Abstract
Necrotizing enterocolitis (NEC) characterized by inflammatory intestinal necrosis is a major cause of mortality and morbidity in newborns. Deep RNA sequencing (RNA-Seq) has recently emerged as a powerful technology enabling better quantification of gene expression than microarrays with a lower background signal. A total of 10 transcriptomes from 5 pairs of NEC lesions and adjacent normal tissues obtained from preterm infants with NEC were analyzed. As a result, a total of 65 genes (57 down-regulated and 8 up-regulated) revealed significantly different expression levels in the NEC lesion compared to the adjacent normal region, based on a significance at fold change ≥ 1.5 and P ≤ 0.05. The most significant gene, DPF3 (P < 0.001), has recently been reported to have differential expressions in colon segments. Our gene ontology analysis between NEC lesion and adjacent normal tissues showed that down-regulated genes were included in nervous system development with the most significance (P = 9.3 × 10−7; Pcorr = 0.0003). In further pathway analysis using Pathway Express based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) database, genes involved in thyroid cancer and axon guidance were predicted to be associated with different expression (Pcorr = 0.008 and 0.020, respectively). Although further replications using a larger sample size and functional evaluations are needed, our results suggest that altered gene expression and the genes' involved functional pathways and categories may provide insight into NEC development and aid in future research.
Keywords: Necrotizing Enterocolitis, RNA-Seq, Gene Expression
Graphical Abstract
INTRODUCTION
Necrotizing enterocolitis (NEC) characterized by intestinal ischemia and necrosis is one of the most common gastrointestinal emergencies in premature infants with very low birth weights (VLBWs), i.e., those who weigh less than 1,500 g (1,2,3). NEC affects about 5%–14% of VLBW neonates. Since NEC is a life-threatening gastrointestinal disease and an unpredictable surgical emergency, the overall mortality from NEC is high, ranging from 25% to 40% (4,5). Many researchers have tried to determine the precise pathophysiology of NEC by examining the various mechanisms that influence NEC development, such as the interaction between intraluminal microbiology and enteral nutrition and the change in inflammatory response by proinflammatory cytokines (6,7,8,9,10,11). Although several studies have been conducted and their associated hypotheses tested, substantial evidence to confirm risk factors (and effective therapies) for NEC has yet to be determined (12,13), other than prematurity and low birth weight (14).
Although several studies suggest that genetic factors affect NEC development in preterm infants with a potential susceptibility to NEC (15,16), the mechanisms underlying NEC are not fully understood. Previously, an NEC mice model has been used in many studies to establish the causes of or risk factors for human NEC due to several limitations in using human subjects, such as necrosis of human intestinal tissues and nonspecific inflammatory changes (17). However, although the animal models contribute to our understanding of disease mechanisms, limitations of reliability and reproducibility make the use of such models controversial (18,19).
Since transcripts are crucial intermediaries between the genome and the proteome, the detection of global gene expression is an important method for understanding molecular mechanisms of diseases and specific functions of particular tissues. Although microarray-based gene expression analysis has become the primary high-throughput platform in the past decade, this method has certain limitations, including high background noise and low resolution (20). Recently, RNA sequencing (RNA-Seq), using high-throughput next-generation sequencing methods, has become a powerful technology providing robust quantification of gene expression levels with a low background signal and high resolution (21,22).
MATERIALS AND METHODS
Study subjects
Study subjects were collected from Seoul National University Bundang Hospital, Gyemyoung University Dongsan Hospital, National Gyeongsang University Hospital, Donga University Hospital, and CHA University Bundang Hospital in Korea. The study protocol was approved by the institutional review board of the hospital, and written informed consent was provided by guardians of all patients. Premature infants at less than 32 weeks of gestational age and less than 1,500 g birth weight were enrolled in this study.
Two tissue sections (NEC lesion and adjacent normal regions) from the resected small bowel segment were collected as follows: 1) an NEC lesion that showed perforation or necrosis; and 2) adjacent normal tissue. Next, the tissues were immediately stored in liquid nitrogen until RNA extraction at −80°C; a portion of the tissues was evaluated by histological examination. Before RNA extraction, the mucosal layers of each tissue section were collected by the pediatric surgeon who performed the operation on the infants.
RNA isolation and quality control
Total RNA was extracted from the dissected tissue sections (NEC lesion and adjacent normal regions) with the Nucleo-Spin-RNA-II-Kit (Macherey-Nagel, Düren, Germany) according to the manufacturer's protocol. RNA integrity and purity were analyzed with the Experion™ automated electrophoresis system (Bio-Rad, Hercules, CA, USA) with the Experion RNA StdSens chip. The mRNA was extracted and purified from the total RNA with the TruSeq stranded mRNA HT sample preparation kit (Illumina, Inc., San Diego, CA, USA), and this was followed by a purity check with Qubit 2.0 fluorometer (Life Technologies, Waltham, MA, USA) before proceeding to cDNA synthesis.
RNA-Seq and data analysis
RNA-Seq was carried out in order to identify differentially expressed genes in the NEC lesion and adjacent normal tissues. The isolated mRNA from 4 μg total RNA using oligo-dT magnetic beads was fragmented and primed at 94°C for 8 minutes, and then prepared for sequencing according to the protocol of the TruSeq stranded mRNA HT sample preparation kit (Illumina, Inc.). The resulting complementary DNA (cDNA) libraries were sequenced on the Illumina MiSeq system with 75 bp paired-end reads using the Illumina MiSeq sequencing kit v3 (150 cycles; Illumina, Inc.) following the manufacturer's instructions. Image processing and base calling were performed using the Illumina Real Time Analysis Software RTA v1.9.35. FastQ sequences were aligned to the human genome database (NCBI37/hg19) using TopHat v.2.0.12 with default parameters. The reads were mapped using the gene models as provided in the annotation GTF file (GRCh37.75). Gene expression values were determined using Cufflinks 2.2.1 release (http://cole-trapnell-lab.github.io/cufflinks/), and the FPKM (fragments per kilobase of exon per million fragments mapped) values were calculated for each transcript. Cufflinks default settings were adopted, and FPKM values were computed by summing the values of different transcripts of the same gene. To identify differentially expressed genes, a fold change over 1.5 between any pairwise comparisons was applied. For statistical analysis, data were examined by t-test.
Ethics statement
The present study protocol was reviewed and approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB No. B-1404-245-008). Informed consent was submitted by guardians of all patients.
RESULTS
Patients
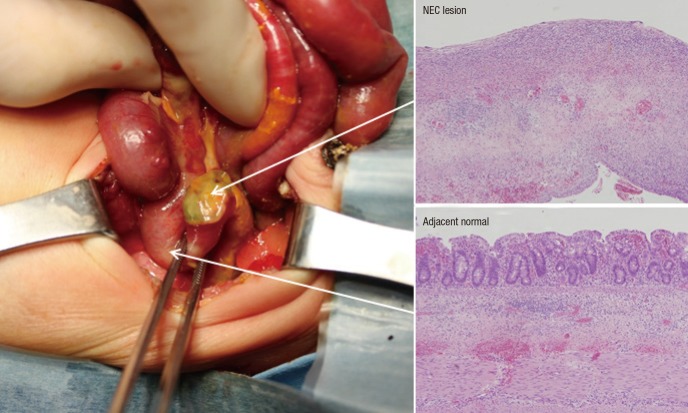
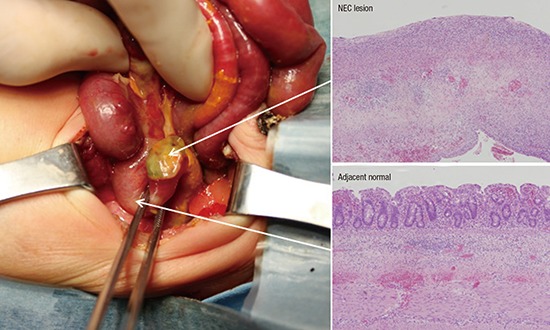
A total of 5 NEC patients, with median gestational ages of 26 weeks and 2 days, birth weight of 922 g, and birth height of 34.2 cm, were enrolled in this study. None of the patients showed other congenital anomalies in the perinatal period, and the mothers had no antenatal/perinatal problems except for premature delivery. The exploratory laparotomy was performed in the NEC patients at a median gestational age of 29 weeks and 1 day. The operations included a segmental resection with temporary ileostomy or primary anastomosis. All patients recovered from NEC and survived without complications. Following surgery, a histological examination of NEC lesion and adjacent normal tissues from the patients was performed (Fig. 1).
Fig. 1.
NEC tissues for experiment and histological features. Arrows indicate 2 tissue sections (NEC lesion and adjacent normal regions) from the resected small bowel segment and each histological examination.
NEC = necrotizing enterocolitis.
RNA-Seq analysis and gene expression comparison
To investigate the gene expression profiles for NEC development in preterm infants, RNA-Seq analysis using the Illumina MiSeq system was performed. Mapping of sequences resulted in an average read count of 11.75 × 106 (± 4.36×106) in 10 RNA samples composed of those from 2 paired small-bowel sections (NEC lesion and adjacent normal tissues) from each of 5 NEC patients. Of the 23,972 tested genes, a total of 65 genes (57 down-regulated and 8 up-regulated) were observed to have significantly different expression levels in the comparison between NEC lesion and adjacent normal tissues, based on a significance at fold change ≥ 1.5 and P ≤ 0.05 (Table 1). As a housekeeping gene, GAPDH was measured as 1,320.16 in NEC lesion and 1,255.20 in adjacent normal tissue (fold change = 1.05).
Table 1. Down-/up-regulated genes in comparison of NEC lesion and adjacent normal tissues.
| No. | Down-/up-regulation | Gene | Locus | Expression level (FPKM, average) | Fold change (NEC lesion/adjacent normal) | P value | |
|---|---|---|---|---|---|---|---|
| NEC lesion (n = 5) | Adjacent normal (n = 5) | ||||||
| 1 | Down | HTR3A | Chr11:113845796-113861034 | 0.71 | 2.43 | 0.29 | 0.030 |
| 2 | Down | CPLX2 | Chr5:175223609-175311023 | 0.38 | 1.09 | 0.35 | 0.040 |
| 3 | Down | PCP4 | Chr21:41239346-41301322 | 12.98 | 36.34 | 0.36 | 0.050 |
| 4 | Down | PCSK2 | Chr20:17206751-17465222 | 0.39 | 1.08 | 0.36 | 0.030 |
| 5 | Down | ST8SIA6 | Chr10:17362675-17496254 | 0.53 | 1.39 | 0.38 | 0.050 |
| 6 | Down | SLC5A7 | Chr2:108602994-108630443 | 1.52 | 3.98 | 0.38 | 0.050 |
| 7 | Down | PCDHGA6 | Chr5:140753650-140892546 | 0.44 | 1.11 | 0.40 | 0.050 |
| 8 | Down | TMIE | Chr3:46742822-46752413 | 0.45 | 1.13 | 0.40 | 0.050 |
| 9 | Down | DNAJC6 | Chr1:65730376-65881552 | 1.69 | 4.18 | 0.40 | 0.030 |
| 10 | Down | ELAVL4 | Chr1:50513685-50667540 | 5.64 | 13.81 | 0.41 | 0.050 |
| 11 | Down | SULT4A1 | Chr22:44220386-44258378 | 2.24 | 5.44 | 0.41 | 0.040 |
| 12 | Down | ATRNL1 | Chr10:116853123-117708496 | 0.59 | 1.38 | 0.43 | 0.050 |
| 13 | Down | RET | Chr10:43572516-43625797 | 3.38 | 7.74 | 0.44 | 0.040 |
| 14 | Down | SPOCK2 | Chr10:73818791-73848790 | 4.04 | 9.01 | 0.45 | 0.007 |
| 15 | Down | PTGES3L | Chr17:41120104-41132545 | 1.65 | 3.66 | 0.45 | 0.040 |
| 16 | Down | PTPRR | Chr12:71031852-71314584 | 1.71 | 3.76 | 0.45 | 0.030 |
| 17 | Down | CHODL | Chr21:19289656-19639687 | 0.79 | 1.73 | 0.46 | 0.010 |
| 18 | Down | NRXN3 | Chr14:78636715-80334633 | 2.96 | 6.47 | 0.46 | 0.050 |
| 19 | Down | PIRT | Chr17:10725791-10741418 | 0.73 | 1.58 | 0.46 | 0.050 |
| 20 | Down | SCUBE1 | Chr22:43599228-43739394 | 0.57 | 1.21 | 0.47 | 0.020 |
| 21 | Down | BZRAP1-AS1 | Chr17:56402810-56431088 | 1.07 | 2.25 | 0.48 | 0.020 |
| 22 | Down | CAMK4 | Chr5:110559946-110820748 | 1.90 | 3.99 | 0.48 | < 0.001 |
| 23 | Down | ACTG2 | Chr2:74120092-74146780 | 155.56 | 322.23 | 0.48 | 0.050 |
| 24 | Down | FAM226B | ChrX:72161567-72163589 | 0.54 | 1.11 | 0.49 | 0.040 |
| 25 | Down | LOC151174 | Chr2:239133753-239140318 | 0.64 | 1.31 | 0.49 | 0.050 |
| 26 | Down | MBNL1-AS1 | Chr3:151980404-151987415 | 2.53 | 5.05 | 0.50 | 0.050 |
| 27 | Down | IQCH-AS1 | Chr15:67695948-67814182 | 1.46 | 2.90 | 0.50 | 0.020 |
| 28 | Down | CDK5R1 | Chr17:30814104-30818271 | 1.13 | 2.23 | 0.51 | 0.010 |
| 29 | Down | FOXN3-AS1 | Chr14:89883697-89886137 | 0.94 | 1.85 | 0.51 | 0.030 |
| 30 | Down | DPF3 | Chr14:73136659-73360809 | 0.53 | 1.04 | 0.51 | < 0.001 |
| 31 | Down | PTPRZ1 | Chr7:121513158-121702090 | 2.98 | 5.70 | 0.52 | 0.020 |
| 32 | Down | SH3GL2 | Chr9:17578952-17797122 | 0.73 | 1.37 | 0.53 | 0.050 |
| 33 | Down | SEPT6 | ChrX:118749687-118827333 | 14.02 | 26.02 | 0.54 | 0.050 |
| 34 | Down | CTNND2 | Chr5:10971951-11904110 | 0.76 | 1.41 | 0.54 | 0.020 |
| 35 | Down | BAI3 | Chr6:69345631-70099403 | 1.35 | 2.48 | 0.54 | 0.040 |
| 36 | Down | RUNDC3A | Chr17:42385926-42396038 | 4.46 | 8.18 | 0.55 | 0.040 |
| 37 | Down | ARNT2 | Chr15:80696691-80890277 | 1.91 | 3.42 | 0.56 | 0.030 |
| 38 | Down | SEMA4F | Chr2:74881354-74910981 | 0.71 | 1.26 | 0.56 | 0.020 |
| 39 | Down | FAM161B | Chr14:74399694-74417117 | 1.15 | 2.04 | 0.56 | 0.050 |
| 40 | Down | RPS6KL1 | Chr14:75370656-75389145 | 0.63 | 1.11 | 0.57 | 0.020 |
| 41 | Down | GRIP1 | Chr12:66741210-67072925 | 0.89 | 1.56 | 0.57 | 0.020 |
| 42 | Down | MACROD2 | Chr20:13976145-16033841 | 0.47 | 0.82 | 0.57 | 0.010 |
| 43 | Down | VAMP1 | Chr12:6571403-6579843 | 3.88 | 6.76 | 0.57 | 0.040 |
| 44 | Down | C3orf70 | Chr3:184795837-184870802 | 5.02 | 8.49 | 0.59 | 0.040 |
| 45 | Down | RBM38 | Chr20:55966453-55984386 | 12.64 | 20.81 | 0.61 | 0.020 |
| 46 | Down | TSPAN11 | Chr12:31079837-31149537 | 0.68 | 1.11 | 0.61 | 0.020 |
| 47 | Down | LRRC4C | Chr11:40135750-41481186 | 1.73 | 2.81 | 0.62 | 0.030 |
| 48 | Down | BCL11A | Chr2:60678301-60780633 | 2.83 | 4.47 | 0.63 | 0.050 |
| 49 | Down | MANEAL | Chr1:38259773-38267278 | 1.49 | 2.33 | 0.64 | 0.050 |
| 50 | Down | MOXD1 | Chr6:132617193-132722664 | 5.43 | 8.39 | 0.65 | 0.030 |
| 51 | Down | JAZF1 | Chr7:27870192-28220437 | 7.10 | 10.87 | 0.65 | 0.050 |
| 52 | Down | TENM3 | Chr4:183245136-183724177 | 1.61 | 2.43 | 0.66 | 0.010 |
| 53 | Down | PCDHB2 | Chr5:140474236-140476964 | 1.10 | 1.65 | 0.67 | 0.050 |
| 54 | Down | PODXL2 | Chr3:127348001-127391653 | 4.85 | 7.29 | 0.67 | 0.040 |
| 55 | Down | KLHL23 | Chr2:170590355-170608396 | 8.80 | 13.15 | 0.67 | 0.050 |
| 56 | Down | GPC4 | ChrX:132435063-132549205 | 13.27 | 19.31 | 0.69 | 0.030 |
| 57 | Down | PRKCE | Chr2:45879042-46415129 | 3.62 | 5.26 | 0.69 | 0.050 |
| 58 | Up | WT1 | Chr11:32409321-32457081 | 1.48 | 0.39 | 3.82 | 0.050 |
| 59 | Up | SPAG4 | Chr20:34203808-34208965 | 1.65 | 0.84 | 1.97 | 0.030 |
| 60 | Up | ADAMTS14 | Chr10:72432558-72522195 | 3.87 | 2.05 | 1.89 | 0.040 |
| 61 | Up | GHRLOS | Chr3:10322635-10335133 | 1.02 | 0.55 | 1.85 | 0.030 |
| 62 | Up | DSTNP2 | Chr12:6993845-6994950 | 4.34 | 2.35 | 1.85 | 0.020 |
| 63 | Up | ZNF503-AS2 | Chr10:77161285-77168740 | 1.07 | 0.68 | 1.57 | 0.030 |
| 64 | Up | GBAP1 | Chr1:155183615-155197325 | 5.97 | 3.91 | 1.53 | 0.030 |
| 65 | Up | MCOLN1 | Chr19:7587495-7598895 | 9.50 | 6.22 | 1.53 | 0.020 |
| GAPDH* | Chr12:6643584-6647537 | 1320.16 | 1255.20 | 1.05 | - | ||
“-” in the fold change indicates down regulation.
NEC = necrotizing enterocolitis, Chr = chromosome.
*GAPDH indicates a housekeeping gene.
Among the differentially expressed genes in NEC lesions compared to the adjacent normal region, double PHD fingers 3 (DPF3, P < 0.001) and calcium/calmodulin-dependent protein kinase IV (CAMK4, P < 0.001) showed relatively robust association signals of upregulation, whereas downregulated genes showed weak signals (Table 1). In addition, 3 genes (PCP4, PTPRR, and WT1), which were recently reported as potential genes of NEC, were also observed to be differentially expressed in the NEC lesion (Table 1).
Ontology and pathway analyses of differentially expressed genes in NEC
To assess the biological functions of the differentially expressed genes in NEC lesions and adjacent normal tissues, this study performed a gene ontology analysis using the WEB-based GEne SeT AnaLysis Toolkit (http://bioinfo.vanderbilt.edu/webgestalt/). As a result, 16 gene ontology categories (14 in biological processes and 2 in the cellular component) were predicted to affect NEC development in humans (Table 2), with the most significant signal at nervous system development (P = 9.3 × 10-7; Pcorr = 0.0003). In additional pathway analysis using Pathway Express (http://vortex.cs.wayne.edu/projects.htm) based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) database, genes involved in thyroid cancer and axon guidance showed significant associations (Table 3, Pcorr = 0.008 and 0.02, respectively).
Table 2. Gene ontology analysis of differentially expressed genes in comparison of NEC lesion and adjacent normal tissues.
| Down-/up-regulation | Category | Gene ontology category | Observed genes | Observed genes number | Expected genes number | Ratio of enrichment | Significance of enrichment | |
|---|---|---|---|---|---|---|---|---|
| P value | Corrected P value | |||||||
| Down | Biological process | Nervous system development | DPF3, LRRC4C, PCSK2, MACROD2, RET, PCP4, PCDHB2, CDK5R1, BCL11A, NRXN3, SEMA4F, SH3GL2, PTPRR, PTPRZ1, CPLX2, ARNT2, SPOCK2 | 17 | 4.71 | 3.61 | 9.3 × 10−7 | < 0.001 |
| Down | Biological process | Neuron cell-cell adhesion | CTNND2, CDK5R1, RET | 3 | 0.03 | 91.44 | 4.1 × 10−6 | 0.001 |
| Down | Biological process | System development | DPF3, LRRC4C, PCSK2, MACROD2, RET, CAMK4, PCP4, PCDHB2, CHODL, CDK5R1, RBM38, BCL11A, NRXN3, BAI3, SH3GL2, SEMA4F, SCUBE1, PTPRR, CPLX2, PTPRZ1, ARNT2, TMIE, SPOCK2 | 23 | 9.63 | 2.39 | 6.0 × 10−6 | 0.002 |
| Down | Biological process | Synaptic transmission | NRXN3, SH3GL2, VAMP1, HTR3A, CPLX2, CAMK4, GRIP1, SLC5A7, PCDHB2, CTNND2 | 10 | 1.78 | 5.62 | 7.2 × 10−6 | 0.003 |
| Down | Biological process | Single-multicellular organism process | DPF3, LRRC4C, PCSK2, MACROD2, RET, CAMK4, SLC5A7, PCP4, PCDHB2, CHODL, ACTG2, CDK5R1, RBM38, BCL11A, NRXN3, BAI3, SH3GL2, VAMP1, SEMA4F, SCUBE1, PTPRR, HTR3A, CPLX2, PTPRZ1, GRIP1, ARNT2, TMIE, SPOCK2, CTNND2 | 29 | 15.34 | 1.89 | 1.2 × 10−5 | 0.004 |
| Down | Biological process | Multicellular organismal process | DPF3, LRRC4C, PCSK2, MACROD2, RET, CAMK4, SLC5A7, PCP4, PCDHB2, CHODL, ACTG2, CDK5R1, RBM38, BCL11A, NRXN3, BAI3, SH3GL2, VAMP1, SEMA4F, SCUBE1, PTPRR, HTR3A, CPLX2, PTPRZ1, GRIP1, ARNT2, TMIE, SPOCK2, CTNND2 | 29 | 15.43 | 1.88 | 1.4 × 10−5 | 0.005 |
| Down | Biological process | Anatomical structure development | DPF3, LRRC4C, PCSK2, MACROD2, RET, CAMK4, PCP4, PCDHB2, CHODL, CDK5R1, RBM38, BCL11A, NRXN3, BAI3, SH3GL2, SEMA4F, SCUBE1, PTPRR, CPLX2, PTPRZ1, ARNT2, TMIE, SPOCK2, CTNND2 | 24 | 11.02 | 2.18 | 1.7 × 10−5 | 0.006 |
| Down | Biological process | Multicellular organismal development | DPF3, LRRC4C, PCSK2, MACROD2, RET, CAMK4, PCP4, PCDHB2, CHODL, CDK5R1, RBM38, BCL11A, NRXN3, BAI3, SH3GL2, SEMA4F, SCUBE1, PTPRR, CPLX2, PTPRZ1, ARNT2, TMIE, SPOCK2, CTNND2 | 24 | 11.15 | 2.15 | 2.1 × 10−5 | 0.007 |
| Down | Biological process | Transmission of nerve impulse | NRXN3, SH3GL2, VAMP1, HTR3A, CPLX2, CAMK4, GRIP1, SLC5A7, PCDHB2, CTNND2 | 10 | 2.01 | 4.98 | 2.1 × 10−5 | 0.007 |
| Down | Biological process | Multicellular organismal signaling | NRXN3, SH3GL2, VAMP1, HTR3A, CPLX2, CAMK4, GRIP1, SLC5A7, PCDHB2, CTNND2 | 10 | 2.05 | 4.87 | 2.5 × 10−5 | 0.009 |
| Down | Biological process | Neurotransmitter secretion | NRXN3, SLC5A7, VAMP1, CPLX2 | 4 | 0.27 | 14.78 | < 0.001 | 0.030 |
| Down | Biological process | Cell-cell signaling | NRXN3, SH3GL2, SEMA4F, VAMP1, HTR3A, CPLX2, CAMK4, GRIP1, SLC5A7, PCDHB2, CTNND2 | 11 | 3.00 | 3.66 | < 0.001 | 0.030 |
| Down | Cellular component | Synapse | SEMA4F, VAMP1, SEPT6, HTR3A, CPLX2, GRIP1, CDK5R1, CTNND2 | 8 | 1.20 | 6.64 | 2.2 × 10−5 | 0.002 |
| Down | Cellular component | Synapse part | GRIP1, SEMA4F, VAMP1, SEPT6, CTNND2, CDK5R1, HTR3A | 7 | 0.91 | 7.68 | 3.0 × 10−5 | 0.002 |
| Up | Biological process | Extracellular structure organization | ADAMTS14, WT1 | 2 | 0.06 | 34.50 | 0.001 | 0.030 |
| Up | Biological process | Extracellular matrix organization | ADAMTS14, WT1 | 2 | 0.06 | 34.67 | 0.001 | 0.030 |
Gene ontology categories with corrected P value of enrichment significance below 0.05 are shown.
NEC = necrotizing enterocolitis.
Table 3. Potential pathways affected by differentially expressed genes in comparisons of NEC lesion and adjacent normal tissues.
| Pathway name | Differentially regulated genes | Input genes in pathway, % | Impact factor | Corrected P value |
|---|---|---|---|---|
| Thyroid cancer | RET | 1.587 | 6.914 | 0.008 |
| Axon guidance | LRRC4C, SEMA4F | 3.175 | 5.740 | 0.020 |
Corrected P value is obtained using the classical hypergeometric model (32).
NEC = necrotizing enterocolitis.
DISCUSSION
Acquired conditions of diffuse necrotic injury to the intestinal segments are known to affect NEC development. Abnormal bacterial colonization and formula feeding have also been implicated as predisposing factors for NEC in humans (23,24). In addition, potential associations between NEC and environmental factors (such as microbiome, microbiome-intestinal reaction to breast milk or formula milk feeding, vaginal or cesarean section mode of delivery, and antibiotics) have been reported (10,11,23,24,25). Interestingly, a significant reduction of NEC in infants who were fed breast milk, compared to those who were fed formula, has been reported (26). Thus, many neonatologists have gone to great effort to manage the microbiome to prevent NEC development. Many neonatologists in Korea have changed their management protocols for preterm infants and observed a decreased incidence of NEC during the last few years.
NEC development may be multifactorial with the interplay between intrinsic and extrinsic factors. In addition, the main risk factor for NEC development in premature infants is thought to be intestinal immaturity (23,27), suggesting that intrinsic risk factors may be more important because premature infants have had a short exposure time to external environments. In this study, we hypothesized that global gene expression profiling may reveal distinct genetic differences between NEC lesion and adjacent normal region. Although candidate genes in this study did not reach great values of significance, several potential genes (such as DPF3 and CAMK4) with relatively robust association signals were identified (P < 0.001). These markers may have a role in NEC development. However, further replication and evaluation studies are needed.
As noted, this study showed relatively robust association signals at DPF3 and CAMK4. DPF3, which is known as an epigenetic key factor for the development of heart and muscle tissue, has been reported to play a role in the neuronal differentiation process and also to take part in the disassembly of muscular fibers (28). In the different colon segments of Hirschsprung's disease, the gene product of DPF3 has been observed to be lowly expressed in a stenotic segment, whereas it is highly expressed in proximal anastomosis (29), suggesting that DPF3 may be dysregulated in colonic diseases such as NEC. In the case of CAMK4, although a direct association between CAMK4 and NEC has not been reported, several connections in the literature related to necrosis can be found. In particular, CAMK4 was observed to be involved in the necrosis factor (NF)-kappaB mediated signaling pathway in human endothelial cells (30). These previous results and our findings suggest that dysregulated expressions of genes identified in this study may contribute to NEC development.
Recently, the first RNA-Seq for gene expression profiling in NEC was reported (31). This first RNA-Seq study used ileum tissues from preterm patients with other diseases for the control, and several genes associated with immune functions (in particular, genes associated with Crohn's disease) were identified as contributing factors to NEC development, together with other candidate genes. When compared to our results, PCP4 and PTPRR were overlapped; however, no connections in the literature related to NEC or related cellular functions (such as necrosis) could be found. Therefore, further studies are required to elucidate the association between these potential genes and NEC development.
So as to remove the heterogeneity of genetic background, this study excluded non-Korean parents. However, the study also has several limitations, such as insufficient sample size and lack of functional evaluation. The small sample size was due to the overall decreased incidence of NEC. In addition, normal tissues from the small bowel segment in infants without NEC or related diseases would have been ideal for the comparison analysis; however, it was limited to obtain these normal tissues. Although the first RNA-Seq analysis study of NEC used the ileum for the normal control (31), this study used adjacent normal tissues, and we do not rule out the possible effect of congenital diseases (such as small intestinal perforation, intestinal atresia, etc.).
In conclusion, despite study limitations, our preliminary results have identified potential involvements of certain genes (such as DPF3 and CAMK4) in NEC development, suggesting that these genetic factors, perhaps together with epigenetic factors such as microbiomes and breast milk, may have a role in NEC development in humans. Further validation studies are needed to determine clinical applications of these potential targets.
Footnotes
Funding: This study was supported by grants from the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT and Future Planning (NRF-2014R1A1A1005096 and NRF-2012M3A9D1054450) and by the Ministry of Education, Science and Technology (2009-0093822).
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conceptualization: Jung K, Koh I, Shin HD. Data curation: Jung K, Park T, Nam SH, Jung SM, Sio CA, Kim SY, Jung E, Lee B, Kim HR, Jung SE, Choi CW, Kim BI, Jung E. Funding acquisition: Investigation: Jung K, Koh I, Shin HD. Koh I, Kim JH, Cheong HS, Shin E. Writing - original draft: Jung K, Koh I, Shin HD.
References
- 1.Bisquera JA, Cooper TR, Berseth CL. Impact of necrotizing enterocolitis on length of stay and hospital charges in very low birth weight infants. Pediatrics. 2002;109:423–428. doi: 10.1542/peds.109.3.423. [DOI] [PubMed] [Google Scholar]
- 2.Blakey JL, Lubitz L, Campbell NT, Gillam GL, Bishop RF, Barnes GL. Enteric colonization in sporadic neonatal necrotizing enterocolitis. J Pediatr Gastroenterol Nutr. 1985;4:591–595. doi: 10.1097/00005176-198508000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Hintz SR, Kendrick DE, Stoll BJ, Vohr BR, Fanaroff AA, Donovan EF, Poole WK, Blakely ML, Wright L, Higgins R, NICHD Neonatal Research Network Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005;115:696–703. doi: 10.1542/peds.2004-0569. [DOI] [PubMed] [Google Scholar]
- 4.Holman RC, Stoll BJ, Clarke MJ, Glass RI. The epidemiology of necrotizing enterocolitis infant mortality in the United States. Am J Public Health. 1997;87:2026–2031. doi: 10.2105/ajph.87.12.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemons JA, Bauer CR, Oh W, Korones SB, Papile LA, Stoll BJ, Verter J, Temprosa M, Wright LL, Ehrenkranz RA, et al. Very low birth weight outcomes of the National Institute of Child health and human development neonatal research network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics. 2001;107:E1. doi: 10.1542/peds.107.1.e1. [DOI] [PubMed] [Google Scholar]
- 6.Hunter CJ, Upperman JS, Ford HR, Camerini V. Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC) Pediatr Res. 2008;63:117–123. doi: 10.1203/PDR.0b013e31815ed64c. [DOI] [PubMed] [Google Scholar]
- 7.Morowitz MJ, Poroyko V, Caplan M, Alverdy J, Liu DC. Redefining the role of intestinal microbes in the pathogenesis of necrotizing enterocolitis. Pediatrics. 2010;125:777–785. doi: 10.1542/peds.2009-3149. [DOI] [PubMed] [Google Scholar]
- 8.Neu J, Mihatsch W. Recent developments in necrotizing enterocolitis. JPEN J Parenter Enteral Nutr. 2012;36:30S–35S. doi: 10.1177/0148607111422068. [DOI] [PubMed] [Google Scholar]
- 9.Sharma R, Hudak ML. A clinical perspective of necrotizing enterocolitis: past, present, and future. Clin Perinatol. 2013;40:27–51. doi: 10.1016/j.clp.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sim K, Shaw AG, Randell P, Cox MJ, McClure ZE, Li MS, Haddad M, Langford PR, Cookson WO, Moffatt MF, et al. Dysbiosis anticipating necrotizing enterocolitis in very premature infants. Clin Infect Dis. 2015;60:389–397. doi: 10.1093/cid/ciu822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortese R, Lu L, Yu Y, Ruden D, Claud EC. Epigenome-Microbiome crosstalk: a potential new paradigm influencing neonatal susceptibility to disease. Epigenetics. 2016;11:205–215. doi: 10.1080/15592294.2016.1155011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christensen RD, Lambert DK, Baer VL, Gordon PV. Necrotizing enterocolitis in term infants. Clin Perinatol. 2013;40:69–78. doi: 10.1016/j.clp.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Stoll BJ. Epidemiology of necrotizing enterocolitis. Clin Perinatol. 1994;21:205–218. doi: 10.1016/S0095-5108(18)30341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanto WP, Jr, Wilson R, Breart GL, Zierler S, Purohit DM, Peckham GJ, Ellison RC. Perinatal events and necrotizing enterocolitis in premature infants. Am J Dis Child. 1987;141:167–169. doi: 10.1001/archpedi.1987.04460020057026. [DOI] [PubMed] [Google Scholar]
- 15.Chan KY, Leung KT, Tam YH, Lam HS, Cheung HM, Ma TP, Lee KH, To KF, Li K, Ng PC. Genome-wide expression profiles of necrotizing enterocolitis versus spontaneous intestinal perforation in human intestinal tissues: dysregulation of functional pathways. Ann Surg. 2014;260:1128–1137. doi: 10.1097/SLA.0000000000000374. [DOI] [PubMed] [Google Scholar]
- 16.Ng PC, Chan KY, Poon TC. Biomarkers for prediction and diagnosis of necrotizing enterocolitis. Clin Perinatol. 2013;40:149–159. doi: 10.1016/j.clp.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Tian R, Liu SX, Williams C, Soltau TD, Dimmitt R, Zheng X, De Plaen IG. Characterization of a necrotizing enterocolitis model in newborn mice. Int J Clin Exp Med. 2010;3:293–302. [PMC free article] [PubMed] [Google Scholar]
- 18.Hackam DG, Redelmeier DA. Translation of research evidence from animals to humans. JAMA. 2006;296:1731–1732. doi: 10.1001/jama.296.14.1731. [DOI] [PubMed] [Google Scholar]
- 19.van der Worp HB, Howells DW, Sena ES, Porritt MJ, Rewell S, O’Collins V, Macleod MR. Can animal models of disease reliably inform human studies? PLoS Med. 2010;7:e1000245. doi: 10.1371/journal.pmed.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ioannidis JP, Allison DB, Ball CA, Coulibaly I, Cui X, Culhane AC, Falchi M, Furlanello C, Game L, Jurman G, et al. Repeatability of published microarray gene expression analyses. Nat Genet. 2009;41:149–155. doi: 10.1038/ng.295. [DOI] [PubMed] [Google Scholar]
- 21.Conesa A, Madrigal P, Tarazona S, Gomez-Cabrero D, Cervera A, McPherson A, Szcześniak MW, Gaffney DJ, Elo LL, Zhang X, et al. A survey of best practices for RNA-seq data analysis. Genome Biol. 2016;17:13. doi: 10.1186/s13059-016-0881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368:1271–1283. [Google Scholar]
- 24.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336:1519–1523. doi: 10.1016/0140-6736(90)93304-8. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, Sun J, Antonopoulos DA, Chang EB, Claud EC. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 2009;3:944–954. doi: 10.1038/ismej.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Repa A, Thanhaeuser M, Endress D, Weber M, Kreissl A, Binder C, Berger A, Haiden N. Probiotics (Lactobacillus acidophilus and Bifidobacterium infantis) prevent NEC in VLBW infants fed breast milk but not formula. Pediatr Res. 2015;77:381–388. doi: 10.1038/pr.2014.192. [corrected] [DOI] [PubMed] [Google Scholar]
- 27.Claud EC. Neonatal necrotizing enterocolitis -inflammation and intestinal immaturity. Antiinflamm Antiallergy Agents Med Chem. 2009;8:248–259. doi: 10.2174/187152309789152020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lange M, Kaynak B, Forster UB, Tönjes M, Fischer JJ, Grimm C, Schlesinger J, Just S, Dunkel I, Krueger T, et al. Regulation of muscle development by DPF3, a novel histone acetylation and methylation reader of the BAF chromatin remodeling complex. Genes Dev. 2008;22:2370–2384. doi: 10.1101/gad.471408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H, Luo Y, Li S, Wang S, Wang N, Jin X. Expression profiles of HA117 and its neighboring gene DPF3 in different colon segments of Hirschsprung’s disease. Int J Clin Exp Pathol. 2014;7:3966–3974. [PMC free article] [PubMed] [Google Scholar]
- 30.You J, Peng W, Lin X, Huang QL, Lin JY. PLC/CAMK IV-NF-kappaB involved in the receptor for advanced glycation end products mediated signaling pathway in human endothelial cells. Mol Cell Endocrinol. 2010;320:111–117. doi: 10.1016/j.mce.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 31.Tremblay É, Thibault MP, Ferretti E, Babakissa C, Bertelle V, Bettolli M, Burghardt KM, Colombani JF, Grynspan D, Levy E, et al. Gene expression profiling in necrotizing enterocolitis reveals pathways common to those reported in Crohn’s disease. BMC Med Genomics. 2016;9:6. doi: 10.1186/s12920-016-0166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Draghici S, Khatri P, Tarca AL, Amin K, Done A, Voichita C, Georgescu C, Romero R. A systems biology approach for pathway level analysis. Genome Res. 2007;17:1537–1545. doi: 10.1101/gr.6202607. [DOI] [PMC free article] [PubMed] [Google Scholar]