina, myocardial infarction and heart failure) and cerebrovascular diseases (stroke) (4) the studies that that have discussed the effect of mental disorders on sleep duration and sleep quality (5) the studies in which participants used any kind of sleeping pills. Thirty-two studies met the inclusion criteria for final review from an initial search result of 186 articles (Fig. 1 for search algorithm). The authors stated that no financial support was given and that no off-label or investigational use of a drug or device was performed as part of this research.
Sleep duration typically defines as “the total amount of sleep obtained, either during the nocturnal sleep episode or across the 24-hour period”19). In the studies discussed in this review, the sleep duration was measured either via subjective methods, including self-reported questionnaires and/or Pittsburg Sleep Quality Index (PSQI) or via objective measures, which include actigraphy and/or polysomnography or by both subjective and objective methods20, 21). The subjective sleep quality is defined as “one's perception that they fall asleep easily, get sufficient duration so as to wake up feeling rested, and can make it through their day without experiencing excessive daytime sleepiness” 22). Using actigraphy to objectively measure sleep quality, the objective sleep quality is defined by sufficient duration (> 7 hrs), high efficiency (> 85%), and low fragmentation (< 25)23). Using polysomnography (PSG) the sleep architecture is added to the equation, whereby the objective sleep quality is defined not only by sufficient duration, high efficiency, and low fragmentation, but also by proper staging of sleep (i.e., cycling through non-REM (stages 1–4) and REM)23). In this review, the sleep quality was measured via subjective methods, including Pittsburg Sleep Quality Index (PSQI) and/or self-reporting of insomnia vs non-insomnia, shift vs non-shift work, arousal index, or via objective measures, which include actigraphy and/or polysomnography or by both subjective and objective methods20, 21). The Pittsburgh Sleep Quality Index (PSQI) is an effective tool used to measure the quality and patterns of sleep in adults. It differentiates “poor” from “good” sleep quality by measuring seven sleep components that include subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medications, and daytime dysfunction over the last month24). Sleep fragmentation is defined as “an index of restlessness during the sleep period expressed as a percentage”. It is calculated as movement index, a fragmentation index, and total sleep fragmentation Index. The higher the sleep fragmentation index, the more sleep is disrupted25).
Among subclinical cardiovascular disease (CVD) measures, the coronary artery calcium (CAC) was measured by either electron beam computed tomography (EBCT) or multidetector computed tomographic (MDCT) scanner. Increased calcium deposition in coronary arteries is related to the presence of atherosclerotic process and increased plaque burden12). The carotid intima-media thickness (CIMT) was measured using high-resolution B-mode ultrasonography on carotid arteries and increased intima media thickness in carotid artery is related to carotid atherosclerosis and future increased risk of CVD13). The endothelial function and/or microvascular function was measured by flow mediated dilation (FMD) and/or peripheral arterial tone (PAT) and/or iontophoresis and/or nailfold capillaroscopy. The FMD is based on an ultrasound test that measures the changes in the arterial diameter based on their blood flow such as the brachial, radial or femoral arteries. The decrease in the flow mediated dilatation is closely related to endothelial cells dysfunction that leads to increased risk of vessels wall injury causing both the early and late mechanisms of atherosclerosis that include increased leukocyte adherence, platelet activation, and vascular smooth muscle proliferation and decreased production of nitric oxide14). The PAT is calculated by EndoPAT device (Itamar Medical, Israel) that involves measuring the pulse amplitude in the fingertip at rest and following the induction of reactive hyperemia. The EndoPAT device consists of a fingertip plethysmograph capable of sensing volume changes in the digit with each arterial pulsation. The volume changes in the fingertip are documented digitally as pulse amplitude that can be tracked over time. The expected response is a post occlusion increase of the PAT signal amplitude and the PAT score is provided automatically by the system's software and is basically the ratio between the post- to pre-occlusion average signal size, corrected for systemic changes and baseline level26). Iontophoresis of acetylcholine (ACh) and sodium nitroprusside (SNP) is a tool used to determine microvascular endothelial function. Two laser-Doppler probes specifically designed with an embedded sponge for skin iontophoresis of agonists are fixed 5 cm apart to the skin with an adhesive patch to measure the endothelium-dependent and endothelium-independent vasodilatation to determine the endothelial function27). The nailfold capillary video microscopy of the dorsal skin of the third finger is used to determine the endothelial function. The measurements are conducted after at least 30 min of rest and with a minimum hand temperature of 28°C. The microvascular measurements include the duplicate measurements of capillary density at the base of the interphalanx at rest and after 4 min of arterial occlusion28). The arterial stiffness was measured via pulse wave velocity (PWV), and/or ankle brachial index (ABI). The PWV is the time the arterial pressure wave takes to travel a distance between two arterial sites and is measured distance (cm)/time (seconds). Higher value of PWV is directly correlated to the arterial stiffness and when measured over the aorta, it represents an independent risk factor for increased cardiovascular disease15). There are different methods to measure the PWV that include brachial artery PWV (baPWV), carotid-femoral PWV (cfPWV), heart-femoral PWV (hfPWV) and femoral-ankle PWV (faPWV) and these reflect properties of central, peripheral or mixed properties of both central and peripheral arterial stiffness. All studies used in this review discussed the relationship of sleep parameters with baPWV which is considered as an important parameter of both central and peripheral arterial stiffness29). ABI is a measure reflective of systemic atherosclerosis and predict the stiffness in the vessels. The ABI is the ratio of the systolic blood pressure at the ankle to the average systolic blood pressure at the right arm. The lower the value of ABI below 1.0 correlates with the severity of arterial stiffness30).
Results
Thirty-two studies met the final selection criteria and discussed the relationship of subjective (self-report) and objective (actigraphy and/or polysomnography) sleep duration as well as subjective and objective sleep quality with non-invasive markers of subclinical CVD. Sleep duration and sleep quality are two distinct parameters of sleep, and these are discussed separately along with each non-invasive marker of subclinical CVD in respective tables. Five studies evaluated association of sleep parameters with coronary artery calcium (CAC)17, 31–34), eight evaluated association with carotid intima-media thickness (CIMT)2, 35–41), thirteen evaluated association with endothelial function42–54), and six studies assessed relationship with arterial stiffness17, 55–58). Kim et al. 2015 studied both the subjective sleep duration and subjective sleep quality with both CAC as well as PWV in same paper so the results of CAC and PWV outcomes were stated separately in the relevant tables17). Table 1 shows the baseline characteristics of all the studies used in this review.
Table 1. Baseline characteristics of studies used in systematic review.
| First Author, Publication Year, | Country | Study Design | Study Population N (age;% female) | Sleep Measurement (Subjective/Self-reported) | Sleep Measurement (Objective) | Non-invasive CVD measurement |
|---|---|---|---|---|---|---|
| King et al. (33), 2008 | United States | Cohort | 495 (40 ± 4; 59%) | Self-Report (PSQI) | Actigraphy | CAC (Agatston score)- electron beam CT |
| Matthews et al. (31), 2011 | United States | Cross-sectional | 195 (60; 50%) | Self-Report (PSQI) | Actigraphy and PSG | CAC (Agatston score)- electron beam CT |
| Matthews et al. (32), 2013 | United States | Cross-sectional | 512 (50 ± 3; 100%) | Self-Report (PSQI) | NR | CAC (Agatston score)- electron beam CT |
| Lutsey et al (34), 2015 | United States | Cross-sectional | 1465 (68; 54%) | NR | Actigraphy and PSG | CAC (Agatston score)- electron beam CT |
| Kim et al, 2015 (17) | Korea | Cross-sectional | 29203 (41.8 ± 7.3; 18.6%) | Self-Report (PSQI) | NR | CAC (Agatston score)- electron beam CT |
| Wolff et al. (35), 2008 | Germany | Cross-sectional | 2383 (45 − 81; 51%) | Self-Report | NR | CIMT (mm)-B-mode ultrasound |
| Abe et al. (36), 2011 | Japan | Cross-Sectional | 2214 (64 ± 10; 52%) | Self-Report questionnaire | NR | CIMT (mm)-B-mode ultrasound |
| Nakazaki et al. (38), 2012 | Japan | Cross-Sectional | 86 (74 ± 5; 71%) | Self-Report (PSQI), | Actigraphy | CIMT (mm)-B-mode ultrasound |
| Sands et al. (39), 2012 | United States | Cohort | 617 (37 − 52; 58%) | NR | Actigraphy | CIMT (mm)-B-mode ultrasound |
| Nagai et al. (2), 2013 | Japan | Cross-Sectional | 201 (80 ± 6; 75%) | Self-Report questionnaire | NR | CIMT (mm)-B-mode ultrasound |
| Ma et al. (37), 2013 | United States | Cross-sectional | 257 (42 ± 9; 26%) | Self-Report (PSQI) | Actigraphy | CIMT (mm)-B-mode ultrasound |
| Ramos-Sepulveda et al. (41), 2010 | United States | Cross-sectional | 1605 (65 ± 8; 60%) | Self-Report (HRSD) | NR | CIMT (mm)-B-mode ultrasound |
| Schwartz et al. (40) 2102 | United States | Cross sectional | 126 (55; 89%) | NR | Actigraphy | CIMT (mm)-B-mode ultrasound |
| Behl et al. (43), 2014 | United States | Cohort | 684 (48 ± 11; 68%) | Self-Report (PSQI and ESS) | EF-FMD | |
| Cooper et al. (42), 2014 | United States | Cross-sectional | 100 (36 ± 10; 43%) | Self-Report (PSQI) and PSG | EF-FMD | |
| Calvin et al. (44), 2014 | United States | Cross-sectional | 16 (18 to 40; 37.5%) | NR | PSG | EF-FMD |
| Takase et al, 2004 (45) | Japan | Cross-sectional | 30 (21.7 ± 1.1; 0%) | Self-Report | NR | EF-FMD |
| Wehrens et al, 2012 (46) | United Kingdom | Cross-sectional | 25 (25 − 45; 0%) | Self-report questionnaire | NR | EF-FMD |
| Schmidt et al, 2013 (47) | Germany | Cross-sectional | 75 (20 − 54; 61.3%) | NR | Actigraphy | EF-FMD |
| Strand et al. (48), 2012 | Norway | Cross-sectional | 4739 (50 ± 13; 45.2%) | Self-report questionnaire | NR | EF-FMD |
| Suessenbacher et al. (49), 2011 | Austria | Cross-sectional | 48 (43 ± 5; 0%) | Self-report questionnaire | NR | EF-PAT index |
| Dettoni et al. (50), 2012 | Brazil | Cross-sectional | 13 (31 ± 2; 0%) | NR | Actigraphy | EF (dorsal hand vein technique) |
| Sauvet et al. (51), 2009 | France | Cross-sectional | 12 (29 ± 3; 0%) | Self-report | NR | EF (Iontophoresis of Acetylcholine & Na- nitroprusside) |
| Bonsen et al. (52), 2015 | Netherlands | Cross-sectional | 259 (42; 55%) | Self-report (SWEL) | NR | EF-Nailfold capillaroscopy |
| Weil et al. (53), 2010 | United States | Cross-sectional | 80 (56.6 ± 1.2; 39%) | Self-Report | NR | EF-Endothelin (ET)-1 levels |
| Weil et al. (54), 2011 | United States | Cross-sectional | 37 (58 ± 1.5; 41%) | Self-Report | NR | EF-Endothelial progenitor cells (EPCs) functions |
| Yoshioka et al. (55), 2011 | Japan | Cross-sectional | 4268 (48 ± 7; 20%) | Self-Report | NR | AS-baPWV |
| Sunbul et al. (56), 2014 | Turkey | Cross-sectional | 42 (30 ± 5; 57%) | Self-report questionnaire | NR | AS-baPWV |
| Tsai et al. (57), 2014 | Taiwan | Cross-sectional | 3, 508 (age 20 − 87, 40%) | Self-Report | NR | AS-baPWV |
| Osonoi et al, 2015 (68) | Japan | Cross-sectional | 724 (57.8 ± 8.6; 37.1%) | Self-Report (PSQI) | NR | AS-baPWV |
| Yamaki et al. 2015, | Japan | Cross-sectional | 101 (70.0 ± 10; 46.5%) | Self-Report (PSQI) | NR | AS-ABI |
Abbreviation: NR: Not – reported; SWEL: Sleep Wake Experience List questionnaire
Association of Subjective and Objective Sleep Parameters with Coronary Artery Calcium (CAC)
Table 2a shows the association of subjective and objective sleep duration with CAC. Kim et al. showed that association of subjective sleep duration with CAC is “U” shaped with stronger association of short subjective sleep duration with increased CAC. King et al. showed that objective longer sleep duration is better predictor of CAC as compared to objective shorter sleep duration with 0.66 times increase in CAC with each hour increase in sleep duration. All other studies did not show any significant association of sleep duration with CAC.
Table 2a. Association of Subjective/ Self-reported and Objective Sleep Duration with Coronary Artery Calcium (CAC).
| First Author, Publication Year, Study Type |
Sleep Measurement | Non-Invasive Subclinical CVD assessment tools. |
Results | Comments |
|---|---|---|---|---|
| Subjective/Self-reported Sleep Duration | ||||
| King et al. (33), 2008 | Self-Report (PSQI) | CAC | Logistic regression of incident CAC [OR (95% CI): Self-reported sleep per hour (Adja): 0.87 (0.67–1.13), p = 0.30 |
|
| Matthews et al. (31), 2011 | Self-Report (PSQI), | CAC | Self-reported Sleep duration (hr) among CAC groups: CAC= 0: 6.41 (1.25) CAC= 1 – 99: 6.51 (1.18) CAC= 100 +: 6.48 (1.19), p = 0.88 |
|
| Matthews et al. (32), 2013 | Self-Report (PSQI) | CAC | No significant association between longer self-reported sleep duration and increased CAC in linear (p = 0.19) or logit models (p = 0.06) |
Women only sample |
| Kim et al, 2015 (17) | Self-Report (PSQI) | CAC | Sleep duration vs. CAC Score (Adjb): OR (95% CI) ≤ 5 h: 1.50 (1.17 – 1.93); p: 0.002 6 h: 1.34 (1.10 – 1.63); p: 0.002 7 h: 1.00 (Reference) 8 h: 1.37 (0.99 – 1.89); p: ≥ 0.05 ≥ 9 h: 1.72 (0.90 – 3.28); p: 0.002 |
The association between sleep duration and CAC is “U-shaped”. |
| Objective Sleep Duration | ||||
| King et al. (33), 2008 | Actigraphy | CAC | Logistic regression of incident CAC [OR (95% CI): Actigraph measured sleep per hour (Adjc): 0.66 (0.48 – 0.92), p = 0.01 | |
| Matthews et al. (31), 2011 | Actigraphy, Polysomnography | CAC | Objective sleep duration (hr) among CAC groups: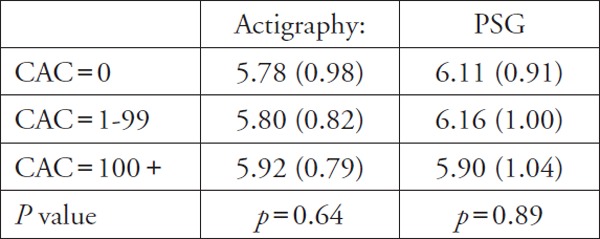
|
Sleep duration both by Actigraphy and PSG was not significant |
| Lutsey et al (34), 2015 | Polysomnography and actigraphy | CAC | Sleep duration (h) vs CAC ≥ 400: PR (95% CI): ≤ 6.65h: 0.75 (0.55–1.03), p = 0.08 6.65–7.4 h: Ref ≥ 7.40h: 0.84 (0.56–1.26), p = 0.40 |
Fully adjusted model is not related to high prevalence of CAC. |
Adjusted for race, sex, age, smoking, education, and apnea risk
Adjusted for age, sex, study center, year of visit, education, marital status, depression, smoking status, alcohol consumption, physical activity, body mass index, fasting glucose, systolic blood pressure, diastolic blood pressure, height, and hear rate.
Adjusted for race, sex, age, smoking, education, apnea risk, BMI, HDL, LDL, BP, DM
The association of sleep quality with CAC is less robust with only one study among the total of three showed that poor sleep quality is associated with increased CAC among women only. Objective sleep quality only in the form of arousal index was directly associated with increased CAC as, higher the arousal index, higher the risk of being in a CAC group of CAC ≥ 400 Agatston units (Table 2b)
Table 2b. Association of Subjective/ Self-reported and Objective Sleep Quality with Coronary Artery Calcium (CAC).
| First Author, Publication Year, Study Type | Sleep Measurement | Non-Invasive Subclinical CVD assessment tools. | Results | Comments |
|---|---|---|---|---|
| Subjective/Self-reported Sleep Quality | ||||
| King et al. (33), 2008 | Self-Report (PSQI) | CAC |
Logistic regression of incident CAC: (OR; 95%CI): PSQI Score (per SD = 2.9 points) (Adjd): 1.21 (0.88–1.65), p = 0.24 Epworth Score (per SD = 4.0 points) (Adjd): 1.26 (0.96, 1.66), p = 0.10 |
|
| Matthews et al. (31), 2011 | Self-Report (PSQI) | CAC | Self-reported sleep quality or efficiency did not differ by CAC groups (p > 0.15) | Apnea/Hypopnea Index was associated with increased CAC scores (p = 0.04). |
| Matthews et al. (32), 2013 | Self-Report (PSQI) | CAC | Poor sleep quality was not associated with odds of being in a higher coronary artery calcification (CAC) group. | Women only |
| Kim et al, 2015 (17) | Self-Report (PSQI) | CAC |
CAC Score by sleep quality: Adjb OR (95% CI): Good vs. Poor sleep quality: Men: 1.00 (Reference) vs. 1.10 (0.86–1.42) Women: 1.00 (Reference) vs. 2.46 (1.30–4.65); p ≤ 0.05 |
Poor subjective sleep quality was associated with CAC in women only in both crude and adjusted model. |
| Objective Sleep Quality | ||||
| King et al. (33), 2008 | Actigraphy | CAC |
Logistic regression of incident CAC: (OR; 95%CI) Fragmentation Index (per SD = 7.7 points)d: 1.07 (0.80, 1.42), p = 0.66 |
|
| Matthews et al. (31), 2011 | Actigraphy, Polysomnography | CAC | Sleep quality or efficiency measures did not differ by CAC groups (CAC = 0 vs CAC > 0); p > 0.05 | |
| Lutsey et al (34), 2015 | Polysomnography and actigraphy | CAC |
Sleep quality vs CAC ≥ 400: PR (95% CI) Arousal index—1.14 (1.02 to 1.27); p = 0.02 Arousal index—REM: 1.15 (1.02 to 1.29); p = 0.02 Arousal index—NREM: 1.14 (1.02 to 1.28); p = 0.03 Average sleep efficiency%—1.00 (0.89 to 1.13); p = 0.97 Average sleep WAS1O—1.03 (0.91 to 1.17); p = 0.62 |
|
Adjusted for race, sex, age, smoking, education, and apnea risk.
Adjusted for age, sex, study center, year of visit, education, marital status, depression, smoking status, alcohol consumption, physical activity, body mass index, fasting glucose, systolic blood pressure, diastolic blood pressure, height, and heart rate.
Association of Subjective and Objective Sleep Parameters with Carotid Intima Media Thickness (CIMT)
Table 3a showed that subjective and objective sleep duration were associated with CIMT with stronger evidence present for shorter sleep duration with increased CIMT as compared with reference sleep hours (6–8 hours). The population used in these studies was heterogeneous with mostly elderly and police officers. Among all studies assessing the CIMT, the sleep duration group of average 7 hours exhibited lowest systolic blood pressure and Hemoglobin A1c.
Table 3a. Association of Subjective/ Self-reported and Objective Sleep Duration with Carotid Intima-Media Thickness (CIMT).
| First Author, Publication Year, Study Type | Sleep Measurement | Non-Invasive Subclinical CVD assessment tools | Results | Comments |
|---|---|---|---|---|
| Subjective/Self-reported Sleep Duration | ||||
| Wolff et al. (35), 2008 | Self-Report questionnaire | CIMT |
Mean difference (mm) in CIMT across hours of sleep: Adje 5h: 0.038 (0.002, 0.074); p < 0.0 6h: 0.007 (−0.012, 0.027); ns) 7h: 0.001 (−0.015, 0.018); ns) 8h: ref group 9h: 0.022 (0.000, 0.045); ns 10h: 0.043 (0.015, 0.070); p < 0.01 > 11h: 0.065 (0.017, 0.113); p < 0.01 |
The association of sleep duration with mean CIMT was J-shaped. The sleep duration group of 7hrs exhibited lowest CIMT, SBP and HbA1c. |
| Abe et al. (36), 2011 | Self-Report questionnaire | CIMT |
Odds ratio for presence of IMT ≥ 1.2 mm: Adj f ≤ 5h: 1.059 (0.764–1.467), p = ≥ 0.05 6h: ref group ≥ 7h: 1.263 (1.031–1.546), p = 0.024 |
The use of hypnotics like benzodiazepines diminished the association between sleep duration and increased CIMT. |
| Nakazaki et al. (38), 2012 | Self-Report (PSQI) | CIMT | Mean CIMT (mm): < 5h sleep vs. > 7h sleep: (1.3 ± 0.5 vs. 0.9 ± 0.3); p = 0.009 Association of sleep duration by PSQI with CIMTg: β = 0.22, p = 0.05 |
Elderly population. |
| Nagai et al. (2), 2013 | Self-Report questionnaire | CIMT |
CIMT (mm) among sleep groups: < 6 hr: 1.03 ± 0.29 ≤ 6–9 hr: 1.01 ± 0.29 ≥ 9 hr: 1.09 ± 0.35, (p = 0.26) |
Elderly population. |
| Ma et al. (37), 2013 | Self-Report (PSQI) | CIMT |
Self-report sleep duration and Mean CIMT (mm): 3.0–4.9h: 0.657 ± 0.024 5.0–5.9h: 0.615 ± 0.012 6.0–6.9h: 0.624 ± 0.011 7.0–7.9h (Reference): 0.617 ± 0.012 8.0–10: 0.607 ± 0.020, (p for all = 0.830) |
Police officers. |
| Objective Sleep Duration | ||||
| Nakazaki et al. (38), 2012 | Actigraphy | CIMT |
Association of total sleep time by Actigraphy with CIMTg: β = −0.32, p = 0.005 Sleep efficiency (%): Insomnia group vs. non-insomnia group (78.6 ± 11.3 vs. 89.3 ± 7.1); p = < 0.0001 |
Elderly population. |
| Sands et al. (39), 2012 | Actigraphy | CIMT |
Linear regression for sleep duration (h) on CIMT (mm) Adjh: OR (95% CI) Male: −0.026 (−0.047, −0.005); p = 0.02 Female: −0.001 (−0.020, 0.022); p = 0.91 |
Model significant in men only. |
| Ma et al. (37), 2013 | Actigraphy | CIMT |
Actigraphy measured sleep duration and mean CIMT mm (SE) (Adji): 1.7–4.9h: 0.633 ± 0.012 5.0–5.9h: 0.617 ± 0.010 6.0–6.9h: 0.623 ± 0.012 7.0–7.9h (Reference): 0.590 ± 0.019 8.0–10.7: 0.625 ± 0.027 (p for all = 0.692) |
Police officers. |
| Schwartz et al. (40) 2102 | Actigraphy | CIMT |
Sleep duration (h) vs. CIMT (mm): β (95% CI)j Nighttime sleep duration: −0.01 (−0.03, 0.01); p ≥ 0.05 Daytime sleep duration: −0.04 (−0.07, −0.01); p ≤ 0.05 |
Study was done on elderly Alzheimer Caregivers |
Adjusted for lifestyle variables include smoking status (number of cigarettes per day), alcohol consumption, physical activity, and shift work; socioeconomic factors include monthly family income, marital status, primary education, vocational status; and biological risk factors include BMI, lipid profile (total cholesterol/HDL cholesterol ratio), diabetes, previous myocardial infarction and hypertension.
Adjusted for age, sex, LDL-cholesterol, HDL-cholesterol, triglyceride, fasting plasma glucose, HbA1c, fasting insulin, BMI, alcohol intake (never, monthly, weekly, daily), and current smoking
Adjusted for age, gender, BMI, SBP, DBP, PSQI, AHI, lowest SpO2, sleep efficiency, total sleep time, hypertension, hyperlipidemia, diabetes, sleep apnea syndrome and insomnia.
Adjusted for age, race/ethnicity, smoking, education, depression, BMI, systolic blood pressure, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and diabetes
Adjusted for age, gender, race/ethnicity, abdominal height, systolic blood pressure, anti-hypertensivemedications, glucose, low-density lipoprotein, high-density lipoprotein, lipid lowering medications, sleep quality (Pittsburgh Sleep Quality Index global score after removing sleep duration component), perceived stress score, depressive symptoms score, physical activity index, smoking status, shiftwork, and having a second job.
Adjusted for age and gender, education, BMI, physical activity, smoking, hypertension, dyslipidemia, diabetes, history of CVD, Role Overload and depression (CESD-10) scores
Table 3b showed that most of the studies discussed the relationship of subjective sleep duration with CIMT. Only one study discussed association of the objective sleep quality with CIMT and it was significant (p ≤ 0.05). Among these studies insomnia was strongly associated with CIMT as compared to non-insomnia. PSQI as a parameter of sleep quality was discussed only in two studies and was significant only in one study (β = 0.22, p = 0.05).
Table 3b. Association of Subjective/ Self-reported and Objective Sleep Quality with Carotid Intima-Media Thickness (CIMT).
| First Author, Publication Year, Study Type | Sleep Measurement | Non-Invasive Sub-clinical CVD assessment tools. | Results | Comments |
|---|---|---|---|---|
| Subjective/Self-reported Sleep Quality | ||||
| Abe et al. (36), 2011 | Self-Report questionnaire | CIMT | Sleep quality was not significantly associated with CIMT (p = ≥ 0.05) | Japanese population. |
| Nakazaki et al. (38), 2012 | Self-Report (PSQI) | CIMT |
Association of sleep quality by PSQI with CIMTk: β = 0.22, p = 0.05 Mean IMT (mm): Insomnia group vs. non-insomnia group (1.3 ± 0.5 vs. 1.1 ± 0.4); p = 0.03 |
Elderly population only. |
| Nagai et al. (2), 2013 | Self-Report questionnaire | CIMT | Mean IMT (mm): Insomnia group vs. non-insomnia group (1.16 ± 0.37 vs. 1.04 ± 0.30); p < 0.01 | Elderly patients. |
| Ma et al. (37), 2013 | Self-Report (PSQI) | CIMT | PSQI sleep quality score & CIMT (mm): Pearson correlation coefficient (r) = −0.0762; p = 0.235 |
Police officers. |
| Ramos-Sepulveda et al (41), 2010 | Self-Report (HRSD) | CIMT |
Association of insomnia with CIMT (mm)Adjl: OR (95% CI) 1.08 (0.70–1.66); β = −0.0012, p = 0.829 |
Multi-ethnic population |
| Objective Sleep Quality | ||||
| Schwartz et al. (40) 2102 | Actigraphy | CIMT |
Sleep quality vs. CIMT (mm): β (95% CI)j Nighttime WASO: 0.04 (0.001, 0.08), p ≤ 0.05 |
Study was done on elderly Alzheimer Caregivers |
Adjusted for age, gender, BMI, SBP, DBP, PSQI, AHI, lowest SpO2, sleep efficiency, total sleep time, hypertension, hyperlipidemia, diabetes, sleep apnea syndrome and insomnia.
Adjusted for age sex, race LDL, HDL BMI hypertension, diabetes, current smoker and any cardiac disease.
Adjusted for age and gender, education, BMI, physical activity, smoking, hypertension, dyslipidemia, diabetes, history of CVD, Role Overload and depression (CESD-10) scores.
Association of Subjective and Objective Sleep Parameters with Endothelial Functions
Table 4a discussed the subjective and objective sleep duration with endothelial functions. Among total of five studies, only one study discussed the objective sleep duration and endothelial functions and it was not significant. Among the other four studies, only two studies showed the significant association of subjective sleep duration with endothelial dysfunctions.
Table 4a. Association of Subjective/ Self-reported and Objective Sleep Duration with Endothelial Function (EF).
| First Author, Publication Year, Study Type | Sleep Measurement | Non-Invasive Subclinical CVD assessment tools. | Results | Comments |
|---|---|---|---|---|
| Subjective/Self-reported Sleep Duration | ||||
| Behl et al. (43), 2014 | Self-Report (PSQI) | EF (FMD) | Total PSQI score was unrelated to brachial artery FMD; p = ≥ 0.05 | |
| Bonsen et al. (52), 2015 | Self-Report questionnaire | EF (nailfold Capillaroscopy) | Sleep duration & endothelial function: regression coefficient 95% CI Men: β = −0.79, 95% CI (−7.20 to 5.63), p = 0.81 Women: β = −11.17, 95% CI (−21.80 to −0.55); p = 0.04 |
Significant only in women |
| Weil et al. (53), 2010 | Self-Report questionnaire | EF [Endothelin (ET)-1 levels] | Sleep duration and endothelial function as ET-1-mediated vasoconstrictor tone: (Correlation) r = −0.32; p < 0.01 | Increased ET-1 vasoconstrictor activity positively correlated with CVD risks. |
| Weil et al. (54), 2011 | Self-Report questionnaire | EF [endothelial progenitor cells (EPCs) functions] | There were no significant correlations between sleep duration and any endothelial progenitor cells (EPCs) measure. | Circulating endothelial progenitor cells (EPCs) are vital to endogenous vascular repair processes and cardiovascular health. |
| Objective Sleep Duration | ||||
| Cooper et al. (42), 2014 | Polysomnography | EF (FMD) | Total sleep time (TST) was not associated with FMD: (Correlation) r = 0.03; p = ≥ 0.05 | |
Table 4b discussed the subjective and objective sleep quality with endothelial functions. Most of the studies showed a significant association of low sleep quality with reduced endothelial functions. The low sleep quality was expressed variably in these studies by PSQI quality scores, acute sleep deprivation, difficulties initiating sleep or maintaining sleep, REM sleep, REM latency, noise level during sleep, chronic stress vs no stress, shift vs. non-shift workers.
Table 4b. Association of Subjective/ Self-reported and Objective Sleep Quality with Endothelial Function (EF).
| First Author, Publication Year, Study Type | Sleep Measurement | Non-Invasive Sub-clinical CVD assessment tools. | Results | Comments |
|---|---|---|---|---|
| Subjective/ Self-reported Sleep Quality | ||||
| Behl et al. (43), 2014 Cohort study | Self-Report (PSQI) | EF (FMD) | Low PSQI quality component was associated with lower FMD; p = 0.038 Higher ESS scores were associated with lower FMD; p = 0.026 |
|
| Cooper et al. (42), 2014 Cross-sectional | Self-Report (PSQI) | EF (FMD) |
Total PSQI Scores and FMD%:
β = −0.22; p = 0.024 % REM sleep and FMD%: β = 0.25; p = 0.022 REM latency and FMD%: β = −0.2; p = 0.036 |
|
| Takase et al, 2004 (45) | Self-report | EF (FMD) | FMD (%): Controls vs. Chronic stress: 7.4 ± 3.0 vs 3.7 ± 2.3; p < 0.05 |
Only male sample |
| Wehrens et al, 2012 (46) | Self-report questionnaire | EF (FMD) | There were no significant effects of day, time, group, or interactions on%FMD among both shift and non-shift workers. | Only male sample |
| Strand et al. (48) 2102 Cross-sectional | Self-report questionnaire | EF (FMD) | There was no evidence for an association of having difficulties initiating sleep or maintaining sleep with FMD in both genders. | |
| Suessenbacher et al. (49), 2011 | Self-report questionnaire | EF (PAT index) | PAT index: shift vs non-shift workers: 1.73 ± 0.4 vs 1.94 ± 0.5; p = 0.03 |
Only male sample |
| Sauvet et al. (51), 2009 | Self-Report | EF (Iontophoresis of Ach & Nanitroprusside) | The endothelium-dependent and – independent cutaneous vascular conductance were significantly decreased after 29 h of TSD (P ≤ 0.05). | 40 hours of total sleep deprivation. |
| Bonsen et al. (52), 2015 | Self-report (SWEL) | EF (Nailfold capillaroscopy) |
Sleep quality & endothelial function: regression coefficient 95% CI Men: β −3.81, 95% CI (−9.45, 1.83); p = 0.18 Women: β 2.11, 95% CI (−3.14, 7.36); p = 0.43 |
|
| Objective Sleep Quality | ||||
| Cooper et al. (42), 2014 Cross-sectional | Polysomnography | EF (FMD) |
% REM sleep and FMD%:
β = 0.25; p = 0.022 REM latency and FMD%: β = −0.2; p = 0.036 |
|
| Calvin et al. (44), 2014 Cross-sectional | Polysomnography (PSG) | EF (FMD) |
FMD%: Acclimation phase vs. Experimental phase Sleep restriction group: 8.6 ± 4.6% vs 5.2 ± 3.4%; p = 0.01 Control group: 5.0 ± 3.0% vs 6.73 ± 2.9%; p = 0.10 Group differences: −4.40%. 95% CI (−7.00, −1.81); p = 0.003 |
Smaller sample |
| Schmidt et al, 2013 (47) | Actigraphy | EF (FMD) |
FMD% among groups: Control: 10.4 + 3.8%; Noise30 dB: 9.7 + 4.1%; Noise60 dB: 9.5 + 4.3%; p = 0.052 A monotone dose-dependent effect of noise level on FMD was significant (p = 0.020) |
There was a priming effect of noise, i.e. the blunting in FMD was particularly evident when subjects were exposed first to 30 and then to 60 noise events (p = 0.006). |
| Dettoni et al. (50), 2012 | Wrist actigraphy | EF (dorsal hand vein technique) |
Endothelial dependent venodilatation in sleep groups: Control sleep (> 7h): 100 ± 22% Partial sleep deprivation (< 5 h): 41 ± 20%); p < 0.05 |
5 nights of partial sleep deprivation was sufficient to cause significant venous endothelial dysfunction. |
Association of Subjective and Objective Sleep Parameters with Arterial Stiffness
Table 5a discussed the association of subjective sleep duration with arterial stiffness. All studies showed that subjective long sleep duration was associated with increased arterial stiffness; however, only one study showed a relationship between subjective short sleep duration and arterial stiffness and other studies failed to demonstrate such association.
Table 5a. Association of Subjective/ Self-reported and Objective Sleep Duration with Arterial Stiffness.
| First Author, Publication Year, Study Type | Sleep Measurement | Non-Invasive Subclinical CVD assessment tools. | Results | Comments |
|---|---|---|---|---|
| Subjective/Self-reported Sleep Duration | ||||
| Yoshioka et al. (55), 2011 | Self-Report questionnaire | baPWV | Association of sleep duration and mean baPWV: PRC (95%CI) Adjm: ≤ 5h, 6h, 8 h, p = ≥ 0.05 ≥ 9hr: 44.69 (17.69–71.69); p < 0.01 |
In gender stratified analyses, ≥ 9h sleep duration was associated with increased baPWV in males only (p < 0.01). |
| Tsai et al. (57), 2014 | Self-Report questionnaire | baPWV |
Relationship of sleep duration with arterial stiffness: Adjn
|
|
| Kim et al. (17), 2015 | Self-Report (PSQI) | baPWV |
Sleep duration (h) vs PWV (cm/s) (Adjb): OR (95% CI) < 5h: 6.7 (0.75–12.6) 6h: 2.9 (−1.7 to 7.4) 7h: Reference (0.0) 8h: 10.5 (4.5–16.5) 9h: 9.6 (−0.7 to 19.8); p = 0.019 |
The association between sleep duration and baPWV was U-shaped |
| Objective Sleep Duration | ||||
| Not even a single study have described the association of objective sleep duration with Arterial Stiffness. | ||||
Adjusted for age, sex, SBP, hypertension, HR, biological risk factors (BMI, TC, log TG, HDL-C, and FBS), lifestyle factors (education, exercise, smoking, and alcohol consumption), and occupational factors (occupation, working hours, shift work, days off, and job strain)
Adjusted for age, BMI, eGFR, Hypertension, DM, TC/HDL-C, sleep duration, smoking, alcohol drinking, regular exercise, and snoring ≥ 3/week
Adjusted for age, sex, study center, year of visit, education, marital status, depression, smoking status, alcohol consumption, physical activity, body mass index, fasting glucose, systolic blood pressure, diastolic blood pressure, height, and heart rate.
In Table 5b, all studies that discussed the subjective association of sleep quality with arterial stiffness, showed a strong positive association of bad sleep quality with arterial stiffness in terms of increased brachial artery pulse wave velocity (baPWV) and decreased ankle brachial index (ABI). There was no study in this review that reported the association of objective sleep quality with arterial stiffness.
Table 5b. Association of Subjective/ Self-reported and Objective Sleep Quality with Arterial Stiffness.
| First Author, Publication Year | Sleep Measurement | Non-Invasive Subclinical CVD assessment tools. | Results | Comments |
|---|---|---|---|---|
| Subjective/Self-reported Sleep Quality | ||||
| Sunbul et al. (56), 2014 | Self-Report questionnaire | baPWV |
PWV cm/sec.: [Sleep Deprivation (SD) vs Regular Sleep (RS)]: 5.33 ± 0.46 vs 5.15 ± 0.26; p = 0.001 Augmentation index (AIx)s: [SD vs RS ]: 20.5 ± 11.9 vs 26.0 ± 8.4%; p = 0.008 |
Turkish population. |
| Kim et al, 2015 (17) | Self-Report (PSQI) | baPWV |
PWV cm/sec: [Good vs. Poor sleep quality] 95% CI Adjb: Men: 0.0 (Ref.) vs. 7.6 (1.2 to 13.9) Women: 0.0 (Ref.) vs. 2.7 (−5.3 to 10.7) |
Poor subjective sleep quality was associated with increase in PWV in men only in adjusted model. |
| Osonoi et al, 2015 (68) | Self-Report (PSQI) | baPWV |
PWV cm/s: [Among sleep quality groups] (95% CI) Adj o Good: 1523 (1500, 1547) Average: 1570 (1533, 1606) Poor: 1604 (1547, 1661); p < 0.05 |
Poor sleep quality associated with higher PWV |
| Yamaki et al. 2015 | Self-Report (PSQI) | ABI | PSQI sleep quality score and ABI (rho = −0.31); p < 0.005 | Lower the ABI, more the arterial stiffness. |
| Objective Sleep Quality | ||||
| Not even a single study have described the association of objective sleep Quality with Arterial Stiffness. | ||||
Adjusted for age, sex, study center, year of visit, education, marital status, depression, smoking status, alcohol consumption, physical activity, body mass index, fasting glucose, systolic blood pressure, diastolic blood pressure, height, and heart rate.
Adjusted for age, gender, BMI, morningness eveningness questionnaire, Beck Depression inventory, energy intake, alcohol intake, current smoking, physical activity, systolic BP, HbA1c, total cholesterol, HDL-cholesterol, triglyceride and sleep duration
Discussion
This systematic review investigated the association of subjectively and/or objectively measured sleep duration as well as sleep quality with non-invasive markers of sub-clinical cardiovascular disease (CVD). According to this review, subjective short sleep duration was associated with CAC and CIMT, but variably associated with endothelial dysfunction (ED) and arterial stiffness, however subjective long sleep duration was associated with CAC, CIMT and arterial stiffness, but variably associated with ED. Objective short sleep duration was positively associated with CIMT, variably with CAC but not associated with ED. Objective long sleep duration was variably associated with CAC and CIMT but not associated with ED. Poor subjective sleep quality was significantly associated with ED & arterial stiffness but variably associated with CAC & CIMT. Poor objective sleep quality was significantly associated with CIMT, and ED but variably associated with CAC (Table 6)
Table 6. Snapshot of association of “Non-Invasive Subclinical CVD assessment tools” with Sleep Duration and Sleep Quality.
| Non-Invasive Subclinical CVD assessment tools | Subjective sleep duration Vs Reference Sleep Duration (6–8 hrs) |
Objective sleep duration Vs Reference Sleep Duration (6–8 hrs) |
Subjective sleep quality | Objective sleep quality | ||
|---|---|---|---|---|---|---|
| Short subjective sleep duration | Long subjective sleep duration | Short objective sleep duration | Long objective sleep duration | |||
| CAC | Association present | Association present | Association variable | Association variable | Association variable | Association variable |
| CIMT | Association present | Association present | Association present | Association variable | Association variable | Association present |
| Endothelial function | Association variable | Association variable | Lack of association | Lack of association | Association present | Association present |
| Arterial stiffness | Association variable | Association present | NR | NR | Association present | NR |
NR = Not reported.
Association variable: In this review some studies showed positive and some studies showed negative and some studies showed lack of an association between sleep duration and sleep quality with Non-Invasive Subclinical CVD assessment tools.
Association present: In this review most of the studies showed presence of an association between sleep duration and sleep quality with No-Invasive Subclinical CVD assessment tools.
Lack of association: In this review most of the studies showed lack of an association between sleep duration and sleep quality with Non-Invasive Subclinical CVD assessment tools.
Subjective Sleep Quality: Expressed as PSQI, good vs PSQI bad sleep quality, insomnia vs non-insomnia, shift vs. non-shift work, arousal index, sleep efficiency (%), REM/Non-REM sleep.
There is a growing body of literature linking sleep duration with CVD outcomes. Sabanayagham et al. revealed a higher odds of myocardial infarction and stroke in subjects that slept ≤ 5 h and ≥ 9h59). In 2011, Cappuccio et al. performed a systematic review showing that both short and long sleep durations were associated with a greater relative risk of coronary heart disease and stroke60). Sleep quality as assessed by insomnia and sleep deprivation have also been linked with adverse CVD outcomes. Westerlund et al. revealed that insomnia itself was not related to CVD risk, but when considered together with short sleep duration, there was a higher risk of overall cardiovascular events3). The exact mechanism by which compromised sleep quality lead to CVD is not clear, but possible explanations include changes in hormones and inflammatory markers, lipid levels, glucose tolerance/metabolism, sympathetic nervous system and subclinical atherosclerosis61). Cappuccio et al. performed another systematic review and meta-analysis in 2010 of 10 cohort studies showing that sleep ≤ 5–6 h and > 8–9h were associated with higher relative risk of developing Type 2 DM62). Gangwisch et al. showed that ≤ 5 hours of sleep significantly increased the risk of hypertension63). Dettoni et al. showed that five nights of partial sleep deprivation can significantly cause a trigger in the sympathetic activity and venous endothelial dysfunction and this evidence is in alignment with the association between short sleep and increased cardiovascular risk in other epidemiological studies50).
Many animal studies have also found sleep deprivation causes an increase in endothelin levels. Endothelin is a potent vasoconstrictor that has been implicated in the pathogenesis of hypertension64). Meier-Ewert et al. revealed that both total (88 hrs of no sleep) and short-term partial sleep (4.2 hrs of sleep) deprivation resulted in elevated high-sensitivity CRP concentrations, compared to those who slept 8.2 hours65). Sauvet et al. demonstrated that total sleep deprivation in rats induces a reduction in endothelial-dependent vasodilation independent of blood pressure and sympathetic activity64). Carreras et al. showed in mice that long-term sleep fragmentation lead to vascular endothelial dysfunction, mild increase in blood pressure, vascular elastic fiber disruption and disorganization, and increased recruitment of inflammatory cells66). According to a review by Elliott et al. sleep deprivation can cause death sooner than food deprivation in both Drosophila and rats, further favoring importance of regular sleep habits67).
Our review provided mixed results in terms of subjective and/or objective short and/or long sleep duration and sleep quality with sub-clinical CVD, with a greater percentage of studies showing a relationship with sub-clinical CVD. However, only some studies in this review failed to demonstrate such an association31, 32, 37, 43) and one possible reason for such results could be due to the use of subjective/self-report versus objectively measured sleep parameters to assess the association with subclinical CVD causing biased results. Lauderdale et al. found a correlation between self-reported and objectively measured sleep duration of 0.45, which is generally considered as a moderate correlation. In current review, while one study reports a “J-shaped” association35), the other two study reports a “U-shaped” association17, 37) between sleep duration and sub-clinical CVD outcomes. Some studies report a positive association of sleep duration with sub-clinical CVD only with short sleep duration; some only with long sleep duration; some studies show a positive association with both short and long duration33, 38, 39, 57) but almost all studies favor the duration of the reference sleep (average 6–8 hours of regular daily sleep). To our surprise, some studies have even shown long sleep hours is related to decrease in CAC33), decrease in CIMT38–40), decrease in PWV56) and one study by Yoshioka et al.55) even showed that sleep duration of ≤ 6 hours was associated with a significant decrease in PWV and thus produced a great source of heterogeneity in the review. Although most of the studies in the current review demonstrate that sleep parameters are associated with early sub-clinical CVD, there is, as yet, no consensus on the biological pathways linking these processes. It is entirely possible, given the complexity of factors responsible for sleep habits that sleep disturbances may be an epiphenomenon of subclinical and clinical CVD rather than the proximate cause1). The population used in these studies is highly heterogeneous based on age and gender differences, and thus no consensus can be made about the particular nature of subclinical CVD burden due to changes in the sleep parameters in specific age groups and gender.
Our systematic review findings need to be considered in light of the following limitations. The metaanalysis for this review is not applicable because the studies included in our review are highly heterogeneous in population, outcome reporting and sleep duration and quality assessment groups, precluding a quantitative or meta-analytical synthesis of evidence. Some studies reported only subjective measures of sleep duration and sleep quality, some only objective measures, and some both. Not all studies used similar/standardized subjective and objectives measures of sleep duration and quality. Some studies used simple questionnaire to measure sleep duration and quality, some used Pittsburgh Sleep Quality Index (PSQI), some used actigraphy and some used polysomnography creating further heterogeneity in assessment of predictor variable. Furthermore, the reference/control sleep duration used in these studies was not a standardized duration, some studies used 6 hours as reference sleep duration, some used 7 hours, some used 8 hours and some studies even used 6–8 hours as reference sleep duration. The assessment of subjective and objective sleep quality was also different among the studies used in this review. Some studies used insomnia vs. non-insomnia, some used shift vs non-shift work, some used fragmentation index and/or arousal index and/or REM/Non-REM sleep to assess the sleep quality. A few studies that examined both subjective and objective sleep parameters demonstrated different outcomes depending on the nature of the predictor variable used in their analysis (subjective versus objective).
Some of the studies included in our review are of questionable methodological quality, which may be another source of observation bias. The population discussed in this systematic review ranges from 20 to 87 years and due to heterogeneous nature of these studies we could not conclude what age groups were at more risk of subclinical CVD based on their sleep disturbances, thus more focused studies directed to specific age group are needed to further clarify this concern. Furthermore, ethnic-specific variations in sleep parameters and association with subclinical CVD have not been discussed and compared in these studies and thus question the need of further studies discussing the comparison of subclinical CVD burden in the multi-ethnic populations. Another concern is that most of the studies used in this review are cross sectional and as a result, causality cannot be determined. Our findings show the need for follow up studies to assess the rapid progression of subclinical CVD with sleep disturbances. This will further clarify the association of sleep parameters with an increased subclinical and clinical CVD burden. Finally, the implications of the associations demonstrated in our review are unclear. Should individuals with sleep disturbances be considered candidates for subclinical CVD screening? Most sleep disturbance treatment focuses on lifestyle modification, which is beneficial for CVD. However, in scenarios where one is found to have these findings, should one be a candidate for aggressive preventive pharmacotherapy such as Statins? What will be the most cost effective method for screening? Unfortunately, no consensus exists in this specific area.
Conclusion
In conclusion, based on our systematic review, individuals with sleep disturbances, both quantitative and qualitative, have an accelerated subclinical CVD burden in the form of augmented Coronary Artery Calcium (CAC), increased Carotid Intima Media Thickness (CIMT), impaired endothelial function, and augmented arterial stiffness. Table 6 provides a snapshot of association of non-Invasive Subclinical CVD assessment tools with sleep duration and sleep quality. These findings suggest that association between sleep disturbances and increased CVD risk is exemplified by early changes in sub-clinical CVD status. Important issues that need further exploration include the public awareness of regular sleep habits, and potential consequences of compromised sleep duration and quality in terms of causing hypertension, high cholesterol and risk of diabetes. There is a need to identify appropriate screening and treatment strategies across different group of populations with sleep disturbances and whether these preventive approaches can slow progression of sub-clinical CVD and reduction in clinical events. Comprehensive studies aimed at answering these key questions can facilitate generation of information to guide preventive efforts in this population.
Conflict of Interests
M. Aziz, SS. Ali, S. Das, A. Younus, R. Malik, M. Latif, Choudhry H, D. Anugula, G. Abbas, Joseph S, JV Elizondo, E Veledar, and K. Nasir stated that no conflict of interest exists. No financial support was given and that no off-label or investigational use of a drug or device was performed as part of this research.
Author Contributorship
Conception and design: Aziz, M; Veledar, E; Nasir, K. Analysis and interpretation: Aziz, M; Das. S; Ali, SS. Data collection: Latif, M; Malik, R. Table formation: Aziz, M; Younus, A; Choudhry, H; Writing the article: Aziz, M; Das. S; Ali. SS; Salami, J. Critical revision of the article: Elizondo JV; Anugula, D; Younus, A; Final approval of the article: Aziz, M; Abbas, G; Nasir, K. Obtained funding: N/A. Overall responsibility: Aziz, M; Nasir, K
Abbreviations Frequently used in this Review
CAC = Coronary Artery Calcium, CIMT = Carotid Intima Media Thickness, ED = Endothelial dysfunction, AS = Arterial stiffness, baPWV = brachial artery Pulse Wave Velocity, PSQI = Pittsburgh Sleep Quality Index, PSG = Polysomnography
References
- 1). Colten HR, Altevogt BM, Research IoMUCoSMa : Sleep Physiology. 2006; [Google Scholar]
- 2). Nagai M, Hoshide S, Nishikawa M, Shimada K, Kario K: Sleep duration and insomnia in the elderly: associations with blood pressure variability and carotid artery remodeling. Am J Hypertens, 2013; 26: 981-989 [DOI] [PubMed] [Google Scholar]
- 3). Westerlund A, Bellocco R, Sundstrom J, Adami HO, Akerstedt T, Trolle Lagerros Y: Sleep characteristics and cardiovascular events in a large Swedish cohort. Eur J Epidemiol, 2013; 28: 463-473 [DOI] [PubMed] [Google Scholar]
- 4). Cappuccio FP, D'Elia L, Strazzullo P, Miller MA: Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep, 2010; 33: 585-592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Meisinger C, Heier M, Löwel H, Schneider A, Döring A: Sleep Duration and Sleep Complaints and Risk of Myocardial Infarction in Middle-aged Men and Women from the General Population: The MONICA/KORA Augsburg Cohort Study. Sleep, 2007; 30: 1121-1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Knutson KL, Spiegel K, Penev P, Van Cauter E: The metabolic consequences of sleep deprivation. Sleep medicine reviews, 2007; 11: 163-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Buxton OM, Marcelli E: Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Social science & medicine (1982), 2010; 71: 1027-1036 [DOI] [PubMed] [Google Scholar]
- 8). Cappuccio FP, D'Elia L, Strazzullo P, Miller MA: Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes care, 2010; 33: 414-420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Gangwisch JE, Malaspina D, Babiss LA, Opler MG, Posner K, Shen S, Turner JB, Zammit GK, Ginsberg HN: Short sleep duration as a risk factor for hypercholesterolemia: analyses of the National Longitudinal Study of Adolescent Health. Sleep, 2010; 33: 956-961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Beihl DA, Liese AD, Haffner SM: Sleep duration as a risk factor for incident type 2 diabetes in a multiethnic cohort. Ann Epidemiol, 2009; 19: 351-357 [DOI] [PubMed] [Google Scholar]
- 11). Bosomworth NJ: Practical use of the Framingham risk score in primary prevention: Canadian perspective. Canadian Family Physician, 2011; 57: 417-423 [PMC free article] [PubMed] [Google Scholar]
- 12). Almasi A, Pouraliakbar H, Sedghian A, Karimi MA, Firouzi A, Tehrai M: The value of coronary artery calcium score assessed by dual-source computed tomography coronary angiography for predicting presence and severity of coronary artery disease. Pol J Radiol, 2014; 79: 169-174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Holland Z, Ntyintyane LM, Raal FJ, Gill GV: Carotid intima–media thickness is a predictor of coronary artery disease in South African black patients. Cardiovasc J Afr, 2009; 20: 237-239 [PMC free article] [PubMed] [Google Scholar]
- 14). Cai H, Harrison DG: Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res, 2000; 87: 840-844 [DOI] [PubMed] [Google Scholar]
- 15). Cecelja M, Chowienczyk P: Role of arterial stiffness in cardiovascular disease. JRSM Cardiovascular Disease, 2012; 1: cvd.2012.012016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Stang A, Dragano N, Poole C, Moebus S, Mohlenkamp S, Schmermund A, Siegrist J, Erbel R, Jockel KH: Daily siesta, cardiovascular risk factors, and measures of subclinical atherosclerosis: results of the Heinz Nixdorf Recall Study. Sleep, 30: 1111-1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Kim CW, Chang Y, Zhao D, Cainzos-Achirica M, Ryu S, Jung HS, Yun KE, Choi Y, Ahn J, Zhang Y, Rampal S, Baek Y, Lima JA, Shin H, Guallar E, Cho J, Sung E: Sleep Duration, Sleep Quality, and Markers of Subclinical Arterial Disease in Healthy Men and Women. Arterioscler Thromb Vasc Biol, 2015; 35: 2238-2245 [DOI] [PubMed] [Google Scholar]
- 18). Ali SS, Oni ET, Warraich HJ, Blaha MJ, Blumenthal RS, Karim A, Shaharyar S, Jamal O, Fialkow J, Cury R, Budoff MJ, Agatston AS, Nasir K: Systematic review on noninvasive assessment of subclinical cardiovascular disease in obstructive sleep apnea: new kid on the block! Sleep Med Rev, 2014; 18: 379-391 [DOI] [PubMed] [Google Scholar]
- 19). Buysse DJ: Sleep Health: Can We Define It? Does It Matter? Sleep, 2014; 37: 9-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Hashmi AM, Khawaja IS, Butt Z, Umair M, Naqvi SH, Jawad Ul H: The Pittsburgh Sleep Quality Index: validation of the Urdu translation. J Coll Physicians Surg Pak, 2014; 24: 123-126 [PubMed] [Google Scholar]
- 21). Buysse DJ, Hall ML, Strollo PJ, Kamarck TW, Owens J, Lee L, Reis SE, Matthews KA: Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. J Clin Sleep Med, 2008; 4: 563-571 [PMC free article] [PubMed] [Google Scholar]
- 22). Krystal AD, Edinger JD: Measuring sleep quality. Sleep Med, 2008; 9 Suppl 1: S10-17 [DOI] [PubMed] [Google Scholar]
- 23). Landry GJ, Best JR, Liu-Ambrose T: Measuring sleep quality in older adults: a comparison using subjective and objective methods. Frontiers in Aging Neuroscience, 2015; 7: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ: The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res, 1989; 28: 193-213 [DOI] [PubMed] [Google Scholar]
- 25). Judge D: What is Sleep Fragmentation and how is it calculated? 2016-07-04 19:38:58: [Google Scholar]
- 26). Axtell AL, Gomari FA, Cooke JP: Assessing Endothelial Vasodilator Function with the Endo-PAT 2000. J Vis Exp, 2010; 2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Puissant C, Abraham P, Durand S, Humeau-Heurtier A, Faure S, Leftheriotis G, Mahe G: Assessment of endothelial function by acetylcholine iontophoresis: impact of inter-electrode distance and electrical cutaneous resistance. Microvasc Res, 2014; 93: 114-118 [DOI] [PubMed] [Google Scholar]
- 28). Wijnstok N, Hoekstra T, Eringa E, Smulders Y, Twisk J, Serne E: The relationship of body fatness and body fat distribution with microvascular recruitment: The Amsterdam Growth and Health Longitudinal Study. Microcirculation, 2012; 19: 273-279 [DOI] [PubMed] [Google Scholar]
- 29). Choo J, Shin C, Barinas-Mitchell E, Masaki K, Willcox BJ, Seto TB, Ueshima H, Lee S, Miura K, Venkitachalam L, Mackey RH, Evans RW, Kuller LH, Sutton-Tyrrell K, Sekikawa A: Regional pulse wave velocities and their cardiovascular risk factors among healthy middle-aged men: a cross-sectional population-based study. BMC Cardiovascular Disorders, 2014; 14: 5-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). van Popele NM, Grobbee DE, Bots ML, Asmar R, Topouchian J, Reneman RS, Hoeks AP, van der Kuip DA, Hofman A, Witteman JC: Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke, 2001; 32: 454-460 [DOI] [PubMed] [Google Scholar]
- 31). Matthews KA, Strollo PJ, Jr., Hall M, Mezick EJ, Kamarck TW, Owens JF, Buysse DJ, Reis SE: Associations of Framingham risk score profile and coronary artery calcification with sleep characteristics in middle-aged men and women: Pittsburgh SleepSCORE study. Sleep, 2011; 34: 711-716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Matthews KA, Everson-Rose SA, Kravitz HM, Lee L, Janssen I, Sutton-Tyrrell K: Do reports of sleep disturbance relate to coronary and aortic calcification in healthy middle-aged women?: Study of women's health across the nation. Sleep Med, 2013; 14: 282-287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). King CR, Knutson KL, Rathouz PJ, Sidney S, Liu K, Lauderdale DS: Short sleep duration and incident coronary artery calcification. Jama, 2008; 300: 2859-2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Lutsey PL, McClelland RL, Duprez D, Shea S, Shahar E, Nagayoshi M, Budoff M, Kaufman JD, Redline S: Objectively measured sleep characteristics and prevalence of coronary artery calcification: the Multi-Ethnic Study of Atherosclerosis Sleep study. Thorax, 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Wolff B, Volzke H, Schwahn C, Robinson D, Kessler C, John U: Relation of self-reported sleep duration with carotid intima-media thickness in a general population sample. Atherosclerosis, 2008; 196: 727-732 [DOI] [PubMed] [Google Scholar]
- 36). Abe T, Aoki T, Yata S, Okada M: Sleep duration is significantly associated with carotid artery atherosclerosis incidence in a Japanese population. Atherosclerosis, 2011; 217: 509-513 [DOI] [PubMed] [Google Scholar]
- 37). Ma CC, Burchfiel CM, Charles LE, Dorn JM, Andrew ME, Gu JK, Joseph PN, Fekedulegn D, Slaven JE, Hartley TA, Mnatsakanova A, Violanti JM: Associations of objectively measured and self-reported sleep duration with carotid artery intima media thickness among police officers. Am J Ind Med, 2013; 56: 1341-1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38). Nakazaki C, Noda A, Koike Y, Yamada S, Murohara T, Ozaki N: Association of insomnia and short sleep duration with atherosclerosis risk in the elderly. Am J Hypertens, 2012; 25: 1149-1155 [DOI] [PubMed] [Google Scholar]
- 39). Sands MR, Lauderdale DS, Liu K, Knutson KL. Matthews KA Eaton CB Linkletter CD Loucks EB: Short sleep duration is associated with carotid intima-media thickness among men in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Stroke, 2012; 43: 2858-2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Schwartz JAM, Ancoli-Israel S, Hovell MF, Patterson R, Natarajan L MS, Grant I: Napping and Less Disturbed Nighttime Sleep Associated with Reduced Carotid Intima-Media Thickness in Alzheimer Caregivers. 2012; 5: 43-50 [Google Scholar]
- 41). Ramos-Sepulveda A, Wohlgemuth W, Gardener H, Lorenzo D, Dib S, Wallace DM, Nolan B, Boden-Albala B, Elkind MS, Sacco RL, Rundek T: Snoring and insomnia are not associated with subclinical atherosclerosis in the Northern Manhattan Study. Int J Stroke, 5: 264-268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). Cooper DC, Ziegler MG, Milic MS, Ancoli-Israel S, Mills PJ, Loredo JS, Von Kanel R, Dimsdale JE: Endothelial function and sleep: associations of flow-mediated dilation with perceived sleep quality and rapid eye movement (REM) sleep. J Sleep Res, 2014; 23: 84-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43). Behl M, Bliwise D. Veledar E Cunningham L Vazquez J Brigham K Quyyumi A: Vascular endothelial function and self-reported sleep. Am J Med Sci, 2014; 347: 425-428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44). Calvin AD, Covassin N, Kremers WK, Adachi T, Macedo P, Albuquerque FN, Bukartyk J, Davison DE, Levine JA, Singh P, Wang S, Somers VK: Experimental sleep restriction causes endothelial dysfunction in healthy humans. J Am Heart Assoc, 2014; 3: e001143-e001143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45). Takase B, Akima T, Uehata A, Ohsuzu F, Kurita A: Effect of chronic stress and sleep deprivation on both flow-mediated dilation in the brachial artery and the intracellular magnesium level in humans. Clinical cardiology, 2004; 27: 223-227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46). Wehrens SM, Hampton SM, Skene DJ: Heart rate variability and endothelial function after sleep deprivation and recovery sleep among male shift and non-shift workers. Scand J Work Environ Health, 2012; 38: 171-181 [DOI] [PubMed] [Google Scholar]
- 47). Schmidt FP, Basner M, Kroeger G, Weck S, Schnorbus B, Muttray A, Sariyar M, Binder H, Gori T, Warnholtz A, Muenzel T: Effect of nighttime aircraft noise exposure on endothelial function and stress hormone release in healthy adults. Eur Heart J, 2013; 34: 3508-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48). Strand LB, Laugsand LE, Skaug EA, Ellingsen O, Madssen E, Wisloff U, Vatten L, Janszky I: Insomnia and endothelial function - the HUNT 3 fitness study. PloS one, 2012; 7: e50933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49). Suessenbacher A, Potocnik M, Dorler J, Fluckinger G, Wanitschek M, Pachinger O, Frick M, Alber HF: Comparison of peripheral endothelial function in shift versus nonshift workers. Am J Cardiol, 2011; 107: 945-948 [DOI] [PubMed] [Google Scholar]
- 50). Dettoni JL, Consolim-Colombo FM, Drager LF, Rubira MC, Cavasin de Souza SBP, Irigoyen MC, Mostarda C, Borile S, Krieger EM, Moreno H, Jr., Lorenzi-Filho G: Cardiovascular effects of partial sleep deprivation in healthy volunteers. J Appl Physiol, 2012; 113: 232-236 [DOI] [PubMed] [Google Scholar]
- 51). Sauvet F, Leftheriotis G, Gomez-Merino D, Langrume C, Drogou C, Van Beers P, Bourrilhon C, Florence G, Chennaoui M: Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol, 2010; 108: 68-75 [DOI] [PubMed] [Google Scholar]
- 52). Bonsen T, Wijnstok NJ, Hoekstra T, Eringa EC, Serne EH, Smulders YM, Twisk JW: Sleep quality and duration are related to microvascular function: the Amsterdam Growth and Health Longitudinal Study. J Sleep Res, 2015; 24: 140-147 [DOI] [PubMed] [Google Scholar]
- 53). Weil BR, Mestek ML, Westby CM, Van Guilder GP, Greiner JJ, Stauffer BL, DeSouza CA: Short sleep duration is associated with enhanced endothelin-1 vasoconstrictor tone. Can J Physiol Pharmacol, 2010; 88: 777-781 [DOI] [PubMed] [Google Scholar]
- 54). Weil BR, Maceneaney OJ, Stauffer BL, Desouza CA: Habitual short sleep duration and circulating endothelial progenitor cells. J Cardiovasc Dis Res , 2011; 2: 110-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55). Yoshioka E, Saijo Y, Kita T, Okada E, Satoh H, Kawaharada M, Kishi R: Relation between self-reported sleep duration and arterial stiffness: a cross-sectional study of middle-aged Japanese civil servants. Sleep, 2011; 34: 1681-1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56). Sunbul M, Kanar BG, Durmus E, Kivrak T, Sari I: Acute sleep deprivation is associated with increased arterial stiffness in healthy young adults. Sleep Breath, 2014; 18: 215-220 [DOI] [PubMed] [Google Scholar]
- 57). Tsai TC, Wu JS, Yang YC, Huang YH, Lu FH, Chang CJ: Long sleep duration associated with a higher risk of increased arterial stiffness in males. Sleep, 2014; 37: 1315-1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58). Yamaki M, Sato T, Fujii H: Lower ankle-brachial index is associated with poor sleep quality in patients with essential hypertension. Am J Cardiovasc Dis, 2015; 5: 77-82 [PMC free article] [PubMed] [Google Scholar]
- 59). Sabanayagam C, Shankar A: Sleep duration and cardiovascular disease: results from the National Health Interview Survey. Sleep, 2010; 33: 1037-1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60). Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA: Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J, 2011; 32: 1484-1492 [DOI] [PubMed] [Google Scholar]
- 61). Miller MA: Association of inflammatory markers with cardiovascular risk and sleepiness. J Clin Sleep Med, 2011; 7: S31-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62). Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, Miller MA: Meta-analysis of short sleep duration and obesity in children and adults. Sleep, 2008; 31: 619-626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63). Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D: Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension, 2006; 47: 833-839 [DOI] [PubMed] [Google Scholar]
- 64). Sauvet F, Florence G, Van Beers P, Drogou C, Lagrume C, Chaumes C, Ciret S, Leftheriotis G, Chennaoui M: Total Sleep Deprivation Alters Endothelial Function in Rats: A Nonsympathetic Mechanism. Sleep, 2014; 37: 465-473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65). Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM: Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol, 2004; 43: 678-683 [DOI] [PubMed] [Google Scholar]
- 66). Carreras A, Zhang SX, Peris E, Qiao Z, Gileles-Hillel A, Li RC, Wang Y, Gozal D: Chronic Sleep Fragmentation Induces Endothelial Dysfunction and Structural Vascular Changes in Mice. Sleep, 2014; 37: 1817-1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67). Elliott AS, Huber JD, O'Callaghan JP, Rosen CL, Miller DB: A review of sleep deprivation studies evaluating the brain transcriptome. SpringerPlus, 2014; 3: 728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68). Osonoi Y, Mita T, Osonoi T, Saito M, Tamasawa A, Nakayama S, Someya Y, Ishida H, Kanazawa A, Gosho M, Fujitani Y, Watada H: Poor sleep quality is associated with increased arterial stiffness in Japanese patients with type 2 diabetes mellitus. BMC endocrine disorders, 2015; 15: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]


