Abstract
Aim: Atherosclerosis is a kind of chronic inflammatory disease. A crucial pathology change of atherosclerosis is the migration of activated VSMCs to the intima where they interact with leukocytes by expressing adhesion molecules, including intercellular cell adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1). Moreover, monocyte chemoattractant protein-1 (MCP-1) expressed by VSMCs plays an important role in recruiting monocytes and macrophages. Leech (Whitmania pigra Whitman) is a traditional Chinese medicine to treat cardiovascular diseases including atherosclerosis, however previous research has rarely reported the molecular mechanism for its curative effect. Thus, our study focuses on the effects of leech extracts on the expression of inflammatory factors, adhesion molecules and MCP-1 in rat VSMCs.
Methods: In our present study, wound-healing assay and Boyden chamber model were applied to evaluate the anti-migration effect of LEE (Leech Enzyme Extracts) on LPS induced VSMCs. The anti-adhesion effect was assessed using DiI-labeled THP-1 and RAW264.7.
Results: LEE suppressed LPS-induced VSMCs migration and decreased the chemotaxis and adhesive capacity of THP-1 and RAW264.7 to LPS-stimulated VSMCs. LEE also attenuated the upregulation of a variety of pro-atherosclerotic factors by inhibiting the phosphorylation of p38 MAPK. LEE was also observed to prevent NF-κB p65 nuclear localization using immune-fluorescent staining.
Conclusions: In conclusion, LEE suppresses LPS-induced upregulation of inflammatory factors, adhesion molecules and MCP-1 in rat VSMCs mainly via inhibiting the p38 MAPK/NF-κB pathways, thus partly uncovered LEE's molecular mechanisms for its therapeutic effect on atherosclerosis.
Keywords: Atherosclerosis, Vascular smooth muscle cells, Vascular cell adhesion molecule-1, Intercellular cell adhesion molecule-1, Mitogen-activated protein kinase (MAPKs)
Introduction
Atherosclerosis is a chronic inflammatory disease. A crucial pathology change of atherosclerosis is the migration of activated vascular smooth muscle cells (VSMCs) to the intima where they interact with leukocytes by expressing adhesion molecules, including intercellular cell adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1). Moreover, monocyte chemoattractant protein-1 (MCP-1) expressed by VSMCs plays an important role in recruiting monocytes and macrophages. Studies on the inflammatory processes of atherosclerosis have uncovered several mechanisms that could lead to potential therapies1). Many known risk factors are in close relation with the pathological process of atherosclerosis2). Inflammatory signaling alters the behavior of endothelial cells and VSMCs, which could recruits more inflammatory cells to promote lesion formation. VSMCs and macrophages are in direct contact, and a variety of adhesion molecules are involved in this process, including VCAM-1 and ICAM-13–6). Thus, the VSMCs are capable of retaining macrophages in the lesion of atherosclerosis.
Pro-inflammatory factors produced by VSMCs, endothelial cells, macrophages and T cells can promote the progression of atherosclerosis7). In VSMCs, some of the most important are platelet-derived growth factor (PDGF), macrophage migration inhibitory factor (MIF), inducible NOS (iNOS) and MCP-18, 9).
Leech has been widely used as a traditional Chinese medicine in cardiovascular diseases10–12). Our previous studies have investigated the anti-atherosclerosis effect of LEE (leech enzyme extracts) from Whitmania pigra Whitman using ApoE-/- mice, and found the treatment of LEE could obviously attenuate the area of atherosclerosis lesion. This effect is dose dependent and mainly a result of a reduced invasion of macrophages in the artery walls13). However, it remains unclear whether LEE can affect the abilities of VSMCs in recruiting and retaining macrophages. Therefore, our present study was aimed at uncovering the effects of LEE on the expression pro-inflammatory mediators, adhesion molecules and MCP-1 in VSMCs.
Materials and Methods
Materials
Antibodies for TLR4, MCP-1, iNOS, ICAM-1, VCAM-1, p38MAPK, p-p38MAPK, JNK1/2, p-JNK 1/2, ERK1/2, p-ERK1/2, β-actin were used for western blot. Trizol reagent, Revert Aid™ First Strand cDNA Synthesis Kit and Dream Taq™ PCR Master Mix were used for RT-PCR. NF-κB nuclear translocation assay kit, total nitric oxide assay kit and 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI), were from Beyotime Institute of Biotechnology (Shanghai, China). Positive drug simvastatin (SIM) was obtained from National Institutes for Food and Drug Control (Beijing, China). LEE was obtained from the International Biotechnology Research and Development Center of Shandong University at Weihai using our previous procedure13).
VSMCs Isolation and LEE Treatment
Male Sprague-Dawley rats were obtained from the School of Medicine at Shandong University. VSMCs were isolated from the thoracic aorta and cultured in DMEM containing 10% FBS, 100 U/mL penicillin and 100 µg/mL streptomycin14). LEE was obtained from the International Biotechnology Research and Development Center of Shandong University at Weihai using a previous procedure13).
VSMCs were subjected to six conditions: (1) LPS (1.0 µg/mL) as model control; (2) LPS(1.0 µg/mL) + SIM(10 µM) as positive control; (3) LPS(1.0 µg/mL) + LEE(50 µg/mL); (4) LPS(1.0 µg/mL) + LEE(100 µg/mL); (5) LPS(1.0 µg/mL) + LEE(200 µg/mL); (6) LPS(1.0 µg/mL) + LEE(400 µg/mL).
THP-1 and RAW264.7 Cell Culture
Human monocytic cell line THP-1 and murine monocyte-macrophage-like cell line RAW264.7 were obtained from the Institute of Biochemistry and Cell Biology in Shanghai and cultured in DMEM medium containing 10% FBS, 100 U/mL penicillin and 100 µg/mL streptomycin. Both these cell lines are commonly used to study the biology of monocyte and macrophage15, 16).
Wound-healing Assay in vitro
The VSMCs were grown to confluence in 24-well plates. After starving in serum-free DMEM medium for 24 h, a sterile pipette tip was used to create a straight scratch through the center of each well17). Cells were incubated with or without LPS, SIM and LEE. The wounds were photographed and analyzed at 48 h after the scratch.
The VSMCs Migration Assay
A modified Boyden chamber with an 8 µm pore size was used for the VSMCs migration assay18). Briefly, 100 µl of serum-free cell suspension (2Ü104 cells) was added to the upper surface of the chamber whose basement membrane had been embedded with Matrigel. The lower chambers were filled with DMEM (500 µl) supplemented with LPS, LPS + SIM or LPS + LEE. The chambers were incubated at 37°C to allow for cell migration. After removal of the cells on the upper surface, the cells on the lower surface were fixed with 4% paraformaldehyde. Crystal violet (0.1%) was used to stain the migration cells. Five random areas of cells per membrane were counted using a microscope.
The THP-1 and RAW264.7 Chemotaxis Assay
The lower chambers were filled with DMEM supplemented with culture supernatant of the VSMCs which had been incubated in relevant conditions according to the planned six groups mentioned up. THP-1 cells were added to the top of the chamber. After incubation for 12 h at 37°C, the upper chambers were removed, and then THP-1 cells in the lower chambers were observed under phase contrast microscope after conventional centrifugal operation. As for RAW264.7, after removal of the cells on the upper surface, the cells on the lower surface were fixed with 4% paraformaldehyde. Crystal violet (0.1%) was used to stain the migration cells. Five random areas per membrane were counted using a microscope.
The THP-1 and RAW264.7 Adhesion Assay
The cell adhesion assay was performed as described previously19). The VSMCs were treated with LPS or LPS with SIM or LPS with LEE in 24-well plates. THP-1 or RAW264.7 monocytes (2.5 Ü 105 cells/mL) labeled with DiI was added to each well. After incubation for 2 h, the unbound cells were aspirated. Five fields were captured using a fluorescence microscope and the numbers of adherent cells were counted.
RT-PCR
Total RNA was isolated using Trizol reagent, and was quantified spectrophotometrically at a ratio of 260–280 nm. RT-PCR was performed by using an RT-PCR System kit according to the protocol, with primers for rat TLR4, TNF-α, iNOS, MCP-1, ICAM-1, VCAM-1, and β-actin. The PCR amplification procedure was performed with denaturation at 95°C for 30 s, annealing at Tm for 30 s and DNA extending at 72°C for 60 s. A total of 30 amplification cycles were carried out. The PCR products were electrophoresed in 1.2% agarose gel. After staining with ethidium bromide, the total cDNA was determined by UV spectroscopy. β-Actin was utilized as a house-keeping gene as indicated. Primers for RT-PCR are listed in Table 1.
Table 1. Primer sequences for RT-PCR and their expected product size.
| Target | Primer sequence (5′ to 3′) | Product size (bp) |
|---|---|---|
| TLR-4 | F: GGCA TCAT CTTC ATTG TCCT TG R: AGCA TTGT CCTC CCAC TCG |
111 |
| iNOS | F: TTCA GGTA TGCG GTAT TTGG R: GTTG GAAG TGTA GCGT TTCG |
249 |
| TNF-α | F: CTTA TCTA CTCC CAGG TTCT CTCA A R: GAGA CTCC TCCC AGGT ACAT GG |
200 |
| β-actin | F: AGAC CTTC AACA CCCC AG R: CACG ATTT CCCT CTCA GC |
254 |
| MCP-1 | F: ATGC AGGT CTCT GTCA CGCT R: GGTG CTGA AGTC CTTA GGGT |
345 |
| ICAM-1 | F: AAAC GGGA GATG AATG GT R: TCTG GCGG TAAT AGGT GTA |
184 |
| VCAM-1 | F: GGAG ACAC TGTC ATTA TCTC CTG R: TCCT TTCA TGTT GGCT TTTC TTGC |
336 |
F indicates forward primer; R indicates reverse primer.
Western Blot
The VSMCs were lysed with RIPA, and the supernatants were collected after centrifugation at 10 000 rpms for 5 min at 4°C. Protein concentrations were measured using the BCA protein assay kit using bovine serum albumin. Samples were separated by SDS-PAGE and transferred to a polyvinylidene difluoride (PVDF) membrane. After blocking with 5% fat-free milk in TBST (20 mM Tris–HCl (pH 7.6), 150 mM NaCl, 0.1% Tween 20), the PVDF membrane was incubated with primary antibodies at 4°C overnight. Then the PVDF membrane was washed three times in TBST before incubating with horseradish peroxidase-conjugated secondary antibodies for 120 min. The optical densities of bands were quantified by using Image J. β-Actin was the endogenous control, and results were expressed relative to control.
Immuno-fluorescent Staining
The VSMCs were fixed in 4% paraformaldehyde and incubated overnight at 4°C with rabbit anti-NF-κB p65 IgG. Cell samples were washed and further incubated with biotinylated anti-rabbit IgG for 1 h. After washing, the cell samples were incubated with 4′,6-diamidino-2-phenylindole (DAPI) for 30 min. NF-κB-positive cells were counted in three separate sections using a fluorescent microscope.
Statistical Analysis
Data were described as mean ± SD. Differences between groups were assessed by one-way ANOVA. Statistical tests were done with SPSS software (International Business Machines Corporation, USA). A value of P <0.05 was considered as the criteria of statistical significance. #P <0.05 vs control, *P <0.05 vs LPS, **P <0.01 vs LPS, ΔP <0.05 vs LPS + SIM.
Results
VSMCs Wound-healing Assay
There were two terms we needed to give new meanings to: Healing rate and anti-migration rate. The following two formulas could account for the implications of these two terms: Healing rate = (the initial gap width − the later gap width)/the initial gap width; anti-migration rate = (the later gap width of group X − the later gap width of group control)/the initial gap width of group X. Compared with the normal control group, the mobility of the LPS and the SIM group differed greatly. More importantly, the potential performance of LEE was equal to that of SIM (10 µM) in inhibiting VSMCs migration in the concentration of 200 µg/mL (Fig. 1A and 1C).
Fig. 1.
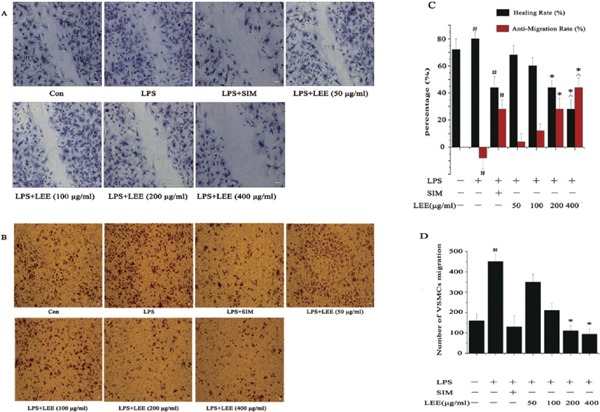
The anti-migration effects of LEE
(A) LEE inhibited wound healing in LPS-induced VSMCs. Bar = 50 µm. (B) LEE inhibited LPS-induced VSMCs migrating in a Boyden chamber assay. Bar = 50 µm. (C) The healing rate and anti-migration rate of the wound-healing assay. (D) Number of VSMCs migration. #P <0.05 vs control, *P <0.05 vs LPS, ΔP <0.05 vs LPS + SIM. Data are from three independent experiments.
LEE Attenuates VSMCs Migration and Decreases the Chemotaxis of THP-1 and RAW264.7
The modified Boyden chamber was involved in these experiments. The anti-migration effect of LEE was consistent with the result of the VSMC scratch healing assay in the previous section. The migration rate of VSMCs could be reduced to below that of the control group with LEE of 200 µg/mL (Fig. 1B and 1D).
In order to study whether LEE has an influence to the chemotaxis of THP-1 or RAW264.7 to LPS-stimulated VSMCs, we proposed a parameter on the chemotaxis inhibition rate, which was a function of the cell migration rate. The calculation method for MIR (migration inhibition rate) = 1 − (the number of cell migration in X group − the number of cell migration in blank control)/(the number of cell migration in model group − the number of cell migration in blank control). LEE (200 µg/mL) could decrease the chemotaxis of THP-1 and RAW264.7 to LPS-stimulated VSMCs, and the inhibition rate of those two experiments were 60% and 80% respectively (Fig. 2).
Fig. 2.
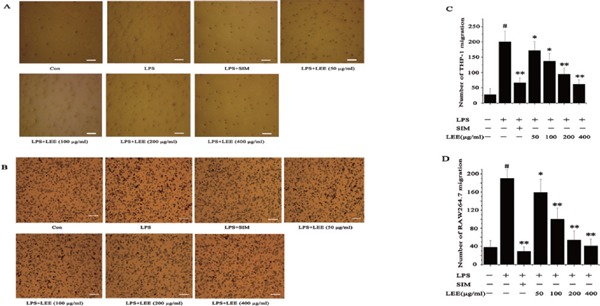
The anti-chemotaxis effects of LEE
(A) Inhibition of chemotaxis of THP-1 cells to LPS-stimulated VSMCs by LEE in a Boyden chamber assay. Bar = 100 µm. (B) Inhibition of chemotaxis of RAW264.7 cells to LPS-stimulated VSMCs by LEE in a Boyden chamber assay. Bar = 100 µm. (C) Number of THP-1 migration. (D) Number of RAW264.7 migration. #P <0.05 vs control, *P <0.05 vs LPS, **P <0.01 vs LPS. Data are from three independent experiments.
LEE Decreases the Adhesion of THP-1 and RAW264.7 to LPS-stimulated VSMCs
The calculation method for the adhesion inhibition rate (AIR) was similar to that of MIR. The formula for AIR = 1 − (the number of cell adhesion in X group − the number of cell adhesion in blank control)/(the number of cell adhesion in model group – the number of cell adhesion in blank control). Our data clearly showed that the number of adherent cells gradually reduced with the increase of LEE concentration. LEE (200 µg/mL) could decrease the adhesion of THP-1 and RAW264.7 to LPS-stimulated VSMCs, and the inhibition rate of those two experiments were 76% and 220% respectively (Fig. 3).
Fig. 3.
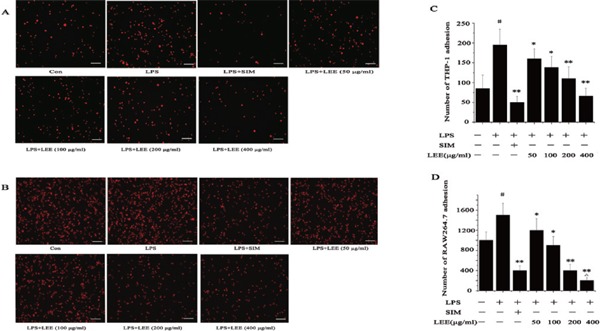
The anti-adhesion effects of LEE
(A) Inhibition of adhesion of THP-1 cells to LPS-stimulated VSMCs by LEE. Bar = 100 µm. (B) Inhibition of adhesion of RAW264.7 cells to LPS-stimulated VSMCs by LEE. Bar = 100 µm. (C) Number of THP-1 adhesion. (D) Number of RAW264.7 adhesion. #P <0.05 vs control, *P <0.05 vs LPS, **P <0.01 vs LPS, ΔP <0.05 vs LPS + SIM. Data are from three independent experiments.
Effect of LEE on Expression of Adhesion Molecule and MCP-1 in VSMCs
LPS stimulation caused an increased secretion of inflammatory factor, adhesion molecule and MCP-1. The LEE inhibited the upregulation of TLR4, TNF-α and iNOS in VSMCs at the mRNA and protein level. However, LEE could probably specialize in reducing the expression of ICAM-1, VCAM-1 and MCP-1 compared with the inhibitory effect on inflammatory factors, particularly as even the highest concentration of LEE would not be able to reduce the generation of TNF-α mRNA significantly. In other words, if SIM was taken into consideration, the anti-inflammatory effect of LEE would be slightly less than SIM. In spite of this, LEE could reduce the expression of ICAM-1, VCAM-1 and MCP-1 to normal levels (Fig. 4, 5).
Fig. 4.
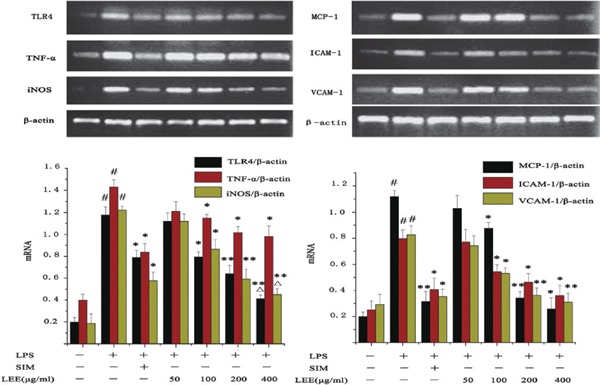
Inhibitory effect of LEE on LPS-induced mRNA production in VSMCs
Total RNA was extracted and detected by RT-PCR. β-actin was chosen as a reference. #P <0.05 vs control, *P <0.05 vs LPS, **P <0.01 vs LPS, ΔP <0.05 vs LPS + SIM. Data are from three independent experiments.
Fig. 5.
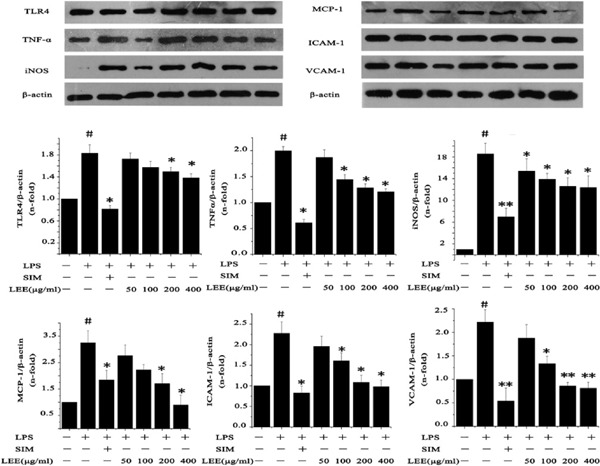
Inhibitory effect of LEE on LPS induced proteins production in VSMCs
VSMCs were pretreated with LPS (1 µg/mL) for 24 h, and then treated with LEE for another 24 h. Total protein was extracted and then detected by western blotting. #P <0.05 vs control, *P <0.05 vs LPS, **P <0.01 vs LPS. Data are from three independent experiments.
LEE Prevents the NF-κB Translocation to Nucleus
The activation of NF-κB contributes to inflammation status by promoting the expression of inflammation genes. To verify NF-κB p65 nuclear translocation, we analyzed VSMCs by fluorescence microscopy and the depth of the magenta color illustrated the degree of p65 translocation. Our results revealed that LPS significantly increased nuclear translocation of NF-κB p65 which could be prevented by LEE treatment (Fig. 6).
Fig. 6.
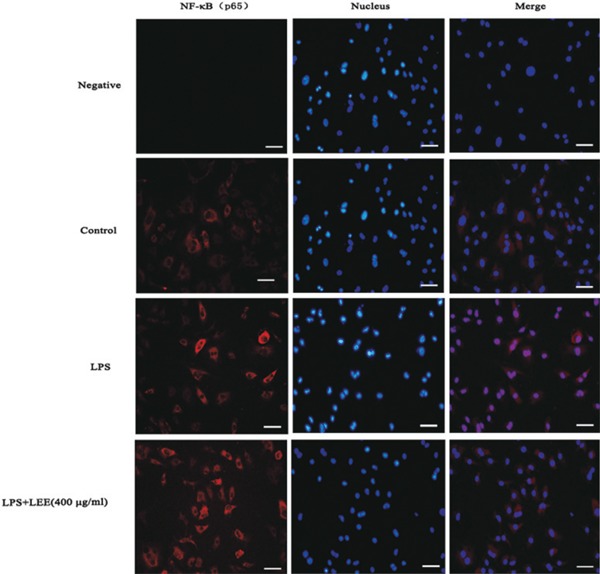
Effect of LEE on nuclear accumulation of p65 induced by LPS in VSMCs
Confluent VSMCs monolayers were grown on coverslips followed by being incubated with different conditions in the 24-hole plates for 48 h. Then cells were fixed, permeabilized, and stained with anti-p65 antibody and a secondary antibody conjugated to fluorescent red. The DNA was stained using DAPI. The negative control (p65 antibody not added) and a blank control are both included.
Effect of MAPK and NF-κB Inhibitors
The SB203580 (p38 MAPK inhibitor), U0126 (ERK inhibitor), SP600125 (JNK inhibitor) and PDTC (the NF-κB inhibitor) inhibited the expression of proteins mentioned above in the LPS-induced VSMC. These results indicated that these signaling pathways are involved in the LPS-induced inflammation process (Fig. 7).
Fig. 7.
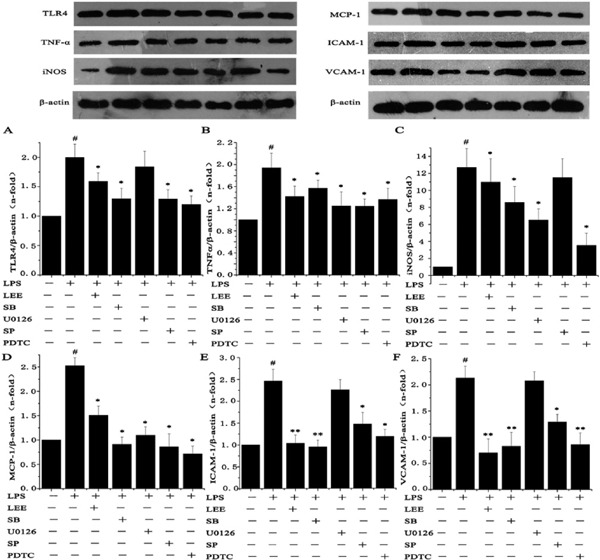
Effect of inhibitors to MAPKs and NF-κB on LPS-induced proteins upregulation
VSMCs were pre-treated with SB203580 (25 µmol/L), U0126 (20 µmol/L), SP600125 (15 µmol/L) and PDTC (80 µmol/L) for 1 h before adding LEE (400 µg/mL), and then incubated with 1 µg/mL LPS for another 24 h. #P <0.05 vs control, *P <0.05 vs LPS, **P <0.01 vs LPS. Data are from three independent experiments.
LEE Inhibits LPS-induced Phosphorylation of p38 MAPK, ERK and JNK in VSMCs
LPS-induced phosphorylation of ERK1/2, p38 MAPK and JNK1/2 in VSMCs was attenuated by LEE treatment to a different degree. Our results showed that the p38 MAPK signaling pathway was involved in LEE's inhibition in VSMCs while the JNK is only slightly involved in this process (Fig. 8).
Fig. 8.
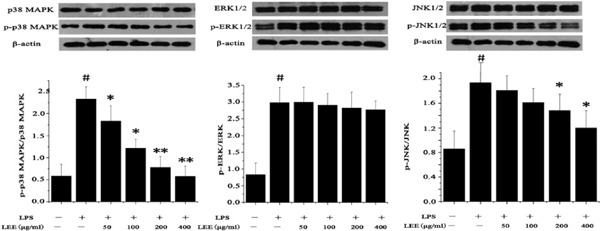
Effect of LEE on MAPK signaling pathway in LPS-stimulated VSMCs
Cells were stimulated with LPS for 12 h, and then were treated with LEE for 24 h. Western blot analysis was subsequently used to detect the expression of p38 MAPK, ERK and JNK. Phosphorylated proteins were also detected. #P <0.05 vs control, *P <0.05 vs LPS, **P <0.01 vs LPS. Data are from three independent experiments.
Discussion
Atherosclerosis is a chronic inflammatory disease. Studies have revealed that endothelial cells, VSMCs and inflammatory cells are involved in atherosclerosis pathological process7). The migration and proliferation of VSMCs play an important role in the process of intimal thickening, which is an important pathogenic change of atherosclerosis. Leech (Whitmania pigra Whitman) is a traditional Chinese medicine included in Chinese Pharmacopoeia (2010) to treat cardiovascular diseases including atherosclerosis, however previous research has rarely reported the molecular mechanism for its curative effect. Thus, our study focuses on the effects of leech extracts on the expression of inflammatory factors, adhesion molecules and MCP-1 in rat VSMCs.
The VSMCs express the cellular adhesion molecules and chemokines in atherosclerosis8). Thus, effective block of these proteins may help to ameliorate the development of atherosclerosis. In this study, we revealed that LEE is capable of inhibiting LPS-induced upregulation of adhesion molecules ICAM-1, VCAM-1 and one chemokine MCP-1 in VSMCs in a concentration-dependent manner. In parallel with these results, migration and adhesion of THP-1 and RAW 264.7 to LPS-activated VSMCs was markedly decreased by LEE. To our knowledge, this is the first report on the inhibiting effect of LEE on adhesion molecules and MCP-1 expression in VSMCs.
Although NO production by the enzyme eNOS has been found to be beneficial in preventing atherosclerosis20), high NO production will increase oxidative stress, which is a risk factor for atherosclerosis21). Thus, inhibition of iNOS and subsequent overproduction of NO could be helpful to treat atherosclerosis. Our results showed that LEE inhibited iNOS both at the mRNA and protein level in LPS-stimulated VSMCs. Additionally, NO production was suppressed by LEE, providing a novel molecular mechanism for its anti-atherosclerotic effects.
Chemokine and their receptors play an important role in the pathogenesis of atherosclerosis. Toll-like receptor 4 (TLR4) is a kind of pattern recognition receptor which can activate inflammatory signaling pathways when combined with LPS22–25). TLR4 is overexpressed in human atherosclerotic plaques, and TLR4 gene knockout mice showed reduced plaques area and vulnerability26). In our study, LEE inhibited LPS-induced upregulation of TLR4, which could activate following signal transduction that triggers inflammatory responses. MCP-1 and TNF-α are, respectively, the major chemokine and pro-inflammatory cytokines involved in atherosclerosis27–29). Consistent with previous reports, we found that LPS increased TNF-α and MCP-1 expression in VSMCs. More importantly, LEE inhibited LPS-induced upregulation of these two factors in a dose-dependent manner.
Mitogen-activated protein kinases (MAPKs) are a group of signaling molecules that regulate inflammatory, proliferation, apoptosis and differentiation through activating several downstream transcription factors30, 31). The NF-κB signaling pathway can be activated by several atherogenic stimuli, serving as a regulator in inflammatory genes32, 33). Thus, NF-κB activation pathway can be recognized as a potential therapeutic target for atherosclerosis in clinical applications34). NF-κB is partially dependent on the phosphorylation of MAPKs35). Our data support that LEE markedly inhibited the phosphorylation of p38 MAPK and the nuclear translocation of NF-κB in LPS-stimulated VSMCs.
A limitation of the study was that the LEE used in this study are crude extracts from leech. In light of this the present study provides a preliminary explanation for the widespread clinical application of leech (Whitmania pigra Whitman) in the treatment of atherosclerosis. In further studies, focus should be put on the separation and purification procedures, which are necessarily required to give more information on the specific effective constituents included in LEE. In conclusion, our results showed that LEE markedly inhibited LPS-induced overproduction of inflammatory factors, adhesion molecules and MCP-1 in VSMCs. Additionally, LEE's beneficial effects on atherosclerosis are at least partially depending on p38 MAPK/NF-κB signaling pathway.
Acknowledgments
This study does not bear any conflict. This work was supported by the National Natural Science Foundation of China (No. 81371455) and Shandong Provincial Natural Science Foundation, China (2015ZRE 27131).
COI
All authors approved the final submission and declare that no potential competing interests exist.
References
- 1). Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat. Med. 2011; 17: 1410-1422 [DOI] [PubMed] [Google Scholar]
- 2). Libby P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012; 32: 2045-2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Mestas J, Ley K. Monocyte-Endothelial Cell Interactions in the Development of Atherosclerosis. Trends Cardiovasc. Med. 2008; 18: 228-232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Koga J, Aikawa M. Crosstalk between macrophages and smooth muscle cells in atherosclerotic vascular diseases. Vascul. Pharmacol. 2012; 57: 24-28 [DOI] [PubMed] [Google Scholar]
- 5). Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2008; 28: 812-819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007; 27: 2292-2301 [DOI] [PubMed] [Google Scholar]
- 7). Wong BW, Meredith A, Lin D, McManus BM. The Biological Role of Inflammation in Atherosclerosis. Can. J. Cardiol. 2012; 28: 631-641 [DOI] [PubMed] [Google Scholar]
- 8). Rudijanto A. The role of vascular smooth muscle cells on the pathogenesis of atherosclerosis. Acta Med. Indones. 2007; 39: 86-93 [PubMed] [Google Scholar]
- 9). Lacolley P, Regnault V, Nicoletti A, Li Z, Michel JB. The vascular smooth muscle cell in arterial pathology: A cell that can take on multiple roles. Cardiovasc. Res. 2012; 95: 194-204 [DOI] [PubMed] [Google Scholar]
- 10). Chen WQ, Zhong L, Zhang L, Ji XP, Zhao YX, Zhang C, Jiang H, Wu YL, Zhang Y. Chinese medicine tongxinluo significantly lowers serum lipid levels and stabilizes vulnerable plaques in a rabbit model. J. Ethnopharmacol. 2009; 124: 103-110 [DOI] [PubMed] [Google Scholar]
- 11). Yin HQ, Wang B, Zhang JD, Lin HQ, Qiao Y, Wang R, Liu FY. Effect of traditional Chinese medicine Shu-Mai-Tang on attenuating TNFα-induced myocardial fibrosis in myocardial ischemia rats. J. Ethnopharmacol. 2008; 118: 133-139 [DOI] [PubMed] [Google Scholar]
- 12). Zhang YH, Liu JT, Wen BY, Liu N. Mechanisms of inhibiting proliferation of vascular smooth muscle cells by serum of rats treated with Dahuang Zhechong pill. J. Ethnopharmacol. 2009; 124: 125-129 [DOI] [PubMed] [Google Scholar]
- 13). Wang Y, Zhao X, Wang YS, Song SL, Liang H, Ji AG. An extract from medical leech improve the function of endothelial cells in vitro and attenuates atherosclerosis in ApoE null mice by reducing macrophages in the lesions. Biochem. Biophys. Res. Commun. 2014; 455: 119-125 [DOI] [PubMed] [Google Scholar]
- 14). Griendling KK, Taubman MB, Akers M, Mendlowitz M, Alexander RW. Characterization of phosphatidylinositol-specific phospholipase C from cultured vascular smooth muscle cells. J. Biol. Chem. 1991; 266: 15498-15504 [PubMed] [Google Scholar]
- 15). Ma S, Zhang D, Lou H, Sun L, Ji J. Evaluation of the anti-inflammatory activities of tanshinones isolated from Salvia miltiorrhiza var. alba roots in THP-1 macrophages. J. Ethnopharmacol. 2016; 188: 193-199 [DOI] [PubMed] [Google Scholar]
- 16). Choi HJ, Chung TW, Kim JE, Jeong HS, Joo M, Cha J, Kim CH, Ha KT. Aesculin inhibits matrix metalloproteinase-9 expression via p38 mitogen activated protein kinase and activator protein 1 in lipopolysachride-induced RAW 264.7 cells. Int. Immunopharmacol. 2012; 14: 267-274 [DOI] [PubMed] [Google Scholar]
- 17). Guo J, Li L, Wu YJ, Yan Y, Xu XN, Wang SB, Yuan TY, Fang LH, Du GH. Inhibitory Effects of Brazilin on the Vascular Smooth Muscle Cell Proliferation and Migration Induced by PDGF-BB. Am. J. Chin. Med. 2013; 41: 1283-1296 [DOI] [PubMed] [Google Scholar]
- 18). Zhu L, Sun G, Zhang H, Zhang Y, Chen X, Jiang X, Jiang X, Krauss S, Zhang J, Xiang Y, Zhang CY. PGC-1α is a key regulator of glucose-induced proliferation and migration in vascular smooth muscle cells. PLoS One. 2009; 4: 1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Cho Y, Lee SE, Lee HC, Hur J, Lee S, Youn SW, Lee J, Lee HJ, Lee TK, Park J, Hwang SJ, Kwon YW, Cho HJ, Oh BH, Park YB, Kim HS. Adipokine resistin is a key player to modulate monocytes, endothelial cells, and smooth muscle cells, leading to progression of atherosclerosis in rabbit carotid artery. J. Am. Coll. Cardiol. 2010; 57: 99-109 [DOI] [PubMed] [Google Scholar]
- 20). Förstermann U, Sessa WC. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012; 33: 829-837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Li H, Horke S, Förstermann U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis. 2014; 237: 208-219 [DOI] [PubMed] [Google Scholar]
- 22). Jovanović I, Zivković M, Djurić T, Popović M, Alavantić D, Stanković A. CXCL16 in Vascular Pathology Research: from Macro Effects to microRNAs. J. Atheroscler. Thromb. 2015; 22: 1012-1024 [DOI] [PubMed] [Google Scholar]
- 23). Doyle SL, O'Neill LA. Toll-like receptors: From the discovery of NF-κB to new insights into transcriptional regulations in innate immunity. Biochem. Pharmacol. 2006; 72: 1102-1113 [DOI] [PubMed] [Google Scholar]
- 24). Den Dekker WK, Cheng C, Pasterkamp G, Duckers HJ. Toll like receptor 4 in atherosclerosis and plaque destabilization. Atherosclerosis. 2010; 209: 314-320 [DOI] [PubMed] [Google Scholar]
- 25). Eguchi K, Manabe I. Toll-like receptor, lipotoxicity and chronic inflammation: the pathological link between obesity and cardiometabolic disease. J. Atheroscler. Thromb. 2014; 21: 629-639 [DOI] [PubMed] [Google Scholar]
- 26). Meng Z, Yan C, Deng Q, Gao D, Niu X. Curcumin inhibits LPS-induced inflammation in rat vascular smooth muscle cells in vitro via ROS-relative TLR4-MAPK/NF-κB pathways. Acta. Pharmacol. Sin. 2013; 34: 901-911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat. Immunol. 2011; 12: 204-212 [DOI] [PubMed] [Google Scholar]
- 28). Coll B, Alonso-Villaverde C, Joven J. Monocyte chemoattractant protein-1 and atherosclerosis: Is there room for an additional biomarker? Clin. Chim. Acta. 2007; 383: 21-29 [DOI] [PubMed] [Google Scholar]
- 29). Kleinbongard P, Heusch G, Schulz R. TNFα in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol. Ther. 2010; 127: 295-314 [DOI] [PubMed] [Google Scholar]
- 30). Wang Z, Castresana MR, Newman WH. NF-κB is required for TNF-α-directed smooth muscle cell migration. FEBS. Lett. 2001; 508: 360-364 [DOI] [PubMed] [Google Scholar]
- 31). Kyriakis JM, Avruch J. Mammalian MAPK Signal Transduction Pathways Activated by Stress and Inflammation: A 10-Year Update. Physiol. Rev. 2012; 92: 689-737 [DOI] [PubMed] [Google Scholar]
- 32). Peng SC, Wong DS, Tung KC, Chen YY, Chao CC, Peng CH, Chuang YJ, Tang CY. Computational modeling with forward and reverse engineering links signaling network and genomic regulatory responses: NF-kappaB signaling-induced gene expression responses in inflammation. BMC. Bioinformatics. 2010; 11: 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). De Winther MP, Kanters E, Kraal G, Hofker MH. Nuclear factor κB signaling in atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2005; 25: 904-914 [DOI] [PubMed] [Google Scholar]
- 34). Madonna R, De Caterina R. Relevance of new drug discovery to reduce NF-κB activation in cardiovascular disease. Vascul. Pharmacol. 2012; 57: 41-47 [DOI] [PubMed] [Google Scholar]
- 35). Tak PP, Firestein GS. NF-κB in defense and disease NF-κB: a key role in inflammatory diseases. J. Clin. Invest. 2001; 107: 7-11 [DOI] [PMC free article] [PubMed] [Google Scholar]


