Abstract
Aim: The Japan Atherosclerosis Society (JAS) guidelines for the prevention of atherosclerotic diseases 2012 (JAS2012) proposed lipid management targets; however, less data is available regarding the attainment rates of each target in community-based settings. Therefore, we assessed the attainment rates of lipid management targets among subjects who underwent Japanese specific health checkups.
Methods: A total of 85,716 subjects (male = 29,282, 34.2%) aged 40–74 years who underwent specific health checkups from 2012 to 2014 in Kanazawa city, Japan, were included in this study. We evaluated the attainment rates of the lipid management targets according to the JAS2012 guideline and investigated the clinical characteristics of the subjects without achieving the targets.
Results: The target for LDL cholesterol (LDL-C) was the least attained in all risk categories, 89, 72, 50, and 34% for category I, II, III, and secondary prevention, respectively, in 2014. In addition, these rates inversely correlated with the grade of risk categories (p-value for trends <0.001). Attainment rate of the LDL-C target in the suspected chronic kidney disease (CKD) group was significantly lower than in the groups with diabetes, stroke, or absolute risk in category III (49.2, 60.3, 63.5, 54.4%, respectively, p-value <0.001 for each). Moreover, the attainment rate of the LDL-C target was significantly lower in subjects that did not receive lipid-lowering therapy than in those who received it in the secondary prevention (27.7 and 40.6%, respectively, p-value <0.001).
Conclusions: Lipid management is inadequate in community-based settings, particularly, in subjects with CKD and secondary prevention.
Keywords: JAS guideline 2012, LDL cholesterol, Specific health checkup
Introduction
The Japan Atherosclerosis Society (JAS) has proposed guidelines for the prevention of atherosclerotic diseases in 2012 (JAS 2012), including lipid management, dividing the subjects into four categories based on the absolute cardiovascular disease risk1). JAS2012 has contributed to cardiovascular disease prevention in subjects with primary prevention as well as with secondary prevention. Several studies have shown that the attainment rates of lipid management targets were up to 50% in hospital-based settings2, 3). However, not much data is available regarding attainment rates in community-based settings. Therefore, we aimed to assess the attainment rates of lipid management targets determined by JAS2012 among subjects who underwent specific health checkups initiated in community-based medical checkups in Japan.
Methods
Study Subjects
A total of 85,716 subjects (men = 29,282, 34.2%) aged 40–74 years who underwent specific health checkups from 2012 to 2014 in Kanazawa city, Japan, and who had no missing data were included in this study. Most of the subjects visited general practitioners in clinics in Kanazawa city. All data were collected and anonymized by the Kanazawa Medical Association.
Ethical Considerations
This study was approved by the Ethics Committee of Kanazawa Medical Association and Kanazawa University and carried out in accordance with the Declaration of Helsinki (2008) of the World Medical Association. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all subjects for being included in the study.
Data collection in Specific Medical Checkup
Eligible participants visited a clinic and responded to a questionnaire regarding past history of stroke, cardiac disease, kidney disease, lifestyle habits, such as smoking, alcohol intake, walking, and medications for hypertension, diabetes, and dyslipidemia. Measurements included standard medical checks, such as measurement of height, weight, waist circumference, blood pressure, fasting blood glucose, hemoglobin A1c, total cholesterol, triglyceride, serum highdensity lipoprotein (HDL) cholesterol (HDL-C), lowdensity lipoprotein (LDL) cholesterol (LDL-C), serum creatinine, dipstick urine test for proteinuria, hematuria, and urinary sugar. Proteinuria was coded as negative, plus and minus, 1 plus, 2 plus, and 3 plus. Serum creatinine levels were measured using the enzymatic method. Glomerular filtration rate was calculated using the formula of the Japanese Society of Nephrology4). Hypertension was defined as a blood pressure of ≥ 140/90 mmHg or if the subject was receiving hypotensive medication. Diabetes was defined as the patient having 1) hemoglobin A1c ≥ 6.5% and blood glucose ≥ 126 at fasting, 2) hemoglobin A1c ≥ 6.5% and blood glucose ≥ 200 at non-fasting, or 3) hypoglycemic medication. The presence of suspected chronic kidney disease (CKD) was defined according to the CKD classification based on the combination of estimated glomerular filtration rate (eGFR) and dipstick proteinuria findings5). We could not definitively determine the presence of CKD based on our cross-sectional design, although the definition of CKD includes a situation where kidney injury continues for over 3 months. Accordingly, we investigated the proportion of subjects fulfilling this CKD criteria at least twice for the subjects suspected as having CKD in 2012. Of these, only the subjects whose follow-up data were available either in 2013 or in 2014 were included to validate our inclusion criteria as CKD.
Risk Assessments and Categorization According to JAS Guideline 2012
Next, we classified subjects into four categories according to the JAS2012. The methodology of the risk classification procedure according to the JAS2012 has been described previously1). Briefly, subjects were assessed if they were secondary prevention cases with any histories of coronary artery disease (CAD). If not, then they were assigned to risk category III when the following criteria were fulfilled: (1) diabetes, (2) suspected CKD, and (3) non-cardiogenic cerebral infarction. After these assignments, remaining subjects were assigned to risk categories I, II, and III based on the absolute risk of CAD death estimated by NIPPON-DATA 806) and on the existence of an additional risk factor (hypo-HDL cholesterolemia).
Evaluations
We evaluated the attainment rates of the lipid management targets according to the JAS2012 guidelines for each risk category and compared these rates among each category. We employed LDL-C values calculated using the Friedewald formula if the measurements were of fasting and TG < 400 mg/dl. Otherwise, we used LDL-C values directory measured. In addition, we investigated the trends of those attainment rates from 2012 to 2014. Furthermore, we investigated the characteristics of the subjects who did not attain the LDL-C target.
Statistical Analysis
Jonckheere-Terpstra trend test was used to assess the trends of the attainment rates among each category. The chi-squared test was used to compare the attainment rates over time. P-value < 0.05 was considered statistically significant, and all tests were two-tailed. All analyses were performed with R statistical software.
Results
Characteristics of Study Subjects
Clinical characteristics of study subjects are shown in Tables 1–3. As expected, category I comprises younger subjects than present in any other categories (p-value <0.001). In addition, we observed significant trends in category I, II, III, and secondary prevention for other basic characteristics, such as, sex (the proportion of male = 17.6, 28.1, 46.1, and 52.4%, respectively, in 2014, p-value for trend < 0.001), body mass index (mean = 21.9, 22.4, 23.4, and 23.4 kg/m2, respectively, in 2014, p-value for trend < 0.001), and waist circumference (mean = 78.9, 81.9, 84.5, and 84.9 cm, respectively, in 2014, p-value for trend < 0.001). As much as a quarter of the subjects were classified as having suspected CKD.
Table 1. Characteristics of the study subjects (2012).
| All (N = 27,370) |
Category I (N = 3,761) |
Category II (N = 12,144) |
Category III (N = 9,384) |
Secondary prevention (N = 2,081) |
|
|---|---|---|---|---|---|
| Age (yr) | 64.3 ± 8.3 | 50.1 ± 6.2 | 67.2 ± 4.5 | 65.5 ± 7.4 | 68.0 ± 5.3 |
| Male (%) | 9312 (34.0) | 640 (17.0) | 3,419 (28.2) | 4,191 (44.6) | 1,062 (51.0) |
| Body mass index (kg/m2) | 22.8 ± 3.3 | 22.0 ± 3.4 | 22.5 ± 3.0 | 23.3 ± 3.5 | 23.5 ± 3.4 |
| Waist circumference (cm) | 82.5 ± 9.4 | 79.1 ± 9.5 | 81.9 ± 8.9 | 84.0 ± 9.7 | 85.2 ± 9.3 |
| Total cholesterol (mg/dl) | 202.2 ± 33.3 | 203.9 ± 33.7 | 205.7 ± 31.4 | 199.5 ± 34.6 | 191.4 ± 33.1 |
| Triglyceride (mg/dl) | 122.4 ± 82.4 | 107.6 ± 86.1 | 116.3 ± 67.2 | 134.3 ± 95.4 | 131.1 ± 85.7 |
| HDL cholesterol (mg/dl) | 60.2 ± 15.1 | 66.1 ± 14.8 | 61.9 ± 14.0 | 56.5 ± 15.5 | 56.3 ± 13.4 |
| LDL cholesterol (mg/dl) | 119.0 ± 29.8 | 118.5 ± 31.0 | 121.2 ± 28.7 | 118.1 ± 30.8 | 111.2 ± 28.6 |
| Smoking (%) | 3,366 (12.3) | 544 (14.5) | 1,189 (9.8) | 1,380 (14.7) | 253 (12.2) |
| Suspected CKD (%) | 6,927 (25.3) | 0 (0) | 0 (0) | 6,150 (65.5) | 777 (37.3) |
| Stroke (%) | 1,263 (4.6) | 0 (0) | 0 (0) | 1,015 (10.8) | 248 (11.9) |
| Coronary artery disease (%) | 2,081 (7.6) | 0 (0) | 0 (0) | 0 (0) | 2,081 (100) |
| Hypertension (%) | 9,272 (33.9) | 395 (10.5) | 3,860 (31.8) | 3,912 (41.7) | 1,105 (53.1) |
| Diabetes (%) | 2,512 (9.2) | 0 (0) | 0 (0) | 2,189 (23.3) | 323 (15.5) |
| Lipid-lowering therapy (%) | 6,504 (23.8) | 348 (9.3) | 3,086 (25.4) | 2,394 (25.5) | 676 (32.5) |
| Hypertriglyceridemia (%) | 6,410 (23.4) | 615 (16.4) | 2,476 (20.4) | 2,745 (26.4) | 574 (27.6) |
| Low HDL cholesterolemia (%) | 1,726 (6.3) | 0 (0) | 133 (0.1) | 1,373 (14.6) | 220 (10.6) |
| Hypertriglyceridemia and low HDL cholesterolemia (%) | 1,082 (4.0) | 0 (0) | 95 (0.1) | 860 (9.2) | 127 (6.1) |
CKD: Chronic kidney disease, Hypertriglyceridemia was defined as triglyceride ≥ 150 mg/dl. Low HDL cholesterolemia was defined as HDL cholesterol <40 mg/dl
Table 3. Characteristics of the study subjects (2014).
| All (N = 29,344) |
Category I (N = 4,213) |
Category II (N = 12,911) |
Category III (N = 10,038) |
Secondary prevention (N = 2,182) |
|
|---|---|---|---|---|---|
| Age (yr) | 64.5 ± 8.5 | 49.9 ± 6.1 | 67.6 ± 4.5 | 66.0 ± 7.5 | 68.3 ± 5.6 |
| Male (%) | 10,132 (34.5) | 742 (17.6) | 3,627 (28.1) | 4,619 (46.1) | 1,144 (52.4) |
| Body mass index (kg/m2) | 22.7 ± 3.4 | 21.9 ± 3.5 | 22.4 ± 3.1 | 23.4 ± 3.6 | 23.4 ± 3.5 |
| Waist circumference (cm) | 82.6 ± 9.6 | 78.9 ± 9.6 | 81.9 ± 9.0 | 84.5 ± 9.9 | 84.9 ± 9.8 |
| Total cholesterol (mg/dl) | 201.5 ± 34.1 | 202.3 ± 33.9 | 204.1 ± 33.0 | 200.8 ± 35.0 | 190.3 ± 33.0 |
| Triglyceride (mg/dl) | 120.0 ± 80.7 | 103.3 ± 69.3 | 115.3 ± 68.9 | 131.3 ± 92.5 | 128.1 ± 99.6 |
| HDL cholesterol (mg/dl) | 62.2 ± 15.7 | 67.6 ± 15.3 | 63.8 ± 14.7 | 58.7 ± 16.0 | 58.1 ± 16.0 |
| LDL cholesterol (mg/dl) | 122.0 ± 30.8 | 120.5 ± 31.3 | 124.3 ± 29.6 | 121.6 ± 31.9 | 113.1 ± 29.9 |
| Smoking (%) | 3,616 (12.3) | 618 (14.7) | 1,279 (9.9) | 1,460 (14.5) | 259 (11.9) |
| Suspected CKD (%) | 7,486 (25.5) | 0 (0) | 0 (0) | 6,681 (66.6) | 805 (36.9) |
| Stroke (%) | 1,396 (4.8) | 0 (0) | 0 (0) | 1,104 (11.0) | 292 (13.4) |
| Coronary artery disease (%) | 2,182 (7.4) | 0 (0) | 0 (0) | 0 (0) | 2,182 (100) |
| Hypertension (%) | 10,047 (34.2) | 438 (10.4) | 4,221 (32.7) | 4,198 (41.8) | 1,190 (54.5) |
| Diabetes (%) | 2,812 (9.6) | 0 (0) | 0 (0) | 2,443 (24.3) | 369 (16.9) |
| Lipid-lowering therapy (%) | 7,163 (24.4) | 358 (8.5) | 3,431 (26.6) | 2,680 (26.7) | 694 (31.8) |
| Hypertriglyceridemia (%) | 6,662 (22.7) | 672 (15.9) | 2,575 (19.9) | 2,828 (28.2) | 587 (26.9) |
| Low HDL cholesterolemia (%) | 1,462 (5.0) | 0 (0) | 115 (0.9) | 1,129 (11.2) | 218 (10.0) |
| Hypertriglyceridemia and low HDL cholesterolemia (%) | 930 (3.2) | 0 (0) | 83 (0.6) | 724 (7.2) | 123 (9.6) |
CKD: Chronic kidney disease, Hypertriglyceridemia was defined as triglyceride ≥ 150 mg/dl. Low HDL cholesterolemia was defined as HDL cholesterol <40 mg/dl
Table 2. Characteristics of the study subjects (2013).
| All (N = 29,002) |
Category I (N = 4,177) |
Category II (N = 13,819) |
Category III (N = 8,878) |
Secondary prevention (N = 2,128) |
|
|---|---|---|---|---|---|
| Age (yr) | 64.3 ± 8.5 | 50.0 ± 6.2 | 67.3 ± 4.5 | 65.4 ± 7.9 | 68.2 ± 5.3 |
| Male (%) | 9,838 (33.9) | 740 (17.7) | 4,222 (30.6) | 3,770 (42.5) | 1,106 (52.0) |
| Body mass index (kg/m2) | 22.7 ± 3.4 | 21.9 ± 3.6 | 22.5 ± 3.2 | 23.2 ± 3.5 | 23.5 ± 3.5 |
| Waist circumference (cm) | 82.5 ± 9.6 | 79.0 ± 9.7 | 82.2 ± 9.1 | 83.9 ± 9.7 | 85.3 ± 9.6 |
| Total cholesterol (mg/dl) | 201.1 ± 33.5 | 201.9 ± 33.0 | 203.0 ± 32.1 | 200.1 ± 35.2 | 189.1 ± 33.0 |
| Triglyceride (mg/dl) | 118.5 ± 78.8 | 99.5 ± 66.0 | 114.1 ± 67.4 | 131.8 ± 95.5 | 128.4 ± 81.4 |
| HDL cholesterol (mg/dl) | 61.1 ± 15.5 | 66.6 ± 14.0 | 62.3 ± 14.3 | 57.5 ± 16.4 | 56.8 ± 15.4 |
| LDL cholesterol (mg/dl) | 120.3 ± 30.3 | 119.8 ± 31.1 | 121.8 ± 29.5 | 120.5 ± 30.9 | 111.1 ± 29.5 |
| Smoking (%) | 3,605 (12.4) | 602 (14.4) | 1,431 (10.4) | 1,299 (14.6) | 273 (12.8) |
| Suspected CKD (%) | 7,715 (26.6) | 0 (0) | 0 (0) | 6,903 (77.8) | 812 (38.2) |
| Stroke (%) | 1,349 (4.7) | 0 (0) | 0 (0) | 1,079 (12.2) | 267 (12.5) |
| Coronary artery disease (%) | 2,128 (7.3) | 0 (0) | 0 (0) | 0 (0) | 2,128 (100) |
| Hypertension (%) | 9,775 (33.7) | 416 (10.0) | 4,583 (33.2) | 3,600 (40.5) | 1,176 (55.3) |
| Diabetes (%) | 2,664 (9.2) | 0 (0) | 0 (0) | 1,024 (11.5) | 331 (15.6) |
| Lipid-lowering therapy (%) | 6,848 (23.6) | 380 (9.1) | 3,655 (26.4) | 2,157 (24.3) | 656 (30.8) |
| Hypertriglyceridemia (%) | 6,375 (22.5) | 584 (14.0) | 2,721 (19.7) | 2,513 (28.3) | 557 (26.2) |
| Low HDL cholesterolemia (%) | 1,709 (5.9) | 0 (0) | 159 (1.2) | 1,318 (14.8) | 232 (11.0) |
| Hypertriglyceridemia and low HDL cholesterolemia (%) | 1,041 (3.6) | 0 (0) | 107 (0.8) | 792 (8.9) | 142 (6.7) |
CKD: Chronic kidney disease, Hypertriglyceridemia was defined as triglyceride ≥ 150 mg/dl. Low HDL cholesterolemia was defined as HDL cholesterol <40 mg/dl
Attainment Rates for Lipid Management Targets
Attainment rates for lipid management targets are illustrated in Fig. 1–3. In 2012, when the current guideline was introduced, the attainment rate of all four lipid management targets (LDL-C, TG, HDL-C, and non-HDL-C) for all the study subjects was 51.5%. When divided into four categories based on JAS2012, an inverse correlation was observed in the attainment rates of all four lipid management targets according to the category (76.3, 60.7, 35.8, and 24.2%, in category I, II, III, and secondary prevention, respectively, p-value for trend < 0.001). Among the four different targets, LDL-C management target was the least attained (67.3%). Similar to the overall trend, an inverse correlation was observed in the attainment rate of LDL-C management target according to the category (90.4, 75.7, 54.3, and 35.2%, in category I, II, III, and secondary prevention, respectively, p-value for trend < 0.001). In 2013, the overall attainment rate of all the four lipid management targets was 52.0%. An inverse correlation was observed in the attainment rates of all the four lipid management targets according to the category (78.3, 60.0, 33.6, and 25.0%, in category I, II, III, and secondary prevention, respectively, p-value for trend < 0.001) in 2012. Further, an inverse correlation was observed in the attainment rates of LDL-C management target according to the category (89.6, 74.3, 51.6, and 36.1, in category I, II, III, and secondary prevention, respectively, p-value for trend < 0.001). In 2014, we observed the same overall trends (75.9, 57.3, 33.5, and 23.2%, in category I, II, III, and secondary prevention, respectively, p-value for trend < 0.001), as well as in LDL-C (89.1, 71.5, 50.0, and 34.3%, in category I, II, III, and secondary prevention, respectively, p-value for trend < 0.001). We could not observe any significant increases in attainment rates in all of lipid management targets (LDL-C, triglyceride, and HDL-C), from 2012 to 2014.
Fig. 1.
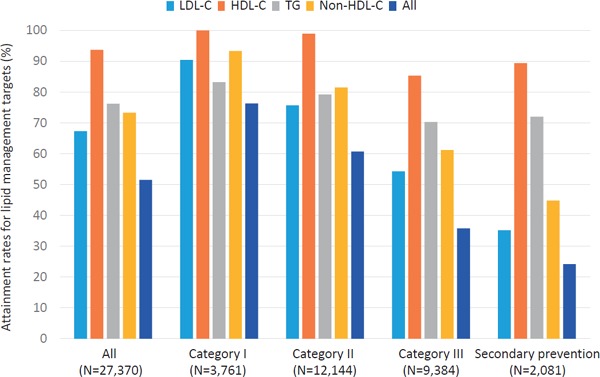
Attainment rates for lipid management targets (2012)
Light blue: LDL-C, Orange: HDL-C, Gray: TG, Yellow: Non-HDL-C, Blue: All
Fig. 3.
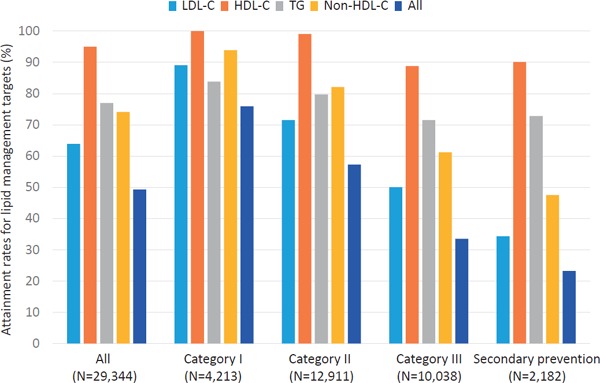
Attainment rates for lipid management targets (2014)
Light blue: LDL-C, Orange: HDL-C, Gray: TG, Yellow: Non-HDL-C, Blue: All
Fig. 2.
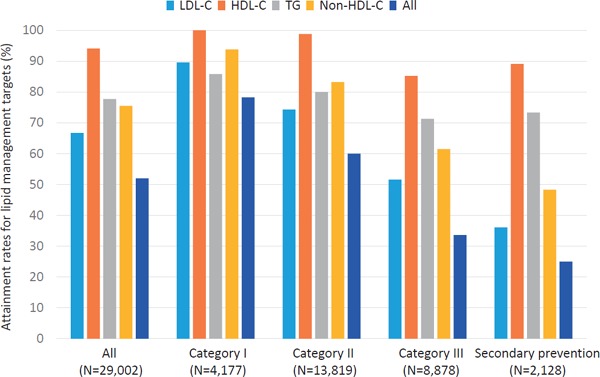
Attainment rates for lipid management targets (2013)
Light blue: LDL-C, Orange: HDL-C, Gray: TG, Yellow: Non-HDL-C, Blue: All
Characteristics of the Subjects that did not Achieve the Targets in Category III and Secondary Prevention Cases
Next, we investigated the characteristics of the subjects who did not attain the management targets, particularly, LDL-C targets using data in 2014 that were the latest and the largest. In category III, the highest risk category in the primary prevention, as many as 5,018 subjects (50.0%) did not attain the LDL-C management target. Among them, as many as 3,392 (67.6%) subjects exhibited CKD (Table 4). The attainment rate of the LDL-C target in the suspected CKD group was significantly lower than in the group with diabetes, stroke, or absolute risk in category III (49.2% vs. 60.3, 63.5, and 54.4%, respectively, p-value < 0.001 for each comparison, Table 4). Moreover, the attainment rate of the LDL-C target was significantly lower in subjects who did not receive lipid-lowering therapy than in those who did as the secondary prevention (27.7 and 40.6%, respectively, p-value < 0.001, Table 5).
Table 4. Comparison of attainment rates of the LDL-C target among risks in category III.
| All (N) | Attained LDL-C target (N, %) |
Did not attain LDL-C target (N, %) |
p-value | |
|---|---|---|---|---|
| Suspected CKD | 6,681 | 3,289 (49.2) | 3,392 (50.8) | reference |
| Diabetes | 2,443 | 1,472 (60.3) | 972 (39.7) | < 0.001 |
| Stroke | 1,104 | 701 (63.5) | 403 (36.5) | < 0.001 |
| Absolute risk | 3,204 | 1,744 (54.4) | 1,460 (45.6) | < 0.001 |
CKD: Chronic kidney disease, LDL-C: LDL cholesterol
Table 5. Comparison of attainment rates of the LDL-C target according to lipid-lowering therapy in secondary prevention cases.
| All (N = 2,182) |
Attained LDL-C target (N, %) |
Did not attain LDL-C target (N, %) |
p-value | |
|---|---|---|---|---|
| On lipid-lowering therapy | 694 | 282 (40.6) | 467 (59.4) | reference |
| No lipid-lowering therapy | 1,488 | 412 (27.7) | 1,021 (68.6) | < 0.001 |
LDL-C: LDL cholesterol
Validation of Suspected CKD as a Substitute for CKD
Among the 6,927 subjects suspected of having CKD in 2012, 4,577 (66.1%) subjects were followed-up either in 2013 or in 2014. We found that 4,236 (92.5%) fulfilled the criteria of CKD diagnosis.
Discussion
Using a large dataset from the community-based specific medical check-ups, we found that 1) the attainment rates of all the four lipid management targets were approximately 50%, 2) the targets of LDL-C were overall the least attained (65%), 3) there were significant inverse trends in the attainment rate of LDL-C management target according to the category, 4) the attainment rate of the LDL-C target in the group with CKD was significantly lower than in the group with diabetes, stroke, or absolute risk in category III, 5) the attainment rate of the LDL-C target was significantly lower in subjects who did not receive lipid-lowering therapy than in those who did as the secondary prevention, and 6) such trends did not change from 2012 to 2014.
In 1992, JAS proposed the initial guideline for the diagnosis and treatment of hyperlipidemia in adults, which is revised every 5 years. These revisions have adopted a number of updated clinical interventional trials and observational cohort studies7–9). On the other hand, only little data investigating the attainment rates of target established by such guidelines are available, most of which are from hospital-based settings2, 3). However, a larger proportion of Japanese population is treated by general practitioners in the community; thus, it is vital to expand such studies to community-based settings. Since 2008, community-based medical checkups named “specific health checkups” have been started in Japan considering our aging society and increases in lifestyle-related diseases. Specific health checkups have great advantages such as financial support provided from the government to a large number of participants and the uniformity of the investigated items10, 11). In this study, we used the dataset collected in Kanazawa city, Japan, which has a population of approximately 500,000.
The proportions of the subjects who attained the LDL-C targets seem to be lower than those previously investigated in hospital-based settings. It is reasonable to see such differences, since the subjects who are treated in hospitals should have much more complications than those treated in clinics in communities, leading to their more intensive therapies.
It is of note that the subjects with suspected CKD, classified into category III, were quite undertreated, although known to be highly associated with CAD12), and that the majority of secondary prevention cases without achieving LDL-C target did not receive any lipid-lowering therapies. Furthermore, such trends unchanged during the study period despite of the intensive effort of enlightenment of this guideline by JAS, at least in community-based settings. It seems that general practitioners in the community tended to treat patients aiming for the normal range (LDL-C = 120 to 140 mg/dl), rather than to attain the targets determined by the guideline. The concept of “the lower, the better” in LDL-C level has been widely accepted, and such a situation will be achievable using new drugs, such as proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor13). However, awareness itself to attain the current target should be the first step in the prevention of CAD.
This study has several limitations. First, this study investigated the population living in Kanazawa area, not using nation-wide dataset, which could potentially affect the results. However, we believe that large sample size could dilute such bias. Second, information of peripheral artery disease, family history of premature CAD, and impaired glucose tolerance are lacking in our data, which could potentially underestimate the risk category. However, such underestimations of risk category do not likely to reduce the proportions of undertreated subjects in category III nor in secondary prevention cases. Third, the definition of diabetes includes only “definite” diabetes, leaving the possibility of missing subjects who require oral glucose tolerance and other diagnostic tests. In addition, we could not determine definite CKD because of the cross-sectional nature of the study design. However, we found that most subjects suspected as having CKD eventually fulfilled the criteria of CKD, and thus, the essential conclusions of this study would not change. Fourth, a fair proportion of subjects exhibited hyper LDL-cholesterolemia ≥ 180 mg/dl (∼4% of the subjects), among whom there should be some patients with familial hypercholesterolemia (FH) whose lipid management targets are set more strictly. In this regard, we need to consider special notification of possibility for FH in the reports, since the prevalence of FH in general population has been shown to be more frequent than previously considered14). Fourth, large proportions of individuals were receiving checkups regularly (17,734 among 29,002 [61%] individuals were overlapped between 2012 and 2013, and 18,298 among 29,334 [62%] individuals were overlapped between 2013 and 2014), which could lead some potential bias. However, this fact rather makes us confident that the attainment rates of lipid management target unchanged during these periods. Finally, detailed information regarding lipid-lowering therapies is lacking in this study. However, we want to emphasize the fact that high-risk patients in category III or in secondary prevention group are not treated adequately, instead of the way of treatment.
In conclusion, lipid management is inadequate in the community-based settings, especially in the subjects with CKD and secondary prevention cases.
Acknowledgments
We express special thanks to Mr. Yoshitaka Sakikawa (Kanazawa Medical Association) for his assistance of data management.
Conflict of Interest Statement
None.
Source of Finding
A part of this study was supported by a scientific research grant from the Mochida Memorial Foundation.
References
- 1). Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, Egusa G, Hiro T, Hirobe K, Iida M, Kihara S, Kinoshita M, Maruyama C, Ohta T, Okamura T, Yamashita S, Yokode M, Yokote K, Japan Atherosclerosis Society : Executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan -2012 version. J Atheroscler Thromb, 2013; 20: 517-523 [DOI] [PubMed] [Google Scholar]
- 2). Teramoto T, Kashiwagi A, Ishibashi S, Daida H, Japan Lipid Guideline Attainment Program Investigators : Cross-sectional survey to assess the status of lipid management in high-risk patients with dyslipidemia: clinical impact of combination therapy with ezetimibe. Curr Ther Res Clin Exp, 2012; 73: 1-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Teramoto T, Kawamori R, Miyazaki S, Teramukai S, Sato Y, Okuda Y, Shirayama M: Lipid and blood pressure control for the prevention of cardiovascular disease in hypertensive patients: a subanalysis of the OMEGA study. J Atheroscler Thromb, 2015; 22: 62-75 [DOI] [PubMed] [Google Scholar]
- 4). Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, Collaborators developing the Japanese equation for estimated GFR : Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis, 2009; 53: 982-992 [DOI] [PubMed] [Google Scholar]
- 5). Chronic Kidney Disease Prognosis Consortium: Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet, 2010; 375: 2073-2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Okamura T, Tanaka H, Miyamatsu N, Hayakawa T, Kadowaki T, Kita Y, Nakamura Y, Okayama A. Ueshima H NIPPON DATA80 Research Group: The relationship between serum total cholesterol and all-cause or causespecific mortality in a 17.3-year study of a Japanese cohort. Atherosclerosis, 2007; 190: 216-223 [DOI] [PubMed] [Google Scholar]
- 7). Mabuchi H, Kita T, Matsuzaki M, Matsuzawa Y, Nakaya N, Oikawa S, Saito Y, Sasaki J, Shimamoto K, Itakura H, J-LIT Study Group : Japan Lipid Intervention Trial. Large scale cohort study of the relationship between serum cholesterol concentration and coronary events with low-dose simvastatin therapy in Japanese patients with hypercholesterolemia and coronary heart disease: secondary prevention cohort study of the Japan Lipid Intervention Trial (J-LIT). Circ J, 2002; 66: 1096-1100 [DOI] [PubMed] [Google Scholar]
- 8). Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y, Toyota T, Nakaya N, Nishimoto S, Muranaka M, Yamamoto A, Mizuno K, Ohashi Y, MEGA Study Group : Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet, 2006; 368: 1155-1163 [DOI] [PubMed] [Google Scholar]
- 9). Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K, Japan EPA lipid intervention study (JELIS) Investigators : Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet, 2007; 369: 1090-1098 [DOI] [PubMed] [Google Scholar]
- 10). Wakasugi M, Kazama JJ, Narita I, Iseki K, Moriyama T, Yamagata K, Fujimoto S, Tsuruya K, Asahi K, Konta T, Kimura K, Kondo M, Kurahashi I, Ohashi Y, Watanabe T: Association between combined lifestyle factors and nonrestorative sleep in Japan: a cross-sectional study based on a Japanese health database. PLoS One, 2014; 9: e108718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Tsuruya K, Yoshida H, Nagata M, Kitazono T, Hirakata H, Iseki K, Moriyama T, Yamagata K, Yoshida H, Fujimoto S, Asahi K, Kurahashi I, Ohashi Y, Watanabe T: Association of the triglycerides to high-density lipoprotein cholesterol ratio with the risk of chronic kidney disease: analysis in a large Japanese population. Atherosclerosis, 2014; 233: 260-267 [DOI] [PubMed] [Google Scholar]
- 12). Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW, American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention : Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation, 2003; 108: 2154-2169 [DOI] [PubMed] [Google Scholar]
- 13). Hopkins PN, Defesche J, Fouchier SW, Bruckert E, Luc G, Cariou B, Sjouke B, Leren TP, Harada-Shiba M, Mabuchi H, Rabís JP, Carrié A, van Heyningen C, Carreau V, Farnier M, Teoh YP, Bourbon M, Kawashiri MA, Nohara A, Soran H, Marais AD, Tada H, Abifadel M, Boileau C, Chanu B, Katsuda S, Kishimoto I, Lambert G, Makino H, Miyamoto Y, Pichelin M, Yagi K, Yamagishi M, Zair Y, Mellis S, Yancopoulos GD, Stahl N, Mendoza J, Du Y, Hamon S, Krempf M, Swergold GD: Characterization of Autosomal Dominant Hypercholesterolemia Caused by PCSK9 Gain of Function Mutations and Its Specific Treatment With Alirocumab, a PCSK9 Monoclonal Antibody. Circ Cardiovasc Genet, 2015; 8: 823-831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Mabuchi H, Nohara A, Noguchi T, Kobayashi J, Kawashiri MA, Tada H, Nakanishi C, Mori M, Yamagishi M, Inazu A, Koizumi J, Hokuriku FH Study Group : Molecular genetic epidemiology of homozygous familial hypercholesterolemia in the Hokuriku district of Japan. Atherosclerosis, 2011; 214: 404-407 [DOI] [PubMed] [Google Scholar]


