Abstract
The present study aimed to investigate the distribution of total serum immunoglobulin E (IgE) levels in Korean schoolchildren and to evaluate its utility in the prediction of atopy and allergic diseases. A nationwide, cross-sectional survey was conducted in first grade students from randomly selected elementary and middle schools. Total IgE levels were measured by ImmunoCAP. Skin prick tests were performed for 18 common inhalant allergens to determine the presence of atopy. Children aged 12–13 years and parents of children aged 6–7 years were asked to complete questionnaire assessing allergic diseases. The cut-off levels of total IgE were determined by analyzing receiver operating characteristic curves. The median total IgE level was 86.7 kU/L (range: 1.5–4,523.1) in 3,753 children aged 6–7 years and 94.7 kU/L (range: 1.5–3,000.0) in 3,930 children aged 12–13 years. Total IgE concentrations were higher in children with atopy or allergic diseases than in those without (all P < 0.001). At the cut-off value of 127.7 kU/L, sensitivity, specificity, and positive and negative predictive values (PPV and NPV) were 67.1%, 75.4%, 65.4%, and 76.7%, respectively, in elementary schoolchildren. At the cut-off value of 63.0 kU/L, sensitivity, specificity, PPV, and NPV were 81.9%, 66.6%, 75.0%, and 75.1%, respectively, in middle schoolchildren. PPV and NPV were ≥ 70% when cut-offs of 258.8 kU/L and 38.4 kU/L were used for the diagnosis of atopy in 6–7 year-olds and 12–13 year-olds, respectively. This nationwide population-based study provided the first normal reference ranges of total IgE in Korean schoolchildren.
Keywords: Allergy, Atopy, Immunoglobulin E, Schoolchildren, Korea
Graphical Abstract
INTRODUCTION
Allergic diseases such as atopic dermatitis (AD), allergic rhinitis (AR), and asthma are the most common chronic diseases in children. The chronic course and frequent recurrences of allergy symptoms have a negative impact on the quality of life of children and their parents. According to the International Study of Asthma and Allergies in Childhood (ISAAC), the prevalence of AD, rhinoconjunctivitis, and asthma are still increasing in Korean schoolchildren (1).
It is widely assumed that atopy is related to allergic diseases in children, because immunoglobulin E (IgE) plays a pivotal role in the pathogenesis of allergic diseases. Atopic sensitization in children is well-known as an important risk factor of severe asthma, and can be a predisposing factor for the development of asthma and AR (2,3,4). Therefore, measurement of serum total IgE levels has been used to evaluate allergic subjects in clinical practice, and to determine risk factors associated with allergic diseases in various studies. Eligibility and dosing for omalizumab treatment also depend on the serum total IgE concentration (5). However, there have been controversies regarding the relationship between IgE-mediated sensitization and allergic diseases. For example, the prevalence of atopy is higher in middle schoolchildren than in elementary schoolchildren, while the prevalence of allergic diseases is higher in elementary schoolchildren than in middle schoolchildren (1,6). Various environmental factors may account for the discrepancy between the prevalence of atopy and allergic diseases (7,8). Non-atopic asthma and intrinsic AD indicate that not all allergic diseases have an atopic basis (9,10).
Moreover, determination of reference values of total IgE is difficult due to the wide range of levels and diverse factors. Total IgE values are influenced by age, race, gender, geographic area, seasons, exposure to environmental pollutants, and history of certain non-allergic diseases (11,12,13). The clinical utility of total IgE values depends on the establishment of reliable reference values for the respective population. However, there are few studies investigating reference values and clinical utility of total IgE in Korean schoolchildren from the general population. Therefore, we conducted this study to investigate the distribution of total serum IgE levels in Korean schoolchildren and to evaluate its utility for the prediction of atopy or allergic diseases.
MATERIALS AND METHODS
Study design and population
A nationwide, cross-sectional survey was conducted in children aged 6–7 years and 12–13 years between October and November 2010. To obtain a representative population, the survey was performed in first-grade students from 45 elementary schools and 40 middle schools in Korea. The sampling method followed a stratified 2-stage cluster sampling according to the method described in our previous study (6,14). First, every school in Korea was stratified according to geographic region and school type. We chose sample schools using the systematic probability proportional to size sampling procedure. To fulfill the required sample size, replacement schools were chosen to substitute the nonparticipating schools. In the second stage, 3 classes were selected randomly from the first grade of each sample school. All children and their parents in the sample classes were asked to participate. Response rates among parents of children aged 6–7 years and children aged 12–13 years were 92.1% and 93.8%, respectively.
Measurements
Children aged 12–13 years and parents of children aged 6–7 years were asked to complete a Korean-translated modified version of the ISAAC questionnaire assessing AD, AR, and asthma. We defined symptoms suggestive of allergic diseases as follows: 1) those who had experienced itchy eczema episodes, or a problem with nasal symptoms or wheezing unrelated to a cold within 12 months of the survey; 2) those who had been diagnosed with AD, AR, or asthma by a physician in their lifetime; and 3) those who had received treatment for AD, AR, or asthma within 12 months of the survey.
On the day of the survey, blood samples were taken to measure total IgE levels. Total IgE levels were determined by the Immuno-CAP (Thermo Fisher Scientific, Waltham, MA, USA) system. A skin prick test (SPT) was performed using 18 common inhalant allergens including Dermatophagoides pteronyssinus, D. farinae, Tyrophagus putrescentiae, cockroach, cat, dog, alder, birch, oak, Japanese cedar (Lofarma, Milan, Italy), orchard grass, Bermuda grass, timothy, mugwort, ragweed, Japanese hop, Alternaria alternata, and Aspergillus fumigatus. Histamine was used as a positive control and normal saline as a negative control. Unless otherwise stated, allergens were provided by Allergopharma, Reinbek, Germany. The largest and perpendicular diameter of the wheal for each of the allergens was measured, and the following value was calculated: (largest + perpendicular diameter)/2. A test was regarded as positive if the value calculated was ≥ 3 mm and controls showed adequate reactions. Atopy was defined as a positive response to a SPT of at least one allergen.
Statistical analysis
We used SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) for statistical analyses. Plotting the IgE frequencies on an arithmetic scale demonstrated a highly skewed distribution and logarithmic scale approximated a normal distribution. Therefore, total IgE levels were logarithmically transformed for analyses. The Wilcoxon rank sum test was used to investigate the association of total IgE levels with gender, the presence of allergic diseases, and atopy. Performance characteristics, such as sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated. The cut-off levels of total IgE were determined by 3 methods as follows: 1) the level to maximize (sensitivity + specificity) for predicting the presence of allergic diseases or atopy in the receiver operating characteristic (ROC) curves; 2) the level of indicating 80% risk of atopy; and 3) the level to maximize (PPV + NPV) with both PPV and NPV greater than 70% for identifying atopy. A P value of < 0.05 was considered significant.
Ethics statement
This study was approved by the Institutional Review Board of Dankook University (DKUH IRB 2010-09-0260) and written informed consent was obtained from each parent prior to his/her child's participation in the study.
RESULTS
Study population
Total serum IgE levels and SPT were tested in 3,753 children aged 6–7 years and 3,930 children aged 12–13 years. The prevalence of positive SPT response to at least one inhalant allergen was 41.0% in children aged 6–7 years, and 55.0% in children aged 12–13 years (Table 1). The lifetime prevalence of asthma, AR, and AD was 10.2%, 37.0%, and 35.7%, respectively in children aged 6–7 years. The lifetime prevalence of asthma, AR, and AD was 7.2%, 29.1%, and 23.4%, respectively in children aged 12–13 years.
Table 1. Characteristics of study population.
| Characteristics | Age group | |
|---|---|---|
| 6–7 yr (n = 3,753) | 12–13 yr (n = 3,930) | |
| Gender | ||
| Male | 1,892 (50.4) | 1,947 (49.5) |
| Female | 1,861 (49.6) | 1,983 (50.5) |
| No. of allergens showing positive | ||
| SPT results | ||
| 0 | 2,214 (59.0) | 1,768 (45.0) |
| 1 | 315 (8.4) | 383 (9.7) |
| 2 | 729 (19.4) | 797 (20.3) |
| ≥ 3 | 495 (13.2) | 982 (25.0) |
| Diagnosis, ever | ||
| Asthma | 384 (10.2) | 286 (7.3) |
| AR | 1,391 (37.1) | 1,162 (29.6) |
| AD | 1,339 (35.7) | |
| Symptom, within the last 12 months | ||
| Asthma | 368 (9.8) | 322 (8.2) |
| AR | 1,622 (43.2) | 1,649 (42.0) |
| AD | 762 (20.3) | 501 (12.7) |
| Treatment, within the last 12 months | ||
| Asthma | 144 (3.8) | 62 (1.6) |
| AR | 1,077 (28.7) | 788 (20.1) |
| AD | 573 (15.3) | 342 (8.7) |
| Paternal history of allergic diseases | 1,079 (28.8) | 692 (17.6) |
| Maternal history of allergic diseases | 1,230 (32.8) | 856 (21.8) |
Values are presented as number of children (%).
SPT = skin prick test, AR = allergic rhinitis, AD = atopic dermatitis.
Total IgE ranged from 1.5–4,523.1 kU/L in elementary schoolchildren and from 1.5–3,000 kU/L in middle schoolchildren. The median total IgE level was 86.7 kU/L (75th percentile, 292.6 kU/L; 90th percentile, 698.5 kU/L; 95th percentile, 1,200.7 kU/L) in elementary schoolchildren (Table 2). The median total IgE level was 94.7 kU/L (75th percentile, 284.3 kU/L; 90th percentile, 625.1 kU/L; 95th percentile, 990.7 kU/L) in middle schoolchildren (Table 3). Boys had significant higher total IgE levels than girls in both elementary and middle schoolchildren (all P < 0.001) (Tables 2 and 3). Total IgE levels were higher in children who had a positive SPT response than in those who did not (all P < 0.001 at both age groups) (Tables 2 and 3). In addition, total IgE concentrations were higher in children with allergic diseases than in those without allergic diseases (all P < 0.001 at both age groups) (Tables 2 and 3).
Table 2. Total serum IgE levels in children aged 6–7 years.
| Characteristics | No. | Geometric mean | 25th | Median | 75th | 90th | 95th | P value |
|---|---|---|---|---|---|---|---|---|
| Overall | 3,753 | 89.0 | 28.5 | 86.7 | 292.6 | 698.5 | 1,200.7 | |
| Gender | < 0.001 | |||||||
| Male | 1,892 | 112.6 | 36.2 | 123.3 | 368.8 | 784.0 | 1252.2 | |
| Female | 1,861 | 70.1 | 23.4 | 63.6 | 208.8 | 594.9 | 1099.3 | |
| Atopy* | < 0.001 | |||||||
| Yes | 1,539 | 220.5 | 85.5 | 246.5 | 586.8 | 1247.0 | 1871.1 | |
| No | 2,214 | 47.4 | 17.9 | 44.7 | 124.9 | 308.8 | 509.7 | |
| Diagnosis of allergic diseases, ever | < 0.001 | |||||||
| Yes | 2,208 | 106.2 | 32.8 | 108.3 | 351.7 | 820.4 | 1480.4 | |
| No | 1,545 | 69.2 | 24.3 | 63.3 | 210.5 | 506.7 | 876.7 | |
| Symptom of allergic diseases, within the last 12 months | < 0.001 | |||||||
| Yes | 2,048 | 121.7 | 36.0 | 127.2 | 386.7 | 853.0 | 1485.9 | |
| No | 1,698 | 56.4 | 23.2 | 59.9 | 184.4 | 457.3 | 851.1 | |
| Treatment for allergic diseases, within the last 12 months | < 0.001 | |||||||
| Yes | 1,468 | 126.9 | 36.0 | 129.7 | 402.4 | 913.6 | 1697.6 | |
| No | 2,284 | 65.3 | 25.8 | 69.4 | 220.3 | 559.9 | 925.2 | |
IgE = immunoglobulin E, SPT = skin prick test.
*Defined as at least a positive SPT response.
Table 3. Total serum IgE levels in children aged 12–13 years.
| Characteristics | No. | Geometric mean | 25th | Median | 75th | 90th | 95th | P value |
|---|---|---|---|---|---|---|---|---|
| Overall | 3,930 | 90.6 | 30.8 | 94.7 | 284.3 | 625.1 | 990.7 | |
| Gender | < 0.001 | |||||||
| Male | 1,947 | 101.4 | 37.1 | 112.2 | 297.8 | 670.0 | 1042.0 | |
| Female | 1,983 | 81.1 | 26.1 | 80.6 | 272.3 | 589.6 | 945.9 | |
| Atopy* | < 0.001 | |||||||
| Yes | 2,162 | 191.0 | 83.2 | 206.1 | 451.9 | 888.0 | 1340.7 | |
| No | 1,768 | 36.3 | 14.5 | 34.6 | 90.0 | 217.6 | 382.5 | |
| Diagnosis of allergic diseases, ever | < 0.001 | |||||||
| Yes | 1,796 | 123.2 | 43.1 | 141.2 | 396.2 | 836.6 | 1326.8 | |
| No | 2,134 | 69.9 | 25.0 | 71.1 | 202.5 | 447.0 | 715.8 | |
| Symptom of allergic diseases, within the last 12 months | < 0.001 | |||||||
| Yes | 1,931 | 125.6 | 44.1 | 143.5 | 390.7 | 818.4 | 1323.8 | |
| No | 1,979 | 65.9 | 23.6 | 67.2 | 190.2 | 442.7 | 729.3 | |
| Treatment for allergic diseases, within the last 12 months | < 0.001 | |||||||
| Yes | 1,027 | 145.3 | 54.5 | 172.4 | 468.1 | 945.3 | 1495.4 | |
| No | 2,893 | 76.6 | 26.5 | 78.3 | 224.6 | 513.4 | 837.6 | |
IgE = immunoglobulin E, SPT = skin prick test.
*Defined as at least a positive SPT response.
In ROC analysis of total IgE for predicting the diagnosis of allergic diseases in their lifetime, the area under curve (AUC) was 0.578 (95% confidence interval [CI], 0.560–0.597) at the value of 92.7 kU/L in 6–7 year-olds and 0.610 (95% CI, 0.592–0.628) at the value of 238.8 kU/L in 12–13 year-olds. In addition, ROC analysis for predicting recent symptoms or treatment showed the AUC of 0.609 (95% CI, 0.591–0.627) and 0.587 (95% CI, 0.568–0.606) at the values of 86.5 and 230.4 kU/L in 6–7 year-olds, and the AUC of 0.623 (95% CI, 0.606–0.641) and 0.624 (95% CI, 0.604–0.645) at the values of 132.7 and 143.5 kU/L in 12–13 year-olds.
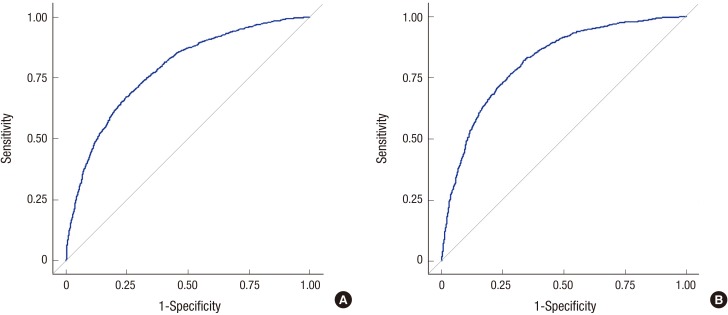
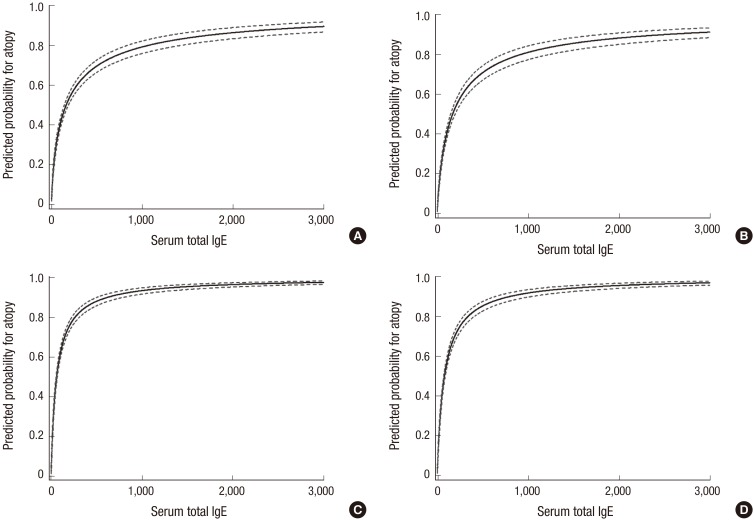
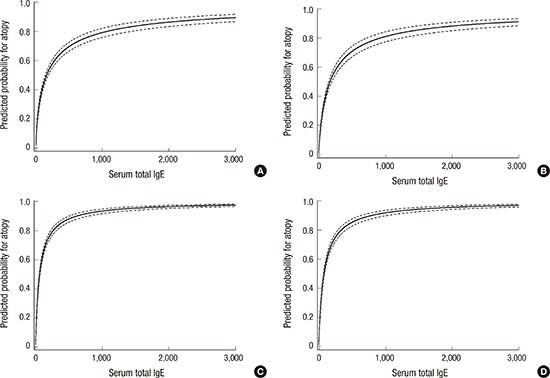
In ROC analysis of total IgE for predicting atopy, the AUC was 0.784 (95% CI, 0.769–0.798) in children aged 6–7 years and 0.817 (95% CI, 0.803–0.830) in children aged 12–13 years. At the optimal cut-off value of 127.7 kU/L, sensitivity, specificity, PPV, and NPV were 67.1%, 75.4%, 65.4%, and 76.7%, respectively, in children aged 6–7 years (Fig. 1). At the optimal cut-off value of 63.0 kU/L, sensitivity, specificity, PPV, and NPV were 81.9%, 66.6%, 75.0%, and 75.1%, respectively, in children aged 12–13 years (Fig. 1). Total IgE concentrations indicating 80% risk of atopy were calculated to be 984.2 kU/L in children aged 6–7 years and 295.6 kU/L in children aged 12–13 years (Fig. 2). Total IgE levels of 258.8 kU/L had a PPV of 74.2%, a NPV of 71.2%, a sensitivity of 48.7%, and a specificity of 88.2% in children aged 6–7 years. In children aged 12–13 years, total IgE levels of 38.4 kU/L presented a PPV of 70.0%, NPV of 81.6%, sensitivity of 90.3%, and specificity of 52.8%.
Fig. 1.
ROC curves of serum total IgE levels, indicating the sensitivity and specificity of total IgE levels for predicting atopy in (A) children aged 6–7 years and (B) children aged 12–13 years.
ROC = receiver operating characteristic, IgE = immunoglobulin E.
Fig. 2.
Predicted probability of atopy for total IgE with 95% confidence intervals (dotted line) in (A) boys and (B) girls aged 6 to 7 years, and (C) boys and (D) girls aged 12–13 years. Two elementary school children were excluded from the analysis, because their total IgE levels were outliers (> 3,000 kU/L).
IgE = immunoglobulin E.
DISCUSSION
Serum total IgE levels have been widely used to aid in the diagnosis of allergic disease, although the reference values are affected by various factors, such as age, sex, race, and geographic area (13,15,16,17,18). Its usefulness depends on the establishment of reliable reference values for the particular population and age groups, therefore cut-off value should be derived from the most recent and geographically defined normalized reference values. To our knowledge, this is the first nationwide, large population-based study to show the distribution of the total IgE levels in Korean schoolchildren. This study showed that total IgE levels discriminate Korean schoolchildren with and without atopy, with the optimal cut-off of 127.7 kU/L in 6–7 year-olds and 63.0 kU/L in 12–13 year-olds.
Notably, our present study demonstrated that the total IgE levels of Korean schoolchildren were higher than those previously established in Western countries (11,19,20). Geometric mean values of total IgE were reported to be 51.8 kU/L and 54.4 kU/L in US children aged 6–11 and 12–15 years, respectively (11). An European study showed that the geometric mean values of total IgE were 51.9 kU/L in 5–7 year-olds and 62.2 kU/L in 11–14 year-olds (20). However, a study among Taiwanese children aged 5–18 years reported geometric mean total IgE levels of 91.1 kU/L, which were similar to those of our study (17). A previous Korean study reported a geometric mean total IgE level of 80.5 kU/L in preschool children aged 3–6 years (15). However, no information has been available on reference values of total IgE in Korean schoolchildren. These findings indicated that reference values of total IgE were similar in Asian children, although age differences were observed among studies. In addition, results from the previous study in Korean preschool children and the present study indicate that total IgE levels gradually increase with age. These results are in agreement with those of previous studies that reported total IgE levels increased after birth, with maximum values achieved after 10 years of age, although there is controversy about the exact age at which peak total IgE is achieved (16,18,19,21).
With respect to sex, several previous studies have demonstrated that total IgE levels were higher in males than in females (11,13,15,22). We also found that boys had significantly higher total IgE levels than girls. Boys are more likely to have a number of atopic diseases such as rhinitis and asthma in childhood (23,24). A birth cohort study showed distinctive patterns of cytokine responses between girls and boys (13). Boys exhibited higher interferon-gamma (IFN-gamma), interleukin (IL)-5, and IL-13 responses, indicating that different amounts and direction of cytokine responses may lead to sex-specific atopic characteristics during childhood. Sex hormones are also implicated in immune development such as the maturation of B lymphocytes, but the effect of hormonal influences on the development of atopy in young children remains poorly understood (25,26). Further investigations are therefore needed to examine gender differences in allergic diseases and the underlying mechanism.
As found in the present study, many studies have also shown that total IgE concentrations tend to be higher in patients with allergic diseases compared with non-allergic individuals (11,15,16,17,18,19). A study in the US reported that the geometric mean for total IgE level was significantly higher among asthmatic subjects than non-asthmatics subjects (81.1 vs. 40.8 kU/L) (11). In a previous Korean study, the atopic group had higher total IgE levels than the non-atopic group (158.00 kU/L vs. 52.75 kU/L) (15). Therefore, there has been considerable interest in the validity of total IgE level as diagnostic tool for allergy. However, the diagnostic value of total IgE levels seems to be limited, although many patients with allergic disorders have elevated levels of total IgE (12,17,19,27,28,29,30,31,32). A Taiwanese study calculated that the cut-off value of total IgE for discriminating between an atopy and non-atopy group was 77.7 kU/L, with sensitivity and specificity of 82.3% and 87.1%, respectively (17). A Saudi Arabian study also reported a total IgE specificity of 83.4% for the allergic group, but sensitivity was only 61.3% when the cut-off level was 195 kU/L (32).
Previous studies showed the relationship between asthma and total IgE levels in the absence of sensitization to specific allergens, supporting the existence of non-atopic individuals with asthma (33,34). Total IgE levels were found to be associated with the prevalence of asthma, even in subjects without the presence of allergen-specific IgE (33,34). However, this current study confirms that total IgE level alone is insufficient for the diagnosis of allergy and atopy. Our results showed that upper levels of total IgE in the non-atopic group could not be used to predict atopy because of the wide overlap in total IgE levels between atopic and non-atopic subjects. The optimal cut-off of 127.7 kU/L had a sensitivity of 67.1% and a specificity of 75.4% in children aged 6–7 years. In children aged 12–13 years, the cut-off of 63.0 kU/L presented a sensitivity of 81.9% and a specificity of 66.6%. These results demonstrated that the sensitivity and specificity of the cut-off value of total IgE were not satisfactory to rely on positive results to predict atopy. Nevertheless, an important finding in our study is that both PPV and NPV exhibited sufficiently high values (≥ 70%) when the cut-off value of 258.8 kU/L was used for diagnosis of atopy in 6–7 year-olds, suggesting that clinicians could use values of total IgE to predict or rule out the likelihood of atopy in elementary schoolchildren. Although the cut-off value of 38.4 kU/L showed both PPV and NPV greater than 70% for identifying atopy in 12–13 year-olds, the point was lower than the median indicating the limited usefulness.
Our study had some limitations that must be considered. The most important limitation stems from the questionnaire-based study design, which introduces the possibility of recall bias regarding the diagnosis of allergic diseases. In addition, as total IgE levels vary by season, the seasonal variation of total IgE level should be taken into consideration (35). The present study was conducted during autumn to estimate interindividual differences of total IgE levels in one season. However, our study population was a nationally representative sample of Korean schoolchildren through 2-stage cluster sampling and a random selection method, which made the results more reliable and meaningful than previous studies. Moreover, subjects in the present study were not chosen on the basis of disease or from hospital settings, but were selected from the community.
In conclusion, this nationwide large population-based study provides the first normal reference ranges of total IgE in Korean schoolchildren. Total serum IgE level was higher in children with atopy or allergic diseases. The cut-off levels of 258.8 kU/L in 6–7 year-olds and 38.4 kU/L in 12–13 year-olds for the prediction of atopy showed sufficiently high values (≥ 70%) in Korean children.
ACKNOWLEDGMENT
We thank the entire survey team, including the field supervisors, field workers, and pediatricians.
Footnotes
Funding: This research was supported by a fund (2010E3303400) from Research of Korea Centers for Disease Control and Prevention.
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conceptualization: Kim HY, Choi J, Ahn K, Hahm MI, Lee SY, Kim WK, Chae Y, Park YM, Han MY, Lee KJ, Kwon HJ, Kim S, Yoo H, Kim J. Data curation: Kim HY, Choi J, Kim J. Formal analysis: Lee KJ, Kwon HJ, Kim S, Yoo H. Funding acquisition: Kwon HJ. Investigation: Kim HY, Choi J, Ahn K, Hahm MI, Lee SY, Kim WK, Chae Y, Park YM, Han MY. Writing - review & editing: Kim HY, Choi J, Kim J.
References
- 1.Ahn K, Kim J, Kwon HJ, Chae Y, Hahm MI, Lee KJ, Park YM, Lee SY, Han M, Kim WK. The prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in Korean children: nationwide cross-sectional survey using complex sampling design. J Korean Med Assoc. 2011;54:769–778. [Google Scholar]
- 2.Lu KD, Phipatanakul W, Perzanowski MS, Balcer-Whaley S, Matsui EC. Atopy, but not obesity is associated with asthma severity among children with persistent asthma. J Asthma. 2016;53:1033–1044. doi: 10.3109/02770903.2016.1174259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Illi S, von Mutius E, Lau S, Niggemann B, Grüber C, Wahn U, Multicentre Allergy Study (MAS) group Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368:763–770. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 4.Codispoti CD, Levin L, LeMasters GK, Ryan P, Reponen T, Villareal M, Burkle J, Stanforth S, Lockey JE, Khurana Hershey GK, et al. Breast-feeding, aeroallergen sensitization, and environmental exposures during infancy are determinants of childhood allergic rhinitis. J Allergy Clin Immunol. 2010;125:1054–1060.e1. doi: 10.1016/j.jaci.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowe PJ, Renard D. Omalizumab decreases IgE production in patients with allergic (IgE-mediated) asthma; PKPD analysis of a biomarker, total IgE. Br J Clin Pharmacol. 2011;72:306–320. doi: 10.1111/j.1365-2125.2011.03962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J, Hahm MI, Lee SY, Kim WK, Chae Y, Park YM, Han MY, Lee KJ, Kwon HJ, Jung JA, et al. Sensitization to aeroallergens in Korean children: a population-based study in 2010. J Korean Med Sci. 2011;26:1165–1172. doi: 10.3346/jkms.2011.26.9.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Subbarao P, Mandhane PJ, Sears MR. Asthma: epidemiology, etiology and risk factors. CMAJ. 2009;181:E181–E190. doi: 10.1503/cmaj.080612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn K. The role of air pollutants in atopic dermatitis. J Allergy Clin Immunol. 2014;134:993–999. doi: 10.1016/j.jaci.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 9.Pugliarello S, Cozzi A, Gisondi P, Girolomoni G. Phenotypes of atopic dermatitis. J Dtsch Dermatol Ges. 2011;9:12–20. doi: 10.1111/j.1610-0387.2010.07508.x. [DOI] [PubMed] [Google Scholar]
- 10.Dannemiller KC, Gent JF, Leaderer BP, Peccia J. Indoor microbial communities: influence on asthma severity in atopic and nonatopic children. J Allergy Clin Immunol. 2016;138:76–83.e1. doi: 10.1016/j.jaci.2015.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gergen PJ, Arbes SJ, Jr, Calatroni A, Mitchell HE, Zeldin DC. Total IgE levels and asthma prevalence in the US population: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2009;124:447–453. doi: 10.1016/j.jaci.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simoni M, Biavati P, Baldacci S, Carrozzi L, Pedreschi M, Di Pede F, Sapigni T, Viegi G. The Po River Delta epidemiological survey: reference values of total serum IgE levels in a normal population sample of North Italy (8-78 yrs) Eur J Epidemiol. 2001;17:231–239. doi: 10.1023/a:1017929831911. [DOI] [PubMed] [Google Scholar]
- 13.Uekert SJ, Akan G, Evans MD, Li Z, Roberg K, Tisler C, Dasilva D, Anderson E, Gangnon R, Allen DB. Sex-related differences in immune development and the expression of atopy in early childhood. J Allergy Clin Immunol. 2006;118:1375–1381. doi: 10.1016/j.jaci.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Han Y, Seo SC, Lee JY, Choi J, Kim KH, Woo SY, Kim EH, Kwon HJ, Cheong HK, et al. Association of carbon monoxide levels with allergic diseases in children. Allergy Asthma Proc. 2016;37:e1–7. doi: 10.2500/aap.2016.37.3918. [DOI] [PubMed] [Google Scholar]
- 15.Kim EJ, Kwon JW, Lim YM, Yoon D, Seo JH, Chang WS, Kim HY, Park JW, Cho SH, Hong SJ, et al. Assessment of total/specific IgE levels against 7 inhalant allergens in children aged 3 to 6 years in Seoul, Korea. Allergy Asthma Immunol Res. 2013;5:162–169. doi: 10.4168/aair.2013.5.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Amici M, Ciprandi G. The age impact on serum total and allergen-specific IgE. Allergy Asthma Immunol Res. 2013;5:170–174. doi: 10.4168/aair.2013.5.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tu YL, Chang SW, Tsai HJ, Chen LC, Lee WI, Hua MC, Cheng JH, Ou LS, Yeh KW, Huang JL, et al. Total serum IgE in a population-based study of Asian children in Taiwan: reference value and significance in the diagnosis of allergy. PLoS One. 2013;8:e80996. doi: 10.1371/journal.pone.0080996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbee RA, Halonen M, Lebowitz M, Burrows B. Distribution of IgE in a community population sample: correlations with age, sex, and allergen skin test reactivity. J Allergy Clin Immunol. 1981;68:106–111. doi: 10.1016/0091-6749(81)90167-6. [DOI] [PubMed] [Google Scholar]
- 19.Carosso A, Bugiani M, Migliore E, Antò JM, DeMarco R. Reference values of total serum IgE and their significance in the diagnosis of allergy in young European adults. Int Arch Allergy Immunol. 2007;142:230–238. doi: 10.1159/000097025. [DOI] [PubMed] [Google Scholar]
- 20.Flohrs K, Brüske I, Thiering E, Rzehak P, Wichmann HE, Heinrich J. Temporal changes in total serum immunoglobulin E levels in East German children and the effect of potential predictors. Int Arch Allergy Immunol. 2012;158:27–34. doi: 10.1159/000329855. [DOI] [PubMed] [Google Scholar]
- 21.Lindberg RE, Arroyave C. Levels of IgE in serum from normal children and allergic children as measured by an enzyme immunoassay. J Allergy Clin Immunol. 1986;78:614–618. doi: 10.1016/0091-6749(86)90078-3. [DOI] [PubMed] [Google Scholar]
- 22.Kulig M, Tacke U, Forster J, Edenharter G, Bergmann R, Lau S, Wahn V, Zepp F, Wahn U. Serum IgE levels during the first 6 years of life. J Pediatr. 1999;134:453–458. doi: 10.1016/s0022-3476(99)70203-9. [DOI] [PubMed] [Google Scholar]
- 23.Hahm MI, Kim J, Kwon HJ, Chae Y, Ahn K, Lee HY. Exposure to mould allergens and rhinoconjunctivitis in Korean children. Pediatr Allergy Immunol. 2016;27:290–298. doi: 10.1111/pai.12520. [DOI] [PubMed] [Google Scholar]
- 24.Chae Y, Hahm MI, Ahn K, Kim J, Kim WK, Lee SY, Park YM, Han MY, Lee KJ, Kwon HJ. Indoor environmental factors associated with wheezing illness and asthma in South Korean children: phase III of the International Study of Asthma and Allergies in Childhood. J Asthma. 2014;51:943–949. doi: 10.3109/02770903.2014.930879. [DOI] [PubMed] [Google Scholar]
- 25.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update. 2005;11:411–423. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- 26.Muñoz-Cruz S, Togno-Pierce C, Morales-Montor J. Non-reproductive effects of sex steroids: their immunoregulatory role. Curr Top Med Chem. 2011;11:1714–1727. doi: 10.2174/156802611796117630. [DOI] [PubMed] [Google Scholar]
- 27.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125:S73–S80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campos A, Reyes J, Blanquer A, Liñares T, Torres M. Total serum IgE: adult reference values in Valencia (1981-2004). Usefulness in the diagnosis of allergic asthma and rhinitis. Allergol Immunopathol (Madr) 2005;33:303–306. doi: 10.1016/s0301-0546(05)73247-x. [DOI] [PubMed] [Google Scholar]
- 29.Carsin AE, Zock JP, Jarvis D, Basagaña X, Heinrich J, Toren K, Janson C, Anto JM, Sunyer J. Serum total immunoglobulin E is a surrogate of atopy in adult-onset asthma: a longitudinal study. Int Arch Allergy Immunol. 2013;160:387–392. doi: 10.1159/000342464. [DOI] [PubMed] [Google Scholar]
- 30.Satwani H, Rehman A, Ashraf S, Hassan A. Is serum total IgE levels a good predictor of allergies in children? J Pak Med Assoc. 2009;59:698–702. [PubMed] [Google Scholar]
- 31.Potaczek DP, Nasta Ek M, Wojas-Pelc A, Undas A. The relationship between total serum IgE levels and atopic sensitization in subjects with or without atopic dermatitis. Allergol Int. 2014;63:485–486. doi: 10.2332/allergolint.13-LE-0660. [DOI] [PubMed] [Google Scholar]
- 32.Al-Mughales JA. Diagnostic utility of total IgE in foods, inhalant, and multiple allergies in Saudi Arabia. J Immunol Res. 2016;2016:1058632. doi: 10.1155/2016/1058632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–277. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 34.Sunyer J, Antó JM, Castellsagué J, Soriano JB, Roca J, The Spanish Group of the European Study of Asthma Total serum IgE is associated with asthma independently of specific IgE levels. Eur Respir J. 1996;9:1880–1884. doi: 10.1183/09031936.96.09091880. [DOI] [PubMed] [Google Scholar]
- 35.Carlsson M, Thorell L, Sjölander A, Larsson-Faria S. Variability of total and free IgE levels and IgE receptor expression in allergic subjects in and out of pollen season. Scand J Immunol. 2015;81:240–248. doi: 10.1111/sji.12270. [DOI] [PubMed] [Google Scholar]