Abstract
Aim: After the Great East Japan Earthquake, over 160,000 residents near the Fukushima Daiichi Nuclear Power Plant were forced to evacuate due to a nuclear accident. Health problems in these evacuees have since become major issues. We examined the association between evacuation and incidence of metabolic syndrome (METS) among residents in Fukushima.
Methods: We conducted a cohort study among residents aged 40–74 years without METS at the time of the disaster in Fukushima. Among 20,269 residents who met the inclusion criteria before the disaster, 8,547 residents (3,697 men and 4,850 women; follow-up proportion: 42.2%) remained available for follow-up examinations after the disaster by the end of March 2013. The main outcome was incidence of METS, defined by guidelines from the Japanese committee, using data from the Comprehensive Health Check before and after the disaster. We divided participants by evacuation status and compared outcomes between groups. Using a logistic regression model, we estimated the odds ratio for incidence of METS, adjusting for potential confounders, age, gender, waist circumference, exercise habit, and alcohol consumption.
Results: Incidence of METS was higher in evacuees (men 19.2%, women 6.6%) than in non-evacuees (men 11.0%, women 4.6%). Evacuees had higher body mass index, waist circumference, triglycerides, and fasting plasma glucose after the disaster than non-evacuees. We found a significant association between evacuation and incidence of METS (adjusted odds ratio 1.72, 95% confidence interval; 1.46–2.02).
Conclusion: This is the first study to demonstrate that evacuation after a disaster is associated with increased incidence of METS.
Keywords: Metabolic syndrome, Disaster, Evacuation, Life style
Introduction
The Great East Japan Earthquake of a magnitude 9.0 and subsequent tsunami on March 11, 2011 struck the Fukushima Daiichi Nuclear Power Plant and a nuclear accident occurred. Then, Fukushima prefecture suffered a triple disaster. Following the disaster, Japanese government designated the 20 km radius area around the Fukushima Daiichi Nuclear Power Plant as a “restricted area” with compulsory evacuation. The Japanese government subsequently designated a 20 to 30 km radius area around the plant as an “evacuation-prepared area in case of emergency,” and near the 30 km radius area where high level radiation exposure (> 20 mSv/y) was expected as “deliberate evacuation areas.” As a result of the governmental directions, about 146,000 residents of Hirono-machi, Narahamachi, Tomioka-machi, Kawauchi-mura, Okumamachi, Futaba-machi, Namie-machi, Katsurao-mura, and Iitate-mura, and some residents of Tamura City, Minami-Soma City, Kawamata-machi, and Date City were forced to evacuate their homes (Fig. 1) regardless of the presence or absence of direct damage from the earthquake and tsunami. Residents living outside the government-designated area of about 20,000 people evacuated voluntary. In total, over 160,000 of residents in Fukushima Prefecture evacuated in May 2011. Namely, many of them were refugees from the nuclear disaster, not direct victims of the earthquake or tsunami. Since this incident, health problems in evacuees have become major issues.
Fig. 1.
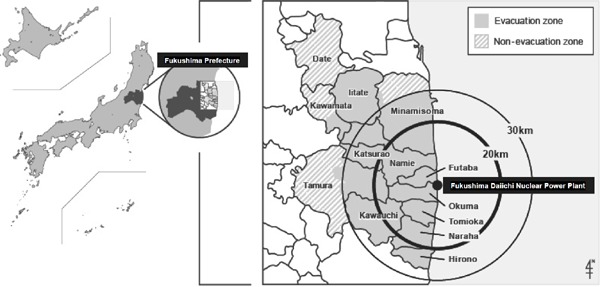
Geography of the evacuation zone, non-evacuation zone, and the Fukushima Daiichi Nuclear Power Plant
Previous studies have reported that the incidence of cardiovascular diseases1–3) is increased after a natural disaster. Further, a recent study showed that incidence of out-of-hospital cardiac accidents in the month directly following the 2011 earthquake in Iwate, Miyagi, and Fukushima Prefectures4, 5) or incidence of heart failure in Fukushima Prefecture six months after that disaster6) was significantly higher than incidence in the previous years. However, while acute effects of a disaster on incidence of cardiovascular diseases have been established, long-term effects are poorly understood.
Prolonged evacuation from the government-designated evacuation zones may lead to changes in lifestyle for evacuees, including altered diet, physical exercise habits, smoking status, and alcohol consumption. Evacuees may therefore be more susceptible than non-evacuees to cardiovascular risk factors, such as visceral obesity, hypertension, diabetes mellitus, and dyslipidemia7) and metabolic syndrome, which is cluster of these risk factors and has been reported to be associated with low level of plasma adiponectin and higher risk of incidence of cardiovascular diseases8, 9). However, few reports have been published concerning the influence of post-disaster evacuation on metabolic syndrome.
Here, in order to clarify the impact of change in living environment due to forced unreasonable evacuation from the nuclear power point accident after a natural disaster on health status, we investigated the association between evacuation following the 2011 Great East Japan Earthquake and incidence of metabolic syndrome among residents in Fukushima Prefecture, Japan.
Materials and Methods
Study Population and Design
Participants in this study were residents living in evacuation-designated areas within and around 30 km of the Fukushima Daiichi Nuclear Power Plant during the 2011 Great East Japan Earthquake. Cities and townships involved were Tamura City, Minamisoma City, Kawamata-machi, Hirono-machi, Naraha-machi, Tomioka-machi, Kawauchi-mura, Okuma-machi, Futaba-machi, Namie-machi, Katsurao-mura, Iitate-mura, and Date City, with total populations of 278,276 in 2010. Following the disaster, the government designated the 20 km radius area around the Fukushima Daiichi Nuclear Power Plant as a “restricted area” with compulsory evacuation. The government subsequently designated a 20 to 30 km radius area around the plant as an “evacuation-prepared area in case of emergency,” and near the 30 km radius area where high level radiation exposure (> 20 mSv/y) was expected as “deliberate evacuation areas.” As a result, the governmental directions forced all residents of Hirono-machi, Narahamachi, Tomioka-machi, Kawauchi-mura, Okumamachi, Futaba-machi, Namie-machi, Katsurao-mura, and Iitate-mura, and some residents of Tamura City, Minami-Soma City, Kawamata-machi, and Date City to evacuate their homes (Fig. 1, Evacuation zone). The other areas of Tamura City, Minami-Soma City, Kawamata-machi, and Date City were defined as a non-evacuation zone in the present study (Fig. 1, Non-evacuation zone). All residents of Hirono-machi, Naraha-machi, Tomioka-machi, Kawauchi-mura, Okuma-machi, Futaba-machi, Namie-machi, Katsuraomura, and Iitate-mura, and residents in part of Tamura City, Minamisoma City, Kawamata-machi, and Date City were forced to evacuate their homes under governmental direction following the earthquake in March 2011.
Residents from these areas aged ≥ 40 years have received annual health checkups since 2008 with a focus on metabolic syndrome for people covered by national health insurance. All analyses in this study were limited to residents aged 40–74 years. Between 2008 and 2010, 31,252 residents (14,022 men and 17,230 women, mean age 67 years) from these areas participated in the health checkups.
Detailed methods of the Comprehensive Health Check (CHC) and the Fukushima Health Management Survey (FHMS) have been described previously10). The exclusion criteria for our study were missing data for abdominal circumference (n = 950) or plasma glucose (n = 5,986) and having metabolic syndrome before the disaster in 2010 (n = 4,047). After excluding residents who met those criteria, 20,269 participants remained for analysis. Follow-up examinations were conducted from June 2011 to the end of March 2013 as a part of the CHC, a further 11,722 subjects were excluded for not receiving these follow-up examinations, leaving 8,547 residents (3,697 men and 4,850 women; follow-up proportion: 42.2%) with complete anthropometric and laboratory results before and after the disaster (mean follow-up time, 1.33 years). For analyses, we used data from the most recent year before the earthquake (2008–2010) and from the earliest year after (2011–2012) to define changes in health status before and after the disaster.
Ethical Considerations
Informed consent was obtained from the community representatives to conduct an epidemiological study based on guidelines of the Council for International Organizations of Medical Science11). Our study was approved by the Ethics Committee of Fukushima Medical University (No. 1916). All participants in the Fukushima Health Management Survey provided written informed consent at our follow-up survey, and evidence of this consent is retained in a secure storage facility.
Definitions and Data Collection
During clinical examination, trained observers evaluated blood pressure and took anthropometric measurements using standard protocols and techniques. Participants were advised to avoid smoking, alcohol, caffeinated beverages, and exercise for at least 30 min before their blood pressure measurement. Body weight and height were also measured during these examinations. Weight was measured with subjects wearing light indoor clothing without shoes, and height was without shoes. Waist circumference (WC) was measured above the navel at minimal respiration.
Various patient characteristics were examined under overnight fasting conditions: including height; weight; waist circumference (WC); body mass index (BMI); blood pressure; and levels of aspartate amino-transferase (AST), alanine aminotransferase (ALT), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), HbA1c, fasting plasma glucose (FPG), urine protein, and urine sugar. Additional measurements included serum creatinine levels, hematocrit levels, hemoglobin levels, estimated glomerular filtration rate, uric acid levels, urinary testing for occult blood, and peripheral blood counts, which evaluated the numbers of red blood cells, platelets, and white blood cells. An interviewer inquired about change in body weight of 3 kg or more within the previous year, exercise habits (1, to continue 30 minutes of exercise twice a week or more for more than one year; 2, walking for one hour a day or equivalent physical activity), sleeping quality, cigarette smoking status, and weekly alcohol intake (current at ≥ 44 g/day, current at < 44 g/day, never, or former). Participants that consumed ≥ 44 g ethanol or more per day were classified as being current excessive drinkers because previous study reported that increase in risk of all causes mortality or coronary risk was observed among current excessive drinkers8, 9).
We defined metabolic syndrome based on the definition of the Japanese committee for establishing diagnostic criteria for metabolic syndrome12), as follows: visceral obesity (waist circumference ≥ 85 cm in men and ≥ 90 cm in women) in addition to the presence of at least two of the following three abnormalities: plasma TG ≥ 150 mg/dL and/or HDL-C < 40 mg/dL or being treated for dyslipidemia; systolic blood pressure ≥ 130 mmHg and/or diastolic blood pressure ≥ 85 mmHg or being treated for hypertension; and fasting plasma glucose ≥ 110 mg/dl (6.1 mmol/L) or being treated for diabetes. BMI was calculated as weight (kg) divided by the square of the height (m2).
Statistical Analysis
The participants were divided into two groups: evacuees (n = 3,019) and non-evacuees (n = 5,528). Changes in body weight, BMI, and proportion of overweight before and after the disaster between two groups were compared using Student's t-test or a Wilcoxon rank sum test. We used logistic regression analyses to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for the association between evacuation and incidence of metabolic syndrome after adjusting for potential confounders. The logistic regression model include the following variables: age (continuous), evacuation status (evacuee or non-evacuee), waist circumference (continuous), ≥ 3 kg weight change during 1 year (yes, or no), physical activity at least ≥ 30 minutes/day and ≥ 2 times/week for over a year (yes or no), walking at least ≥ 1 hour/day (yes or no), good sleeping quality (yes or no), smoking status (current, never, or former), and alcohol consumption (current at ≥ 44 g/day, current at < 44 g/day, never, or former). Following initial analyses, we repeated analyses after stratification by age (≥ 65 years or < 65 years).
SAS version 9.3 (SAS Institute, Cary, NC, USA) was used for analyses. All probability values for statistical tests were two-tailed, and P < 0.05 was regarded as statistically significant.
Results
Clinical Characteristics
The baseline clinical characteristics of the 8,547 participants (3,697 men and 4,850 women; follow-up proportion: 42.2%; mean follow-up period, 1.33 years) are listed in Table 1. Among men, body weight, BMI, and waist circumference were higher at baseline in the evacuee group than in the non-evacuee group, while only body weight and BMI were higher at baseline for women in the evacuee group versus the non-evacuee group. In addition, the evacuee group had a higher proportion of marked body weight change (≥ 3 kg/year) in the year before baseline than the non-evacuee group.
Table 1. Baseline characteristics of subjects before the Great East Japan Earthquake.
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| Non-evacuee | Evacuee | P values i | Non-evacuee | Evacuee | P values i | |
| Participants, n | 2443 | 1254 | 3085 | 1765 | ||
| Incidence of metabolic syndrome | 270 | 241 | <0.001 | 144 | 116 | 0.005 |
| Age, years | 62.4 (7.7) | 62.3 (7.3) | 0.757 | 62.3 (7.4) | 61.5 (7.7) | <0.001 |
| Height, cm | 163.5 (6.3) | 163.8 (6.2) | 0.125 | 151.2 (5.9) | 151.8 (5.7) | <0.001 |
| Weight, kg | 61.2 (8.7) | 62.3 (9.0) | <0.001 | 52.0 (7.7) | 53.0 (7.7) | <0.001 |
| BMIa, kg/m2 | 22.9 (2.7) | 23.2 (2.8) | 0.002 | 22.7 (3.1) | 23.0 (3.1) | 0.009 |
| Waist circumference, cm | 82.5 (7.6) | 83.1 (7.7) | 0.017 | 82.0 (8.2) | 82.3 (8.2) | 0.161 |
| SBPb mmHg, | 129.2 (15.8) | 129.9 (17.2) | 0.225 | 127.8 (16.6) | 126.9 (17.0) | 0.067 |
| DBPc, mmHg | 78.6 (10.0) | 78.1 (10.4) | 0.169 | 76.4 (10.1) | 74.9 (10.2) | <0.001 |
| Triglyceride, mg/dL | 103.7 (72.9) | 101.9 (63.4) | 0.438 | 94.5 (47.6) | 91.8 (47.6) | 0.062 |
| HDL-Cd, mg/dL | 58.4 (15.0) | 59.1 (14.7) | 0.217 | 63.2 (14.1) | 63.7 (14.6) | 0.239 |
| LDL-Ce, mg/dL | 117.6 (29.1) | 119.4 (30.3) | 0.070 | 127.6 (29.6) | 127.3 (30.3) | 0.766 |
| FPGf, mg/dL (25%–75%) | 96 (90–102) | 94 (89–102) | 0.003 | 94 (88–100) | 91 (86–98) | <0.001 |
| HbA1c | 5.0 (0.6) | 5.1 (0.6) | 0.067 | 5.1 (0.5) | 5.1 (0.5) | 0.338 |
| ≥ 3-kg weight gain during 1 year, % | 18.7 | 22.1 | 0.015 | 17.2 | 21.9 | <0.001 |
| Exercise habit 1g, % | 29.9 | 34.1 | 0.010 | 29.5 | 31.2 | 0.200 |
| Exercise habit 2h, % | 39.3 | 41.6 | 0.171 | 32.9 | 36.4 | 0.014 |
| Good sleep, % | 78.1 | 77.7 | 0.792 | 72.4 | 72.4 | 0.987 |
| Never or past smoker, % | 70.8 | 70.0 | 0.632 | 95.8 | 94.5 | 0.030 |
| Never or past drinker, % | 12.1 | 13.9 | 0.244 | 43.2 | 42.3 | 0.097 |
| Current drinker, % | ||||||
| <44 g/day | 46.5 | 44.6 | 54.8 | 54.6 | ||
| ≥ 44 g/day | 41.4 | 41.6 | 2.1 | 3.1 | ||
All values in parentheses, except for FPG, represent the standard deviation.
Body Mass Index
Systolic Blood Pressure
Diastolic Blood Pressure
High-Density-Lipoprotein-Cholesterol
Low-Density-Lipoprotein-Cholestrol
Fasting Plasma Glucose
Physical activity at least ≥ 30 minutes/day and ≥ 2 times/week for over a year
Walking at least ≥ 1 hour/day
P values were calculated by t-test (continuous variables), Wilcoxon rank sum test (continuous variables), or chi-squared test (categorical variables).
Changes in Clinical Characteristics Before and After the Disaster by Evacuation Status
Table 2 shows changes in clinical characteristics before and after the disaster by evacuation status. For both sexes, we observed a greater increase after the earthquake in weight, BMI, waist circumference, and TG and fasting plasma glucose levels in the evacuee group than in the non-evacuee group. In addition, while the proportion of subjects reporting good sleeping quality decreased across both groups, the reduction was more marked among evacuees than non-evacuees.
Table 2. Changes in clinical characteristics before and after disaster by evacuation status.
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| Non-evacuee | Evacuee | P values i | Non-evacuee | Evacuee | P values i | |
| Participants, n | 2443 | 1254 | 3085 | 1765 | ||
| Incidence of metabolic syndrome, n | 270 | 241 | 144 | 116 | ||
| Δ weight, kg | 0.4 | 2.0 | <0.001 | 0.3 | 0.8 | <0.001 |
| Δ BMIa, kg/m2 | 0.1 | 0.8 | <0.001 | 0.2 | 0.4 | <0.001 |
| Δ waist circumference, cm | 0.3 | 2.0 | <0.001 | −0.2 | 0.4 | <0.001 |
| Δ SBPb, mmHg | 2.2 | 2.5 | 0.575 | 2.2 | 2.0 | 0.780 |
| Δ DBPc, mmHg | 0.7 | 1.4 | 0.054 | 0.7 | 1.8 | 0.002 |
| Δ Triglyceride, mg/dL | 9.1 | 18.1 | <0.001 | 4.4 | 12.4 | <0.001 |
| Δ HDL-Cd, mg/dL | 0.0 | −2.8 | <0.001 | −0.3 | −1.2 | <0.001 |
| Δ LDL-Ce, mg/dL | 0.4 | −17.2 | <0.001 | −0.5 | 3.2 | <0.001 |
| Δ FPGf, mg/dL | 0.0 | 3.0 | <0.001 | 0.0 | 3.0 | <0.001 |
| Δ HbA1c | 0.0 | 0.0 | <0.001 | −0.1 | 0.0 | 0.006 |
| Δ ≥ 3-kg weight gain during 1 year, % | 0.0 | 16.3 | 1.6 | 18.6 | ||
| Δ Exercise habit 1g, % | 0.2 | −0.2 | 0.2 | −1.6 | ||
| Δ Exercise habit 2h, % | −0.3 | −0.6 | −2.0 | −3.0 | ||
| Δ Good sleep, % | −2.2 | −7.7 | −0.3 | −13.8 | ||
| Δ Never or past smoker, % | 3.2 | 3.9 | 0.5 | 0.8 | ||
| Δ Never or past drinker, % | −0.4 | 0.7 | −0.8 | 3.8 | ||
| Δ Current drinker, % | ||||||
| <44 g/day | 1.3 | 1.6 | 0.8 | −3.8 | ||
| ≥ 44 g/day | −0.9 | −2.3 | 0.0 | 0.0 | ||
Body Mass Index
Systolic Blood Pressure
Diastolic Blood Pressure
High-Density-Lipoprotein-Cholesterol
Low-Density-Lipoprotein-Cholestrol
Fasting Plasma Glucose
Physical activity at least ≥ 30 minutes/day and ≥ 2 times/week for over a year
Walking at least ≥ 1 hour/day
P values of difference-in-difference were calculated by t-test (continuous variables).
Impact of Evacuation on Incidence of Metabolic Syndrome After the Disaster
Crude incidence proportion of metabolic syndrome was significantly higher in the evacuee group [men: 19.2% (241/1254), women: 6.6% (116/1765)], than in the non-evacuee group [men: 11.0% (270/2443), women: 4.6% (144/3085)].
Logistic regression analysis revealed a significant association between evacuation and incidence of metabolic syndrome, even after adjusting for potential confounders of age, baseline clinical characteristics, and life style [adjusted OR (95% CI): 1.72 (1.46–2.02); Table 3]. We performed an additional subgroup analysis by gender and found that the significant association between evacuation and incidence of metabolic syndrome persisted in both men and women [adjusted OR (95% CI) in men: 1.89 (1.55–2.31), in women: 1.45 (1.10–1.92); Table 4]. Another subgroup analysis by age (< 65 and ≥ 65 years) also revealed a significant association between evacuation and incidence of metabolic syndrome across both age groups [adjusted OR (95% CI) for < 65 years of age: 1.72 (1.46–2.02), for ≥ 65 years of age: 1.60 (1.26–2.03); Table 5].
Table 3. Association between evacuation status and incidence of metabolic syndrome.
| ORs (95% CIs) |
||
|---|---|---|
| Crude | Multivariate | |
| Evacuee (ref: non-evacuee) | 1.66 (1.43–1.92) | 1.72 (1.46–2.02) |
| Women (ref: men) | 0.35 (0.30–0.41) | 0.35 (0.29–0.43) |
| Age (1-year increase) | 1.02 (1.004–1.03) | 1.02 (1.01–1.03) |
| Waist circumference (1-cm increase) | 1.13 (1.12–1.14) | 1.14 (1.12–1.15) |
| ≥ 3-kg weight change during 1 year (ref: no) | 1.58 (1.33–1.87) | 1.17 (0.97–1.41) |
| Exercise 1a (ref: no) | 1.13 (0.96–1.32) | 1.09 (0.90–1.32) |
| Exercise 2b (ref: no) | 1.08 (0.92–1.25) | 0.97 (0.81–1.17) |
| Good sleep (ref: no) | 1.15 (0.96–1.37) | 0.94 (0.78–1.14) |
| Current smoker (ref: never or former smoker) | 1.55 (1.29–1.87) | 1.24 (1.01–1.54) |
| Current drinker (ref: never or former drinker) | ||
| <44 g/day | 1.06 (0.88–1.28) | 0.85 (0.70–1.05) |
| ≥ 44 g/day | 2.27 (1.86–2.77) | 1.12 (0.87–1.43) |
CI, confidence interval; OR, odds ratio
Physical activity at least ≥ 30 minutes/day and ≥ 2 times/week for over a year
Walking at least ≥ 1 hour/day
Table 4. ORs (95% CIs) for incidence of metabolic syndrome after the disaster (gender subgroup analysis); Relationship between evacuation and incidence of metabolic syndrome.
| ORs (95% CIs) |
||||
|---|---|---|---|---|
| Men |
Women |
|||
| Crude | Multivariate | Crude | Multivariate | |
| Evacuee (ref: non-evacuee) | 1.92 (1.59–2.31) | 1.89 (1.55–2.31) | 1.44 (1.12–1.85) | 1.45 (1.10–1.92) |
| Age (1-year increase) | 1.01 (0.997–1.02) | 1.02 (1.004–1.03) | 1.02 (1.002–1.04) | 1.03 (1.01–1.05) |
| Waist circumference (1-cm increase) | 1.11 (1.10–1.13) | 1.11 (1.10–1.13) | 1.17 (1.15–1.19) | 1.17 (1.15–1.19) |
| ≥ 3-kg weight change during 1 year (ref: no) | 1.44 (1.16–1.80) | 1.14 (0.90–1.45) | 1.81 (1.37–2.39) | 1.22 (0.89–1.68) |
| Exercise 1a (ref: no) | 1.08 (0.88–1.21) | 1.01 (0.80–1.29) | 1.18 (0.90–1.54) | 1.20 (0.87–1.66) |
| Exercise 2b (ref: no) | 1.03 (0.85–1.25) | 1.02 (0.81–1.28) | 0.97 (0.74–1.26) | 0.91 (0.67–1.26) |
| Good sleep (ref: no) | 1.02 (0.82–1.29) | 0.91 (0.71–1.17) | 1.13 (0.85–1.51) | 1.02 (0.74–1.40) |
| Current smoker (ref: never or former smoker) | 0.98 (0.80–1.21) | 1.18 (0.94–1.48) | 1.27 (0.74–2.17) | 1.30 (0.67–2.50) |
| Current drinker (ref: never or former drinker) | ||||
| <44 g/day [can you clarify why you changed the cut-off point? This is unclear] | 0.81 (0.60–1.10) | 0.86 (0.62–1.18) | 0.88 (0.69–1.14) | 0.86 (0.65–1.13) |
| ≥ 44 g/day | 1.17 (0.87–1.57) | 1.16 (0.85–1.58) | 0.57 (0.21–1.58) | 0.65 (0.22–1.95) |
CI, confidence interval; OR, odds ratio
Physical activity at least ≥ 30 minutes/day and ≥ 2 times/week for over a year
Walking at least ≥ 1 hour/day
Table 5. ORs (95% CIs) for incidence of metabolic syndrome after the disaster (age subgroup analysis); Relationship between evacuation and incidence of metabolic syndrome.
| ORs (95% CIs) |
||||
|---|---|---|---|---|
| <65 years |
≥ 65 years |
|||
| Crude | Multivariate | Crude | Multivariate | |
| Evacuee (ref: non-evacuee) | 1.76 (1.44–2.16) | 1.83 (1.47–2.29) | 1.56 (1.26–1.94) | 1.60 (1.26–2.03) |
| Women (ref: men) | 0.35 (0.28–0.43) | 0.32 (0.24–0.42) | 0.36 (0.29–0.45) | 0.37 (0.28–0.49) |
| Age (1-year increase) | 1.02 (1.01–1.04) | 1.04 (1.02–1.06) | 0.98 (0.93–1.02) | 0.96 (0.92–1.01) |
| Waist circumference (1-cm increase) | 1.13 (1.12–1.15) | 1.14 (1.12–1.16) | 1.13 (1.11–1.15) | 1.14 (1.12–1.16) |
| ≥ 3-kg weight change during 1 year (ref: no) | 1.71 (1.37–2.14) | 1.26 (0.98–1.62) | 1.47 (1.13–1.92) | 1.05 (0.78–1.42) |
| Exercise 1a (ref: no) | 1.04 (0.82–1.32) | 0.91 (0.68–1.21) | 1.15 (0.92–1.42) | 1.27 (0.98–1.66) |
| Exercise 2b (ref: no) | 1.05 (0.84–1.31) | 1.09 (0.84–1.42) | 1.05 (0.85–1.30) | 0.89 (0.68–1.15) |
| Good sleep (ref: no) | 1.16 (0.92–1.46) | 0.96 (0.74–1.24) | 1.08 (0.83–1.42) | 0.91 (0.67–1.22) |
| Current smoker (ref: never or former smoker) | 1.49 (1.17–1.89) | 1.15 (0.87–1.51) | 1.79 (1.34–2.40) | 1.42 (1.02–1.97) |
| Current drinker (ref: never or former drinker) | ||||
| <44 g/day | 0.91 (0.70–1.17) | 0.73 (0.55–0.97) | 1.27 (0.97–1.67) | 0.99 (0.73–1.33) |
| ≥ 44 g/day | 1.80 (1.37–2.38) | 0.92 (0.65–1.30) | 2.99 (2.23–4.00) | 1.29 (0.89–1.86) |
CI, confidence interval; OR, odds ratio
Physical activity at least ≥ 30 minutes/day and ≥ 2 times/week for over a year
Walking at least ≥ 1 hour/day
Discussion
In our analysis of the influence of evacuation following the 2011 Great East Japan Earthquake on health status in residents of Fukushima, we examined the changes in health status from before the disaster to after using data from CHC conducted annually in Japan for all people aged ≥ 40 years, including residents who lived in the evacuation zone. After adjusting for potential confounders, we found significant associations between evacuee status and incidence of metabolic syndrome. In addition, we further found that weight, BMI, and waist circumference increased more dramatically following the disaster among evacuees than among non-evacuees (Table 2). Taken together, these results suggest that refugee life might lead to lifestyle-related disease, and we should therefore carefully monitor the health of evacuees following a disaster.
Changes in lifestyle following a disaster may render evacuees susceptible to a number of cardiovascular risk factors13–15), such as hypertension, impaired glucose tolerance/diabetes mellitus, and dyslipidemia. Although previous studies have shown a reduction in control of glucose metabolism16–18) and blood pressure17, 19–22) among victims of natural disasters such earthquakes and hurricanes, few studies have examined the long-term effects of evacuation following a disaster on metabolic syndrome due to lifestyle changes when subjects have a sufficient food supply in chronic phase (versus suffering from malnutrition due to a temporarily insufficient food supply in very acute phase)7, 23–25).
A previous study in evacuees in Fukushima after the Great East Japan Earthquake reported increases in body weight and proportion of overweight/obesity26), and another study reported increases in BMI and waist circumference associated with glycemic and lipid parameters and blood pressure27). These previous reports support our present findings of an association between evacuation and incidence of metabolic syndrome. Lifestyle-related diseases may develop after a disaster because evacuees experience difficulty in maintaining the same lifestyle they previously enjoyed, due to loss of jobs and social connectedness in the wake of evacuation. In these situations, long-term effects of evacuation on health status have become more important with respect to overall health of an individual than short-term effects.
Since we did not evaluate detailed lifestyle changes in food intake and social activity before and after the earthquake in the present study, potential mechanisms by which evacuation might influence development of metabolic syndrome remain unclear. Overall, exercise habits in evacuees did not change markedly before and after the disaster, although a recent study in Fukushima reported that prevalence of habitual physical activity was lower in evacuees living apart from their community in rental housing/apartments than in those living in evacuation shelters or temporary housing together with their community28). Further, results of the FHMS showed that evacuees after the 2011 earthquake tended to be less physically active and have greater alcohol intake than before the earthquake29). Never or past smoker in evacuees were decreased after the disaster, although the 2014 National Health and Nutrition Survey reported proportion of smoker of Fukushima prefecture in 2012 was 39.7%, which was higher than that before disaster 2010 of 23%. Previous studies reported that smoking is a risk factor for the development the metabolic syndrome30) and is a major determinant of cardiovascular diseases, such as ischemic heart disease and stroke, among middle-aged Japanese men and women, in particular among smokers31). Taken together, these previous findings suggest that health-related behaviors may be an important factor mediating the influence of evacuation on metabolic syndrome and to prevent ischemic heart disease and stroke.
A mental health survey through the FHMS has also suggested that evacuation might lead to either or both anxiety or trauma32), which evacuees are particularly vulnerable to due to their persistent stress exposure (long stays away from home, reduced exercise, sudden disruption of social environment, and changes in dietary pattern). Anxiety and trauma may also increase prevalence of sleep disorder in evacuees, which has been associated with each component of metabolic syndrome33–35), including incidence of obesity36–39), particularly abdominal adiposity40), impaired glucose metabolism41–43), and hypertension39, 44, 45).
Several limitations to the present study warrant mention. First, we did not evaluate socio-economic status, which may influence several parameters associated with metabolic syndrome. Indeed, a previous study reported that disorderly conditions after Hurricane Katrina (USA) were associated with increased BMI46). The influence of socio-economic factors may modify the association between evacuation and changes in body weight and BMI. Second, we only included participants who received a comprehensive health check both before and after the disaster; such participants are typically particularly health-conscious individuals and may therefore have lent some bias to our findings. Third, the mean follow-up time of 1.33 years may not have been sufficient to examine longterm effects of evacuation on health outcomes. However, given that many Fukushima residents may be under evacuation order indefinitely, ongoing monitoring of their health status may lend greater clarity to our findings.
United Nations Scientific Committee on the Effects of Atomic Radiation reported no discernible increase in incidence of radiation-related health effects among exposed members of the public or their descendants based on the data of low or very low doses to the general public, both those incurred during the first year and estimated for their lifetime47). Therefore, the most important health effect is not on direct radiation effects, but on indirect effects of life style change due to forced evacuation from the nuclear accident or the fear and stigma related to the risk perception of the radiation exposure or rumor. Evacuation to avoid exposure from ionized radiation has increased the risk of cardiovascular diseases of the evacuees via the increase of incidence of metabolic syndrome. Based on these results, we should make best effort to prevent the incidence of METS associated with evacuation via support for the life style improvement of the evacuees, such as food intake48) or exercise habit49, 50).
In conclusion, evacuation was associated with increased incidence of metabolic syndrome in Fukushima prefecture after the 2011 Great East Japan Earthquake. Even today, four years after the disaster, over 100,000 residents in Fukushima remain under evacuation order. Given this observed connection between evacuee status and metabolic syndrome, we may conclude that these individuals are at increased risk of cardiovascular events15). Ongoing monitoring will be necessary to stem this tide of increasing incidence of life-style related diseases due to long-term evacuation. The present study is the first to examine the influence of evacuation on incidence of metabolic syndrome, and our findings may aid physicians in protecting the health of evacuees. These data derived from the Fukushima disaster will help guard against similar effects in future cataclysms.
Acknowledgments
This survey was supported by the National Health Fund for Children and Adults Affected by the Nuclear Incident. The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the Fukushima Prefecture government.
Conflict of Interest Disclosure
All authors declare no conflicts of interest.
Financial Disclosure
No financial disclosures were reported by the authors of this paper.
Appendix
The Fukushima Health Management Survey Group Hitoshi Ohto, Masafumi Abe, Shunichi Yamashita, Kenji Kamiya, Seiji Yasumura, Mitsuaki Hosoya, Akira Ohtsuru, Akira Sakai, Shinichi Suzuki, Hirooki Yabe, Masaharu Maeda, Shirou Matsui, Keiya Fujimori, Tetsuo Ishikawa, Tetsuya Ohira, Tsuyoshi Watanabe, Hiroaki Satoh, Hitoshi Suzuki, Yukihiko Kawasaki, Atsushi Takahashi, Kotaro Ozasa, Gen Kobashi, Shigeatsu Hashimoto, Satoru Suzuki, Toshihiko Fukushima, Sanae Midorikawa, Hiromi Shimura, Hirofumi Mashiko, Aya Goto, Kenneth Eric Nollet, Shinichi Niwa, Hideto Takahashi, and Yoshisada Shibata
References
- 1). Kario K, Ohashi T. Increased coronary heart disease mortality after the Hanshin-Awaji earthquake among the older community on Awaji Island. Tsuna Medical Association. J Am Geriatr Soc. 1997; 45: 610-613 [DOI] [PubMed] [Google Scholar]
- 2). Kario K, Matsuo T, Kayaba K, Soukejima S, Kagamimori S, Shimada K. Earthquake-induced cardiovascular disease and related risk factors in focusing on the Great Hanshin-Awaji Earthquake. J Epidemiol Jpn Epidemiol Assoc. 1998; 8: 131-139 [DOI] [PubMed] [Google Scholar]
- 3). Watanabe H, Kodama M, Okura Y, Aizawa Y, Tanabe N, Chinushi M, et al. Impact of earthquakes on Takotsubo cardiomyopathy. JAMA. 2005; 294: 305-307 [DOI] [PubMed] [Google Scholar]
- 4). Kitamura T, Kiyohara K, Iwami T. The great east Japan earthquake and out-of-hospital cardiac arrest. N Engl J Med. 2013; 369: 2165-2167 [DOI] [PubMed] [Google Scholar]
- 5). Nozaki E, Nakamura A, Abe A, Kagaya Y, Kohzu K. Sato K et al. Occurrence of cardiovascular events after the 2011 Great East Japan Earthquake and tsunami disaster. Int Heart J. 2013; 54: 247-253 [DOI] [PubMed] [Google Scholar]
- 6). Yamauchi H, Yoshihisa A, Iwaya S, Owada T, Sato T, Suzuki S, et al. Clinical Features of Patients With Decompensated Heart Failure After the Great East Japan Earthquake. Am J Cardiol. 112: 94-99 [DOI] [PubMed] [Google Scholar]
- 7). Dowd JB, Zajacova A. Long-term obesity and cardiovascular, inflammatory, and metabolic risk in U.S. adults. Am J Prev Med. 2014; 46: 578-584 [DOI] [PubMed] [Google Scholar]
- 8). Takahara M, Katakami N, Kaneto H, Noguchi M, Shimomura I. Contribution of Visceral Fat Accumulation and Adiponectin to the Clustering of Metabolic Abnormalities in a Japanese Population. J Atheroscler Thromb. 2014; 21: 543-553 [PubMed] [Google Scholar]
- 9). Ouchi N: Adipocytokines in Cardiovascular and Metabolic Diseases. J Atheroscler Thromb, 2016; 23: 645-654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Yasumura S, Hosoya M, Yamashita S, Kamiya K, Abe M, Akashi M, et al. Study protocol for the Fukushima Health Management Survey. J Epidemiol Jpn Epidemiol Assoc. 2012; 22: 375-383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). International guidelines for ethical review of epidemiological studies. Law Med Health Care Publ Am Soc Law Med. 1991; 19: 247-258 [PubMed] [Google Scholar]
- 12). Matsuzawa Y. Metabolic syndrome--definition and diagnostic criteria in Japan. J Atheroscler Thromb, 2005; 12: 301. [DOI] [PubMed] [Google Scholar]
- 13). Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT: The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA, 2002; 288: 2709-2716 [DOI] [PubMed] [Google Scholar]
- 14). Kondo T, Osugi S, Shimokata K, Honjo H, Morita Y, Yamashita K, Maeda K, Muramatsu T, Shintani S, Matsushita K, Murohara T: Metabolic syndrome and all-cause mortality, cardiac events, and cardiovascular events: a follow-up study in 25,471 young- and middle-aged Japanese men. Eur J Cardiovasc Prev Rehabil Off J Eur Soc Cardiol Work Groups Epidemiol Prev Card Rehabil Exerc Physiol. 2011; 18: 574-580 [DOI] [PubMed] [Google Scholar]
- 15). Nakamura T, Tsubono Y, Kameda-Takemura K, Funahashi T, Yamashita S, Hisamichi S, Kita T, Yamamura T, Matsuzawa Y, Group of the Research for the Association between Host Origin and Atherosclerotic Diseases under the Preventive Measure for Work-related Diseases of the Japanese Labor Ministry : Magnitude of sustained multiple risk factors for ischemic heart disease in Japanese employees: a case-control study. Jpn Circ J. 2001; 65: 11-17 [DOI] [PubMed] [Google Scholar]
- 16). Satoh H, Ohira T, Hosoya M, Sakai A, Watanabe T, Ohtsuru A, Kawasaki Y, Suzuki H, Takahashi A, Kobashi G, Ozasa K, Yasumura S, Yamashita S, Kamiya K, Abe M: Evacuation after the Fukushima Daiichi Nuclear Power Plant Accident Is a Cause of Diabetes: Results from the Fukushima Health Management Survey. J Diabetes Res, 2015; 2015: 627390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Kamoi K, Tanaka M, Ikarashi T, Miyakoshi M: Effect of the 2004 Mid Niigata Prefecture earthquake on glycemic control in type 1 diabetic patients. Diabetes Res Clin Pract. 2006; 74: 141-147 [DOI] [PubMed] [Google Scholar]
- 18). Fonseca VA, Smith H, Kuhadiya N, Leger SM, Yau CL, Reynolds K, Shi L, McDuffie RH, Thethi T, John-Kalarickal J: Impact of a natural disaster on diabetes: exacerbation of disparities and long-term consequences. Diabetes Care, 2009; 32: 1632-1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Minami J, Kawano Y, Ishimitsu T, Yoshimi H, Takishita S: Effect of the Hanshin-Awaji earthquake on home blood pressure in patients with essential hypertension. Am J Hypertens, 1997; 10: 222-225 [DOI] [PubMed] [Google Scholar]
- 20). Satoh M, Kikuya M, Ohkubo T, Imai Y. Acute and subacute effects of the great East Japan earthquake on home blood pressure values. Hypertension, 2011; 58: e193-194 [DOI] [PubMed] [Google Scholar]
- 21). Ogawa S, Ishiki M, Nako K, Okamura M, Senda M, Sakamoto T, Ito S: Effects of the Great East Japan Earthquake and huge tsunami on glycaemic control and blood pressure in patients with diabetes mellitus. BMJ Open, 2012; 2: e000830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Tanaka K, Nakayama M, Tani Y, Watanabe K, Asai J, Hayashi Y, Asahi K, Watanabe T: The great East Japan earthquake: blood pressure control in patients with chronic kidney disease. Am J Hypertens, 2012; 25: 951-954 [DOI] [PubMed] [Google Scholar]
- 23). Sun J, Huo J, Zhao L, Fu P, Wang L, Song P, Fang Z, Chang S, Yin S, Zhang J, Ma G: The nutritional status of young children and feeding practices two years after the Wenchuan Earthquake in the worst-affected areas in China. Asia Pac J Clin Nutr, 2013; 22: 100-108 [DOI] [PubMed] [Google Scholar]
- 24). Ayoya MA, Heidkamp R, Ngnie-Teta I, Pierre JM, Stoltzfus RJ. Child malnutrition in Haiti: progress despite disasters. Glob Health Sci Pract. 2013; 1: 389-396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Dong C, Ge P, Ren X, Zhao X, Fan H, Yin SA, Weiderpass E: Evaluating the micronutrient status of women of child-bearing age living in the rural disaster areas one year after Wenchuan Earthquake. Asia Pac J Clin Nutr, 2014; 23: 671-677 [DOI] [PubMed] [Google Scholar]
- 26). Ohira T, Hosoya M, Yasumura S, Satoh H, Suzuki H, Sakai A, Ohtsuru A, Kawasaki Y, Takahashi A, Ozasa K, Kobashi G, Kamiya K, Yamashita S, Abe M, Fukushima Health Management Survey Group : Effect of Evacuation on Body Weight After the Great East Japan Earthquake. Am J Prev Med, 2016; 50: 553-560 [DOI] [PubMed] [Google Scholar]
- 27). Tsubokura M, Takita M, Matsumura T, Hara K, Tanimoto T, Kobayashi K, Hamaki T, Oiso G, Kami M, Okawada T, Tachiya H: Changes in metabolic profiles after the Great East Japan Earthquake: a retrospective observational study. BMC Public Health, 2013; 13: 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Nagai M, Ohira T, Yasumura S, Takahashi H, Yuki M, Nakano H, Wen Z, Yabe H, Ohtsuru A, Maeda M, Takase K, Fukushima health Management Survey Group : Association between evacuation condition and habitual physical activity in Great East Japan Earthquake evacuees: The Fukushima Health Management Survey. Nihon Koshu Eisei Zasshi, 2016; 63: 3-10 [DOI] [PubMed] [Google Scholar]
- 29). Yabe H, Suzuki Y, Mashiko H, Nakayama Y, Hisata M, Niwa S, Yasumura S, Yamashita S, Kamiya K, Abe M, Mental Health Group of the Fukushima Health Management Survey : Psychological distress after the Great East Japan Earthquake and Fukushima Daiichi Nuclear Power Plant accident: results of a mental health and lifestyle survey through the Fukushima Health Management Survey in FY2011 and FY2012. Fukushima J Med Sci, 2014; 60: 57-67 [DOI] [PubMed] [Google Scholar]
- 30). Nakanishi N, Takatorige T, Suzuki K: Cigarette smoking and the risk of the metabolic syndrome in middle-aged Japanese male office workers. Ind Health, 2005; 4: 295-301 [DOI] [PubMed] [Google Scholar]
- 31). Chei CL, Yamagishi K, Tanigawa T, Kitamura A, Imano H, Kiyama M, Sato S, Iso H: Metabolic Syndrome and the Risk of Ischemic Heart Disease and Stroke among Middle-Aged Japanese. Hypertens Res, 2008; 31: 1887-1894 [DOI] [PubMed] [Google Scholar]
- 32). The 11th Prefectural Oversight Committee Meeting for Fukushima Health Management Survey | Fukushima Radiation and HealthFukushima Radiation and Health [Internet]. [cited 2015 Nov 1] Available from: http://fmu-global.jp/survey/proceedings-of-the-11th-prefectural-oversight-committee-meeting-for-fukushima-healthmanagement-survey/
- 33). Li M, Liu X, Zhang L, Scientific publications in public, environmental and occupational health journals by authors from China, Japan and Korea in East Asia : A 10-year literature survey from 2003 to 2012. Int J Occup Med Environ Health, 2015; 28: 663-673 [DOI] [PubMed] [Google Scholar]
- 34). Chang JH, Huang PT, Lin YK, Lin CE, Lin CM, Shieh YH, Lin YC: Association between sleep duration and sleep quality, and metabolic syndrome in Taiwanese police officers. Int J Occup Med Environ Health. 2015; 28: 1011-1023 [DOI] [PubMed] [Google Scholar]
- 35). Iftikhar IH, Donley MA, Mindel J, Pleister A, Soriano S, Magalang UJ: Sleep Duration and Metabolic Syndrome. An Updated Dose-Risk Metaanalysis. Ann Am Thorac Soc, 2015; 12:1364-1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). Chaput J-P, Després J-P, Bouchard C, Tremblay A: Short sleep duration preferentially increases abdominal adiposity in adults: preliminary evidence. Clin Obes, 2011; 1: 141-146 [DOI] [PubMed] [Google Scholar]
- 37). Fogelholm M, Kronholm E, Kukkonen-Harjula K, Partonen T, Partinen M, Härmä M: Sleep-related disturbances and physical inactivity are independently associated with obesity in adults. Int J Obes Int J Obes (Lond), 2007; 31: 1713-1721 [DOI] [PubMed] [Google Scholar]
- 38). Hairston KG, Bryer-Ash M, Norris JM, Haffner S, Bowden DW, Wagenknecht LE: Sleep duration and fiveyear abdominal fat accumulation in a minority cohort: the IRAS family study. Sleep, 2010; 33: 289-295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39). Patel SR, Blackwell T, Redline S, Ancoli-Israel S, Cauley JA, Hillier TA, Lewis CE, Orwoll ES, Stefanick ML, Taylor BC, Yaffe K, Stone KL, Osteoporotic Fractures in Men Research Group; Study of Osteoporotic Fractures Research Group : The association between sleep duration and obesity in older adults. Int J Obes (Lond), 2008; 32: 1825-1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Suzuki Y, Yabe H, Yasumura S, Ohira T, Niwa S, Ohtsuru A, Mashiko H, Maeda M, Abe M. Psychological distress and the perception of radiation risks: the Fukushima health management survey. Bull World Health Organ, 2015; 93: 598-605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Briançon-Marjollet A, Weiszenstein M, Henri M, Thomas A, Godin-Ribuot D, Polak J. The impact of sleep disorders on glucose metabolism: endocrine and molecular mechanisms. Diabetol Metab Syndr. 2015; 7: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). Keckeis M, Lattova Z, Maurovich-Horvat E, Beitinger PA, Birkmann S, Lauer CJ, Wetter TC, Wilde-Frenz J, Pollmächer T: Impaired glucose tolerance in sleep disorders. PloS One, 2010; 5: e9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43). Tuomilehto H, Peltonen M, Partinen M, Lavigne G, Eriksson JG, Herder C, Aunola S, Keinänen-Kiukaanniemi S, Ilanne-Parikka P, Uusitupa M, Tuomilehto J, Lindström J, Finnish Diabetes Prevention Study Group : Sleep duration, lifestyle intervention, and incidence of type 2 diabetes in impaired glucose tolerance: The Finnish Diabetes Prevention Study. Diabetes Care, 2009; 32: 1965-1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44). Meng L, Zheng Y, Hui R: The relationship of sleep duration and insomnia to risk of hypertension incidence: a meta-analysis of prospective cohort studies. Hypertens Res, 2013; 36: 985-995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45). Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D: Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension, 2006; 47: 833-839 [DOI] [PubMed] [Google Scholar]
- 46). Arcaya M, James P, Rhodes JE, Waters MC, Subramanian SV: Urban sprawl and body mass index among displaced Hurricane Katrina survivors. Prev Med, 2014; 65: 40-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47). United Nations Scientific Committee on the Effects of Atomic Radiation: UNSCEAR 2013 Report. Volume I: Report to the General Assembly, Annex A: Levels and effects of radiation exposure due to the nuclear accident after the 2011 great east-Japan earthquake and tsunami. United Nations Scientific Committee on the Effects of Atomic Radiation; New York: United Nations, 2014 [Google Scholar]
- 48). Shen J, Wilmot KA, Ghasemzadeh N, Molloy DL, Burkman G, Mekonnen G, Gongora MC, Quyyumi AA, Sperling LS: Mediterranean Dietary Patterns and Cardiovascu lar Health. Annu Rev Nutr, 2015 17; 35: 425-449 [DOI] [PubMed] [Google Scholar]
- 49). Nagasawa Y, Yamamoto R, Shinzawa M, Hasuike Y, Kuragano T, Isaka Y, Nakanishi T, Iseki K, Yamagata K, Tsuruya K, Yoshida H, Fujimoto S, Asahi K, Moriyama T, Watanabe T: Body Mass Index Modifies an Association between Self-Reported Regular Exercise and Proteinuria. J Atheroscler Thromb, 2016; 23: 402-412 [DOI] [PubMed] [Google Scholar]
- 50). Wada T, Fukumoto T, Ito K, Hasegawa Y, Osaki T, Ban H: Of the Three Classifications of Healthy Lifestyle Habits, Which One is the Most Closely Associated with the Prevention of Metabolic Syndrome in Japanese? Intern Med, 2009; 48: 647-655 [DOI] [PubMed] [Google Scholar]


