Abstract
Human serum microRNAs (miRNAs) have been shown to serve as disease fingerprints for predicting survival of cancer patients. However, the roles of specific miRNAs involved in gastric cancer (GC) are largely unknown. In this study, miRNA profiling was performed on sera obtained from six patients in good- and poor-survival groups. Expression of miR-423-3p was validated by quantitative RT-PCR in another 67 GC serum samples and paired normal and cancerous gastric tissues. Luciferase reporter assays were used to identify the target gene Bcl-2-interacting mediator of cell death (Bim). As a result, between the good-survival and poor-survival groups, the expression of nine serum miRNAs was altered more than two-fold. Among these, miR-423-3p was significantly increased in the poor-survival group, and its overexpression in GC tissues predicted poor survival in 119 patients with GC. miR-423-3p was found to promote cell proliferation, migration, and invasion in cell lines and animal models. Mechanistically, knockdown of the autophagy-related gene (Atg) 7 rescued the GC-promoting effect of miR-423-3p. In conclusion, miR-423-3p activates oncogenic and Beclin-1-dependent autophagy and promotes GC progression by reducing the expression of Bim. The newly identified miR-423-3p-Bim axis might be a potential therapeutic target in GC.
Keywords: microRNA-423-3p, Bim, autophagy, gastric cancer
Xu et al. performed genome-wide serum miRNA expression analysis and discovered and validated gastric cancer-specific miR-423-3p. Moreover, miR-423-3p activates oncogenic autophagy and promotes GC progression by reducing expression of Bim. The newly identified miR-423-3p-Bim axis might be a potential therapeutic target in GC.
Introduction
Gastric cancer (GC) is the fourth most common solid cancer and the third leading cause of cancer mortality worldwide.1 Despite its declining incidence and improved surgical and adjuvant treatment approaches, the 5-year survival rate of GC remains very low.2 Further understanding of molecular mechanisms in GC is urgently needed for the development of novel diagnostic or therapeutic targets.
Autophagy is a highly conserved cellular catabolic process delivering long-lived proteins and damaged organelles to lysosomes for degradation.3 It has functions in multiple normal cellular mechanisms, including infection, stress, and cell death.4, 5, 6, 7 It is generally believed that autophagy plays a tumor-suppressing role in the early stages of tumor development, protecting cells from oxidative stress and harmful genomic mutations.8, 9 During tumor growth and progression, autophagy can allow cells to obtain nutrients required by many cancers.10, 11
MicroRNAs (miRNAs) were first discovered as small non-coding RNAs that post-transcriptionally suppress translation or induce mRNA degradation by targeting the 3′ UTRs of target genes.12 There is compelling evidence that they also act as tumor promoters or suppressors, regulating the progression and metastasis of cancers.13, 14 Some miRNAs have also been reported to be involved in autophagy regulation in cancer cells through regulation of autophagy-related genes.15, 16 However, the relationship between dysregulated miRNAs and autophagy modulation in GC remains unclear.
In this study, we screened serum miRNAs from six gastric cancer patients with different prognoses by using GeneChip miRNA to identify a comprehensive set of GC-specific miRNAs, including previously unrecognized miRNAs. miR-423-3p expression was significantly downregulated in the sera of GC patients with good survival compared with those with poor survival. We systematically validated miR-423-3p expression in gastric cancer tissues and its functions in cell lines and animal models. We discovered that the miR-423-3p-Bcl-2-interacting mediator of cell death (Bim) axis plays a predominant role in GC promotion through oncogenic autophagy. Our study provides new insights into the role of dysregulated miRNA in autophagy regulation and the possibility of targeting miRNAs and autophagy in GC therapy.
Results
Serum miRNA Expression Profile Microarray, Microarray-Based Discovery of miR-423-3p, and Validation of miR-423-3p Expression in GC
Microarrays of serum specimens from three GC patients with good survival and three cases with poor survival were performed to detect the expression level of miRNAs. Using a two-fold expression difference as the cut-off level, nine miRNAs were found to be differentially expressed, and miR-423-3p was the most significantly downregulated in the good-survival group compared with the poor-survival group (p < 0.05, Figure S1A; Table S1).
Next, we validated miR-423-3p expression in the sera of GC patients with different prognoses by qRT-PCR analyses. Compared with GC patients in the good-survival group (>60 months, n = 35), miR-423-3p expression was much higher in patients in the poor-survival group (<12 months, n = 32) (p < 0.001; Figure 1A; Table S2). Interestingly, in 119 cancer tissues, the expression level of miR-423-3p was significantly higher than in adjacent normal tissues (p < 0.001; Figure 1B; Table S3). Similarly, levels of miR-423-3p in GC cell lines were also higher than in the human normal gastric epithelial cells GES-1 (p < 0.01; Figure 1C). Furthermore, using R language and Bioconductor approaches, we conducted genome-wide analyses of several GC transcriptome datasets available online. After analyzing the TCGA cohort data, we identified >800 differentially expressed genes from both Zhang’s cohort (GSE63121) and Carvalho J’s cohort (GSE33743).17 Among these changed genes, the level of miR-423-3p was significantly higher in GC (Figure 1D). Together, these results suggested that miR-423-3p is consistently upregulated and may serve as a candidate oncogene in GCs.
Figure 1.
miR-423-3p Is Significantly Upregulated in GC Cell Lines and Tissues
(A) miR-423-3p expression was validated in the serum of GC patients with different prognoses by qRT-PCR analyses (>60 months, n = 35; <12 months, n = 32). (B) Relative expression levels of miR-423-3p in gastric cancer tissues (n = 119) and adjacent normal tissues (n = 119) (p < 0.001). (C) miR-423-3p expression was examined via real-time PCR in gastric cancer cells. U6 snRNA was used as an endogenous control. (D) miR-423-3p is highly expressed in GC patients. Using R language and Bioconductor methods, we analyzed miR-423-3p expression in GC tumors and peri-tumor tissues from Zhang’s cohort (GSE63121) and Carvalho J’s cohort (GSE33743) datasets. *p < 0.05; **p < 0.01; ***p < 0.001.
miR-423-3p Levels in GC Tissue Specimens Are Associated with Clinicopathological Characteristics and Prognosis
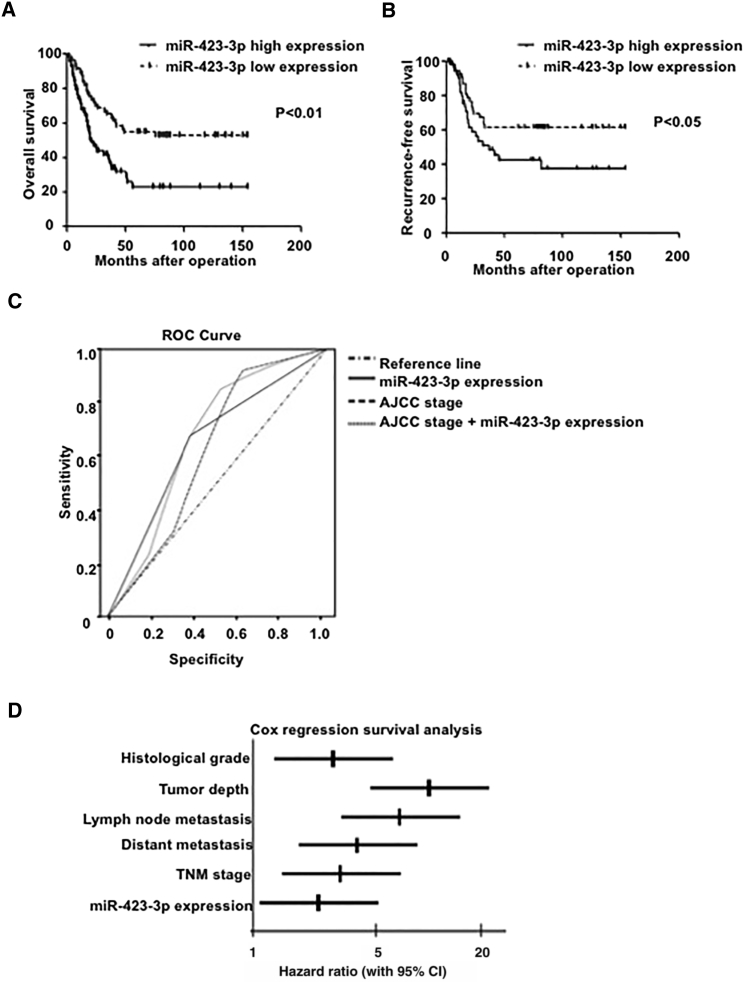
To investigate the clinical significance of miR-423-3p expression in GC, we analyzed miR-423-3p expression in an independent cohort of 119 GC tissue specimens by using quantitative RT-PCR (Table S3). Consistently with the results from our serum validation cohort, miR-423-3p was significantly associated with advanced age, tumor size, tumor invasion, distant metastasis, the American Joint Committee on Cancer 7th edition (AJCC) stage, and chemotherapy. Moreover, Kaplan-Meier analysis indicated that patients with a high miR-423-3p expression level had worse overall survival (OS) and recurrence-free prognosis (RFP) than those with a low expression level (p < 0.01, Figure 2A; p < 0.05, Figure 2B). By considering the AJCC stage or miR-423-3p expression level or a combination of both, we constructed receiver operating characteristic (ROC) curves to predict overall survival (Figure 2C). Strikingly, compared with the AJCC stage, the combination of both factors achieved the highest area under the curve (AUC) value (p < 0.05). Additionally, univariate analysis was conducted to identify the factors that affected the OS and was followed by multivariate analysis, which controlled for potential confounders (Figure 2D; Table S4). Multivariate analysis further confirmed that an increased miR-423-3p level was an independent predictor of shorter OS in GC patients (hazard ratio [HR] = 2.36, 95% confidence interval [CI] 1.10 to 4.86, p < 0.05).
Figure 2.
miR-423-3p Levels Are Associated with Clinicopathological Characteristics and Prognosis in GC Patients
(A) Kaplan-Meier curve of overall survival of GC patients with high and low miR-423-3p levels (p < 0.01). The miR-423-3p level was assessed by qRT-PCR, and the median value of all 119 cases was chosen as the cutoff point for separating miR-423-3p high-expression tumors (n = 61) from miR-423-3p low-expression cases (n = 58). (B) Kaplan-Meier curve of recurrence-free survival of GC patients with high (n = 61) and low (n = 58) miR-423-3p levels (p < 0.05). (C) The receiver operating characteristic (ROC) curves for predicting patient recurrence by using a combination of miR-423-3p and AJCC stage. (D) Different factors (including miR-423-3p expression level, histological grade, tumor depth, lymph node metastasis, and AJCC stage) were analyzed for their association with GC patient overall survival using a Cox regression model. The hazard ratio and 95% CI are plotted for each factor.
miR-423-3p Promotes Cell Proliferation, Migration, and Invasion In Vitro and Tumor Growth In Vivo in GC
To understand the crucial role of miR-423-3p in gastric carcinogenesis, GC cell lines were transfected with either a miR-423-3p mimic or a miR-423-3p inhibitor (Figure S1B). As expected, miR-423-3p overexpression markedly promoted cell proliferation in 3-(4,5-dimethylthiazole-2-yl)-2, 5 biphenyl tetrazolium bromide (MTT) assays (p < 0.01, Figures 3A and S2A), and overexpression of miR-423-3p promoted colony formation ability in GC cells (p < 0.05, Figures 3B, S2B, and S2C). In Transwell and wound-healing assays, the results were similar (p < 0.05, Figures 3B, 3C, and S2B–S2D). Moreover, knockdown of endogenous miR-423-3p expression dramatically inhibited cell proliferation, migration, invasion, and colony formation (Figures 3A and S3A–S3D).
Figure 3.
miR-423-3p Promotes Gastric Cancer Cell Growth, Colony Formation, and Invasion In Vitro and Tumor Growth In Vivo
(A) Overexpression miR-423-3p significantly promoted cell viability (left panel), and knockdown miR-423-3p expression significantly inhibited cell viability (right panel) in MGC803 cells, as demonstrated by MTT assays. (B) Ectopic miR-423-3p expression significantly promoted colony formation and invasiveness in MGC803 cells, as determined by colony formation assays and Transwell assays. (C) Ectopic miR-423-3p expression significantly promoted migration of MGC803 cells, as shown by wound-healing assays. (D and E) Ectopic miR-423-3p expression significantly increased MGC803 cell-mediated tumor volumes in vivo (MGC803/miR-423-3p, n = 7; MGC803/anti-miR-423-3p, n = 7; MGC803/miR-Ctrl, n = 7). *p < 0.05; **p < 0.01; ***p < 0.001.
To evaluate the in vivo effects of miR-423-3p on GC tumor growth, cells (MGC803/miR-423-3p, MGC803/anti-miR-423-3p, and MGC803/Ctrl) were subcutaneously injected into the flanks of nude mice. Real-time PCR revealed the miR-423-3p expression levels in different groups (Figure S4A). In accordance with the results in GC cell lines, anti-miR-423-3p cells formed smaller tumors, whereas the weights and volumes of the tumors formed by the MGC803/miR-423-3p cells were significantly larger than those of the other groups (p < 0.01, Figures 3D and 3E). Similar results were also observed in the MKN45 cell series (Figures S4B and S4C). Collectively, these data demonstrated that miR-423-3p expression strongly promotes gastric tumor cell proliferation, migration, and invasion in vitro and in vivo.
Bim Is a Direct Target of miR-423-3p in GC Cells
Considering that miRNAs function through downregulation of target genes, we searched for the putative target genes of miR-423-3p by using bioinformatics tools, such as TargetScan, miRanda, and PicTar. Analysis of the 3′ UTR of Bim mRNA revealed potential binding sites for miR-423-3p, thus suggesting the existence of a regulatory relationship between miR-423-3p and Bim (Figure 4A). To determine whether Bim is a direct target of miR-423-3p, luciferase activity assays were performed. The full-length (WT-Bim-3′ UTR) and mutant (Mut-Bim-3′ UTR) Bim 3′ UTRs were amplified and directly fused downstream of the luciferase reporter gene in a psiCHECK-2 vector. We then performed luciferase reporter assays to verify a direct interaction between miR-423-3p and the 3′ UTR of Bim. As shown in Figure 4B, miR-423-3p markedly decreased the relative luciferase activity of WT-Bim-3′ UTR in the cells (p < 0.01), whereas the cells transfected with Mut-Bim-3′ UTR did not exhibit decreased luciferase activity in the presence of miR-423-3p. Notably, compared with those of the control cells, the mRNA and protein levels of Bim were significantly decreased in GC cells with an increased dose of miR-423-3p, and levels increased in cells with an increased dose of anti-miR-423-3p (p < 0.05; Figures 4C–4E). Furthermore, immunohistochemical analysis of Bim in animal tumor tissues showed similar results (Figure S4D). Collectively, these results indicated that Bim is a direct target of miR-423-3p in GC cells.
Figure 4.
Bim Is a Direct Target of miR-423-3p in Gastric Cancer Cells
(A) The 3′ UTR of Bim mRNA contains the binding sequences of miR-423-3p. (B) Cotransfection of miR-423-3p and Bim-WT reduced luciferase activity levels in 293T cells, whereas cotransfection of miR-423-3p and Bim-Mut did not reduce these levels. (C) Ectopic miR-423-3p expression significantly reduced the mRNA levels of Bim in gastric cancer cells. (D) Ectopic miR-423-3p expression (mimics) significantly reduced Bim protein levels in gastric cancer cells. (E) Ectopic miR-423-3p expression (inhibitors) significantly increased Bim protein levels in gastric cancer cells. *p < 0.05; **p < 0.01; ***p < 0.001.
Bim Is Involved in Cell Growth and Migration and Is a Functional Target of miR-423-3p
To study the molecular mechanisms of miR-423-3p in tumor promotive activity, we sought out its gene targets by interrogating the interaction between miR-423-3p and Bim in GC cells. First, we investigated whether miR-423-3p exerted its effects through the regulation of Bim. Notably, knockdown of Bim promoted the proliferation and migration of GC cells, thus resembling the promotive effects of miR-423-3p overexpression in GC cells. Moreover, we rescued cell growth and invasion in GC cells by reintroduction of Bim (Figures S5A–S5C, S6B, and S6C). In addition, as shown in Figures S6A and S6D, the overexpression of Bim in SGC7901 cells also clearly rescued the migration ability induced by miR-423-3p. These results indicate that Bim is a functional target of miR-423-3p in GC cells.
miR-423-3p Activates Autophagy Mediated by Bim in GC Cells
To explore the association between miR-423-3p and autophagy in GC cells, we detected LC3 levels in a panel of GC cell lines. Notably, the LC3 level was higher in GC cells than that in normal gastric epithelial cells (GES-1) in the LH conditions (Figure S7A). As shown in Figures S7B–S7D, we also found that the levels of LC3 markedly increased after miR-423-3p transfection in GC cells. In contrast, when we inhibited miR-423-3p in GC cells, the levels of LC3 dramatically decreased (Figures S7E–S7G). Moreover, fluorescence microscopy analysis revealed that autophagy was activated when miR-423-3p was upregulated in GC cells (Figure S8A). Consistently, LC3 expression in GC cells transfected with miR-423-3p (MGC803/miR-423-3p) was significantly higher than in those transfected with negative control (MGC803/miR-Ctrl) (Figure S8B–S8D). In the absence of LH conditions, with the anti-autophagy drug, the results were similar (Figure S8E and S8F). In addition, as shown by fluorescence microscopy, overexpression of Bim significantly abolished the level of LC3 and was followed by an increase in miR-423-3p (Figure S9A). Together, these results suggest that miR-423-3p activates autophagy mediated by Bim in GC cells.
Autophagy Induced by miR-423-3p Is Oncogenic and Beclin-1 Dependent in GC Cells
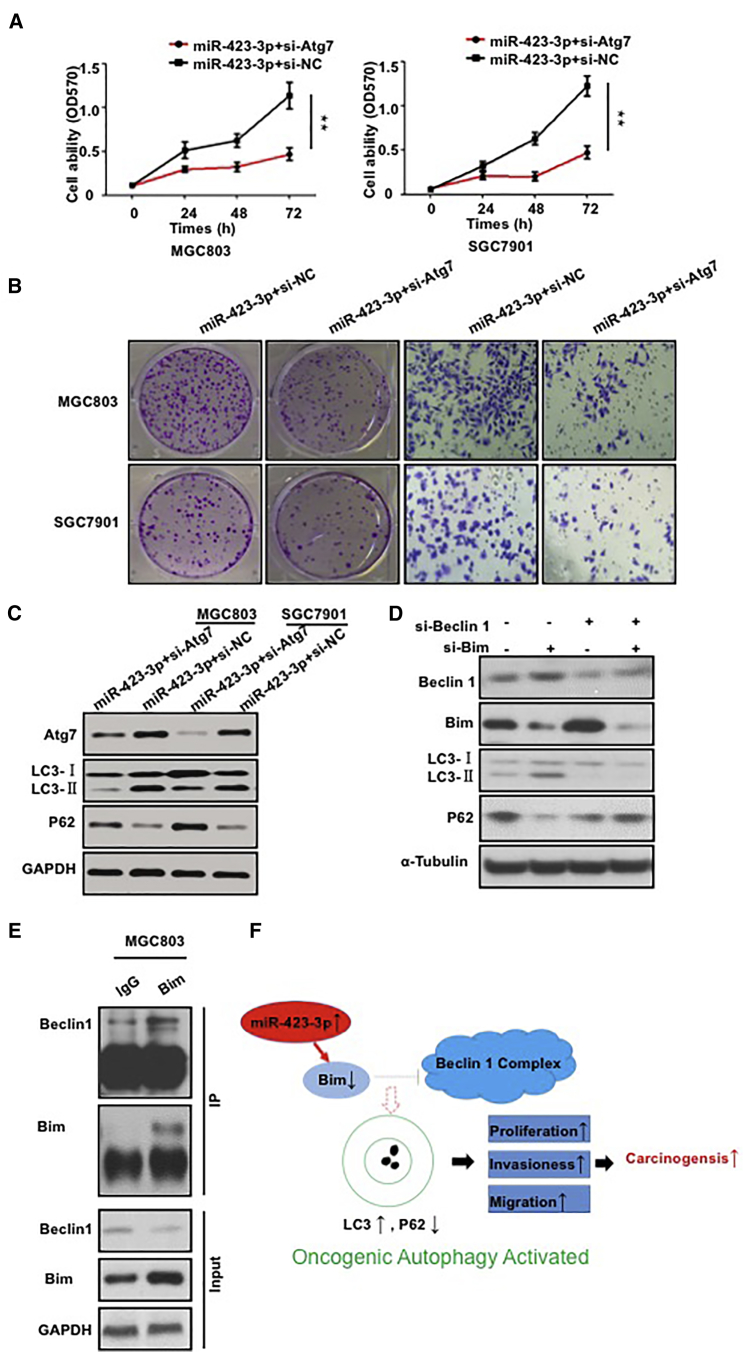
Because autophagy-related gene (Atg) 7 is involved in the maturation phase of autophagy, we abrogated autophagy by decreasing Atg7 expression in stable GC cell lines. The results showed that knockdown of Atg7 expression rescued the increased cell viability, colony formation, and invasion induced by miR-423-3p (Figures 5A–5C), thus suggesting that the autophagy induced by miR-423-3p drives gastric carcinogenesis. Interestingly, when we knocked down Beclin-1 in MGC803 cells, Bim knockdown did not rescue autophagy or increase LC3 levels (Figure 5D). We further confirmed the interaction between endogenous Bim and Beclin1 in GC cells by coimmunoprecipitation (Figures 5E and S9B). The results indicated that the effect of Bim on LC3 formation is Beclin-1 dependent. A model depicting the effect of miR-423-3p on autophagy pathways is presented in Figure 5F.
Figure 5.
Oncogenic Autophagy Is Activated by miR-423-3p Upregulation and Is Beclin 1 Dependent in GC Cells
(A and B) Transfection of MGC803 and SGC7901 cells with miR-423-3p and si-Atg7 significantly reduced the cell viability (A), colony formation ability, and invasion ability (B) compared with those in cells transfected with negative control (miR-423-3p+si-NC). (C) MGC803 and SGC7901 cells transfected with miR-423-3p and si-Atg7 exhibited significantly reduced expression of LC3 compared with that in cells transfected with negative control (miR-423-3p+si-NC), as demonstrated by western blotting. (D) Bim knockdown did not increase LC3 levels when Beclin 1 was knocked down in MGC803 cells. (E) Coimmunoprecipitation (IP) of endogenous Bim and Beclin1 in MGC803 cells. IgG, immunoglobulin G. (F) A model depicting the effect of miR-423-3p on autophagy pathways. miR-423-3p regulates autophagy by targeting the autophagy protein Bim. The resulting decrease in Beclin-1 activity leads to attenuation of LC3 lipidation and inhibits the membrane elongation and completion stage of oncogenic autophagy. *p < 0.05; **p < 0.01; ***p < 0.001.
Discussion
Recently, some studies have suggested that serum miRNAs may serve as noninvasive biomarkers for cancer prognosis.18 Circulating miRNAs are well protected from RNases. Even after being subjected to harsh conditions, they remain stable.19 A relationship between the expression of serum miRNAs and GC survival has been reported.20, 21, 22 However, the analysis of GC-specific serum miRNAs by microarray platforms is still lacking.
In this study, we performed a genome-wide serum miRNA expression analysis on patient groups with either a good or poor prognosis, and eventually discovered miR-423-3p is significantly associated with GC. Moreover, we validated the results in another 63 GC patient serum samples and found that serum miR-423-3p expression is significantly correlated with good- and poor-prognosis groups. Additionally, we discovered that high miR-423-3p expression in GC tissue specimens was a strong independent predictor of short overall survival in patients with GC. Our results clearly demonstrated that miR-423-3p expression in serum and primary tumor tissue can predict the prognosis of GC patients, thus highlighting the specificity of miR-423-3p as an oncogene and a biomarker for GC.
In 2004, miR-423-3p was first described as a mature miRNA in HL-60 leukemia cells.23 Some studies have also found that miR-423-3p expression in serum or tissues is associated with prognosis in prostate cancer, hepatocellular carcinoma, and laryngeal carcinoma,24, 25 in accordance with our findings in GC. However, the exact regulatory mechanism of miR-423-3p in gastric cancer remains unclear.
Currently, we found that overexpression of miR-423-3p significantly promoted GC colony formation, independent growth, migration, and invasion, and we identified Bim as a direct target of miR-423-3p. Restoration of the expression of Bim in GC cells stably expressing miR-423-3p counteracted the tumor-promoting effects of miR-423-3p, whereas the knockdown of Bim in these cells mimicked the tumor-promoting effects of miR-423-3p. Therefore, our results established Bim as a functional target of miR-423-3p during GC tumorigenesis.
Previously, some studies have reported that Bim is involved in the progression of GC and may be a potent prognostic marker.26, 27 For instance, Ha Thi HT has reported that Bim is regulated by tumor necrosis factor α (TNF-α) and transforming growth factor β (TGF-β) and is involved in the apoptosis of GC cells.28 Considering the emerging concept that autophagy is a critical adaptive response that aids in tumor cell survival during microenvironment stresses by reducing apoptosis,29, 30 we identified whether miR-423-3p activates autophagy by mediation of Bim. Indeed, both LC3 expression and fluorescence microscopy analysis showed that GC cells transfected with miR-423-3p exhibited significantly higher levels of autophagy than did control cells. Additionally, we discovered that ectopic miR-423-3p expression did not affect the Akt and mTOR pathway in gastric cancer cells (Figure S9C). Furthermore, we found that overexpression of Bim significantly rescued the proliferation and invasion of the GC cells and was followed by a decrease in the level of LC3, thus suggesting that the miR-423-3p-Bim axis might regulate autophagy in GC cells. These data are consistent with a report stating that Bim can inhibit autophagy.31, 32
Another unexpected finding in our study was that autophagy induced by miR-423-3p was oncogenic in GC cells. Atg7 exhibits homology to the E1 ubiquitin-activating enzymes and regulates autophagosome formation, which is a critical step in autophagy.33, 34 In our study, knockdown of Atg7 expression rescued the cell growth, colony formation, and invasion induced by miR-423-3p. These results showed that miR-423-3p increases the proliferation, invasiveness, and aggressiveness of GC through regulation of oncogenic autophagy.
Recently, oncogenic autophagy has been given considerable attention. For example, Ying et al. have demonstrated that activation of autophagy contributes to cell proliferation in bladder cancer cells.35 Shen et al. have identified a role of SMYD3 in autophagy activation and a role of oncogenic autophagy in the progression of bladder cancer.36 The underlying mechanism may be that metabolic stress often strongly induces autophagy in cancer cells, and autophagy activation is required for tumor cells to survive metabolic stress.10 In addition, the current study revealed that autophagy induced by the miR-423-3p-Bim axis is Beclin-1 dependent. Pioneering studies by Luo et al. demonstrated that Bim interacts with Beclin-1, and this interaction is stimulated by LC8. Hence, Bim inhibits autophagy by recruiting Beclin-1 to microtubules, which was mediated via bridging Beclin-1 and LC8.8, 32
Considering the functional role of autophagy in GC, we speculate that regulating autophagy and sensitizing the cells to metabolic stress should be an approach for GC therapy. Indeed, it has been found that facilitating the process of autophagy prolongs survival relative to placebo in patients with metastatic renal cell carcinoma.37, 38 Additionally, the prevention and treatment of GC-related Helicobacter pylori infection via regulation of autophagy has been reported. Thus, it would be interesting to determine whether the oncogenic autophagy inhibition of the miR-423-3p-Bim axis may have clinical applications in the management of GC.
To the best of our knowledge, this is the first study in which a genome-wide serum miRNA expression analysis, based on good- and poor-survival groups, was performed to discover GC-specific miRNAs. We demonstrated that the promotion of oncogenic autophagy, regulated by the miR-423-3p-Bim axis, contributes to GC-promoting effects. Our results suggest that the miR-423-3p-Bim axis is a new biomarker and potential target for treatment of GC, and it may have important clinical applications in cancer diagnosis and treatment.
Materials and Methods
miRNA Expression Profiling, Human Tissue Samples, and Cell Lines
73 serum samples from gastric cancer patients treated at the Cancer Center of Sun Yat-Sen University (SYSUCC) (Guangzhou, China) between July 2002 and July 2003 were analyzed. Six samples were selected for the miRNA microarray analysis (>60 months, n = 3; <12 months, n = 3), and another 67 GC patients with different prognoses (>60 months, n = 35; <12 months, n = 32) were used as a validated group. The patient characteristics are summarized in Table S2. Patients who had survival times of more than 60 months were identified as the good-survival group, and those with survival times of less than 12 months were identified as the poor-survival group.
The experiments on the six serum samples, using miRNA expression profile microarrays, were performed by CapitalBio (CapitalBioCorp). Procedures are described in detail on the website of CapitalBio (http://www.capitalbio.com). Briefly, the procedures included total RNA extraction, quality control, miRNA isolation, FlashTag biotin labeling of miRNAs, hybridization to an Affymetrix GeneChip microarray, and microarray washing, staining, and scanning. When the miRNA expression changed by at least ± 2-fold and the P value was less than 0.05, the miRNA was considered to be significantly differentially expressed and was selected for qPCR validation.
GC tissue specimens and paired normal tissues for validation were obtained from 119 GC patients who received surgery at the Sun Yat-Sen University Cancer Center between 2005 and 2010. The study was approved by the ethics committee of the Sun Yat-Sen University Cancer Center, and written, informed consent was obtained from all participants. None of the patients had received any treatment prior to the surgery. All patients were followed up regularly at an interval of 3 months following the operation, and clinicopathological characteristics were collected, including age, gender, histological grade, tumor size, tumor depth, lymph node metastasis, distant metastasis, and AJCC stage. The median follow-up time was 47.7 months and ranged from 6 to 154 months. Overall survival was defined as the time from the date of the operation to the date of death or last contact.
HEK293 cells were obtained from ATCC (the American Type Culture Collection), and the cells were maintained in DMEM with 10% fetal bovine serum (FBS) (12800–017, GIBCO). Human GC cell lines (BGC823, MGC803, SGC7901, and MKN45) and the normal gastric epithelial cell line GES-1 were purchased from the Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, and were cultured at 37°C with RPMI-1640 medium containing 10% fetal calf serum (31800–022, GIBCO).
RNA Extraction and Real-Time PCR Analysis
Total RNA was extracted from tissue samples and cells with an RNeasy RNA Mini Kit (QIAGEN, http://www.qiagen.com). For the measurement of miR-423-3p, a Hairpin-it micro RNA and U6 snRNA Normalization Real-Time PCR Quantitation kit (GenePharma, http://www.genepharma.com) was used according to the manufacturer’s instructions. U6 small RNA was used as the reference. In addition, the average expression level of miR-423-3p was normalized against U6, as described previously.24 For the detection of Bim and Atg 7 mRNA, first-strand cDNA was synthesized using AMV Reverse Transcriptase (Promega), and GAPDH was used as the internal control. The following primers were used for quantitative PCR:
Bim
Forward:
5′-TGCAGTTGCTTCAGTACCCATAAT-3′,
Reverse:
5′-ATCCCCGTGTACTTTCCCATCATAAT-3′;
Atg 7
Forward:
5′-TTGACATGAGTGCTCCCACC-3′,
Reverse:
5′-AGACAGAGGGCAGGATAGCA-3′;
GAPDH
Forward:
5′-TGCACCACCAACTGCTTAGC-3′,
Reverse:
5′-GGCATGGACTGTGGTCATGAG-3′;
U6
Forward: 5′-TGCACCACCAACTGCTTAGC-3′,
Reverse: 5′-GGCATGGACTGTGGTCATGAG-3′.
Real-time PCR was performed with a Bio-Rad CFX96 qPCR system, and the data were analyzed using the 2-ΔCT method or the 2-ΔΔCT method.
miRNA-Related Reagents and Transfection
miRNA mimics, inhibitors, negative controls, inhibitor negative controls, and Bim small-interfering RNA (siRNA) were purchased from GenePharma.
Bim siRNA: 5′-GACCGAGAAGGUAGACAAUUU-3′,
Negative control (Bim): 5′-UUCUCCGAACGUGUCACGUTT-3′;
hsa-miR-423-3p mimics:
Forward: 5′-AGCUCGGUCUGAGGCCCCUCAGU-3′,
Reverse: 5′-UGAGGGGCCUCAGACCGAGCUUU-3′;
Negative control (mimics):
Forward: 5′-UUCUCCGAACGUGUCACGUTT-3′,
Reverse: 5′-ACGUGACACGUUCGGAGAATT-3′;
hsa-miR-423-3p inhibitors:
5′-ACUGAGGGGCCUCAGACCGAGCU-3′;
Negative control (inhibitors):
5′-CAGUACUUUUGUGUAGUACAA-3′.
Transfections with 100 nM miRNAs and siRNAs were performed using Lipofectamine RNAiMAX Transfection Reagent (Life Technologies) according to the manufacturer’s instructions.
To restore Bim expression, the cells stably overexpressing miR-423-3p were transfected with a psiCHECK-2-Bim plasmid, which contained the coding sequences but lacked the 3′ UTR. Transfection with 1 μg/mL of plasmid was performed using Lipofectamine-2000 Transfection Reagent (Invitrogen) according to the manufacturer’s instructions. The medium was changed 6 hr after transfection, and the cells were collected after being cultured for 36 hr. Transfections were performed as previously described.39
Cell Proliferation, Migration, and Invasion Assays
Cell viabilities were assessed with MTT assays. The spectrophotometric absorbance at 570 nm was detected for each sample, and the experiments were performed in triplicate and repeated three times. A colony formation assay was performed as previously described.40 Briefly, cells were seeded in a six-well plate 36 hr after transfection and cultured for 2 weeks in RPMI 1640 medium containing 10% FBS (GIBCO). Colonies were fixed and dyed with 0.1% crystal violet (1 mg/mL), and the number of colonies with over 50 cells was counted. Cell invasion was evaluated using a Transwell assay with Matrigel (8-μm pore; BD Biosciences). The procedures were performed as previously described. The experiments were repeated three times. Wound-healing assays were performed to detect cell migration. The cells were seeded in six-well plates, and an artificial wound was created using a 200-μL pipette tip. The wound closure was observed after 24 hr, and images were obtained. We measured the fraction of cell coverage across the line to assess the relative migration distance.
Luciferase Assay
Luciferase assays were performed as described previously. Briefly, a 60-bp fragment of the wild-type Bim 3′ UTR containing the putative miR-423-3p binding site or a mutant Bim 3′ UTR sequence was cloned into a psiCHECK-2 vector luciferase plasmid (Ambion). HEK293 cells were transfected with different reporter vectors (p-Luc-3′ UTR-WT-Bim or p-Luc-3′ UTR-mut-Bim) and cotransfected with negative control or miR-423-3p mimics. Luciferase activity levels were measured using a Dual-Luciferase Reporter Assay Kit (Promega) following the manufacturer’s instructions.
Antibodies and Western Blotting
The following antibodies were used in this study: anti-α-tubulin (Sigma), anti-LC3B (Novus Biological), anti-p62/SQSTM1 (Santa Cruz Biotechnology), anti-GAPDH (Abcam, #AB127428), goat anti-mouse secondary antibody (Thermo Scientific), and goat anti-rabbit secondary antibody (Thermo Scientific). Rabbit Bim antibody (CST, #2819), mouse Beclin1 antibody (CST, #4122), rabbit Beclin1 antibody (CST, #3738), rabbit Atg7 antibody (CST, #2631), rabbit Akt antibody (CST, #9272), rabbit mTOR antibody (CST, #2972), rabbit Caspase 3 antibody (CST, #9662), and rabbit PARP antibody (CST, #9542) were purchased from Cell Signaling Technology. Western blotting was performed as described previously.
Low Glucose and Hypoxia Conditions
The cells were cultured in DMEM (low glucose) (GIBCO, Life Technologies, Cat. 11885-084) in a CO2 incubator (Thermo Scientific) and maintained at 94% N2, 5%CO2, and 1% O2.
Bafilomycin A1 Assay
After 24–36 hr of transfection, cells were treated with 100 nM Bafilomycin A1 (Sigma, Cat: 88899-55-2). Then, the cells were subjected to hypoxic conditions.
Confocal Microscopy
Cultured cells were fixed, stained, and observed with a confocal microscope, as described previously.
Preparation of Stable miR-423-3p-Expressing Cells
The lentiviral plasmid pEZX-MR01 expressing miR-423-3p and the negative control miRNA precursor sequences were purchased from GenePharma and were termed pLV-miR-423-3p and pLV-miR-Ctrl, respectively. Recombinant lentivirus for miR-423-3p expression was prepared according to methods described previously. MGC803 and MKN45 cells were infected with recombinant lentivirus (Lenti-miR-423-3p) or control lentivirus (Lenti-GFP-NC) and purified by fluorescence-activated cell sorting for stable miR-423-3p-expressing transfectants and control cells.
Animal Experiments
All mice were obtained from the Laboratory Animal Center of Guangdong province (Guangzhou, China). Nude mice (6-week-old females) were injected subcutaneously in the right flank region with 5 × 106 stable cells. Tumor volume was measured every 5 days for 36 days using the following formula: tumor volume = π/6 (L × W2). Mice were killed on day 36, and the tumors were dissected and analyzed. All animal experiments complied with ethical regulations and were approved by the Subcommittee on Research Animal Care of Sun Yat-Sen University.
Immunohistochemistry
Xenograft tumors were fixed in 4% paraformaldehyde (PFA), embedded in paraffin, sectioned, and stained with H&E. Immunohistochemical staining of paraffin-embedded tumor tissues was performed using Bim (CST, 1:100 dilution) primary antibody and the ABC Elite immune peroxidase kit according to the manufacturers’ instructions.
Statistical Analysis
An SPSS software package (version 19.0, SPSS) was used to conduct the statistical analysis. Student’s t test or a Chi-square test was performed to determine statistical significance. Survival was evaluated using the Kaplan-Meier method with the log-rank test. A Cox proportional hazards model was used for multivariate analysis, which included the age at diagnosis, gender, histological grade, tumor size, tumor depth, lymph node metastasis, distant metastasis, AJCC staging, and miR-423-3p expression. A P value of less than 0.05 was considered statistically significant. Cohort datasets were downloaded from Medline or PubMed. R language and Bioconductor methods were used for the calculation of gene expression and annotation. We used R3.2.2 for further analysis of gene and expression lists.
Author Contributions
P.K., X.Z., Q.G., and L.X. contributed equally to this work. D.X. conceived and designed the experiments. P.K., X.S., Y.C., W.L., Z.Z., and Y.Z. analyzed the data. P.K., Q.G., X.Z., and L.X. contributed reagents, materials, and analysis tools. P.K. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
We thank Drs. Xuan Li and Zifeng Wang for statistical advising and review of the manuscript. The protocol was approved by the Sun Yat-Sen University Cancer Center review board in accordance with Chinese bioethical regulations. All patients provided written informed consent to offer related information in the hospital. This study was supported by the National Science Foundation of China (no. 81172341).
Footnotes
Supplemental Information includes nine figures and four tables and can be found with this article online at http://dx.doi.org/10.1016/j.ymthe.2017.01.013.
Supplemental Information
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Shen L., Shan Y.S., Hu H.M., Price T.J., Sirohi B., Yeh K.H., Yang Y.H., Sano T., Yang H.K., Zhang X. Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol. 2013;14:e535–e547. doi: 10.1016/S1470-2045(13)70436-4. [DOI] [PubMed] [Google Scholar]
- 3.Mizushima N., Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 4.Paludan C., Schmid D., Landthaler M., Vockerodt M., Kube D., Tuschl T., Münz C. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307:593–596. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- 5.Nakai A., Yamaguchi O., Takeda T., Higuchi Y., Hikoso S., Taniike M., Omiya S., Mizote I., Matsumura Y., Asahi M. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat. Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 6.Qu X., Zou Z., Sun Q., Luby-Phelps K., Cheng P., Hogan R.N., Gilpin C., Levine B. Autophagy gene-dependent clearance of apoptotic cells during embryonic development. Cell. 2007;128:931–946. doi: 10.1016/j.cell.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 7.Xie J.M., Li B., Yu H.P., Gao Q.G., Li W., Wu H.R., Qin Z.H. TIGAR has a dual role in cancer cell survival through regulating apoptosis and autophagy. Cancer Res. 2014;74:5127–5138. doi: 10.1158/0008-5472.CAN-13-3517. [DOI] [PubMed] [Google Scholar]
- 8.White E., DiPaola R.S. The double-edged sword of autophagy modulation in cancer. Clinical Cancer Res. 2009;15:5308–5316. doi: 10.1158/1078-0432.CCR-07-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y., Gibson S.B. Is mitochondrial generation of reactive oxygen species a trigger for autophagy? Autophagy. 2008;4:246–248. doi: 10.4161/auto.5432. [DOI] [PubMed] [Google Scholar]
- 10.Mathew R., Karantza-Wadsworth V., White E. Role of autophagy in cancer. Nat. Rev. Cancer. 2007;7:961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen P., Cescon M., Bonaldo P. Autophagy-mediated regulation of macrophages and its applications for cancer. Autophagy. 2014;10:192–200. doi: 10.4161/auto.26927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 13.Berindan-Neagoe I., Monroig Pdel C., Pasculli B., Calin G.A. MicroRNAome genome: a treasure for cancer diagnosis and therapy. CA Cancer J. Clin. 2014;64:311–336. doi: 10.3322/caac.21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao X., Wang P., Liu J., Zheng J., Liu Y., Chen J., Xue Y. Gas5 exerts tumor-suppressive functions in human glioma cells by targeting miR-222. Mol. Ther. 2015;23:1899–1911. doi: 10.1038/mt.2015.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall D.P., Cost N.G., Hegde S., Kellner E., Mikhaylova O., Stratton Y., Ehmer B., Abplanalp W.A., Pandey R., Biesiada J. TRPM3 and miR-204 establish a regulatory circuit that controls oncogenic autophagy in clear cell renal cell carcinoma. Cancer Cell. 2014;26:738–753. doi: 10.1016/j.ccell.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lan S.H., Wu S.Y., Zuchini R., Lin X.Z., Su I.J., Tsai T.F., Lin Y.J., Wu C.T., Liu H.S. Autophagy-preferential degradation of MIR224 participates in hepatocellular carcinoma tumorigenesis. Autophagy. 2014;10:1687–1689. doi: 10.4161/auto.29959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carvalho J., van Grieken N.C., Pereira P.M., Sousa S., Tijssen M., Buffart T.E., Diosdado B., Grabsch H., Santos M.A., Meijer G. Lack of microRNA-101 causes E-cadherin functional deregulation through EZH2 up-regulation in intestinal gastric cancer. J. Pathol. 2012;228:31–44. doi: 10.1002/path.4032. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y., Gu J., Roth J.A., Hildebrandt M.A., Lippman S.M., Ye Y., Minna J.D., Wu X. Pathway-based serum microRNA profiling and survival in patients with advanced stage non-small cell lung cancer. Cancer Res. 2013;73:4801–4809. doi: 10.1158/0008-5472.CAN-12-3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y., Qu A., Liu J., Wang R., Liu Y., Li G., Duan W., Fang Q., Jiang X., Wang L. Serum miR-210 contributes to tumor detection, stage prediction and dynamic surveillance in patients with bladder cancer. PLoS ONE. 2015;10:e0135168. doi: 10.1371/journal.pone.0135168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J., Song Y., Zhang C., Zhi X., Fu H., Ma Y., Chen Y., Pan F., Wang K., Ni J. Circulating MiR-16-5p and MiR-19b-3p as two novel potential biomarkers to indicate progression of gastric cancer. Theranostics. 2015;5:733–745. doi: 10.7150/thno.10305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanco-Calvo M., Tarrío N., Reboredo M., Haz-Conde M., García J., Quindós M., Figueroa A., Antón-Aparicio L., Calvo L., Valladares-Ayerbes M. Circulating levels of GDF15, MMP7 and miR-200c as a poor prognostic signature in gastric cancer. Future Oncol. 2014;10:1187–1202. doi: 10.2217/fon.13.263. [DOI] [PubMed] [Google Scholar]
- 22.Valladares-Ayerbes M., Reboredo M., Medina-Villaamil V., Iglesias-Díaz P., Lorenzo-Patiño M.J., Haz M., Santamarina I., Blanco M., Fernández-Tajes J., Quindós M. Circulating miR-200c as a diagnostic and prognostic biomarker for gastric cancer. J. Transl. Med. 2012;10:186. doi: 10.1186/1479-5876-10-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasashima K., Nakamura Y., Kozu T. Altered expression profiles of microRNAs during TPA-induced differentiation of HL-60 cells. Biochem. Biophys. Res. Commun. 2004;322:403–410. doi: 10.1016/j.bbrc.2004.07.130. [DOI] [PubMed] [Google Scholar]
- 24.Lin J., Huang S., Wu S., Ding J., Zhao Y., Liang L., Tian Q., Zha R., Zhan R., He X. MicroRNA-423 promotes cell growth and regulates G(1)/S transition by targeting p21Cip1/Waf1 in hepatocellular carcinoma. Carcinogenesis. 2011;32:1641–1647. doi: 10.1093/carcin/bgr199. [DOI] [PubMed] [Google Scholar]
- 25.Guan G., Zhang D., Zheng Y., Wen L., Yu D., Lu Y., Zhao Y. microRNA-423-3p promotes tumor progression via modulation of AdipoR2 in laryngeal carcinoma. Int. J. Clin. Exp. Pathol. 2014;7:5683–5691. [PMC free article] [PubMed] [Google Scholar]
- 26.Petrocca F., Visone R., Onelli M.R., Shah M.H., Nicoloso M.S., de Martino I., Iliopoulos D., Pilozzi E., Liu C.G., Negrini M. E2F1-regulated microRNAs impair TGFbeta-dependent cell-cycle arrest and apoptosis in gastric cancer. Cancer Cell. 2008;13:272–286. doi: 10.1016/j.ccr.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Yano T., Ito K., Fukamachi H., Chi X.Z., Wee H.J., Inoue K., Ida H., Bouillet P., Strasser A., Bae S.C. The RUNX3 tumor suppressor upregulates Bim in gastric epithelial cells undergoing transforming growth factor beta-induced apoptosis. Mol. Cell. Biol. 2006;26:4474–4488. doi: 10.1128/MCB.01926-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ha Thi H.T., Lim H.S., Kim J., Kim Y.M., Kim H.Y., Hong S. Transcriptional and post-translational regulation of Bim is essential for TGF-β and TNF-α-induced apoptosis of gastric cancer cell. Biochim. Biophys. Acta. 2013;1830:3584–3592. doi: 10.1016/j.bbagen.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Rouschop K.M., Ramaekers C.H., Schaaf M.B., Keulers T.G., Savelkouls K.G., Lambin P., Koritzinsky M., Wouters B.G. Autophagy is required during cycling hypoxia to lower production of reactive oxygen species. Radiother. Oncol. 2009;92:411–416. doi: 10.1016/j.radonc.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 30.Wilkinson S., Ryan K.M. Growth factor signaling permits hypoxia-induced autophagy by a HIF1alpha-dependent, BNIP3/3L-independent transcriptional program in human cancer cells. Autophagy. 2009;5:1068–1069. doi: 10.4161/auto.5.7.9821. [DOI] [PubMed] [Google Scholar]
- 31.Maiuri M.C., Le Toumelin G., Criollo A., Rain J.C., Gautier F., Juin P., Tasdemir E., Pierron G., Troulinaki K., Tavernarakis N. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 2007;26:2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo S., Garcia-Arencibia M., Zhao R., Puri C., Toh P.P., Sadiq O., Rubinsztein D.C. Bim inhibits autophagy by recruiting Beclin 1 to microtubules. Mol. Cell. 2012;47:359–370. doi: 10.1016/j.molcel.2012.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ravikumar B., Sarkar S., Davies J.E., Futter M., Garcia-Arencibia M., Green-Thompson Z.W., Jimenez-Sanchez M., Korolchuk V.I., Lichtenberg M., Luo S. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol. Rev. 2010;90:1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- 34.Wang H., Wan H., Li X., Liu W., Chen Q., Wang Y., Yang L., Tang H., Zhang X., Duan E. Atg7 is required for acrosome biogenesis during spermatogenesis in mice. Cell Res. 2014;24:852–869. doi: 10.1038/cr.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ying L., Huang Y., Chen H., Wang Y., Xia L., Chen Y., Liu Y., Qiu F. Downregulated MEG3 activates autophagy and increases cell proliferation in bladder cancer. Mol. Biosyst. 2013;9:407–411. doi: 10.1039/c2mb25386k. [DOI] [PubMed] [Google Scholar]
- 36.Shen B., Tan M., Mu X., Qin Y., Zhang F., Liu Y., Fan Y. Upregulated SMYD3 promotes bladder cancer progression by targeting BCLAF1 and activating autophagy. Tumour Biol. 2016;37:7371–7381. doi: 10.1007/s13277-015-4410-2. [DOI] [PubMed] [Google Scholar]
- 37.Nazio F., Strappazzon F., Antonioli M., Bielli P., Cianfanelli V., Bordi M., Gretzmeier C., Dengjel J., Piacentini M., Fimia G.M. mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat. Cell Biol. 2013;15:406–416. doi: 10.1038/ncb2708. [DOI] [PubMed] [Google Scholar]
- 38.Motzer R.J., Escudier B., Oudard S., Hutson T.E., Porta C., Bracarda S., Grünwald V., Thompson J.A., Figlin R.A., Hollaender N., RECORD-1 Study Group Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 39.Deng R., Tang J., Ma J.G., Chen S.P., Xia L.P., Zhou W.J., Li D.D., Feng G.K., Zeng Y.X., Zhu X.F. PKB/Akt promotes DSB repair in cancer cells through upregulating Mre11 expression following ionizing radiation. Oncogene. 2011;30:944–955. doi: 10.1038/onc.2010.467. [DOI] [PubMed] [Google Scholar]
- 40.Chen D.L., Zeng Z.L., Yang J., Ren C., Wang D.S., Wu W.J., Xu R.H. L1cam promotes tumor progression and metastasis and is an independent unfavorable prognostic factor in gastric cancer. J. Hematol. Oncol. 2013;6:43. doi: 10.1186/1756-8722-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.