Abstract
Mucosal-associated invariant T (MAIT) cells and natural killer T (NKT) cells are known to play important roles in autoimmunity, infectious diseases and cancers. However, little is known about the roles of these invariant T cells in multiple trauma. The purposes of this study were to examine MAIT and NKT cell levels in patients with multiple trauma and to investigate potential relationships between these cell levels and clinical parameters. The study cohort was composed of 14 patients with multiple trauma and 22 non-injured healthy controls (HCs). Circulating MAIT and NKT cell levels in the peripheral blood were measured by flow cytometry. The severity of injury was categorised according to the scoring systems, such as Acute Physiology and Chronic Health Evaluation (APACHE) II score, Simplified Acute Physiology Score (SAPS) II, and Injury Severity Score (ISS). Circulating MAIT and NKT cell numbers were significantly lower in multiple trauma patients than in HCs. Linear regression analysis showed that circulating MAIT cell numbers were significantly correlated with age, APACHE II, SAPS II, ISS category, hemoglobin, and platelet count. NKT cell numbers in the peripheral blood were found to be significantly correlated with APACHE II, SAPS II, and ISS category. This study shows numerical deficiencies of circulating MAIT cells and NKT cells in multiple trauma. In addition, these invariant T cell deficiencies were found to be associated with disease severity. These findings provide important information for predicting the prognosis of multiple trauma.
Keywords: Flow Cytometry, Mucosal-Associated Invariant T Cells, Multiple Trauma, Natural Killer T Cells
Graphical Abstract
INTRODUCTION
Multiple trauma is defined as trauma-related damage involving multiple body cavities or body parts with high morbidity and mortality worldwide (1). After severe traumatic damage, the immune system is activated by injured tissues, which subsequently induce a specific series of immune responses: systemic inflammatory response syndrome (SIRS) and compensatory anti-inflammatory response syndrome (CARS). The majority of trauma patients survive the initial SIRS response, follow a period of clinical stability, and manifest the CARS response with suppressed immunity and diminished resistance to infection, leading to resolution without complications (2). Nevertheless, approximately 20% to 30% of the patients having persistent SIRS or CARS will eventually develop the early or late multiple organ dysfunction syndrome (MODS) with an attendant high mortality rate. Given this general paradigm, the interactions between the innate and adaptive immune systems may play an important role in the induction and regulation of both SIRS and CARS in trauma patients (3).
Recent studies have implicated 2 distinct unconventional, innate-like T lymphocytes, such as MR1-restricted mucosal-associated invariant T (MAIT) cells and CD1d-restricted natural killer T (NKT) cells as effectors and/or regulators of inflammatory responses during sepsis (4). These cell types express an invariant T cell receptor (TCR) Vα7.2-Jα33 chain paired with a limited repertoire of Vβ chains or an invariant TCR pair of Vα24-Jα18/Vβ11 chains together with the Vβ chain, respectively (5). Using these unique pairs of TCR chains, MAIT cells recognize bacteria-derived vitamin B2 metabolite antigens presented by the major histocompatibility complex (MHC) class Ib-like related protein MR1 (6), whereas NKT cells react with endogenous and exogenous glycolipid antigens, such as isoglobotrihexosylceramide (iGb3) and α-galactosylceramide (α-GalCer), presented by the MHC class I-like protein CD1d (7). Following antigen recognition, MAIT cells rapidly produce Th1/Th17 cytokines, such as interferon-γ (IFN-γ) and interleukin (IL)-17 (8), whereas NKT cells rapidly produce large amounts of Th1 and Th2 cytokines, such as IFN-γ and IL-4, in an innate-like manner (7). These 2 invariant T cells are known to play a protective role in host immune responses against a variety of infectious pathogens, including certain enterobacterial and mycobacterial species, viruses, fungi, and parasites (9). Therefore, given the general paradigm of trauma, they may be considered attractive targets during the early hyper-inflammatory SIRS phase of trauma when immediate interventions are urgently needed, and also in later anti-inflammatory CARS phases when adjuvant immuno-therapies could potentially reverse the dangerous state of immunosuppression (4).
It is important to assess injury severity because understanding injury severity is essential to predict clinical outcome in patients with multiple trauma. Injury Severity Score (ISS) is one of the foremost and gold standard tests for assessing the degree of injury, which is known to have excellent predictive capability for trauma mortality (10). Recently, a study reported that human lymphocyte subsets were associated with ISS (11). Early changes within lymphocyte population have also been associated with the development of MODS after trauma, as demonstrated by increased CD56dim NK cells and reduced γδlow T cells, which have been identified as key components of the early, innate immune response (12). Furthermore, our previous study also showed that NKT cell deficiency was associated with severity score and outcome in acute cholecystitis (13). Therefore, current evidence suggests that certain innate lymphocyte subsets may play an important role in the immunological response to trauma, requiring their dynamics relevant to clinical outcome in trauma patients. However, little is known about the dynamics and clinical relevance of MAIT and NKT cells in patients with multiple trauma. Thus, the aims of the present study were to measure MAIT and NKT cell levels in the peripheral blood of patients with multiple trauma and to investigate potential relationships between these cell numbers and clinical parameters.
MATERIALS AND METHODS
Study population
The study cohort was composed of 14 patients (6 women and 8 men; mean age ± standard deviation [SD], 51.9 ± 21.0 years) who visited the Chonnam National University Hospital Regional Trauma Center after the occurrence of multiple trauma and 22 non-injured healthy controls (HCs; 9 women and 13 men; 55.7 ± 15.9 years). None of the controls had a documented history of respiratory disorders such as chronic obstructive pulmonary disease (COPD) and pulmonary embolism, autoimmune diseases, pregnancy, infectious diseases, recent surgery, malignancies, chronic liver, renal, or endocrine diseases, or had experienced fever during 72 hours prior to enrollment. Multiple trauma was defined as the presence of injury to more than one body area or system. On admission, heart rate, systolic blood pressure, body temperature, laboratory tests, Acute Physiology and Chronic Health Evaluation (APACHE) II score, Simplified Acute Physiology Score (SAPS) II, and ISS were obtained from the trauma patients.
Monoclonal antibodies (mAbs) and flow cytometry
The following mAbs and reagents were used in this study: fluorescein isothiocyanate (FITC)-conjugated anti-CD3, phycoerythrin (PE)-Cy5-conjugated anti-CD161, FITC-conjugated anti-TCR γδ and PE-conjugated 6B11 (all from Becton Dickinson, San Diego, CA, USA), allophycocyanin (APC)-conjugated anti-TCR Vα7.2 (BioLegend, San Diego, CA, USA) and APC-Alexa Fluor 750-conjugated anti-CD3 (Beckman Coulter, Marseille, France). Cells were stained with combinations of appropriate mAbs for 20 minutes at 4°C. Stained cells were analyzed on a Navios flow cytometer using Kaluza software (Beckman Coulter, Brea, CA, USA).
Isolation of peripheral blood mononuclear cells (PBMCs) and the identification of MAIT and NKT cells
Peripheral venous blood samples were collected in heparin-containing tubes, and PBMCs were isolated by density-gradient centrifugation using Ficoll-Paque Plus solution (Amersham Biosciences, Uppsala, Sweden). MAIT and NKT cells were identified phenotypically as CD3+TCRγδ−Vα7.2+CD161high and CD3+6B11+ cells, respectively, by flow cytometry, as previously described (14).
Statistical analysis
All comparisons of percentages and absolute numbers of MAIT and NKT cells in peripheral blood were performed by analysis of covariance (ANCOVA) after adjusting for age and sex using Bonferroni correction for multiple comparisons. Spearman's correlation coefficients were used to examine relationships between MAIT/NKT cell numbers and clinical or laboratory parameters. P values less than 0.05 were considered statistically significant. Statistical analysis was performed using SPSS version 18.0 software (SPSS Inc., Chicago, IL, USA).
Ethics statement
The study protocol was approved by the Institutional Review Board of Chonnam National University Hospital (IRB No. CNUH-2012-093), and written informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
RESULTS
The clinical and laboratory characteristics of the patients with multiple trauma are summarized in Table 1. Fourteen patients with mutiple trauma were included in this study. The severity of injury was categorised as mild (< 9), moderate (9–14), and severe (> 15), according to the ISS scoring system, which is considered to be the gold standard for evaluating injury severity. Of the 14 trauma patients, 2 patients (14.3%) had mild injury, 3 patients (21.4%) had moderate injury, and 9 patients (64.3%) had severe injury.
Table 1. Clinical and laboratory characteristics of the 14 patients with multiple trauma.
| Parameters | Findings |
|---|---|
| Sex (male/female), No. | 8/6 |
| Age, yr | 51.9 ± 21.0 |
| Clinical variables, score | |
| APACHE II | 8.4 ± 6.4 |
| SAPS II | 26.9 ± 15.2 |
| ISS | 15.2 ± 5.8 |
| ISS categories No. (%) | |
| Mild (< 9) | 2 (14.3) |
| Moderate (9–15) | 3 (21.4) |
| Severe (> 15) | 9 (64.3) |
| Mortality, No. (%) | 3 (21.4) |
| Laboratory variables | |
| Leukocytes, cells/μL | 10,188 ± 3,820 |
| Lymphocytes, cells/μL | 985 ± 554 |
| Neutrophils, cells/μL | 8,445 ± 3,266 |
| Monocytes, cells/μL | 705 ± 426 |
| Hemoglobin, g/dL | 10.8 ± 2.4 |
| Platelets, ×103/μL | 139 ± 44 |
| Bilirubin, mg/dL | 0.8 ± 0.5 |
| BUN, mg/dL | 15.1 ± 6.6 |
| Creatinine, mg/dL | 0.8 ± 0.3 |
| CRP, mg/dL | 11.1 ± 10.4 |
| PaO2, mmHg | 109.4 ± 34.4 |
| Lactate, mmol/L | 2.9 ± 1.6 |
| Bicarbonate, mmol/L | 21.0 ± 3.6 |
| Prothrombin time, INR | 1.34 ± 0.35 |
| SBP, mmHg | 89 ± 23 |
| HR, beat/min | 101 ± 14 |
| Body temperature, ℃ | 36.2 ± 0.4 |
Data shown are mean ± SD not otherwise specified.
APACHE = Acute Physiology and Chronic Health Evaluation, SAPS = Simplified Acute Physiology Score, ISS = Injury Severity Score, BUN = blood urea nitrogen, CRP = C-reactive protein, PaO2 = partial pressure of oxygen in arterial blood, INR = international normalized ratio, SBP = systolic blood pressure, HR = heart rate, SD = standard deviation.
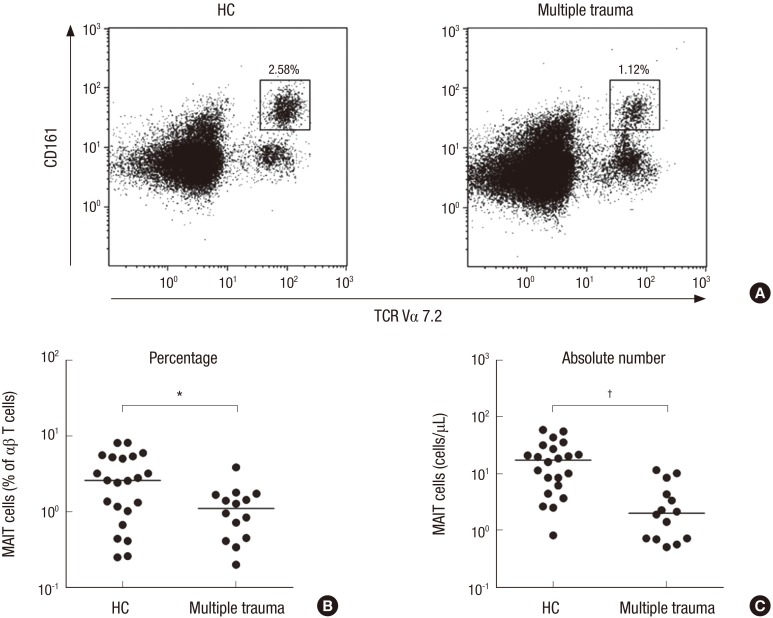
The percentages and absolute numbers of MAIT cells in the peripheral blood samples of the 14 patients with multiple trauma and the 22 HCs were determined by flow cytometry. All comparisons of percentages and absolute numbers of MAIT cells were performed by ANCOVA after adjusting for age and sex using the Bonferroni correction for multiple comparisons, as described in the ‘Patients and Methods’ section. MAIT cells were defined as CD3+TCRγδ− cells expressing TCR Vα7.2 and CD161high (Fig. 1A). Percentages of circulating MAIT cells were significantly lower in patients than in HCs (median 1.12% vs. 2.58%; P < 0.010) (Fig. 1B). Absolute numbers of MAIT cells were calculated by multiplying MAIT cell fractions by CD3+γδ− T cell fractions and total lymphocyte numbers (per microliter of peripheral blood). Patients with multiple trauma had significantly lower absolute numbers of MAIT cells than HCs (median 2.03 vs. 17.27 cells/μL; P < 0.001) (Fig. 1C).
Fig. 1.
Reduced circulating MAIT cell numbers in the peripheral blood of multiple trauma patients. Freshly isolated PBMC from 22 HCs and 14 patients with multiple trauma were stained with APC-Alexa Fluor 750-conjugated anti-CD3, FITC-conjugated anti-TCR γδ, APC-conjugated anti-TCR Vα7.2 and PE-Cy5-conjugated anti-CD161 mAbs and then analyzed by flow cytometry. Percentages of MAIT cells were calculated within a αβ T cell gate. (A) Representative MAIT cell percentages as determined by flow cytometry. (B) MAIT cell percentages among peripheral blood αβ T cells. (C) Absolute MAIT cell numbers (per microliter of blood). Symbols (●) represent individual subjects; horizontal bars show the median.
MAIT = mucosal-associated invariant T, PBMC = peripheral blood mononuclear cell, HCs = healthy controls, APC = allophycocyanin, FITC = fluorescein isothiocyanate, TCR = T cell receptor, PE = phycoerythrin, mAbs = monoclonal antibodies, ANCOVA = analysis of covariance.
*P < 0.01, †P < 0.001 by ANCOVA test.
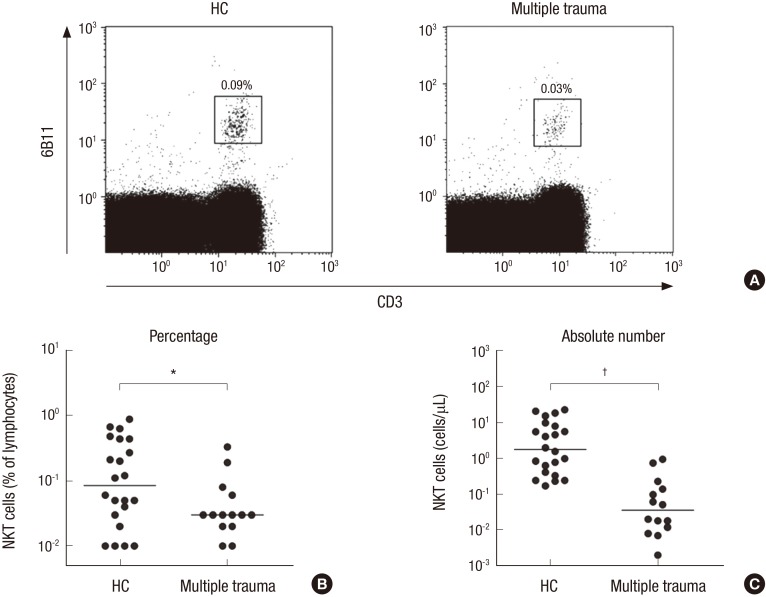
The percentages and absolute numbers of NKT cells in the peripheral blood samples of the 14 patients and 22 HCs were determined by flow cytometry. NKT cells were defined as CD3+6B11+ cells (Fig. 2A). Percentages of circulating NKT cells were significantly lower in patients than in HCs (median 0.03% vs. 0.09%; P < 0.050) (Fig. 2B). Absolute NKT cell numbers were calculated by multiplying NKT cell fractions by total lymphocyte numbers (per microliter of peripheral blood). Patients with multiple trauma had significantly lower absolute NKT cell numbers than HCs (median 0.04 vs. 1.77 cells/μL; P < 0.010) (Fig. 2C).
Fig. 2.
Reduced circulating NKT cell numbers in the peripheral blood multiple trauma patients. Freshly isolated PBMC from 22 HCs and 14 patients with multiple trauma were stained with FITC-conjugated anti CD3, PerCP-conjugated anti-CD4, APC-conjugated anti-CD8α, and PE-conjugated 6B11 mAbs, and then analyzed by flow cytometry. Percentages of NKT cells were calculated within a lymphoid gate. (A) Representative NKT cell percentages as determined by flow cytometry. (B) NKIT cell percentages among peripheral blood lymphocytes. (C) Absolute NKT cell numbers (per microliter of peripheral blood). Symbols (●) represent individual subjects and horizontal lines are median values.
NKT = natural killer T, PBMC = peripheral blood mononuclear cell, HCs = healthy controls, FITC = fluorescein isothiocyanate, APC = allophycocyanin, PE = phycoerythrin, mAbs = monoclonal antibodies, ANCOVA = analysis of covariance.
*P < 0.05, †P < 0.01 by ANCOVA test.
To investigate the clinical relevance of MAIT and NKT cell levels in patients, we explored relationships between the absolute numbers of MAIT cells and NKT cells in peripheral blood with clinical and laboratory parameters using linear regression analysis (Table 2). Because distributions were skewed, the absolute numbers of MAIT cells and NKT cells were log-transformed for the analysis. Linear regression analysis showed that log-transformed MAIT cell numbers were significantly correlated with age, APACHE II, SAPS II, ISS category, hemoglobin, and platelet count (P = 0.024; P = 0.007; P = 0.021; P = 0.005; P = 0.027; and P = 0.028, respectively). Log-transformed NKT cell numbers were found to be significantly correlated with APACHE II, SAPS II, and ISS category (P = 0.013; P = 0.049; and P = 0.015, respectively) (Table 2).
Table 2. Regression coefficients for log-transformed absolute MAIT and NKT cell numbers with respect to clinical and laboratory findings in multiple trauma patients.
| Variables | MAIT | NKT | ||||
|---|---|---|---|---|---|---|
| β | SE | P value | β | SE | P value | |
| Sex (male) | −0.324 | 0.249 | 0.217 | −0.186 | 0.434 | 0.676 |
| Age, yr | −0.013 | 0.005 | 0.024* | −0.019 | 0.009 | 0.058 |
| Mortality | −0.415 | 0.298 | 0.189 | −0.216 | 0.524 | 0.687 |
| APACHE II (score) | −0.050 | 0.016 | 0.007* | −0.078 | 0.027 | 0.013* |
| SAPS II (score) | −0.019 | 0.007 | 0.021* | −0.027 | 0.013 | 0.049* |
| ISS (categories) | −0.435 | 0.129 | 0.005* | −0.651 | 0.228 | 0.015* |
| Leukocytes, cells/µL | 0.032 | 0.025 | 0.231 | 0.037 | 0.043 | 0.410 |
| Neutrophils, cells/µL | 0.037 | 0.028 | 0.215 | 0.050 | 0.048 | 0.318 |
| Lymphocytes, cells/µL | −0.016 | 0.220 | 0.942 | −0.334 | 0.348 | 0.356 |
| Hemoglobin, g/dL | 0.072 | 0.029 | 0.027* | 0.107 | 0.049 | 0.051 |
| Platelets, cells/µL | 0.005 | 0.002 | 0.028* | 0.008 | 0.004 | 0.052 |
| Bilirubin, mg/dL | 0.018 | 0.354 | 0.613 | 0.523 | 0.569 | 0.376 |
| BUN, mg/dL | −0.009 | 0.019 | 0.643 | −0.007 | 0.032 | 0.838 |
| Creatinine, mg/dL | −0.412 | 0.521 | 0.445 | −0.583 | 0.863 | 0.512 |
| CRP, mg/dL | 0.179 | 0.088 | 0.064 | 0.173 | 0.160 | 0.299 |
| PaO2, mmHg | 0.000 | 0.000 | 0.966 | 0.009 | 0.006 | 0.190 |
| Lactate, mmol/L | −0.126 | 0.080 | 0.143 | −0.155 | 0.142 | 0.300 |
| Bicarbonate, mmol/L | 0.055 | 0.035 | 0.141 | 0.062 | 0.061 | 0.328 |
| Prothrombin time, INR | −0.661 | 0.343 | 0.078 | −0.629 | 0.620 | 0.330 |
| SBP, mmHg | 0.007 | 0.006 | 0.225 | 0.011 | 0.009 | 0.254 |
| HR, beat/min | 0.001 | 0.010 | 0.952 | 0.001 | 0.016 | 0.968 |
| Body temperature, °C | 0.200 | 0.351 | 0.580 | 0.665 | 0.554 | 0.253 |
MAIT = mucosal-associated invariant T, NKT = natural killer T, β = regression coefficient, SE = standard error, APACHE = Acute Physiology and Chronic Health Evaluation, SAPS = Simplified Acute Physiology Score, ISS = Injury Severity Score, BUN = blood urea nitrogen, CRP = C-reactive protein, PaO2 = partial pressure of oxygen in arterial blood, INR = international normalized ratio, SBP = systolic blood pressure, HR = heart rate.
*Indicates statistical significance.
DISCUSSION
In the present study, we demonstrated that absolute numbers of circulating MAIT cells were reduced early and dramatically in trauma patients as compared with HCs. The early drop occurred usually a few hours after traumatic injury. Because the reduction in circulating MAIT cell numbers could be due to lower lymphocyte counts, we obtained percentages of MAIT cells after normalization of MAIT cell numbers to lymphocyte counts. Nevertheless, the frequency of MAIT cells still remained significantly lower in the traumatic patients than in HCs. Moreover, the frequencies of CD3+ T cells in the peripheral blood lymphocytes were found to be comparable between multiple trauma patients and HCs (data not shown). These data suggest that MAIT cells follow an independent kinetic pattern in trauma patients. These findings were also observed in several previous studies on infectious diseases, including Vibrio cholerae O1 infection, Pseudomonas aeruginosa infection in cystic fibrosis, acute cholecystitis, scrub typhus, and severe sepsis (4,13,15,16,17). However, the present study lacks a comprehensive exploration of mechanisms that drive the early drop in MAIT cells. Two potential mechanisms, including apoptotic cell death and migration into injured tissues, might explain MAIT cell deficiency in trauma patients.
Another finding of our study was the early reduction in circulating NKT cells in trauma patients. In parallel with our data, a previous study using a murine model showed that following cecal ligation and puncture, murine NKT cells were markedly reduced in the liver nonparenchymal cell population, suggesting that NKT cells play a critical role in the control of the innate immune/systemic inflammatory response and survival in a model of acute septic shock (18). In contrast, our data differ from the findings reported by Grimaldi et al. (19), demonstrating that circulating NKT cell levels were similar between septic patients and HCs. Circulating NKT cell deficiency was also found in infectious diseases and autoimmune or autoinflammatory disorders, such as acute cholecystitis, mycobacterial infection, adult-onset Still's disease, and systemic lupus erythematosus (13,20,21,22). The potential mechanism that drives the early decline in NKT cells following injury in humans and animals remains unclear, but it may be one of the followings mechanisms: invariant TCR internalization, activation-induced cell death, and migration into injured tissues. Further studies are needed to identify the pathomechanism of the decline in NKT cells.
Besides MAIT cells and NKT cells, γδ T cells comprise one of the innate-like T lymphocyte subsets, which are particularly abundant in human blood (23). To determine the specificity of changes in frequency among different innate-like T cell subsets, we next performed an additional analysis of γδ T cell levels in the present study. The analysis revealed that γδ T cell levels were reduced in trauma patients, indicating that after traumatic injury, all major innate-like T cell subsets exhibit similar changes in frequency, irrespective of their specific cell type (data not shown). In contrast with our data, a recent study demonstrated that unlike other innate-like T cells, MAIT cells were specifically reduced in severe infections, in particular, more prominent in non-streptococcal infections (19). These different changes in frequency in innate-like T cells between trauma and sepsis can be explained in part by the possibility that their activation can be differently initiated according to the specific type of danger signals, which originate from endogenous damage-associated molecular patterns (DAMPs) during trauma or exogenous DAMPs during sepsis (24).
Previous studies provided evidence that MAIT and NKT cells have a close lineage relationship and a shared feature (25). In particular, they were found to be highly susceptible to activation-induced cell death (26). We investigated the numerical association between MAIT cells and NKT cells, and it showed that MAIT cell levels were significantly correlated with NKT cell levels in peripheral blood of trauma patients (data not shown). In addition, our univariate analysis showed that MAIT and NKT cell numbers were inversely correlated with clinical risk factors, such as APACHE II, SAPS II, and ISS scores in trauma patients. Furthermore, another interesting finding of our study was the association between MAIT cell numbers and age, hemoglobin level, or platelet count, although there was a weak association between NKT cell numbers and these parameters. Age and/or hemoglobin are known to account for a part of parameters for APACHE II and SAPS II scoring systems (27,28). Collectively, these findings indicate that MAIT and NKT cell levels reflect disease severity and inflammation following trauma. The prognostic values of these invariant T cells were also reported in our previous studies on acute cholecystitis and COPD (13,29). However, the limited sample size precluded a multivariate analysis for clinical relevance of these invariant T cells in trauma patients. Further investigations in large populations are needed to determine the functional role of MAIT and NKT cells in the pathogenesis of multiple trauma.
Summarizing, the present study describes numerical deficiencies of circulating MAIT cells and NKT cells in patients with multiple trauma. In addition, these invariant T cell deficiencies were found to be associated with disease severity.
Footnotes
Funding: This study was supported by the National Research Foundation of Korea funded by the Korean Government (Grants No. 2013R1A2A2A01067956, 2015R1D1A4A01019017, and 2015R1D1A1A01059762) and the Chonnam National University Hospital Biomedical Research Institute (Grants No. BCRI15001-43, CRI13905-22.4, CRI16036-22, and CRI17028-1).
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conceptualization: Jo YG, Choi HJ, Kim JC, Kee SJ, Park YW. Data curation: Jo YG, Choi HJ, Kim JC, Cho YN, Kang JH, Jin HM. Formal analysis: Jo YG, Choi HJ, Kim JC, Cho YN, Jin HM, Kee SJ, Park YW. Investigation: Jo YG, Cho YN, Kee SJ, Park YW. Project administration: Jo YG, Choi HJ, Kim JC, Kee SJ, Park YW. Writing - original draft: Jo YG, Choi HJ, Kim JC, Cho YN, Kee SJ, Park YW.
References
- 1.Karakuş A, Kekeç Z, Akçan R, Seydaoğlu G. The relationship of trauma severity and mortality with cardiac enzymes and cytokines at multiple trauma patients. Ulus Travma Acil Cerrahi Derg. 2012;18:289–295. doi: 10.5505/tjtes.2012.81488. [DOI] [PubMed] [Google Scholar]
- 2.Mannick JA, Rodrick ML, Lederer JA. The immunologic response to injury. J Am Coll Surg. 2001;193:237–244. doi: 10.1016/s1072-7515(01)01011-0. [DOI] [PubMed] [Google Scholar]
- 3.Baue AE, Durham R, Faist E. Systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), multiple organ failure (MOF): are we winning the battle? Shock. 1998;10:79–89. doi: 10.1097/00024382-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Szabo PA, Anantha RV, Shaler CR, McCormick JK, Haeryfar SM. CD1d- and MR1-restricted T cells in sepsis. Front Immunol. 2015;6:401. doi: 10.3389/fimmu.2015.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J Exp Med. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, Bhati M, Chen Z, Kostenko L, Reantragoon R, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491:717–723. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- 7.Van Kaer L. alpha-Galactosylceramide therapy for autoimmune diseases: prospects and obstacles. Nat Rev Immunol. 2005;5:31–42. doi: 10.1038/nri1531. [DOI] [PubMed] [Google Scholar]
- 8.Le Bourhis L, Guerri L, Dusseaux M, Martin E, Soudais C, Lantz O. Mucosal-associated invariant T cells: unconventional development and function. Trends Immunol. 2011;32:212–218. doi: 10.1016/j.it.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Le Bourhis L, Martin E, Péguillet I, Guihot A, Froux N, Coré M, Lévy E, Dusseaux M, Meyssonnier V, Premel V, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010;11:701–708. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- 10.Bolorunduro OB, Villegas C, Oyetunji TA, Haut ER, Stevens KA, Chang DC, Cornwell EE, 3rd, Efron DT, Haider AH. Validating the Injury Severity Score (ISS) in different populations: ISS predicts mortality better among Hispanics and females. J Surg Res. 2011;166:40–44. doi: 10.1016/j.jss.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Hua R, Chen FX, Zhang YM, Zhou ZH, Wang SJ, Liang J. Association of traumatic severity with change in lymphocyte subsets in the early stage after trauma. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2013;25:489–492. doi: 10.3760/cma.j.issn.2095-4352.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Manson J, Cole E, De’Ath HD, Vulliamy P, Meier U, Pennington D, Brohi K. Early changes within the lymphocyte population are associated with the development of multiple organ dysfunction syndrome in trauma patients. Crit Care. 2016;20:176. doi: 10.1186/s13054-016-1341-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JC, Jin HM, Cho YN, Kwon YS, Kee SJ, Park YW. Deficiencies of circulating mucosal-associated invariant T cells and natural killer T cells in patients with acute cholecystitis. J Korean Med Sci. 2015;30:606–611. doi: 10.3346/jkms.2015.30.5.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho YN, Kee SJ, Kim TJ, Jin HM, Kim MJ, Jung HJ, Park KJ, Lee SJ, Lee SS, Kwon YS, et al. Mucosal-associated invariant T cell deficiency in systemic lupus erythematosus. J Immunol. 2014;193:3891–3901. doi: 10.4049/jimmunol.1302701. [DOI] [PubMed] [Google Scholar]
- 15.Kang SJ, Jin HM, Won EJ, Cho YN, Jung HJ, Kwon YS, Kee HJ, Ju JK, Kim JC, Kim UJ, et al. Activation, impaired tumor necrosis factor-alpha production, and deficiency of circulating mucosal-associated invariant T cells in patients with scrub typhus. PLoS Negl Trop Dis. 2016;10:e0004832. doi: 10.1371/journal.pntd.0004832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leung DT, Bhuiyan TR, Nishat NS, Hoq MR, Aktar A, Rahman MA, Uddin T, Khan AI, Chowdhury F, Charles RC, et al. Circulating mucosal associated invariant T cells are activated in Vibrio cholerae O1 infection and associated with lipopolysaccharide antibody responses. PLoS Negl Trop Dis. 2014;8:e3076. doi: 10.1371/journal.pntd.0003076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith DJ, Hill GR, Bell SC, Reid DW. Reduced mucosal associated invariant T-cells are associated with increased disease severity and Pseudomonas aeruginosa infection in cystic fibrosis. PLoS One. 2014;9:e109891. doi: 10.1371/journal.pone.0109891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu CK, Venet F, Heffernan DS, Wang YL, Horner B, Huang X, Chung CS, Gregory SH, Ayala A. The role of hepatic invariant NKT cells in systemic/local inflammation and mortality during polymicrobial septic shock. J Immunol. 2009;182:2467–2475. doi: 10.4049/jimmunol.0801463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grimaldi D, Le Bourhis L, Sauneuf B, Dechartres A, Rousseau C, Ouaaz F, Milder M, Louis D, Chiche JD, Mira JP, et al. Specific MAIT cell behaviour among innate-like T lymphocytes in critically ill patients with severe infections. Intensive Care Med. 2014;40:192–201. doi: 10.1007/s00134-013-3163-x. [DOI] [PubMed] [Google Scholar]
- 20.Kee SJ, Kwon YS, Park YW, Cho YN, Lee SJ, Kim TJ, Lee SS, Jang HC, Shin MG, Shin JH, et al. Dysfunction of natural killer T cells in patients with active Mycobacterium tuberculosis infection. Infect Immun. 2012;80:2100–2108. doi: 10.1128/IAI.06018-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SJ, Cho YN, Kim TJ, Park SC, Park DJ, Jin HM, Lee SS, Kee SJ, Kim N, Yoo DH, et al. Natural killer T cell deficiency in active adult-onset Still’s Disease: correlation of deficiency of natural killer T cells with dysfunction of natural killer cells. Arthritis Rheum. 2012;64:2868–2877. doi: 10.1002/art.34514. [DOI] [PubMed] [Google Scholar]
- 22.Cho YN, Kee SJ, Lee SJ, Seo SR, Kim TJ, Lee SS, Kim MS, Lee WW, Yoo DH, Kim N, et al. Numerical and functional deficiencies of natural killer T cells in systemic lupus erythematosus: their deficiency related to disease activity. Rheumatology (Oxford) 2011;50:1054–1063. doi: 10.1093/rheumatology/keq457. [DOI] [PubMed] [Google Scholar]
- 23.Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB. The burgeoning family of unconventional T cells. Nat Immunol. 2015;16:1114–1123. doi: 10.1038/ni.3298. [DOI] [PubMed] [Google Scholar]
- 24.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359–365. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Greenaway HY, Ng B, Price DA, Douek DC, Davenport MP, Venturi V. NKT and MAIT invariant TCRalpha sequences can be produced efficiently by VJ gene recombination. Immunobiology. 2013;218:213–224. doi: 10.1016/j.imbio.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Gérart S, Sibéril S, Martin E, Lenoir C, Aguilar C, Picard C, Lantz O, Fischer A, Latour S. Human iNKT and MAIT cells exhibit a PLZF-dependent proapoptotic propensity that is counterbalanced by XIAP. Blood. 2013;121:614–623. doi: 10.1182/blood-2012-09-456095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 28.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 29.Kwon YS, Jin HM, Cho YN, Kim MJ, Kang JH, Jung HJ, Park KJ, Kee HJ, Kee SJ, Park YW. Mucosal-associated invariant T cell deficiency in chronic obstructive pulmonary disease. COPD. 2016;13:196–202. doi: 10.3109/15412555.2015.1069806. [DOI] [PubMed] [Google Scholar]