Abstract
Aim: Accumulation level of fluorescent advanced glycation end products (AGEs) in the skin can be measured non-invasively as skin autofluorescence (skin AF) by autofluorescence reader. The aim of this study was to assess possible associations between skin AF and diabetic complications, especially early-stage atherosclerosis, in Japanese type 1 diabetic patients.
Methods: Skin AF was measured by AGE reader® in 105 Japanese type 1 diabetic patients (34 men and 71 women, aged 37.4 ± 12.4 years (± SD)) and 23 age-matched healthy non-diabetic subjects. Ultrasonic carotid intima-media thickness (IMT), ankle-brachial index (ABI), and brachial ankle pulse wave velocity (baPWV) were evaluated as indices of early-stage diabetic macroangiopathy. Urinary albumin-to-creatinine ratio (UACR), the coefficient of variation of R-R intervals (CVR-R), and presence of retinopathy were also evaluated.
Results: Skin AF values were significantly higher in type 1 diabetic patients than in healthy controls (2.07 ± 0.50 (mean ± SD) and 1.90 ± 0.26, respectively, p = 0.024). Skin AF was associated with carotid IMT (r = 0.446, p <0.001) and baPWV (r = 0.450, p <0.001), but not with ABI (r = −0.019, p = 0.8488). Notably, skin AF was an independent risk factor for IMT thickening. Similarly, skin AF was associated with log (UACR) (r = 0.194, p = 0.049) and was an independent risk factor for UACR. Furthermore, skin AF values were significantly higher in patients with diabetic retinopathy than in those without (2.21 ± 0.08 and 1.97 ± 0.06, respectively, p = 0.020).
Conclusions: Skin AF was significantly associated with the presence and/or severity of diabetic complications and was an independent risk factor for carotid atherosclerosis.
Keywords: Advanced glycation end products (AGEs), Skin autofluorescence, Carotid intima-media thickness, Type 1 diabetes
Since diabetic micro- and macrovascular complications lead to impairment of quality of life and are major causes of mortality in patients with type 1 diabetes mellitus, risk stratification and subsequent rapid intervention against them are important in the management of type 1 diabetes mellitus1, 2). Although poor glycemic control is one of the most important risk factors for diabetic complications3, 4), indices of current or recent glycemic control status such as blood glucose levels, glycoalbumin (GA), and glycated hemoglobin A1c (HbA1c) do not fully reflect the risk of diabetic complications and are insufficient for risk stratification1).
Advanced glycation end products (AGEs), a complex group of compounds produced from slowly occurring nonenzymatic glycation of proteins, represent the integration of various vascular risk factors, such as chronic hyperglycemia, insulin resistance, oxidative stress, aging, and smoking5, 6), and play important roles in the development of vascular disease7, 8). Furthermore, since relatively long-term metabolic status affects the formation of AGEs, AGEs are regarded as a marker of so-called “metabolic memory.” The Diabetes Control and Complications Trial/Epidemiology of Diabetes Intervention and Complications (DCCT/EDIC) study revealed that early intensive glycemic control retards the development and progression of diabetic complications in the long term, despite worsening glycemic control4, 9). Interestingly, in that study, long-term intensive treatment of hyperglycemia, as compared to conventional treatment, was associated with lower levels of AGEs in skin collagen10). It was also demonstrated that level of skin AGEs predicts the risk of future 10-year progression of diabetic retinopathy and nephropathy independently of past HbA1c11). Thus, assessment of AGEs accumulated in the skin can improve the predictability of diabetic complications. However, skin biopsy cannot be applied in routine clinical practice, because of its invasive and costly characteristics and technical limitations.
Recently, accumulation level of fluorescent AGEs in the skin has become measurable non-invasively with skin autofluorescence (skin AF) using an autofluorescence reader. Skin AF correlated with accumulation of skin AGEs assessed by skin biopsy12). Several reports described elevation of skin AF in both type 1 and type 2 diabetes compared with healthy controls13–16). Furthermore, in type 1 diabetes, it was reported that skin AF correlated with nephropathy13, 17, 18), neuropathy17, 19), and retinopathy13), suggesting that skin AF may become a surrogate marker candidate for diabetic microangiopathy. However, still few studies have assessed the association between skin AF and macroangiopathy, especially early-stage atherosclerosis, in type 1 diabetes. In addition, it is of note that most of the previous studies dealt with Caucasian subjects, although there seems to be ethnic differences in skin AF values20).
The aims of this study were: (1) to evaluate skin AF in Japanese patients with type 1 diabetes in comparison with healthy controls, and (2) to assess of possible associations between skin AF and diabetic complications, especially early-stage atherosclerosis, in this population.
Research Design and Methods
Study Population
Study subjects were recruited from patients at the Diabetes Clinic of Osaka University Hospital and the Osaka Police Hospital during the period from June 2014 to August 2015. Patients with type 1 diabetes diagnosed by diabetologists were considered eligible as subjects. All patients were diagnosed as type 1 diabetes at the time of presenting with hyperglycemia and/or ketosis in their clinical history, and since that time, all had been under insulin therapy. The patients on dialysis were excluded. Finally,, 105 type 1 diabetes patients were enrolled. As control subjects, 23 age-matched healthy volunteers were also enrolled. The study protocol was approved by the local ethics committee and the study was conducted in accordance with the principles of the Helsinki Declaration. All participants provided written informed consent.
Clinical and Biochemical Assessment
The clinical assessments included a medical history interview, physical examination (height, weight, and resting blood pressure), electrocardiography, and laboratory testing. Smoking status was classified as having a current smoking habit or not. Venous blood samples were taken, and serum total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, serum triglyceride, and HbA1c levels were measured by SRL Inc. (Tokyo, Japan) using standard laboratory protocols. Plasma pentosidine levels were also measured by SRL Inc. (Tokyo, Japan) using enzyme-linked immunosorbent assay (ELISA) (CV = 6.4%).
Assessment of Diabetic Macroangiopathy
Ultrasonic carotid intima-media thickness (IMT), ankle-brachial index (ABI), and brachial ankle pulse wave velocity (baPWV) were evaluated as indices of early-stage diabetic macroangiopathy. B-mode ultrasonography of the carotid artery was performed using an ultrasound machine with a 7.5-MHz linear transducer. In accordance with the guidelines of the Japan Society of Ultrasonics21), all scanning was conducted by experienced physicians using the same ultrasound system and the same measuring method. Scanning of the extracranial common carotid artery, the carotid bulb, and the internal carotid artery in the neck was performed bilaterally from three different longitudinal projections (i.e., anterior-oblique, lateral, and posterior-oblique). The carotid IMT was measured as the distance from the leading edge of the first echogenic line to the leading edge of the second echogenic line. The thickest points of IMT (including plaque lesions) were measured, and the highest value among them was defined as maxIMT and used as the representative value for each individual.
Measurement of ABI and baPWV was performed using the same volume-plethysmographic apparatus as for ABI (BP-203RPE II form PWV/ABI), with subjects in the supine position after at least 5 min of rest. Oscillometric cuffs, each connected to a plethysmographic sensor that determined volume pulse form and to an oscillometric pressure sensor that measured blood pressure, were wrapped on both ankles and upper arms of each subject, and an electrocardiogram electrode was placed on each wrist. The cuffs were simultaneously pressurized to the approximate value of the subject's diastolic pressure so that the pulse volume waveforms could be recorded using semiconductor pressure sensors. The distance between sampling points of baPWV was calculated based on the height of the subject. The path length from the suprasternal notch to the ankle (La) was calculated as: La = 0.8129* height (in cm) + 12.328. The path length from the suprasternal notch to the brachium (Lb) was calculated as: Lb = 0.2195*height − 2.0734. The baPWV was calculated according to the following formula: baPWV = (La − Lb)/Tba, where Tba was the time interval between the wave front of the brachial waveform and that of the ankle waveform22). Two simultaneous measurements of baPWV were recorded, on the right side and left side, respectively, and the higher of these readings was used as the representative value for each individual.
Assessment of Diabetic Microangiopathy
Presence of retinopathy was diagnosed by a diabetologist based on the findings of single-field fundus photography under regular check up by an ophthalmologist, and classified as no diabetic retinopathy (NDR) or simple or more advanced diabetic retinopathy (≥ SDR). The coefficient of variation of R-R intervals (CVR-R) was measured as an index of autonomic nerve function. Presence and severity of diabetic nephropathy were diagnosed based on glomerular filtration rate (GFR) and urine albumin excretion. GFR was estimated using the abbreviated Modification of Diet in Renal Disease (MDRD) for Japanese. Urine albumin excretion was evaluated by turbidimetric immunoassay and expressed as urinary albumin-to-creatinine ratio (UACR).
Measurement of Skin Autofluorescence
Skin autofluorescence was measured by AGE reader (DiagnOptics BV, Groningen, the Netherlands). The detailed measurement protocol was reported previously12). Briefly, the apparatus illuminates the skin with an excitation light source of 300 to 420 nm. Subsequently, emitted light and light reflected from skin are assessed with a spectrometer in the range 300–600 nm. Skin autofluorescence is calculated in arbitrary units (AU) by dividing the area under the curve between 420 and 600 nm (emission spectrum) by the area under the curve between 300 and 420 nm (excitation spectrum). For each subject, skin AF was measured three times in series and the arithmetical mean of those assessments was used. AF was measured on ventral site of the forearm.
Statistical Analysis
All values are reported as mean ± SD, or median for continuous variables, or number with percentage in parentheses for categorical variables. Comparisons between the groups were assessed using an unpaired Student's t-test (or Welch's t-test when two groups were assumed to have unequal population variance) for parametric data, or the Mann–Whitney U-test for nonparametric data. Differences in proportions were tested using the χ2-test or Fisher's exact test. Associations between variables were tested with Pearson's univariate test. Multivariate regression analysis was performed to assess variables significantly associated with an objective variable. In case of multivariate regression analysis for categorical data, we performed logistic regression analysis to derive the odds ratio (OR) and 95% confidence intervals (CI). A two-sided p value < 0.05 was considered statistically significant. All analyses were performed with the use of JMP® pro 11.1.1 (SAS Institute Inc., Cary, NC, USA).
Results
Skin AF Values in Type 1 Diabetic Subjects in Comparison with Healthy Controls
The clinical characteristics of the study subjects are presented in Table 1. Smoking rates, plasma glucose, HbA1c, GA, and BMI levels were significantly higher, and uric acid (UA) levels were significantly lower in subjects with type 1 diabetes as compared to non-diabetic subjects (p <0.05). There was no significant difference between the two groups regarding the other clinical parameters such as age, systolic and diastolic BP, eGFR, total, LDL, and HDL cholesterol, and triglyceride levels. Skin AF values were significantly higher in type 1 diabetic subjects than in healthy controls (2.07 ± 0.50 (mean ± SD) and 1.90 ± 0.26, respectively, p = 0.024).
Table 1. The clinical characteristics of the study subjects.
| controls (n = 23) |
type 1 diabetes (n = 105) |
p | |
|---|---|---|---|
| Age (years) | 34.7 ± 6.2 | 37.4 ± 12.4 | NS |
| female n (%) | 8 (34.8) | 71 (65.7) | 0.006 |
| duration of diabetes (years) | – | 21.9 ± 9.2 | – |
| Smoking n (%) | 2 (8.7) | 35 (34.0) | 0.016 |
| BMI (kg/m2) | 20.6 ± 2.6 | 23.0 ± 3.0 | <0.001 |
| Systolic BP (mmHg) | 114 ± 7 | 117 ± 14 | NS |
| Diastolic BP (mmHg) | 68.9 ± 5.9 | 69.7 ± 9.2 | NS |
| AST (U/L) | 18.5 ± 4.0 | 18.4 ± 5.5 | NS |
| ALT (U/L) | 14.9 ± 6.2 | 15.4 ± 9.3 | NS |
| GGT (U/L) | 20.1 ± 9.6 | 24.4 ± 36.7 | NS |
| Glucose (mg/dL) | 92.8 ± 8.3 | 156.7 ± 75.0 | <0.001 |
| HbA1c (%) | 5.1 ± 0.2 | 7.7 ± 1.4 | <0.001 |
| GA (%) | 13.8 ± 1.1 | 23.5 ± 5.0 | <0.001 |
| TC (mg/dL) | 181.8 ± 23.9 | 186.6 ± 32.2 | NS |
| TG (mg/dL) | 59 (30–623) | 66 (21–757) | NS* |
| HDL-C (mg/dL) | 62.8 ± 13.1 | 67.3 ± 13.2 | NS |
| LDL-C (mg/dL) | 104.1 ± 22.1 | 102.6 ± 26.8 | NS |
| UA (mg/dL) | 5.2 ± 1.5 | 4.4 ± 1.3 | 0.012 |
| Creatinine (mg/dL) | 0.82 ± 0.16 | 0.71 ± 0.17 | 0.005 |
| eGFR (ml/min/1.73 m2) | 81.4 ± 11.3 | 87.5 ± 17.2 | NS |
| UACR (mg/g/Cr) | – | 45.3 ± 320 | – |
| CVR-R (%) | 5.47 ± 2.71 | 3.94 ± 1.84 | 0.001 |
| Nephropathy (no n (%)) | – | 95 (91.3) | – |
| Retinopathy (no n (%)) | – | 58 (59.2) | – |
| maxIMT (mm) | 0.76 ± 0.21 | 1.09 ± 0.48 | 0.002 |
| ABI | 1.07 ± 0.08 | 1.07 ± 0.08 | NS |
| ba-PWV (cm/sec) | 1225 ± 156 | 1318 ± 248 | NS |
| Skin AF (AU) | 1.90 ± 0.26 | 2.07 ± 0.50 | 0.024 |
Data are expressed as mean ± SD, median (range), or number and percentage. Unpaired t-test, Welch's t-test or the *Mann-Whitney U test was performed. P-value <0.05 was considered statistically significant.
Abbreviations: BMI, body mass index; BP, blood pressure; AST, aspartate transaminase; ALT, alanine transaminase; GGT, κ-glutamyltransferase; HbA1c, glycated hemoglobin A1c; GA, glycoalbumin; TC, total cholesterol; TG, serum triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; UA, uric acid; eGFR, estimated glomerular filtration rate; UACR, urinary albumin-to-creatinine ratio; CVR-R, coefficient of variation of R-R intervals; IMT, intima media thickness; ABI, ankle-brachial index; PWV, pulse wave verbosity.
Next, to assess what factors affected skin AF values in the type 1 diabetic subjects, we analyzed associations between skin AF value and clinical parameters. Univariate regression analysis showed that skin AF was significantly associated with age, gender, smoking status, duration of diabetes, HbA1c, GA, aspartate transaminase (AST), γ-glutamyltransferase (GGT), UA, serum Creatinine, and eGFR in the type 1 diabetic subjects (Table 2, Supplementary Fig. 1A). A stepwise multivariate regression analysis including variables that were significantly associated with skin AF in univariate analysis as independent variables demonstrated that age and smoking status were independent determinants of skin AF, while HbA1c and GA were not (Table 2). Interestingly, there was also a statistically significant association between skin AF and the average HbA1c value for a long term (utmost past 10 years) in 67 subjects whose past HbA1c levels were available (Supplementary Fig. 1B). Using this average HbA1c value as an index of glycemic control, instead of the single-point HbA1c value measured at the time of enrollment, showed the average HbA1c to be an independent determinant of skin AF (β = 0.350, p = 0.002), even after adjustment for the other variables.
Table 2. Relative risk factors of skin AF in type 1 diabetes.
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| r | p | β | p | |||
| Age (year) | 0.470 | <0.001 | 0.412 | <0.001 | ||
| Gender (male) | 0.213 | 0.029 | NI | |||
| Duration of diabetes (years) | 0.248 | 0.003 | NI | |||
| Smoking | 0.332 | <0.001 | 0.225 | 0.012 | ||
| BMI (kg/m2) | 0.170 | NS | ||||
| Systolic BP (mmHg) | 0.173 | NS | ||||
| Diastolic BP (mmHg) | 0.153 | NS | ||||
| GGT (U/L) | 0.320 | <0.001 | NI | |||
| Glucose (mg/dL) | 0.118 | NS | ||||
| HbA1c (%) | 0.217 | 0.026 | NI | |||
| TG (mg/dL) | 0.130 | NS | ||||
| LDL-C (mg/dL) | 0.106 | NS | ||||
| HDL-C (mg/dL) | −0.072 | NS | ||||
| UA (mg/dL) | 0.246 | 0.011 | NI | |||
| eGFR (ml/min/1.73 m2) | −0.246 | 0.011 | NI | |||
| R2 | 0.269 | |||||
The threshold of statistical significance was defined as p <0.05.
Gender: male = 1, female = 0, Smoking: yes = 1, no = 0.
Stepwise multivariate regression analysis was performed to determine the predictors of skin AF. The significant (p <0.05) variables in Pearson's univariate analysis were selected for multivariate regression analysis.
Abbreviations: β, partial regression coefficient; NS, not significant; NI, not included in the model; Gender: male = 1, female = 0, Smoking: yes = 1, no = 0; BMI, body mass index; BP, blood pressure; GGT, κ-glutamyltransferase; HbA1c, glycated hemoglobin A1c; GA, glycoalbumin; TG, serum triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; UA, uric acid; eGFR, estimated glomerular filtration rate.
Supplementary Fig. 1.
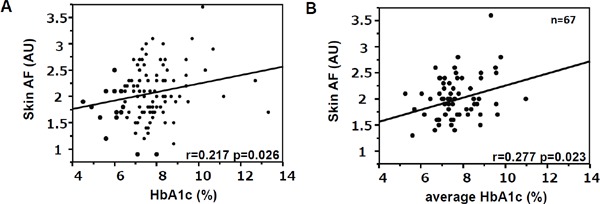
Association between skin AF and (A) single-point HbA1c, (B) average of past HbA1c values. Statistical analysis for association was performed using Pearson's univariate test.
Associations between Skin AF and Diabetic Complications
Skin AF was associated with maxIMT (r = 0.446, p%0.001, Fig. 1A) and baPWV (r = 0.450, p <0.001, Fig. 1B) but not with ABI (r = −0.019, p = 0.8488). Since maxIMT was also associated with other variables such as age, duration of diabetes, systolic BP, GGT, LDL-C, UA, serum Cr, and eGFR, a stepwise multivariate regression analysis was performed to evaluate whether skin AF value was an independent determinant for maxIMT (β = 0.247, p = 0.008), even after adjustment for these variables. This analysis revealed that skin AF value and age were significant independent determinants for maxIMT (Table 3). Furthermore, another multivariate regression analysis revealed that skin AF value was a significant independent determinant for maxIMT (β = 0.201, p = 0.043) even after mandatory adjustment for these variables. On the other hand, skin AF was no longer an independent determinant for baPWV after adjustment for age, duration of diabetes, systolic BP, and eGFR, all of which were significantly associated with baPWV (Supplementary Table 1).
Fig. 1.
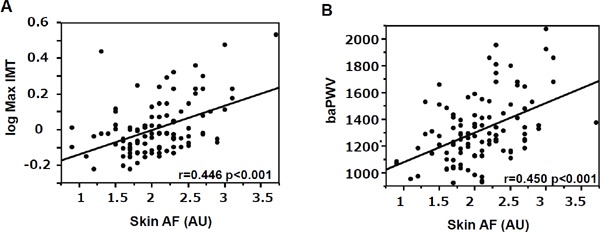
Association between skin AF and (A) maxIMT and (B) baPWV. Statistical analysis for association was performed using Pearson's univariate test.
Table 3. Relative risk factors of maxIMT in type 1 diabetes.
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| r | p | β | p | |||
| Age (year) | 0.538 | <0.001 | 0.422 | <0.001 | ||
| Gender (male) | 0.165 | NS | ||||
| Duration of diabetes (years) | 0.286 | 0.003 | NI | |||
| Smoking | 0.197 | 0.045 | NI | |||
| BMI (kg/m2) | 0.102 | NS | ||||
| Systolic BP (mmHg) | 0.210 | 0.033 | NI | |||
| GGT (U/L) | 0.277 | 0.004 | NI | |||
| HbA1c (%) | 0.116 | NS | ||||
| TG (mg/dL) | 0.108 | NS | ||||
| LDL-C (mg/dL) | 0.196 | 0.048 | NI | |||
| HDL-C (mg/dL) | −0.110 | NS | ||||
| UA (mg/dL) | 0.208 | 0.034 | NI | |||
| eGFR (ml/min/1.73 m2) | −0.357 | <0.001 | NI | |||
| Skin AF (AU) | 0.446 | <0.001 | 0.247 | 0.008 | ||
| R2 | 0.337 | |||||
The threshold of statistical significance was defined as p <0.05.
Gender: male = 1, female = 0, Smoking: yes = 1, no = 0.
Stepwise multivariate regression analysis was performed to determine the predictors of maxIMT. The significant (p<0.05) variables in Pearson's univariate analysis were selected for multivariate regression analysis.
Abbreviations: β, partial regression coefficient; NS, not significant; NI, not included in the model; BMI, body mass index; BP, blood pressure; GGT, κ-glutamyltransferase; HbA1c, glycated hemoglobin A1c; GA, glycoalbumin; TG, serum triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; UA, uric acid; eGFR, estimated glomerular filtration rate.
Although skin AF values were significantly higher in subjects with diabetic retinopathy than in those without it (2.21 ± 0.08 and 1.97 ± 0.06, respectively, p = 0.02), a multiple logistic regression analysis revealed that skin AF was no longer an independent determinant for the presence of diabetic retinopathy after adjustment for duration of diabetes and plasma glucose level (odds ratio; 1.94, 95% confidence interval; 0.70–5.85) (Supplementary Table 2). Similarly, univariate analysis revealed that skin AF was significantly associated with CVR-R (r = −0.342, p <0.001), a multiple regression analysis revealed that skin AF was no longer an independent determinant for CVR-R after adjustment for age and HbA1c (Supplementary Table 3).
Univariate analysis revealed that log (UACR) was associated with gender, HbA1c, and skin AF value. Skin AF was a significant independent determinant for UACR even after adjustment for gender and HbA1c (Supplementary Table 4). Similarly, univariate analysis revealed that there were significant associations between skin AF value and eGFR (r = −0.246, p = 0.0113), while a multiple regression analysis revealed that skin AF is no longer an independent determinant for eGFR after adjustment for age, duration of diabetes, and serum UA levels.
Supplementary Table 1. Relative risk factors of baPWV in type 1 diabetes.
| Univariate |
Multivariate |
||||
|---|---|---|---|---|---|
| r | p | β | p | ||
| Age (year) | 0.691 | <0.001 | 0.529 | <0.001 | |
| Gender (male) | 0.330 | <0.001 | NI | ||
| Duration of diabetes (years) | 0.480 | <0.001 | 0.117 | 0.011 | |
| Smoking | 0.196 | 0.047 | NI | ||
| BMI (kg/m2) | 0.247 | 0.011 | NI | ||
| Systolic BP (mmHg) | 0.519 | <0.001 | 0.37 | <0.001 | |
| GGT (U/L) | 0.218 | 0.026 | NI | ||
| HbA1c (%) | 0.068 | NS | |||
| TG (mg/dL) | 0.144 | NS | |||
| LDL-C (mg/dL) | 0.111 | NS | |||
| HDL-C (mg/dL) | −0.154 | NS | |||
| UA (mg/dL) | 0.298 | 0.002 | NI | ||
| eGFR (ml/min/1.73 m2) | −0.417 | <0.001 | −0.142 | 0.036 | |
| Skin AF (AU) | 0.450 | <0.001 | NI | ||
| R2 | 0.700 | ||||
The threshold of statistical significance was defined as p <0.05.
Gender: male = 1, female = 0, Smoking: yes = 1, no = 0.
Stepwise multivariate regression analysis was performed to determine the predictors of baPWV. The significant (p<0.05) variables in Pearson's univariate analysis were selected for multivariate regression analysis.
Abbreviations: β, partial regression coefficient; NS, not significant; NI, not included in the model; BMI, body mass index; BP, blood pressure; GGT, κ-glutamyltransferase; HbA1c, glycated hemoglobin A1c; GA, glycoalbumin; TG, serum triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; UA, uric acid; eGFR, estimated glomerular filtration rate.
Associations between Plasma Pentosidine and Diabetic Complications
We also assessed associations between diabetic complications and plasma pentosidine, which is a well-known circulating AGE. Although plasma pentosidine levels were not associated with skin AF (r = 0.075, p = 0.414), plasma pentosidine levels were significantly higher in type 1 diabetes than in healthy controls (0.043 ± 0.013 (mean ± SD) and 0.036 ± 0.001 respectively, p < 0.001). However, plasma pentosidine levels were not associated with either single-point or average HbA1c levels. In addition, there were no associations between plasma pentosidine levels and any markers of diabetic macroangiopathy (maxIMT, baPWV, and ABI) or microangiopathy (presence of retinopathy, CVR-R, UACR, and, eGFR) in this population (all p > 0.05).
Discussion
In the present study, we confirmed that skin AF was higher in participants with type 1 diabetes as compared to non-diabetic control subjects and that average past HbA1c level was a determinant of skin AF independent of age, gender, duration of diabetes, and smoking status, although single-point HbA1c was not associated with skin AF after adjustment for confounding factors. These findings are consistent with previous reports13, 16) and support the idea that skin AF as an index of relatively long-term glycemic control (historical glycemic exposure) can be a better marker of diabetic complications than single-point HbA1c.
Notably, the present study revealed that skin AF values are associated with markers of early-stage atherosclerosis such as maxIMT and baPWV. This finding supports the idea that AGEs are accumulated before development of atherosclerosis. On the other hand, there was no significant association between skin AF and ABI in the present study. One possible explanation for this phenomenon is that the ABI values in the patients with type 1 diabetes were not increased in the present study, and thus, cannot be used as an index of atherosclerotic change. Indeed, in this study, mean and SD values of ABI in the patients with type 1 diabetes were similar to those of healthy controls and in almost normal range. Although there have been several studies that evaluated the association between skin AF and early-stage subclinical atherosclerosis in diabetic patients23–27), very few of them were concerned with non-Caucasian patients. Very recently, the associations between skin AF and coronary artery calcification score28) or IMT24) have been shown in Japanese type 2 diabetes mellitus. Our study confirmed the findings of these previous studies and extended them to Japanese patients with type 1 diabetes mellitus. Furthermore, to the best of our knowledge, this is the first study to find that skin AF was an independent risk factor for IMT even after adjustment for the other established risk factors for atherosclerosis. It would be important to demonstrate whether skin AF reflects the risk of atherosclerosis even in its subclinical stage, while several clinical studies have already shown positive association between skin AF and cardiovascular disease15, 29–32). Increased skin AF may reflect early abnormalities in processes involved in atherosclerosis development.
This study also confirmed positive associations between skin AF and presence and/or severity of diabetic microangiopathies. Skin AF values were significantly higher in subjects with diabetic retinopathy and significantly associated with CVR-R, a marker of diabetic neuropathy. However, these associations were no longer statistically significant after adjustment for the established risk factors (e.g., age, duration of diabetes, HbA1c.) These results suggest that much of this association appears to be related to historical glycemic exposure, as shown in the DCCT/EDIC study3).
On the other hand, skin AF was an independent risk factor for UACR even after adjustment for the other established risk factors for nephropathy. This finding is compatible with the experimental studies indicating that AGEs play important roles for development and progression of chronic renal disease, especially diabetic nephropathy33). Although these results were already reported in previous studies based on Caucasian patients13, 17, 18), there are still few reports concerning Asian patients13).
Experimental studies have shown that AGEs and their receptor system (RAGE) play an important role in the development of diabetic vascular complications. AGEs are engaged in vascular complications through the changing of three-dimensional structure of proteins by cross-linking. The binding of AGEs to RAGE is also known to cause phenotypic changes in various cells such as endothelial cells, smooth muscle cells, pericytes, and renal mesangial cells, leading to the pathogenesis of diabetic retinopathy, nephropathy, and macroangiopathies8, 34–43). Thus, AGEs not only represent various metabolic disorders as a marker of “metabolic memory”, but also directly play critical pathogenic roles, which would be reason why skin AF can be a marker of diabetic complications independent of the other established risk factors.
We also assessed association between diabetic complications and pen tosidine, a well-known fluorescent AGE for which causal relationship with renal disease has been reported44, 45). However, the present study found no significant association between serum pentosidine levels and diabetic complications in Japanese patients with type 1 diabetes mellitus. Similar to our result, several studies showed that tissue AGEs are better predictor for vascular disease as compared to circulating AGEs25, 46, 47). One of the reasons might be that tissue AGEs have slower turnover than circulating AGEs48). These findings suggest that skin AF may more useful marker for diabetic complications than circulating AGEs.
There were several limitations in this study. First, the number of study subjects was small. Therefore, to establish the utility of the measurement of skin AF in daily clinical practice, another study with larger sample size should be undertaken. Second, all participants were Japanese, and this may limit the generalizability of the results, since it is reported that there is an ethnic heterogeneity in skin AF20). However, from a different perspective, this report is valuable in that our showed the utility of skin AF in Japanese, since most of the previous studies were concerned with Caucasians. Third, skin AF cannot be measured by non-fluorescent AGEs, such as CML and CEL, because of the characteristics of the measurement principle of the AGE Reader. However, it was already demonstrated that non-fluorescent AGEs accumulated in skin are also strongly correlated with skin AF12). Fourth, although we tried to exclude the influence of the known interfering factors that can affect skin AF value, such as body creams and sunscreen creams49), we could not eliminate the influence of unknown interfering factors. Finally, because of the cross-sectional nature of this study, the associations do not necessarily indicate causality. Further longitudinal studies are necessary to confirm the significance of skin AF in type 1 diabetes.
In conclusion, our study confirmed that skin AF represents historical glycemic exposure and that there were moderately strong associations between skin AF and a number of markers of diabetic complications even in Japanese patients with type 1 diabetes mellitus. Especially, skin AF was an independent risk factor for IMT even after adjustment for the other established risk factors for atherosclerosis. These findings suggest that increased skin AF may reflect early atherosclerotic changes and may be a good predictor of development and progression of diabetic macroangiopathy.
Acknowledgments
Author Contributions S.O., N.K., and M.M. analyzed data and wrote the manuscript. M.T., F.S., D.K., A.K., and T.M. analyzed the data. I.S. contributed to the interpretation of the results and the discussion. All authors reviewed and approved the report. N.K. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Funding Sources: This work was supported by the Grants-in-Aid for Scientific Research from the Japanese Ministry of Education, Science, Sports, Culture and Technology [KAKENHI 25461349].
Conflict of Interest Disclosures
None.
Supplementary Table 2. Relative risk factors of presence of retinopathy in type 1 diabetes.
| Univariate |
Multivariate |
||||
|---|---|---|---|---|---|
| r | p | OR | 95%CI | ||
| Age (year) | 0.051 | NS | |||
| Gender (male) | −0.108 | NS | |||
| Duration of diabetes (years) | 0.433 | <0.001 | 1.19 | 1.10–1.30 | |
| Smoking | −0.046 | NS | |||
| BMI (kg/m2) | 0.034 | NS | |||
| Systolic BP (mmHg) | 0.091 | NS | |||
| GGT (U/L) | −0.033 | NS | |||
| PG (mg/dl) | 0.227 | 0.022 | 1.01 | 1.00–1.02 | |
| HbA1c (%) | 0.140 | NS | |||
| TG (mg/dL) | −0.099 | NS | |||
| LDL-C (mg/dL) | −0.049 | NS | |||
| HDL-C (mg/dL) | 0.128 | NS | |||
| UA (mg/dL) | −0.147 | NS | |||
| eGFR (ml/min/1.73 m2) | −0.146 | NS | |||
| Skin AF (AU) | 0.221 | 0.026 | 2.43 | 0.87–7.44 | |
| R2 | 0.337 | ||||
The threshold of statistical significance was defined as p <0.05.
Gender: male = 1, female = 0, Smoking: yes = 1, no = 0.
Multiple logistic regression analysis was performed to determine the predictors of presence of retinopathy (yes = 1, no = 0). The significant (p <0.05) variables in Pearson's univariate analysis were selected for multiple logistic regression analysis.
Abbreviations: OR, odds ratio; NS, not significant; NI, not included in the model; BMI, body mass index; BP, blood pressure; GGT, κ-glutamyltransferase; HbA1c, glycated hemoglobin A1c; GA, glycoalbumin; TG, serum triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; UA, uric acid; eGFR, estimated glomerular filtration rate.
Supplementary Table 3. Relative risk factors of CVR-R in type 1 diabetes.
| Univariate |
Multivariate |
||||
|---|---|---|---|---|---|
| r | p | β | p | ||
| Age (year) | −0.468 | <0.001 | −0.422 | <0.001 | |
| Gender (male) | −0.124 | NS | |||
| Duration of diabetes (years) | −0.052 | NS | |||
| Smoking | −0.200 | 0.042 | NI | ||
| BMI (kg/m2) | −0.094 | NS | |||
| Systolic BP (mmHg) | −0.144 | NS | |||
| GGT (U/L) | −0.119 | NS | |||
| HbA1c (%) | −0.356 | <0.001 | −0.290 | <0.001 | |
| TG (mg/dL) | −0.099 | NS | |||
| LDL-C (mg/dL) | −0.112 | NS | |||
| HDL-C (mg/dL) | 0.151 | NS | |||
| UA (mg/dL) | −0.163 | NS | |||
| eGFR (ml/min/1.73 m2) | 0.170 | NS | |||
| Skin AF (AU) | −0.342 | <0.001 | NI | ||
| R2 | 0.301 | ||||
The threshold of statistical significance was defined as p <0.05.
Gender: male = 1, female = 0, Smoking: yes = 1, no = 0.
Stepwise multivariate regression analysis was performed to determine the predictors of CVR-R. The significant (p <0.05) variables in Pearson's univariate analysis were selected for multivariate regression analysis.
Abbreviations: β, partial regression coefficient; NS, not significant; NI, not included in the model; BMI, body mass index; BP, blood pressure; GGT, κ-glutamyltransferase; HbA1c, glycated hemoglobin A1c; GA, glycoalbumin; TG, serum triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; UA, uric acid; eGFR, estimated glomerular filtration rate.
Supplementary Table 4. Relative risk factors of log(UACR) in type 1 diabetes.
| Univariate |
Multivariate |
||||
|---|---|---|---|---|---|
| r | p | β | p | ||
| Age (year) | 0.016 | NS | |||
| Gender (male) | −0.260 | 0.008 | −0.318 | 0.001 | |
| Duration of diabetes (years) | −0.094 | NS | |||
| Smoking | 0.044 | NS | |||
| BMI (kg/m2) | −0.069 | NS | |||
| Systolic BP (mmHg) | −0.028 | NS | |||
| GGT (U/L) | 0.084 | NS | |||
| HbA1c (%) | 0.247 | 0.012 | NI | ||
| TG (mg/dL) | −0.001 | NS | |||
| LDL-C (mg/dL) | −0.034 | NS | |||
| HDL-C (mg/dL) | 0.041 | NS | |||
| UA (mg/dL) | 0.128 | NS | |||
| eGFR (ml/min/1.73 m2) | −0.142 | NS | |||
| Skin AF (AU) | 0.194 | 0.049 | 0.264 | 0.007 | |
| R2 | 0.134 | ||||
The threshold of statistical significance was defined as p <0.05.
Gender: male = 1, female = 0, Smoking: yes = 1, no = 0.
Stepwise multivariate regression analysis was performed to determine the predictors of log(UACR). The significant (p <0.05) variables in Pearson's univariate analysis were selected for multivariate regression analysis.
Abbreviations: β, partial regression coefficient; NS, not significant; NI, not included in the model; BMI, body mass index; BP, blood pressure; GGT, κ-glutamyltransferase; HbA1c, glycated hemoglobin A1c; GA, glycoalbumin; TG, serum triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; UA, uric acid; eGFR, estimated glomerular filtration rate.
References
- 1). McVeigh GE, Gibson W, Hamilton PK: Cardiovascular risk in the young type 1 diabetes population with a low 10-year, but high lifetime risk of cardiovascular disease. Diabetes Obes Metab, 2013; 15: 198-203 [DOI] [PubMed] [Google Scholar]
- 2). Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM: All-cause mortality rates in patients with type 1 diabetes mellitus compared with a non-diabetic population from the UK general practice research database, 1992–1999. Diabetologia, 2006; 49: 660-666 [DOI] [PubMed] [Google Scholar]
- 3). The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med, 1993; 329: 977-986 [DOI] [PubMed] [Google Scholar]
- 4). Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B: Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med, 2005; 353: 2643-2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Cerami C, Founds H, Nicholl I, Mitsuhashi T, Giordano D, Vanpatten S, Lee A, Al-Abed Y, Vlassara H, Bucala R, Cerami A: Tobacco smoke is a source of toxic reactive glycation products. Proc Natl Acad Sci U S A, 1997; 94: 13915-13920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Piperi C, Adamopoulos C, Dalagiorgou G, Diamanti-Kandarakis E, Papavassiliou AG: Crosstalk between advanced glycation and endoplasmic reticulum stress: emerging therapeutic targeting for metabolic diseases. J Clin Endocrinol Metab, 2012; 97: 2231-2242 [DOI] [PubMed] [Google Scholar]
- 7). Singh R, Barden A, Mori T, Beilin L: Advanced glycation end-products: a review. Diabetologia, 2001; 44: 129-146 [DOI] [PubMed] [Google Scholar]
- 8). Baumann M: Role of advanced glycation end products in hypertension and cardiovascular risk: human studies. J Am Soc Hypertens, 2012; 6: 427-435 [DOI] [PubMed] [Google Scholar]
- 9). Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med, 2000; 342: 381-389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Monnier VM, Bautista O, Kenny D, Sell DR, Fogarty J, Dahms W, Cleary PA, Lachin J, Genuth S: Skin collagen glycation, glycoxidation, and crosslinking are lower in subjects with long-term intensive versus conventional therapy of type 1 diabetes: relevance of glycated collagen products versus HbA1c as markers of diabetic complications. DCCT Skin Collagen Ancillary Study Group. Diabetes Control and Complications Trial. Diabetes, 1999; 48: 870-880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Genuth S, Sun W, Cleary P, Sell DR, Dahms W, Malone J, Sivitz W, Monnier VM: Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes. Diabetes, 2005; 54: 3103-3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Meerwaldt R, Graaff R, Oomen PH, Links TP, Jager JJ, Alderson NL, Thorpe SR, Baynes JW, Gans RO, Smit AJ: Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia, 2004; 47: 1324-1330 [DOI] [PubMed] [Google Scholar]
- 13). Sugisawa E, Miura J, Iwamoto Y, Uchigata Y: Skin autofluorescence reflects integration of past long-term glycemic control in patients with type 1 diabetes. Diabetes care, 2013; 36: 2339-2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Lutgers HL, Graaff R, Links TP, Ubink-Veltmaat LJ, Bilo HJ, Gans RO, Smit AJ: Skin autofluorescence as a non-invasive marker of vascular damage in patients with type 2 diabetes. Diabetes care, 2006; 29: 2654-2659 [DOI] [PubMed] [Google Scholar]
- 15). Meerwaldt R, Lutgers HL, Links TP, Graaff R, Baynes JW, Gans RO, Smit AJ: Skin autofluorescence is a strong predictor of cardiac mortality in diabetes. Diabetes care, 2007; 30: 107-112 [DOI] [PubMed] [Google Scholar]
- 16). Samborski P, Naskret D, Araszkiewicz A, Niedzwiecki P, Zozulinska-Ziolkiewicz D, Wierusz-Wysocka B: Assessment of skin autofluorescence as a marker of advanced glycation end product accumulation in type 1 diabetes. Pol Arch Med Wewn, 2011; 121: 67-72 [PubMed] [Google Scholar]
- 17). Orchard TJ, Lyons TJ, Cleary PA, Braffett BH, Maynard J, Cowie C, Gubitosi-Klug RA, Way J, Anderson K, Barnie A, Villavicencio S: The association of skin intrinsic fluorescence with type 1 diabetes complications in the DCCT/EDIC study. Diabetes care, 2013; 36: 3146-3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Chabroux S, Canoui-Poitrine F, Reffet S, Mills-Joncour G, Morelon E, Colin C, Thivolet C: Advanced glycation end products assessed by skin autofluorescence in type 1 diabetics are associated with nephropathy, but not retinopathy. Diabetes Metab, 2010; 36: 152-157 [DOI] [PubMed] [Google Scholar]
- 19). Conway BN, Aroda VR, Maynard JD, Matter N, Fernandez S, Ratner RE, Orchard TJ: Skin intrinsic fluorescence correlates with autonomic and distal symmetrical polyneuropathy in individuals with type 1 diabetes. Diabetes care, 2011; 34: 1000-1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Mook-Kanamori MJ, Selim MM, Takiddin AH, Al-Homsi H, Al-Mahmoud KA, Al-Obaidli A, Zirie MA, Rowe J, Gherbi WS, Chidiac OM, Kader SA, Al Muftah WA, McKeon C, Suhre K, Mook-Kanamori DO: Ethnic and gender differences in advanced glycation end products measured by skin auto-fluorescence. Dermatoendocrinol, 2013; 5: 325-330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Terminology and Diagnostic Criteria Committee, Japan Society of Ultrasonics in Medicine Subcommittee for Preparing Guidelines for Ultrasound Diagnosis of Carotid Artery. Standard method for ultrasound evaluation of carotid artery lesions. J Med Ultrason, 2009; 36: 501-518 [Google Scholar]
- 22). Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, Koji Y, Hori S, Yamamoto Y: Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res, 2002; 25: 359-364 [DOI] [PubMed] [Google Scholar]
- 23). den Dekker MA, Zwiers M, van den Heuvel ER, de Vos LC, Smit AJ, Zeebregts CJ, Oudkerk M, Vliegenthart R, Lefrandt JD, Mulder DJ: Skin autofluorescence, a noninvasive marker for AGE accumulation, is associated with the degree of atherosclerosis. PloS one, 2013; 8: e83084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Temma J, Matsuhisa M, Horie T, Kuroda A, Mori H, Tamaki M, Endo I, Aihara K, Abe M, Matsumoto T: Non-invasive Measurement of Skin Autofluorescence as a Beneficial Surrogate Marker for Atherosclerosis in Patients with Type 2 Diabetes. J Med Invest, 2015; 62: 126-129 [DOI] [PubMed] [Google Scholar]
- 25). Llaurado G, Ceperuelo-Mallafre V, Vilardell C, Simo R, Gil P, Cano A, Vendrell J, Gonzalez-Clemente JM: Advanced glycation end products are associated with arterial stiffness in type 1 diabetes. J Endocrinol, 2014; 221: 405-413 [DOI] [PubMed] [Google Scholar]
- 26). Araszkiewicz A, Naskret D, Zozulinska-Ziolkiewicz D, Pilacinski S, Uruska A, Grzelka A, Wegner M, Wierusz-Wysocka B: Skin autofluorescence is associated with carotid intima-media thickness, diabetic microangiopathy, and long-lasting metabolic control in type 1 diabetic patients. Results from Poznan Prospective Study. Microvasc Res, 2015; 98: 62-67 [DOI] [PubMed] [Google Scholar]
- 27). Lutgers HL, Graaff R, de Vries R, Smit AJ, Dullaart RP: Carotid artery intima media thickness associates with skin autofluoresence in non-diabetic subjects without clinically manifest cardiovascular disease. Eur J Clin Invest, 2010; 40: 812-817 [DOI] [PubMed] [Google Scholar]
- 28). Hangai M, Takebe N, Honma H, Sasaki A, Chida A, Nakano R, Togashi H, Nakagawa R, Oda T, Matsui M, Yashiro S, Nagasawa K, Kajiwara T, Takahashi K, Takahashi Y, Satoh J, Ishigaki Y: Association of Advanced Glycation End Products with coronary Artery Calcification in Japanese Subjects with Type 2 Diabetes as Assessed by Skin Autofluorescence. J Atheroscler Thromb, 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Noordzij MJ, Lefrandt JD, Loeffen EA, Saleem BR, Meerwaldt R, Lutgers HL, Smit AJ, Zeebregts CJ: Skin autofluorescence is increased in patients with carotid artery stenosis and peripheral artery disease. Int J Cardiovasc Imaging, 2012; 28: 431-438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Mulder DJ, van Haelst PL, Graaff R, Gans RO, Zijlstra F, Smit AJ: Skin autofluorescence is elevated in acute myocardial infarction and is associated with the one-year incidence of major adverse cardiac events. Neth Heart J, 2009; 17: 162-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Ohnuki Y, Nagano R, Takizawa S, Takagi S, Miyata T: Advanced glycation end products in patients with cerebral infarction. Intern Med, 2009; 48: 587-591 [DOI] [PubMed] [Google Scholar]
- 32). Lutgers HL, Gerrits EG, Graaff R, Links TP, Sluiter WJ, Gans RO, Bilo HJ, Smit AJ: Skin autofluorescence provides additional information to the UK Prospective Diabetes Study (UKPDS) risk score for the estimation of cardiovascular prognosis in type 2 diabetes mellitus. Diabetologia, 2009; 52: 789-797 [DOI] [PubMed] [Google Scholar]
- 33). Mallipattu SK, Uribarri J: Advanced glycation end product accumulation: a new enemy to target in chronic kidney disease? Curr Opin Nephrol Hypertens, 2014; 23: 547-554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Bos DC, de Ranitz-Greven WL, de Valk HW: Advanced glycation end products, measured as skin autofluorescence and diabetes complications: a systematic review. Diabetes Technol Ther, 2011; 13: 773-779 [DOI] [PubMed] [Google Scholar]
- 35). Yamagishi S, Nakamura K, Imaizumi T: Advanced glycation end products (AGEs) and diabetic vascular complications. Curr Diabetes Rev, 2005; 1: 93-106 [DOI] [PubMed] [Google Scholar]
- 36). Yamagishi S, Fujimori H, Yonekura H, Yamamoto Y, Yamamoto H: Advanced glycation endproducts inhibit prostacyclin production and induce plasminogen activator inhibitor-1 in human microvascular endothelial cells. Diabetologia, 1998; 41: 1435-1441 [DOI] [PubMed] [Google Scholar]
- 37). Yamagishi S, Hsu CC, Taniguchi M, Harada S, Yamamoto Y, Ohsawa K, Kobayashi K, Yamamoto H: Receptor-mediated toxicity to pericytes of advanced glycosylation end products: a possible mechanism of pericyte loss in diabetic microangiopathy. Biochem Biophys Res Commun, 1995; 213: 681-687 [DOI] [PubMed] [Google Scholar]
- 38). Yamagishi S, Yamamoto Y, Harada S, Hsu CC, Yamamoto H: Advanced glycosylation end products stimulate the growth but inhibit the prostacyclin-producing ability of endothelial cells through interactions with their receptors. FEBS Lett, 1996; 384: 103-106 [DOI] [PubMed] [Google Scholar]
- 39). Schmidt AM, Hasu M, Popov D, Zhang JH, Chen J, Yan SD, Brett J, Cao R, Kuwabara K, Costache G, et al. Receptor for advanced glycation end products (AGEs) has a central role in vessel wall interactions and gene activation in response to circulating AGE proteins. Proc Natl Acad Sci U S A, 1994; 91: 8807-8811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Tsuji H, Iehara N, Masegi T, Imura M, Ohkawa J, Arai H, Ishii K, Kita T, Doi T: Ribozyme targeting of receptor for advanced glycation end products in mouse mesangial cells. Biochem Biophys Res Commun, 1998; 245: 583-588 [DOI] [PubMed] [Google Scholar]
- 41). Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ, Jr, Chow WS, Stern D, Schmidt AM: Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med, 1998; 4: 1025-1031 [DOI] [PubMed] [Google Scholar]
- 42). Schmidt AM, Hori O, Chen JX, Li JF, Crandall J, Zhang J, Cao R, Yan SD, Brett J, Stern D: Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest 1995; 96: 1395-1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43). Naka Y, Bucciarelli LG, Wendt T, Lee LK, Rong LL, Ramasamy R, Yan SF, Schmidt AM: RAGE axis: Animal models and novel insights into the vascular complications of diabetes. Arterioscler Throm Vasc Biol, 2004; 24: 1342-1349 [DOI] [PubMed] [Google Scholar]
- 44). Sugiyama S, Miyata T, Ueda Y, Tanaka H, Maeda K, Kawashima S, Van Ypersele de Strihou C, Kurokawa K: Plasma levels of pentosidine in diabetic patients: an advanced glycation end product. J Am Soc Nephrol, 1998; 9: 1681-1688 [DOI] [PubMed] [Google Scholar]
- 45). Sell DR, Nagaraj RH, Grandhee SK, Odetti P, Lapolla A, Fogarty J, Monnier VM: Pentosidine: a molecular marker for the cumulative damage to proteins in diabetes, aging, and uremia. Diabetes Metab Rev, 1991; 7: 239-251 [DOI] [PubMed] [Google Scholar]
- 46). Ueno H, Koyama H, Fukumoto S, Tanaka S, Shoji T, Shoji T, Emoto M, Tahara H, Inaba M, Kakiya R, Tabata T, Miyata T, Nishizawa Y: Advanced glycation end products, carotid atherosclerosis, and circulating endothelial progenitor cells in patients with end-stage renal disease. Metabolism, 2011; 60: 453-459 [DOI] [PubMed] [Google Scholar]
- 47). Jiang J, Chen P, Chen J, Yu X, Xie D, Mei C, Xiong F, Shi W, Zhou W, Liu X, Sun S, Zhang P, Yang X, Zhang Y, Zhang Y, Liang X, Zhang Z, Lin Q, Yu Y, Miyata T, Tian J, Liang M, Luo W, Xu X, Hou F: Accumulation of tissue advanced glycation end products correlated with glucose exposure dose and associated with cardiovascular morbidity in patients on peritoneal dialysis. Atherosclerosis, 2012; 224: 187-194 [DOI] [PubMed] [Google Scholar]
- 48). Arsov S, Graaff R, van Oeveren W, Stegmayr B, Sikole A, Rakhorst G, Smit AJ: Advanced glycation end-products and skin autofluorescence in end-stage renal disease: a review. Clin Chem Lab Med, 2014; 52: 11-20 [DOI] [PubMed] [Google Scholar]
- 49). Noordzij MJ, Lefrandt JD, Graaff R, Smit AJ: Dermal factors influencing measurement of skin autofluorescence. Diabetes Technol Ther, 2011; 13: 165-170 [DOI] [PubMed] [Google Scholar]


