Abstract
Aim: Epicardial adipose tissue (EAT) has been suggested as a contributing factor for coronary atherosclerosis based on the previous cross-sectional studies and pathophysiologic background. However, a causal relationship between EAT and the development of non-calcified coronary plaque (NCP) has not been investigated.
Methods: A total of 122 asymptomatic individuals (age, 56.0 ± 7.6 years; male, 80.3%) without prior history of coronary artery disease (CAD) or metabolic syndrome and without NCP or obstructive CAD at baseline cardiac computed tomography (CT) were enrolled. Repeat cardiac CT was performed with an interval of more than 5 years. Epicardial fat volume index (EFVi; cm3/m2) was assessed in relation to the development of NCP on the follow-up CT where the results were classified into “calcified plaque (CP),” “no plaque,” and “NCP” groups.
Results: On the follow-up CT performed with a median interval of 65.4 months, we observed newly developed NCP in 24 (19.7%) participants. Baseline EFVi was significantly higher in the NCP group (79.9 ± 30.3 cm3/m2) than in the CP group (63.7 ± 22.7 cm3/m2; P = 0.019) and in the no plaque group (62.5 ± 24.7 cm3/m2; P = 0.021). Multivariable logistic regression analysis demonstrated that the presence of diabetes (OR, 9.081; 95% CI, 1.682–49.034; P = 0.010) and the 3rd tertile of EFVi (OR, 4.297; 95% CI, 1.040–17.757; P = 0.044 compared to the 1st tertile) were the significant predictors for the development of NCP on follow-up CT.
Conclusions: Greater amount of EAT at baseline CT independently predicts the development of NCP in asymptomatic individuals.
Keywords: Epicardial adipose tissue, Epicardial fat volume, Atherosclerosis, Non-calcified plaque, Computed tomography (CT)
See editorial vol. 24: 254–255
Introduction
Epicardial adipose tissue (EAT) has been suggested to be a potential contributing factor for coronary atherosclerosis1–3). EAT is a type of visceral adipose tissue; it has both paracrine and endocrine effects and is known to secret several proatherogenic cytokines4). Previous cross-sectional studies showed that increased EAT is not only associated with a higher prevalence of coronary artery disease (CAD) but may also be a prognosticator for future cardiovascular events and, eventually, cardiovascular mortality5–8).
The main limitation of previous EAT studies is that most were cross-sectional studies, and therefore, a causal relationship between increased EAT and the progression or development of CAD or coronary plaques cannot be determined5, 6, 9, 10). Several studies have demonstrated a relationship between EAT and the progression of coronary calcification by serial measurement11, 12), but the impact of EAT on the development of high-risk coronary plaque has not been established. The lack of prospective studies defining a causal relationship between EAT and the development of NCP has resulted in skepticism toward EAT measurement in clinical practice, and EAT is considered only an innocent bystander in the atherogenic process13).
In this study, we aimed to investigate the impact of EAT on the development of coronary plaque in order to clarify the missing link between EAT and cardiovascular events. We reviewed records of individuals who had undergone cardiac computed tomography (CT) more than two times, and we identified 122 asymptomatic individuals without prior history of CAD, without metabolic syndrome, and without NCP on baseline CT. We used serial cardiac CT performed with an interval of more than 5 years to investigate whether baseline epicardial fat volume (EFV) is associated with the “de novo” development of NCP.
Methods
Study Population
Between July 2006 and November 2008, we enrolled 162 asymptomatic Korean individuals who underwent general health exams at the Healthcare System Gangnam Center, Seoul National University Hospital and underwent repeat cardiac CT with an interval of at least 5 years between examinations. All subjects were aged 40 years or older and underwent medical examinations and cardiac CT for screening purposes at their requests. Patients with metabolic syndrome as defined according to the World Health Organization's criteria (n = 8) were excluded14). Those with NCP or obstructive CAD (≥ 70% stenosis; n = 32) at baseline cardiac CT were also excluded in order to assess the predictive value of EFV for the development of de novo NCP at follow-up CT. Patients with a prior history of CAD, metabolic syndrome, clinically significant valvular heart disease, or cardiomyopathy were also excluded. Past medical history and current medications for each patient were obtained from medical questionnaires. Hypertension (HTN) was defined as systolic blood pressure (SBP) greater than or equal to 140 mmHg or diastolic blood pressure (DBP) greater than or equal to 90 mmHg without medication or current antihypertensive therapy. Diabetes mellitus (DM) was defined as fasting blood glucose greater than or equal to 126 mg/dL, hemoglobin A1c (HbA1c) greater than or equal to 6.5%, or use of antidiabetic medications. Dyslipidemia was defined as total cholesterol greater than or equal to 240 mg/dL, triglycerides greater than or equal to 200 mg/dL, low-density lipoprotein cholesterol (LDL-c) greater than or equal to 160 mg/dL, high-density lipoprotein cholesterol (HDL-c) less than 40 mg/dL in men and less than 50 mg/dL in women, or use of lipid-lowering medications. Obesity was defined as a body mass index (BMI) greater than or equal to 25 kg/m2, according to the criteria from the World Health Organization's Asia-Pacific guideline15). Central obesity was defined as a waist circumference greater than 102 cm in men and greater than 88 cm in women, according to the current guidelines from the National Cholesterol Education Program (NCEP)16).
This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the institutional review board of the Healthcare System Gangnam Center, Seoul National University Hospital (IRB No. 1604-009-752).
Anthropometric and Laboratory Parameters
Body weight (kg), height (cm), waist circumference (cm), and blood pressures were measured on the day of the medical exam and CT scan. All participants fasted for at least 12 hours before the blood test. Total cholesterol (mg/dL), fasting insulin (µU/mL), triglyceride (mg/dL), HDL-c (mg/dL), LDL-c (mg/dL), fasting blood glucose (mg/dL), HbA1c (%), and high-sensitivity C-reactive protein (hsCRP; mg/L) levels were measured. Homeostatic model assessment for insulin resistance (HOMA-IR) was calculated from fasting glucose and insulin levels using the following equation: HOMA-IR= (fasting blood glucose) × (fasting insulin)/40517).
Cardiac CT and EAT Measurement
After completion of the medical questionnaire, anthropometric measurements and laboratory tests, cardiac CT was performed using either a 16-slice multidetector CT scanner (Somatom Sensation 16; Siemens Medical Solutions, Forchheim, Germany) or a 256-slice multidetector CT scanner (Brilliance iCT 256; Philips Medical Systems, Cleveland, OH, USA), with electrocardiogram-gated dose modulation. A standard scanning protocol was applied, with 128 × 0.625 mm section collimation, 0.27 ms rotation time, 120 kV tube voltage and 800 mA tube current for the 256-slice multidetector CT and a tube voltage of 120 kV, 170 effective mAs, and 0.37 rotation time for the 16-slice CT. Before cardiac CT, heart rate was measured, and if the heart rate was higher than 60 beats per min, participants received 50–100 mg of metoprolol.
For measurement of EFV, the volume of EAT was measured by two different observers with a dedicated computer 3D station (Rapidia; Infinitt, Seoul, Korea), which was described in our previous publication18). Segmentation of the pericardial adipose tissue was achieved by isolating the pericardial adipose tissue and heart from the thorax using specific anatomical landmarks. The superior extent of the volume was determined by the center of the right pulmonary artery as it crossed the midsagittal plane. This point was determined by simultaneously visualizing the right pulmonary artery in the axial and sagittal planes. Inferiorly, the analysis volume was segmented at the level where the coronary sinus drained into the right atrium. Each observer had interactive access to the coronal images to aid in this segmentation step. The anterior border of the volume was defined by the sternum. Special care was taken not to include posterior mediastinal and paravertebral adipose tissues. After segmentation of the heart and EAT from the remainder of the thorax, a threshold of -190 to -30 Hounsfield units (HU) was applied to isolate adipose tissue-containing voxels. The voxels were summed to provide a value for the EFV (cm3), which was then related to body surface area as the EFV index (EFVi; cm3/m2).
All participants without NCP at baseline underwent follow-up cardiac CT for screening purposes. Atherosclerotic lesions were classified as NCP (calcified lesions with at least 130 HU that occupied <50% of the plaque area) and calcified plaque (CP; calcified lesions with at least 130 HU that occupied ≥ 50% of the plaque area). Patients with both NCP and CP or mixed plaques at follow-up CT were categorized as NCP group, given that the presence of NCP, a vulnerable coronary plaque, would indicate a higher risk of future cardiovascular event than that of CP.
Statistical Analysis
We categorized participants on the basis of results of the follow-up CT: no plaque (NP) group, CP group, and NCP group. Categorical variables are presented as frequencies and percentages, and continuous variables are presented as means ± standard deviations or medians with interquartile ranges (IQRs) according to the subgroups. We compared categorical variables using χ2 analysis, and we compared continuous variables using the Student's t-test and analysis of variance (ANOVA) or the Mann Whitney U-test and Kruskall Wallis test, depending on the number of groups assessed. We applied linear regression analysis to evaluate the correlation of EFVi with other baseline parameters. Then, we used multiple linear regression analysis on all variables with P values less than 0.200 by univariable analyses in order to examine the determinants of baseline EFVi. The differences in EFVi according to the results of the follow-up CT were further assessed by one-way analysis of covariance (ANCOVA) with post-hoc comparisons using age, sex, HTN, DM, Framingham risk score, BMI, and waist circumference as covariates. Adjusted odds ratios (ORs) for having NCP on follow-up cardiac CT were calculated by multivariable logistic regression models that included univariable predictors with P values less than 0.200. We used the tertiles of EFVi for the logistic regression analysis, with the 1st tertile of EFVi as the reference. All statistical analyses were performed with SPSS 20.0 software (SPSS Inc, Chicago, IL). A P value less than 0.05 was considered statistically significant.
Results
Baseline Characteristics
Among the total 122 participants with EFVi measurements and without NCP at baseline, follow-up CT was performed with a median interval of 65.4 (IQR, 62.3–73.4 months) months after the baseline CT scan. Baseline characteristics of the study population are summarized according to the results of the follow-up CT (Table 1): NP group (n = 50; 41.0%), CP group (n = 48; 39.3%), and NCP group (n = 24; 19.7%). Compared to the NP group, the CP and NCP groups had older participants and had more males than females. Comorbidities such as HTN, DM, dyslipidemia, and obesity were more common in the CP and NCP groups, than in the NP group. Framingham 10-year risk score was also significantly higher in the CP and NCP groups.
Table 1. Baseline characteristics of participants according to results of follow-up CT.
| Total (n = 122) |
Follow-up cardiac CT |
Overall P | |||
|---|---|---|---|---|---|
| No plaque | Calcified plaque | Non-calcified plaque | |||
| (n = 50) | (n = 48) | (n = 24) | |||
| Age | 56.04 ± 7.62 | 53.54 ± 7.75 | 58.15 ± 7.08† | 57.04 ± 7.14 | 0.008 |
| Age ≥ 65 years | 18 (14.8%) | 3 (6.0%) | 11 (22.9%) | 4 (16.7%) | 0.059 |
| Male sex | 98 (80.3%) | 35 (70.0%) | 42 (87.5%) | 21 (87.5%) | 0.057 |
| HTN | 49 (40.2%) | 17 (34.0%) | 19 (39.6%) | 13 (54.2%) | 0.252 |
| DM | 11 (9.0%) | 1 (2.0%) | 3 (6.2%) | 7 (29.2%) | < 0.001 |
| Dyslipidemia | 21 (17.2%) | 5 (10.0%) | 11 (22.9%) | 5 (20.8%) | 0.208 |
| Smoking | 0.519 | ||||
| - Never | 45 (36.9%) | 21 (42.0%) | 14 (29.2%) | 10 (41.7%) | |
| - Ex-smoker | 54 (44.3%) | 19 (38.0%) | 26 (54.2%) | 9 (37.5%) | |
| - Current smoker | 23 (18.9%) | 10 (20.0%) | 8 (16.7%) | 5 (20.8%) | |
| SBP (mmHg) | 120.7 ± 14.7 | 119.9 ± 15.0 | 119.6 ± 14.1 | 124.1 ± 15.4 | 0.450 |
| DBP (mmHg) | 78.2 ± 10.9 | 76.1 ± 11.6 | 79.1 ± 10.4 | 80.9 ± 11.7 | 0.169 |
| Height (cm) | 167.3 ± 8.2 | 165.6 ± 9.0 | 168.7 ± 7.7 | 168.3 ± 7.0 | 0.157 |
| Weight (kg) | 69.8 ± 10.0 | 67.8 ± 10.0 | 70.6 ± 9.3 | 72.4 ± 10.9 | 0.149 |
| BMI (kg/m2) | 24.9 ± 2.6 | 24.7 ± 2.7 | 24.8 ± 2.5 | 25.5 ± 2.8 | 0.436 |
| BMI ≥ 25 kg/m2 | 53 (43.4%) | 24 (48.0%) | 20 (41.7%) | 9 (37.5%) | 0.661 |
| BSA (m2) | 1.79 ± 0.16 | 1.76 ± 0.17 | 1.81 ± 0.15 | 1.82 ± 0.17 | 0.246 |
| Waist circumference (cm) | 89 ± 7 | 88 ± 7 | 89 ± 7 | 91 ± 7 | 0.318 |
| Central obesity* | 10 (8.2%) | 5 (10.0%) | 3 (6.2%) | 2 (8.3%) | 0.795 |
| Framingham 10-yr risk score (%) | 6 (3–10) | 4 (2–8) | 8 (4–11)† | 7 (3–12)‡ | 0.002 |
| Framingham score ≥ 10% | 35 (28.7%) | 7 (14.0%) | 20 (41.7%) | 8 (33.3%) | 0.009 |
| Total cholesterol (mg/dL) | 195.7 ± 31.4 | 194.9 ± 32.1 | 198.7 ± 31.0 | 191.7 ± 31.5 | 0.675 |
| Triglyceride (mg/dL) | 122.2 ± 61.6 | 124.8 ± 72.9 | 112.0 ± 42.4 | 135.9 ± 65.7 | 0.299 |
| HDL cholesterol (mg/dL) | 53.8 ± 13.2 | 54.4 ± 14.2 | 54.0 ± 12.2 | 52.2 ± 13.3 | 0.808 |
| LDL cholesterol (mg/dL) | 118.0 ± 30.9 | 115.9 ± 32.6 | 123.2 ± 29.2 | 112.7 ± 30.2 | 0.345 |
| Creatinine (mg/dL) | 1.09 ± 0.16 | 1.08 ± 0.18 | 1.10 ± 0.15 | 1.11 ± 0.13 | 0.672 |
| GFR (ml/min/1.73 m2) | 73.2 ± 10.1 | 73.7 ± 10.9 | 72.7 ± 9.1 | 73.0 ± 10.5 | 0.900 |
| HbA1c (%) | 5.95 ± 0.67 | 5.87 ± 0.49 | 5.91 ± 0.52 | 6.19 ± 1.12 | 0.174 |
| FBS (mg/dL) | 98.7 ± 11.1 | 98.0 ± 10.1 | 98.8 ± 12.6 | 100.2 ± 10.4 | 0.796 |
| Fasting insulin (µU/mL) | 10.57 ± 4.84 | 9.60 ± 3.85 | 11.60 ± 6.00 | 10.73 ± 3.92 | 0.357 |
| HOMA-IR | 2.20 ± 1.25 | 1.98 ± 0.89 | 2.40 ± 1.62 | 2.40 ± 1.18‡ | 0.440 |
| hsCRP (mg/L) | 1.13 ± 1.82 | 1.10 ± 1.21 | 1.28 ± 2.39 | 0.95 ± 1.70 | 0.808 |
| Follow-up duration (months) | 65 (62–73) | 65 (63–73) | 65 (62–73) | 66 (63–74) | 0.916 |
Data are shown as mean ± standard deviation, median (interquartile range), or proportions (%). P values were calculated by analysis of covariance with post-hoc comparisons.
Central obesity: waist circumference >120 cm in men and >88 cm in women, according to the guidelines of NCEP ATP III16).
P < 0.01 versus “No plaque” group
P < 0.05 versus “No plaque” group
Abbreviations: CT, computed tomography; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; BSA, body surface area; HOMA-IR, homeostatic model assessment insulin resistance index; hsCRP, high-sensitivity C-reactive protein.
Determinants of Epicardial Fat Volume
To examine the determinants of baseline EFVi, we used linear regression analysis for the EFVi values and logistic regression analysis for the 3rd tertile of EFVi, among the univariable predictors with p values <0.200 (Table 2 and Supplementary Table 1). For the EFVi values, age (B = 0.761; 95% CI, 0.116–1.407; P = 0.021 per 1-year increase) and BMI (B = 4.055; 95% CI, 0.836–7.274; P = 0.014 per 1 kg/m2 increase) were significant determinants (Table 2). Similarly, analysis for the 3rd tertile of baseline EFVi showed that advanced age and higher BMI were significant determinants (Supplementary Table 1).
Table 2. Multiple linear regression analysis for baseline epicardial fat volume index.
| Univariable |
Multivariable |
|||||
|---|---|---|---|---|---|---|
| B | 95% CI | P value | B | 95% CI | P value | |
| Age (per + 1 year) | 0.563 | −0.052–1.179 | 0.072 | 0.761 | 0.116–1.407 | 0.021 |
| Male sex | 3.557 | −8.330–15.445 | 0.555 | |||
| HTN | 6.370 | −3.214–15.955 | 0.191 | 2.657 | −6.783–12.097 | 0.578 |
| DM | 6.583 | −9.897–23.063 | 0.431 | |||
| Dyslipidemia | 4.037 | −8.478–16.552 | 0.524 | |||
| Current smoker | −5.817 | −17.871–6.236 | 0.341 | |||
| BMI (kg/m2) | 4.977 | 3.332–6.622 | <0.001 | 4.055 | 0.836–7.274 | 0.014 |
| Waist circumference (cm) | 1.797 | 1.162–2.432 | <0.001 | 0.330 | −0.900–1.560 | 0.596 |
| Framingham 10-yr risk score (%) | 0.949 | −0.104–2.002 | 0.077 | −0.036 | −1.237–1.166 | 0.953 |
| Total cholesterol (mg/dL) | 0.056 | −0.100–0.213 | 0.479 | |||
| Triglyceride (mg/dL) | −0.001 | −0.081 -0.079 | 0.985 | |||
| LDL-C (mg/dL) | 0.129 | −0.028–0.287 | 0.107 | 0.120 | −0.029–0.270 | 0.113 |
| HDL-C (mg/dL) | −0.300 | −0.669–0.068 | 0.109 | −0.072 | −0.434–0.289 | 0.692 |
| HOMA-IR | 3.141 | −0.879–7.161 | 0.124 | −0.294 | −5.360–4.771 | 0.908 |
| hsCRP (mg/L) | 1.526 | −1.837–4.888 | 0.369 | |||
Abbreviations: HTN, hypertension; DM, diabetes mellitus; BMI, body mass index; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment insulin resistance index; hsCRP, high-sensitivity C-reactive protein.
Supplementary Table 1. Multivariable logistic regression analysis for the 3rd tertile of baseline epicardial fat volume index.
| Univariable |
Multivariable† |
|||||
|---|---|---|---|---|---|---|
| B | 95% CI | P value | B | 95% CI | P value | |
| Age (per + 1 year) | 1.060 | 1.006–1.116 | 0.028 | 1.133 | 1.016–1.265 | 0.025 |
| Male sex | 0.808 | 0.319–2.044 | 0.653 | |||
| HTN | 1.708 | 0.797–3.661 | 0.169 | 0.808 | 0.194–3.365 | 0.770 |
| DM | 2.606 | 0.745–9.118 | 0.134 | 0.454 | 0.053–3.927 | 0.474 |
| Dyslipidemia | 1.617 | 0.619–4.226 | 0.327 | |||
| Current smoker | 1.067 | 0.411–2.770 | 0.895 | |||
| BMI (kg/m2) | 1.795 | 1.407–2.289 | <0.001 | 1.718 | 1.031–2.865 | 0.038 |
| Waist circumference (cm) | 1.206 | 1.109–1.310 | <0.001 | 1.101 | 0.905–1.339 | 0.338 |
| Framingham 10-yr risk score (%) | 1.093 | 1.003–1.190 | 0.042 | 1.016 | 0.852–1.213 | 0.857 |
| Total cholesterol (mg/dL) | 1.006 | 0.993–1.018 | 0.367 | |||
| Triglyceride (mg/dL) | 1.000 | 0.993–1.006 | 0.913 | |||
| LDL-C (mg/dL) | 1.020 | 0.997–1.023 | 0.120 | 1.013 | 0.989–1.037 | 0.282 |
| HDL-C (mg/dL) | 0.988 | 0.959–1.018 | 0.412 | |||
| HOMA-IR | 1.399 | 0.990–1.976 | 0.057 | 0.958 | 0.527–1.742 | 0.888 |
| hsCRP (mg/L) | 7.376 | 0.600–90.681 | 0.119 | 1.099 | 0.769–1.572 | 0.604 |
Central obesity: waist circumference >120 cm in men and >88 cm in women, according to the guidelines of NCEP ATP III.
Low HDL-C: HDL-C levels <40 mg/dL in men and <50 mg/dL in women, according to the guidelines of NCEP ATP III.
adjustment with age (per + 1 year), HTN, DM, BMI (per + 1 kg/m2), waist circumference (per + 1 cm), Framingham 10-year risk score (per + 1%), LDL-C (per + 1 mg/dL), HOMA-IR and hsCRP (per + 1 mg/L).
Abbreviations: HTN, hypertension; DM, diabetes mellitus; BMI, body mass index; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment insulin resistance index; hsCRP, high-sensitivity C-reactive protein.
Differences in EFV According to Follow-up CT Results
In the total population, the mean EFV at baseline was 120.1 ± 49.5 cm3, and the mean of EFVi was 66.4 ± 25.8 cm3/m2 (Table 3). Baseline EFVi was significantly different between subgroups according to the follow-up CT results, but the follow-up EFVi and changes in EFVi from baseline to follow-up were not different between the subgroups. The difference in baseline EFVi between subgroups remained significant even after adjusting for age, sex, HTN, DM, Framingham risk score, and obesity as covariates (adjusted overall P = 0.013; Fig. 1). In the NCP group, the baseline EFVi was 79.9 ± 30.3 cm3/m2, which was significantly higher than in the NP group (62.5 ± 24.7 cm3/m2; adjusted P = 0.019) and in the CP group (63.7 ± 22.7 cm3/m2; adjusted P = 0.021).
Table 3. Baseline and follow-up EFV and EFVi levels according to follow-up CT results.
| Total (n = 122) |
Follow-up cardiac CT |
Overall P | |||
|---|---|---|---|---|---|
| No plaque | Calcified plaque | Non-calcified plaque | |||
| (n = 50) | (n = 48) | (n = 24) | |||
| EFV (cm3) | |||||
| - Baseline | 120.12 ± 49.48 | 110.95 ± 45.80 | 116.74 ± 46.27 | 146.00 ± 55.96*† | 0.013 |
| - Follow-up | 125.97 ± 53.93 | 119.39 ± 50.61 | 122.35 ± 55.59 | 146.93 ± 54.33 | 0.100 |
| - Change | 5.85 ± 2.65 | 8.43 ± 2.94 | 5.61 ± 2.64 | 0.93 ± 1.96 | 0.524 |
| - Change (%) | 7.36 ± 24.26 | 12.12 ± 29.43 | 5.17 ± 21.53 | 1.81 ± 14.57 | 0.168 |
| EFVi (cm3/m2) | |||||
| - Baseline | 66.41 ± 25.84 | 62.52 ± 24.72 | 63.71 ± 22.71 | 79.93 ± 30.30*† | 0.016 |
| - Follow-up | 69.97 ± 28.13 | 67.37 ± 27.28 | 67.01 ± 27.53 | 81.28 ± 29.37 | 0.094 |
| - Change | 3.52 ± 14.57 | 4.86 ± 16.54 | 3.27 ± 14.04 | 1.23 ± 11.03 | 0.601 |
| - Change (%) | 7.52 ± 23.48 | 11.98 ± 28.92 | 5.38 ± 20.29 | 2.51 ± 14.04 | 0.193 |
Data are shown as mean ± standard deviation (SD). P values were calculated by ANOVA with post-hoc comparisons.
P < 0.05 versus “No plaque” group
P < 0.05 versus “Calcified plaque” group
Abbreviations: CT, computed tomography; EFV, epicardial fat volume; EFVi, epicardial fat volume index.
Fig. 1.
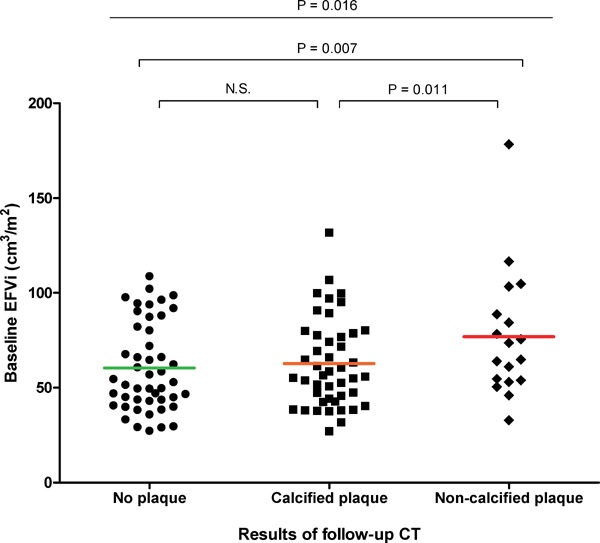
Differences in EFVi according to follow-up CT results
Baseline EFVi was significantly higher in participants who had NCP on follow-up CT than participants who had CP or no plaque on follow-up CT. P-values were calculated by ANCOVA with age, sex, HTN, DM, Framingham risk score, BMI, and central obesity as covariates.
Abbreviations: EFVi, epicardial fat volume index; CT, computed tomography; HTN, hypertension; DM, diabetes mellitus; NCP, non-calcified plaque; CP, calcified plaque; ANCOVA, analysis of covariance; BMI, body mass index.
Impact of EFV on the Development of NCP
Fig. 2 shows that there were significant differences in the proportions of participants in the NP, CP, and NCP groups in each tertile of baseline EFVi (overall P = 0.038). Among the participants in the 3rd tertile of baseline EFVi, 11 participants (29.3%) had NCP on the follow-up CT; 14 participants (22.5%) who were in the 2nd tertile at baseline and 16 participants (7.3%) who were in the 1st tertile at baseline had NCP on the follow-up CT (3rd tertile vs. 1st tertile, P = 0.020; 2nd tertile vs. 1st tertile, P = 0.067).
Fig. 2.
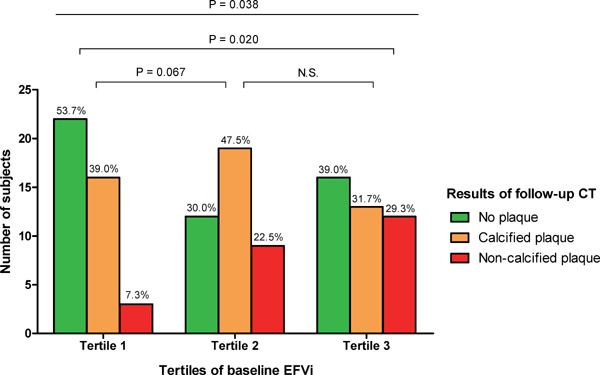
Impact of EFVi on the development of coronary plaque
The development of NCP on follow-up CT was observed more frequently among participants with increased EFVi at baseline. P-values were calculated by the χ2 test.
Abbreviations: EFV, epicardial fat volume; CT, computed tomography; NCP, non-calcified plaque.
We investigated baseline characteristics and baseline EFVi as potential contributing factors for the development of NCP on follow-up CT (Table 4). In the univariable logistic regression analysis, baseline EFVi was significantly associated with NCP on follow-up CT (OR, 1.024; 95% CI, 1.006– 1.042; P = 0.008 per 1 cm3/m2 increase). When the 1st tertile of baseline EFVi was used as the reference group, the 2nd tertile had an OR of 3.677 (95% CI, 0.916–14.765; P = 0.066), and the 3rd tertile had an OR of 5.241 (95% CI, 1.353–20.306; P = 0.017). After adjusting for the univariable factors with a P value of less than 0.200, the baseline EFVi values and the 3rd tertile of EFVi were significantly associated with the development of NCP. The predictive value of the 3rd tertile of baseline EFVi was maintained even after adjusting for age, sex, HTN, DM, dyslipidemia, smoking status, obesity, central obesity, and Framingham risk score (OR, 4.297; 95% CI, 1.040 –17.757; P = 0.044).
Table 4. Factors that contribute to the presence of non-calcified coronary plaque on follow-up CT.
| Univariable |
Multivariable Model 1‡ |
Multivariable Model 2§ |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | |
| Age (per + 1 year) | 1.021 | 0.964–1.082 | 0.472 | ||||||
| Age ≥ 65 years | 1.200 | 0.357–4.038 | 0.768 | ||||||
| Male sex | 1.909 | 0.519–7.022 | 0.331 | ||||||
| HTN | 2.035 | 0.826–5.016 | 0.122 | 1.513 | 0.558–4.106 | 0.416 | 1.712 | 0.561–5.222 | 0.345 |
| DM | 9.767 | 2.552–36.685 | 0.001 | 8.911 | 2.235–35.531 | 0.002 | 9.081 | 1.682–49.034 | 0.010 |
| Dyslipidemia | 1.349 | 0.439–4.139 | 0.601 | ||||||
| Current smoker | 1.170 | 0.385–3.549 | 0.782 | ||||||
| BMI (kg/m2) | 1.123 | 0.939–1.342 | 0.203 | ||||||
| BMI ≥ 25 kg/m2 | 0.736 | 0.294–1.843 | 0.513 | ||||||
| Waist circumference (cm) | 1.041 | 0.973–1.114 | 0.241 | ||||||
| Central obesity* | 1.023 | 0.203–5.158 | 0.978 | ||||||
| Framingham 10-yr risk score (%) | 1.065 | 0.966–1.175 | 0.206 | ||||||
| Framingham risk score <10% | 1.315 | 0.505–3.425 | 0.575 | ||||||
| Total cholesterol (mg/dL) | 0.995 | 0.980–1.010 | 0.496 | ||||||
| Triglyceride (mg/dL) | 1.004 | 0.997–1.011 | 0.238 | ||||||
| LDL-C (mg/dL) | 0.993 | 0.978–1.008 | 0.355 | ||||||
| LDL-C ≥ 160 mg/dL | 0.307 | 0.038–2.490 | 0.269 | ||||||
| HDL-C (mg/dL) | 0.988 | 0.953–1.024 | 0.518 | ||||||
| Low HDL-C** | 1.095 | 0.326–3.677 | 0.884 | ||||||
| HOMA-IR | 1.082 | 0.647–1.811 | 0.763 | ||||||
| HOMA-IR <2.0 | 1.600 | 0.471–5.440 | 0.452 | ||||||
| HOMA-IR <2.5 | 1.833 | 0.555–6.055 | 0.320 | ||||||
| hsCRP (mg/L) | 0.919 | 0.669–1.262 | 0.602 | ||||||
| hsCRP ≥ 1.0 mg/L | 0.626 | 0.213–1.842 | 0.395 | ||||||
| hsCRP ≥ 2.0 mg/L | 0.981 | 0.280–3.435 | 0.976 | ||||||
| Baseline EFVi (cm3/m2)† | 1.024 | 1.006–1.042 | 0.008 | 1.024 | 1.005–1.043 | 0.012 | |||
| - Tertile 1 | Ref | Ref | |||||||
| - Tertile 2 | 3.677 | 0.916–14.765 | 0.066 | 3.606 | 0.844–15.402 | 0.083 | |||
| - Tertile 3 | 5.241 | 1.353–20.306 | 0.017 | 4.297 | 1.040–17.757 | 0.044 | |||
Central obesity: waist circumference >120 cm in men and >88 cm in women, according to the guidelines of NCEP ATP III16).
Low HDL-C: HDL-C levels <40 mg/dL in men and <50 mg/dL in women, according to the guidelines of NCEP ATP III16).
EFVi values were used as a continuous variable in multivariable model 1, and as categorical variables in multivariable model 2; the 1st tertile of EFVi was used as the reference group.
Model 1: adjustment for HTN, DM and baseline EFVi.
Model 2: adjustment for HTN, DM and tertiles of baseline EFVi (1st tertile as reference).
Abbreviations: CT, computed tomography; OR, odds ratio; HTN, hypertension; DM, diabetes mellitus; BMI, body mass index; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment insulin resistance index; hsCRP, high-sensitivity C-reactive protein; EFVi, epicardial fat volume index.
Discussion
Among 122 participants without prior history of CAD and without metabolic syndrome, high EFVi was an independent risk factor for the future development of NCP even after adjustment for traditional cardiovascular risk factors. Given the scarce previous research on the impact of EAT on the “de novo” development of NCP over enough time interval and the lack of direct evidence on the causal relationship between EAT and vulnerable coronary plaque, this study of repeat CT scans among individuals in preclinical stage provides novel implications for cardiovascular prevention and screening. Our findings expand current knowledge related to EAT and cardiovascular risk and reveal that excessive EAT promotes the development of high-risk coronary plaque, which may further contribute to a higher risk of cardiovascular events.
EAT: a Potential Promoter of Coronary Atherosclerosis
EAT refers to the brown adipose tissue located between the myocardium and pericardium; it originates embryologically from the splanchno-pleuric mesoderm associated with the gut2, 19, 20). Compared to white adipose tissue, which has the primary function of storing triglycerides and free fatty acids, EAT is more metabolically active and has distinctive features such as the expression of adiponectin and inflammatory cytokines1, 4, 21). Because there is no separation by a physical fascia, EAT has direct contact with the surface of the myocardium and coronary vessels, and therefore, it is reasonable to assume that EAT could influence coronary atherosclerosis through local paracrine interactions1, 22).
Current understandings of the pathophysiology of EAT and atherosclerosis were strengthened by cross-sectional studies that revealed independent associations between increased EAT and the presence of CAD9, 23, 24). Moreover, several serial studies demonstrated that increased baseline EAT volume was associated with the progression or new development of coronary artery calcification (CAC). In a study by Nakanishi et al., 375 consecutive subjects underwent serial CAC measurements at least 3 to 5 years apart, and the findings revealed that increased EFV was associated with greater progression of CAC and with the development of calcified plaque11). Another study of serial CAC measurements by Yerramasu et al. also reported the independent predictive value of EAT volume for CAC progression12).
In the present study, we showed that baseline EAT was not associated with future development of CP, which may seem conflicting with the aforementioned study results. However, our findings are not against the current evidence on the relationship between EAT and coronary calcification. Although the exact pathophysiologic mechanisms by which EAT causes atherosclerosis are not completely understood yet, growing evidence suggest that EAT promotes the early stages of atherosclerotic plaque formation: proinflammatory and inflammatory cytokines that are produced and secreted by EAT, such as interleukin-1, interleukin-6, tumor necrosis factor (TNF) and monocyte chemoattractant protein 1 (MCP-1), proinflammatory M1 macrophages in EAT, increased oxidative stress, and accumulation of lipids are the potential mechanisms25). In accordance with those preclinical findings, recent studies suggested that EAT might be associated with vulnerable plaque independent of coronary calcification26, 27). Since our study focused on the development of NCP rather than overall calcification, our findings are still in line with previous studies in terms of association between EAT and coronary atherosclerosis. Moreover, our findings have an important clinical significance for the following practical reason.
From the viewpoint of risk prediction and prevention, the usefulness of EAT measurement merely for the prediction of CAC is limited because CAC burden is an indirect reflection of the atherosclerotic process rather than a causative lesion. It should be noted that the presence and progression of CAC cannot always be regarded as direct evidence of cardiovascular risk, since calcification in coronary arteries not only reflects the atherosclerotic process but suggests stable plaque in advanced stages of atherosclerosis28, 29). Moreover, according to recent studies, the progression of CAC seems to be an inevitable process9), and the use of statin therapy may accelerate coronary calcification19,22). Therefore, the relationship between EAT and CAC progression does not support the usefulness of EAT measurement as a determinant of cardiovascular risk or prevention. The direct relationship between EAT and the development of NCP, which is a vulnerable coronary plaque, has required further confirmation.
EAT and the Development of NCP: A Missing Link between the Pathobiologic Process and Clinical Practice
Growing evidence suggests a causal relationship between EAT and cardiovascular risk, but there is no evidence or investigation by serial measurement to support the actual impact of EAT on the development of vulnerable coronary plaque. In a study of intravascular ultrasound (IVUS) examinations, increased EAT volume was significantly associated with necrotic plaque tissue30). Other studies of cardiac CT also showed that higher EAT volume was associated with the presence of NCP31, 32). However, these were cross-sectional studies that cannot infer a causal relationship. The lack of evidence of the relationship between EAT and NCP can be attributed to the pathogenesis of coronary atherosclerosis. Because the development of NCP is rarer than that of CAC and the detection of incident NCP requires longer follow-up periods and larger study populations, most evidence has focused on the development and progression of CAC.
Clinically, the relationship between EAT and NCP development will enhance the additive prognostic value of EAT and will support the use of EAT measurement in practice. EAT is associated with the presence of diabetes, obesity, and metabolic syndrome, so it could be argued that EAT is simply an indicator of atherogenic burden. The counter-arguments against the use of EAT as a measure of disease prevention also include questions of the clinical usefulness of EAT beyond the current available measures such as CAC, myocardial perfusion imaging, and other vascular indices8, 33). If the amount of EAT merely reflects the burden of coronary calcification rather than the direct pathogenesis of coronary atherosclerosis, the measurement of EAT in clinical practice will be significantly limited. Furthermore, the prognostic value of EAT over current CAC measurement is debated8, 33). For EAT measurements to be applied in clinical practice, the connection between the pathobiologic process of EAT and the development of NCP, which actually contributes to cardiovascular events, needs to be clarified, particularly in a serial-measurement study design.
In this study, we showed that baseline EFVi is associated with future development of NCP. The predictive value of EFVi for the development of NCP was significant, even after adjusting for age, BMI, and other traditional cardiovascular risk factors such as waist circumference, HTN, diabetes, dyslipidemia, and Framingham 10-year risk score. Our findings support a causal relationship between EAT and NCP, which indicates that excessive EAT is not only a measure of current metabolic status but an independent prognostic factor for the development of vulnerable coronary plaques.
Clinical Implications and Future Directions
Recent cross-sectional studies showed that, among patients with a CAC score of zero, EAT measurement would have a clinical role in the detection of NCP, which cannot be detected on a CAC scan31). Further, increased EAT volume is associated with necrotic plaque tissue30). In addition to the previous studies, we demonstrated a novel clinical evidence of a causal relationship between EAT and the development of vulnerable coronary plaque. Our findings suggest the clinical usefulness of EAT measurement for risk stratification and its application in preventive measures and support the additional prognostic value of EAT. As a screening tool, EAT measurement would provide simple but important clinical information on the future risk of NCP development and cardiovascular events, especially among asymptomatic individuals.
However, further studies are needed to investigate whether the action mechanism of preventive medications, such as statins, involves the modification of EAT. It has been well-established that the use of statin therapy can reduce the progression of coronary atherosclerosis and stabilize the vulnerability of plaque, all of which contribute to a better prognosis34, 35). Several studies also suggested that the use of statin may lead to a regression of EAT36, 37). Together with the findings of our study, contemporary evidence indicates that it would be worth to investigate whether the benefit of statin therapy through atherosclerosis modulation effect is associated with any changes in EAT. More importantly, it needs to be validated in prospective trials whether preventive therapies guided by EAT measurement could improve clinical outcomes.
Another important clinical issue is the presence of diabetes, which is a well-known risk factor for coronary atherosclerosis and was an independent predictor of NCP development in the present study. Considering the previous studies that showed a relationship between the presence of diabetes or insulin resistance and increased EAT38, 39), it can be assumed that the association between EAT and NCP development in our study might be an epiphenomenon or secondary to the atherogenic effect of diabetes. However, we showed that the increased EAT is an independent predictor of future NCP development, even after adjustment for the presence of diabetes. Also, the level of HOMA-IR was neither significantly higher in NCP group than in the other groups nor an independent predictor of NCP. The interpretation of this finding needs caution, because we excluded the patients with metabolic syndrome from our study population given the strong relationship between EAT and metabolic syndrome25, 38, 40). Therefore, our results should be interpreted as indicating the significant predictive value of EAT for the development of vulnerable plaque but not as suggesting that insulin resistance is not associated with EAT or NCP.
Limitations
Our study has several limitations. First, we could not use imaging methods such as IVUS or optical coherence tomography (OCT) to obtain detailed information of each coronary plaque. However, it should be noted that the study participants were asymptomatic and underwent CCTA as part of a routine healthcare check-up. Because the invasive assessment of coronary plaque confers potential risks, evaluation by IVUS or OCT was determined to be unethical for our study design. Second, we could not evaluate the impact of location-specific EAT on the development of plaques in adjacent coronary arteries. Current evidence suggests an association between the location-specific EAT thickness at the atrioventricular groove and the presence of obstructive CAD41), so evaluation of this relationship would provide interesting insight regarding the pathobiology of EAT on the development of coronary atherosclerosis. Third, we excluded participants with metabolic syndrome from this study, since there is a strong relationship between the presence of metabolic syndrome and EAT. The main purpose of this study was to investigate the causal relationship between EAT and the development of NCP, and our findings addressed this association. Because patients with metabolic syndrome or obesity are the populations of interest for the application of EAT measurement, further studies are required to validate the clinical usefulness of EAT measurement in these populations. Last, our study population consisted of relatively healthy subjects with low risk, and ethical issues may arise from using EAT measurement as a screening tool, unless more evidence confirms substantial gain from undergoing cardiac CT. Radiation doses from cardiac CT have been lowered recently, but there is still marginal exposure to radiation; contrast agent hypersensitivity should also be considered as an additional safety issue.
Conclusions
Among 122 asymptomatic individuals without prior history of CAD and without metabolic syndrome, a high EAT volume at baseline was an independent risk factor for NCP development. The prognostic value of a high EAT volume index for vulnerable coronary plaque was significant even after adjusting for traditional risk factors, BMI, and central obesity. Our findings from serial CT scans suggest a causal relationship between EAT and NCP and support the clinical usefulness of EAT measurement.
Financial Disclosure
Nothing to disclose.
Conflict of Interest
There is no conflict of interest related to this study.
Contributions
Research idea and study design: ICH, HEP, SYC; data acquisition: HEP, SYC; literature search: ICH, HEP; data analysis and interpretation: ICH, HEP, SYC; statistical analysis: ICH, HEP; supervision and mentorship: HEP, SYC. Each author contributed important intellectual content during the writing of the manuscript, and each accepts accountability for the overall work by confirming that questions pertaining to the accuracy or integrity of any portion of the work have been appropriately investigated and resolved. HEP confirms that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
References
- 1). Iacobellis G, Corradi D, Sharma AM: Epicardial adi pose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med, 2005; 2: 536-543 [DOI] [PubMed] [Google Scholar]
- 2). Lee HY, Despres JP, Koh KK: Perivascular adipose tissue in the pathogenesis of cardiovascular disease. Atherosclerosis, 2013; 230: 177-184 [DOI] [PubMed] [Google Scholar]
- 3). Lim S, Meigs JB: Links between ectopic fat and vascular disease in humans. Arterioscler Thromb Vasc Biol, 2014; 34: 1820-1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O'Brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y: Human epicardial adipose tissue is a source of inflammatory mediators. Circulation, 2003; 108: 2460-2466 [DOI] [PubMed] [Google Scholar]
- 5). Nakazato R, Dey D, Cheng VY, Gransar H, Slomka PJ, Hayes SW, Thomson LE, Friedman JD, Min JK, Berman DS: Epicardial fat volume and concurrent presence of both myocardial ischemia and obstructive coronary artery disease. Atherosclerosis, 2012; 221: 422-426 [DOI] [PubMed] [Google Scholar]
- 6). Ueno K, Anzai T, Jinzaki M, Yamada M, Jo Y, Maekawa Y, Kawamura A, Yoshikawa T, Tanami Y, Sato K, Kuribayashi S, Ogawa S: Increased epicardial fat volume quantified by 64-multidetector computed tomography is associated with coronary atherosclerosis and totally occlusive lesions. Circ J, 2009; 73: 1927-1933 [DOI] [PubMed] [Google Scholar]
- 7). Mahabadi AA, Berg MH, Lehmann N, Kalsch H, Bauer M, Kara K, Dragano N, Moebus S, Jockel KH, Erbel R, Mohlenkamp S: Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. J Am Coll Cardiol, 2013; 61: 1388-1395 [DOI] [PubMed] [Google Scholar]
- 8). Forouzandeh F, Chang SM, Muhyieddeen K, Zaid RR, Trevino AR, Xu J, Nabi F, Mahmarian JJ: Does quantifying epicardial and intrathoracic fat with noncontrast computed tomography improve risk stratification beyond calcium scoring alone? Circ Cardiovasc Imaging, 2013; 6: 58-66 [DOI] [PubMed] [Google Scholar]
- 9). Ahn SG, Lim HS, Joe DY, Kang SJ, Choi BJ, Choi SY, Yoon MH, Hwang GS, Tahk SJ, Shin JH: Relationship of epicardial adipose tissue by echocardiography to coronary artery disease. Heart, 2008; 94: e7. [DOI] [PubMed] [Google Scholar]
- 10). de Vos AM, Prokop M, Roos CJ, Meijs MF, van der Schouw YT, Rutten A, Gorter PM, Cramer MJ, Doevendans PA, Rensing BJ, Bartelink ML, Velthuis BK, Mosterd A, Bots ML: Peri-coronary epicardial adipose tissue is related to cardiovascular risk factors and coronary artery calcification in post-menopausal women. Eur Heart J, 2008; 29: 777-783 [DOI] [PubMed] [Google Scholar]
- 11). Nakanishi R, Rajani R, Cheng VY, Gransar H, Nakazato R, Shmilovich H, Otaki Y, Hayes SW, Thomson LE, Friedman JD, Slomka PJ, Berman DS, Dey D: Increase in epicardial fat volume is associated with greater coronary artery calcification progression in subjects at intermediate risk by coronary calcium score: a serial study using non-contrast cardiac CT. Atherosclerosis, 2011; 218: 363-368 [DOI] [PubMed] [Google Scholar]
- 12). Yerramasu A, Dey D, Venuraju S, Anand DV, Atwal S, Corder R, Berman DS, Lahiri A: Increased volume of epicardial fat is an independent risk factor for accelerated progression of sub-clinical coronary atherosclerosis. Atherosclerosis, 2012; 220: 223-230 [DOI] [PubMed] [Google Scholar]
- 13). Wang TD, Chen MF: Thicker epicardial adipose tissue in nonobese hypertensive patients: an innocent bystander or overlooked villain? Am J Hypertens, 2011; 24: 1191-1192 [DOI] [PubMed] [Google Scholar]
- 14). Alberti KG, Zimmet PZ: Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med, 1998; 15: 539-553 [DOI] [PubMed] [Google Scholar]
- 15). Organization WH: International Association for the Study of Obesity, International Obesity Task Force. The Asia-Pacific perspective: redefining obesity and its treatment Sydney: Health Communications, 2000; 15-21 [Google Scholar]
- 16). National Cholesterol Education Program Expert Panel on Detection E and Treatment of High Blood Cholesterol in A: Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation, 2002; 106: 3143-3421 [PubMed] [Google Scholar]
- 17). Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia, 1985; 28: 412-419 [DOI] [PubMed] [Google Scholar]
- 18). Park HE, Choi SY, Kim HS, Kim MK, Cho SH, Oh BH: Epicardial fat reflects arterial stiffness: assessment using 256-slice multidetector coronary computed tomography and cardio-ankle vascular index. J Atheroscler Thromb, 2012; 19: 570-576 [DOI] [PubMed] [Google Scholar]
- 19). Iozzo P: Myocardial, perivascular, and epicardial fat. Diabetes Care, 2011; 34 Suppl 2: S371-379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Ouwens DM, Sell H, Greulich S, Eckel J: The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. J Cell Mol Med, 2010; 14: 2223-2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Iacobellis G, Pistilli D, Gucciardo M, Leonetti F, Miraldi F, Brancaccio G, Gallo P, di Gioia CR: Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine, 2005; 29: 251-255 [DOI] [PubMed] [Google Scholar]
- 22). Cherian S, Lopaschuk GD, Carvalho E: Cellular cross-talk between epicardial adipose tissue and myocardium in relation to the pathogenesis of cardiovascular disease. Am J Physiol Endocrinol Metab, 2012; 303: E937-949 [DOI] [PubMed] [Google Scholar]
- 23). Jeong JW, Jeong MH, Yun KH, Oh SK, Park EM, Kim YK, Rhee SJ, Lee EM, Lee J, Yoo NJ, Kim NH, Park JC: Echocardiographic epicardial fat thickness and coronary artery disease. Circ J, 2007; 71: 536-539 [DOI] [PubMed] [Google Scholar]
- 24). Picard FA, Gueret P, Laissy JP, Champagne S, Leclercq F, Carrie D, Juliard JM, Henry P, Niarra R, Chatellier G, Steg PG: Epicardial adipose tissue thickness correlates with the presence and severity of angiographic coronary artery disease in stable patients with chest pain. PLoS One, 2014; 9: e110005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Iacobellis G: Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol, 2015; 11: 363-371 [DOI] [PubMed] [Google Scholar]
- 26). Nakanishi K, Fukuda S, Tanaka A, Otsuka K, Jissho S, Taguchi H, Yoshikawa J, Shimada K: Persistent epicardial adipose tissue accumulation is associated with coronary plaque vulnerability and future acute coronary syndrome in non-obese subjects with coronary artery disease. Atherosclerosis, 2014; 237: 353-360 [DOI] [PubMed] [Google Scholar]
- 27). Ito T, Suzuki Y, Ehara M, Matsuo H, Teramoto T, Terashima M, Nasu K, Kinoshita Y, Tsuchikane E, Suzuki T, Kimura G: Impact of epicardial fat volume on coronary artery disease in symptomatic patients with a zero calcium score. Int J Cardiol, 2013; 167: 2852-2858 [DOI] [PubMed] [Google Scholar]
- 28). Arbab-Zadeh A, Fuster V: The myth of the “vulnerable plaque”: transitioning from a focus on individual lesions to atherosclerotic disease burden for coronary artery disease risk assessment. J Am Coll Cardiol, 2015; 65: 846-855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Libby P: Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med, 2013; 368: 2004-2013 [DOI] [PubMed] [Google Scholar]
- 30). Yamashita K, Yamamoto MH, Ebara S, Okabe T, Saito S, Hoshimoto K, Yakushiji T, Isomura N, Araki H, Obara C, Ochiai M: Association between increased epicardial adipose tissue volume and coronary plaque composition. Heart Vessels, 2014; 29: 569-577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Tsushima H, Yamamoto H, Kitagawa T, Urabe Y, Tatsugami F, Awai K, Kihara Y: Association of epicardial and abdominal visceral adipose tissue with coronary atherosclerosis in patients with a coronary artery calcium score of zero. Circ J, 2015; 79: 1084-1091 [DOI] [PubMed] [Google Scholar]
- 32). Oka T, Yamamoto H, Ohashi N, Kitagawa T, Kunita E, Utsunomiya H, Yamazato R, Urabe Y, Horiguchi J, Awai K, Kihara Y: Association between epicardial adipose tissue volume and characteristics of non-calcified plaques assessed by coronary computed tomographic angiography. Int J Cardiol, 2012; 161: 45-49 [DOI] [PubMed] [Google Scholar]
- 33). Possner M, Liga R, Gaisl T, Vontobel J, Clerc OF, Mikulicic F, Benz DC, Grani C, Stehli J, Fuchs TA, Dey D, Pazhenkottil AP, Herzog BA, Gaemperli O, Buechel RR, Kaufmann PA: Quantification of epicardial and intrathoracic fat volume does not provide an added prognostic value as an adjunct to coronary artery calcium score and myocardial perfusion single-photon emission computed tomography. Eur Heart J Cardiovasc Imaging, 2015; [DOI] [PubMed] [Google Scholar]
- 34). Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, Davignon J, Erbel R, Fruchart JC, Tardif JC, Schoenhagen P, Crowe T, Cain V, Wolski K, Goormastic M, Tuzcu EM, Investigators A : Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA, 2006; 295: 1556-1565 [DOI] [PubMed] [Google Scholar]
- 35). Wakabayashi K, Nozue T, Yamamoto S, Tohyama S, Fukui K, Umezawa S, Onishi Y, Kunishima T, Sato A, Miyake S, Morino Y, Yamauchi T, Muramatsu T, Hibi K, Terashima M, Suzuki H, Michishita I, investigators T : Efficacy of Statin Therapy in Inducing Coronary Plaque Regression in Patients with Low Baseline Cholesterol Levels. J Atheroscler Thromb, 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). Alexopoulos N, Melek BH, Arepalli CD, Hartlage GR, Chen Z, Kim S, Stillman AE, Raggi P: Effect of intensive versus moderate lipid-lowering therapy on epicardial adipose tissue in hyperlipidemic post-menopausal women: a substudy of the BELLES trial (Beyond Endorsed Lipid Lowering with EBT Scanning). J Am Coll Cardiol, 2013; 61: 1956-1961 [DOI] [PubMed] [Google Scholar]
- 37). Ahmadi N, Nabavi V, Malpeso J, Hajsadeghi F, Ismaeel H: Statin Therapy is Associated with Reduction of Epicardial Adipose Tissues and Coronary Plaque Volumes with Vulnerable Composition, Measured by Computed Tomography Angiography. J Cardiovasc Res 4: 4 doi: http://dx doi org/104172/2324, 2015; 8602: 2 [Google Scholar]
- 38). Wang CP, Hsu HL, Hung WC, Yu TH, Chen YH, Chiu CA, Lu LF, Chung FM, Shin SJ, Lee YJ: Increased epicardial adipose tissue (EAT) volume in type 2 diabetes mellitus and association with metabolic syndrome and severity of coronary atherosclerosis. Clin Endocrinol (Oxf), 2009; 70: 876-882 [DOI] [PubMed] [Google Scholar]
- 39). Iacobellis G, Leonetti F: Epicardial adipose tissue and insulin resistance in obese subjects. J Clin Endocrinol Metab, 2005; 90: 6300-6302 [DOI] [PubMed] [Google Scholar]
- 40). Pierdomenico SD, Pierdomenico AM, Cuccurullo F, Iacobellis G: Meta-analysis of the relation of echocardiographic epicardial adipose tissue thickness and the metabolic syndrome. Am J Cardiol, 2013; 111: 73-78 [DOI] [PubMed] [Google Scholar]
- 41). Wu FZ, Chou KJ, Huang YL, Wu MT: The relation of location-specific epicardial adipose tissue thickness and obstructive coronary artery disease: systemic review and meta-analysis of observational studies. BMC Cardiovasc Disord, 2014; 14: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]


