Abstract
Tumor-associated antigens have emerged as important immunotherapeutic targets in the fight against cancer. Germline tumor antigens, such as WT1, Wilms’ tumor gene 1, are overexpressed in many human malignancies but have low expression in somatic tissues. Recent vaccination approaches to target WT1 have been hampered by poor in vivo immune potency, likely due to the conserved self-antigen nature of WT1. In this study, we use a novel synthetic micro-consensus SynCon DNA vaccine approach with the goal of breaking tolerance and increasing vaccine immune potency. This approach induced new, neo-antigen-like responses that were superior to those induced by native WT1 DNA immunogens for driving T cell immunity and breaking tolerance. Non-human primates (NHPs) vaccinated with SynCon WT1 antigens elicited immune responses against native rhesus WT1 peptides. When delivered by electroporation (EP) in mice, SynCon-based WT1 constructs elicited strong CD4 and CD8 T cell responses (including IFN-γ, CD107a, and TNF-α) to both native and consensus peptides. In addition, SynCon WT1 vaccine-induced antibodies recognized native WT1 in vitro. Vaccination with the SynCon WT1 immunogens was capable of slowing tumor growth in therapeutic models in vivo. These data support the further study of synthetic consensus DNA vaccines for breaking tolerance to important germline antigens.
Keywords: DNA vaccines, immune tolerance, leukemia, neo-antigen, WT1
Breaking tolerance to tumor-associated self-antigens is a major challenge for cancer immune therapy. Here, Walters et al. report a novel DNA vaccine design strategy using consensus sequences to help break tolerance and induce a neo-antigen-like response to the germline antigen WT1.
Introduction
Major advances in the field of immune therapy have unveiled powerful approaches that harness a patient’s immune system to target cancer. Several therapies exist to elicit a de novo T cell immune response against tumor-associated antigens. These include peptide-, DNA-, or cell-based vaccines, such as PROVENGE, a dendritic cell vaccine for men with castration-resistant prostate cancer that was the first U.S. Food and Drug Administration (FDA)-approved therapeutic cancer vaccine.1 Chimeric antigen receptor (CAR) therapy has proven highly effective in patients with B cell lymphoblastic leukemia.2 Additional therapies include checkpoint blockade inhibitors, such as PD-1 and CTLA-4, which remove inhibitory signals to allow T cells to react to tumor neo-antigens and induce tumor shrinkage.3 However, many patients lack T cells that are already primed to tumor-associated antigens and thus require initiation of a de novo immune response.
A study by the National Cancer Institute compiled a list of potentially attractive tumor antigens based on several criteria, including immunogenicity, therapeutic function, and tumor specificity.4 Many of these tumor-associated antigens are considered germline antigens, because they are expressed highly in germ tissues, absent in somatic tissues, and aberrantly activated in human malignancies. For many of these germline antigens, chimeric antigen receptor therapy is not possible because of the lack of cell surface expression of the antigen, and T cell receptor (TCR)-based cellular approaches are human leukocyte antigen (HLA) restricted and cannot be used for most patients. Vaccine-based approaches for these targets are therefore important. However, a major challenge for vaccines targeting germline cancer antigens is breaking tolerance to these self-antigens.
The No. 1 tumor antigen that emerged from the National Cancer Institute list was Wilms’ tumor 1 (WT1). WT1 protein is a self-antigen whose expression is upregulated during embryogenesis but is maintained at lower levels in adult tissues, such as the kidney, spleen, heart, and gonadal cells.5 WT1 is known to bind to and transcriptionally regulate a number of genes, including insulin growth factor 2 (IGF2), platelet-derived growth factor A (PDGF-A), and transforming growth factor β1 (TGF-β1), as well as itself.6 Aside from being a known transcription factor, WT1 is mutated or overexpressed in Wilms’ tumor, an embryonic tumor of the kidney, as well as in most types of adult leukemia, acute myeloid leukemia (AML), chronic myeloid leukemia, acute lymphocytic leukemia, glomerular diseases, and various solid tumors, including lung, pancreatic, thyroid, breast, testicular, and ovarian carcinomas and melanoma.7, 8
Because of its importance as an immune therapy target, many peptide-based vaccination approaches have been developed for WT1. Several major histocompatibility complex (MHC) class I-restricted WT1 epitopes have been identified with high binding affinity to HLA-A*0201 and HLA-A*2402 in leukemia patients.8, 9, 10, 11, 12 Peptide vaccines derived from these epitopes, alone or in combination with Montanide ISA-51, have elicited CD8+ T cell responses and anti-tumor activity in some patients with leukemia, lung, breast disease, glioblastoma, various sarcomas, mesothelioma, or pancreatic cancer in early-phase human clinical trials.8, 13, 14, 15, 16, 17, 18, 19, 20
Although these peptide vaccines have shown some clinical results in early testing, this therapeutic approach is limited because of MHC class or HLA haplotype restrictions in the patient population. Furthermore, peptide vaccines are limited in their ability to activate CD4+ T helper responses and many fail to produce long-term memory responses against antigens. Another vaccination approach that is not HLA restricted is DNA vaccination. This platform has many advantages for cancer immune therapy; however, its immune performance in the past has been poor.21 In this regard, we have shown that optimized DNA vaccines delivered by electroporation (EP) can elicit potent T cell responses against tumor antigens in mice, non-human primates (NHPs), and humans and that such vaccines in humans can reverse neoplastic disease.22, 23, 24
Although there have been a few attempts to target WT1 by DNA vaccine technologies, the results of these prior studies achieved mixed results. Tsuboi and colleagues immunized mice with full-length mouse WT1 plasmid DNA intramuscularly and were able to elicit WT1-specific cytotoxic T lymphocyte (CTL) responses, as well as prolonged survival of mice challenged with WT1-expressing tumor cells.25 However, later studies resorted to toxoid fusion constructs to break tolerance against the encoded epitopes and improve on low native T cell-induced responses to achieve improved immune response in an epitope-specific fashion.26 A more recent study by Tan and colleagues showed that a full-length native WT1 DNA vaccine was capable of inducing an immune response in mice but offered little protection in a mesothelioma tumor challenge model.27 The only existing clinical trial using DNA vaccination to target WT1 used an epitope-based vaccine, which is HLA restricted (NCT: NCT01334060). Thus, there is a need for vaccine approaches targeting WT1 to enhance the diversity of patient populations who can benefit.
In prior studies, matched peptides, a toxoid epitope fusion, or a full-length native murine WT1 was used. Such an immunogen likely has limited capacity to break tolerance for both MHC class I and MHC class II responses against this self-antigen. We therefore sought to improve this vaccine design strategy by introducing mutations in the self-antigen to generate a broader immune target with an improved immune response profile that can induce both CD4 and CD8 T cell responses, an important issue for induction of memory responses. It has been suggested that introduction of mutations in self can induce auto-immunity; however, this strategy has not been broadly applied to current anti-cancer vaccines, and methods for rationally approaching this issue are not in routine study.28 Furthermore, for many antigens, the choice of mutations is unclear.29 We thus developed a synthetic consensus (SynCon) approach in which protein sequences from various species are compared to create a consensus sequence with enhanced capacity to break tolerance, without altering protein structure. Here, we show that our SynCon vaccines targeting WT1 are vastly superior at breaking tolerance compared to a native murine WT1 vaccine, including a codon-optimized version. We also performed matrix mapping to determine which specific mutated epitopes have the ability to break tolerance. Finally, when mice were challenged with tumor cells, animals vaccinated with the SynCon WT1 constructs were able to control tumor challenge, showing reduced tumor burden and increased survival compared to unvaccinated animals. These results support further use of the SynCon technology for additional tumor-associated antigens to break tolerance and elicit an anti-tumor response.
Results
Design and Construction of WT1 DNA Vaccines
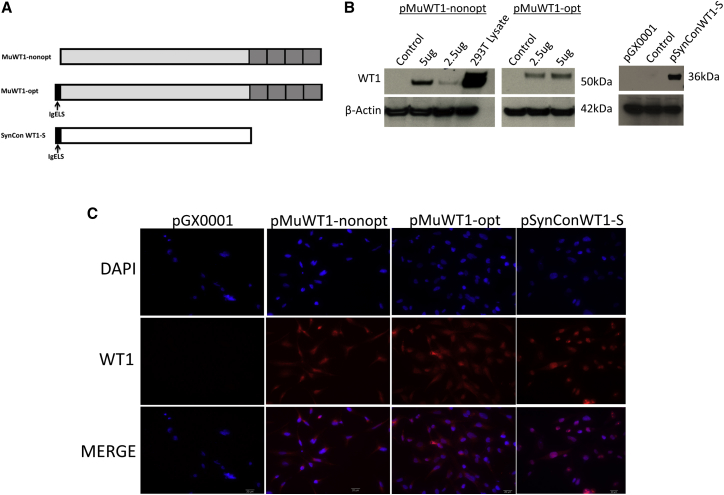
In an effort to break tolerance and generate a robust immune response against the WT1 self-antigen, we generated highly optimized SynCon DNA sequences. Specifically, we analyzed and aligned 16 divergent WT1 protein sequences from the NCBI database to generate a consensus sequence from this collection that shares 96.6% identity with human native WT1. Considering the potential safety issues, we either engineered eight mutations in the first two zinc fingers (four mutations per zinc finger) to abolish its DNA-binding specificity (SynConWT1-L) or removed all four zinc fingers from our SynCon sequence (SynConWT1-S) (Figure 1A; Figure S1A).30 The final SynConWT1-L and SynConWT1-S proteins share 94.7% and 95.2% sequence identities with human germline WT1 protein, and these two antigens share 92.5% and 92.4% sequence identities with mouse native WT1 protein, respectively. Thus, this sequence represents a consensus sequence for humans, mice, and NHPs. We further modified the WT1 consensus sequence by adding a synthetic immunoglobulin E (IgE) leader sequence to the N terminus as previously described.31 The synthetic full-length WT1 gene with mutated zinc fingers (SynConWT1-L) was 1,314 bp in length, and the synthetic WT1 gene lacking the C-terminal zinc fingers (SynConWT1-S) was 936 bp in length. In addition, we developed two mouse WT1 constructs encoding the mouse native WT1 protein: pMuWT1-opt contains a codon- and RNA-optimized full-length mouse WT1 gene with an IgE leader sequence and a Kozak sequence at the N terminus (1,404 bp in length), while pMuWT1-nonopt contains a full-length non-optimized mouse WT1 gene (1,350 bp in length).
Figure 1.
In Vitro Expression of Mouse WT1 and Synthetic WT1 DNA Vaccines
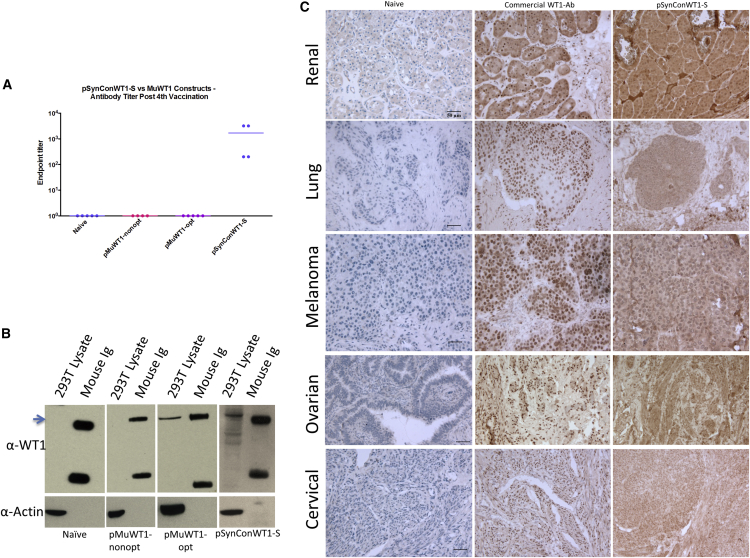
(A) Schematic outline of pMuWT1-opt, pMuWT1-nonopt, and pSynConWT1-S. (B) Detection of pMuWT1-opt, pMuWT1-nonopt, and pSynConWT1-S expression by immunoblotting. (C) Immunofluorescence assay of pMuWT1-opt, pMuWT1-nonopt, and pSynConWT1-S. Transfected RD cells expressing WT1 protein showed typical phalloidin fluorescence using a commercial WT1 polyclonal antibody.
Expression of WT1 DNA Vaccines
To verify the expression of each WT1 construct, human rhabdomyosarcoma (RD) cells were transiently transfected with each construct and expression was analyzed by immunoblotting with a commercial WT1 antibody. Although the RD cells show no basal expression of WT1, the cells transfected with pMuWT1-opt, pMuWT1-nonopt, pSynConWT1-L, and pSynConWT1-S expressed WT1 (Figure 1B; Figure S1B). Because WT1 is expressed in the kidneys, HEK293T cell lysate was used as a positive control. Expression of WT1 by each construct was confirmed by indirect immunofluorescence analysis in RD cells transiently transfected with each construct and then stained with a commercial WT1 antibody (Figure 1C; Figure S1C). As a control, expression was not detected in the cells transfected with the empty expression vector, pGX0001.
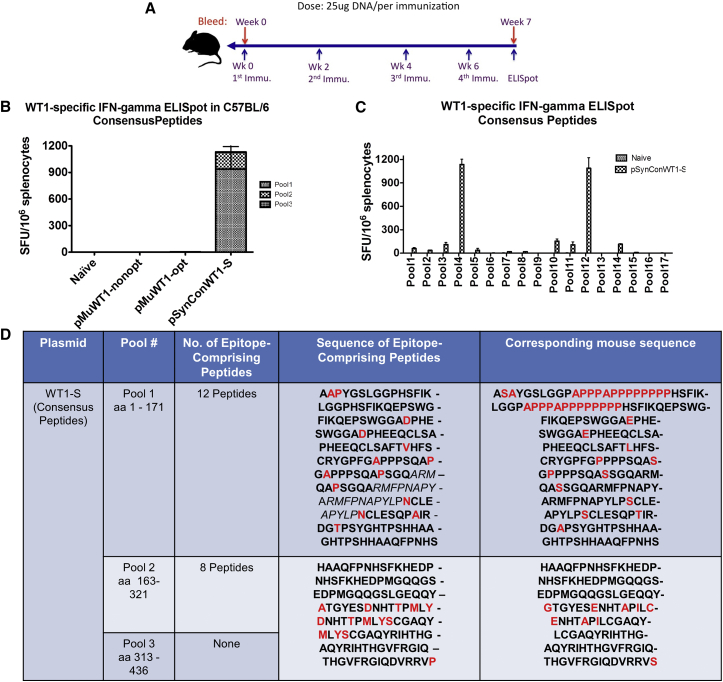
pSynConWT1-S and pSynConWT1-L DNA Vaccines Induce WT1-Specific T Cell Responses Using Matched Consensus Peptides
To determine whether pSynConWT1-S and pSynConWT1-L were immunogenic in mice, C57BL/6 mice were vaccinated intramuscularly, followed by EP with pMuWT1-nonopt, pMuWT1-opt, pSynConWT1-L, or pSynConWT1-S DNA constructs (Figure 2A). We then performed interferon γ (IFN-γ) ELISpots using peptides matched to the SynCon antigen sequences (Figure 2B; Figure S2A). As expected, pMuWT1-nonopt and pMuWT1-opt did not elicit immune responses against consensus peptides (Figure 2B). However, pSynConWT1-S and pSynConWT1-L DNA vaccine induced robust cellular immune responses against consensus peptides of roughly 1,200 spot-forming units (SFUs) per 106 splenocytes for pSynConWT1-S and 800 SFUs per 106 splenocytes for pSynConWT1-L (Figure 2B; Figure S2A). Characterization of the cellular immune responses using a library of consensus WT1 peptides, consisting of 17 matrix pools, revealed that 9 of 17 consensus matrix pools showed more than 20 SFUs per 106 splenocytes in mice immunized with pSynConWT1-S and 10 of 17 consensus matrix pools showed more than 20 SFUs per 106 splenocytes in mice immunized with pSynConWT1-L (Figure 2C; Figure S2B), corresponding to 20 epitope-comprising peptides for pSynConWT1-S (Figure 2D) and 30 epitope-comprising peptides for pSynConWT1-L (Figure S2C). Previous studies have identified the following immunodominant WT1 peptide epitopes: WT137–45 (VLDFAPPGA), WT1126–134 (RMFPNAPYL), and WT1235–243 (CMTWNQMNL) in humans.8, 26, 32, 33 Our analyses identified that the immunodominant human WT1126–134 epitope elicited strong T cell responses after pSynConWT1-S DNA vaccination in mice and that all three of these human epitopes elicited strong T cell responses after pSynConWT1-L DNA vaccination, indicating that similar immunodominant epitopes may be displayed by both mouse and human MHCs (highlighted in italics in Figure 2D; Figure S2C). 20 additional epitope-comprising peptides for pSynConWT1-S and 25 additional epitope-comprising peptides for pSynConWT1-L were identified in our matrix mapping (Figure 2D; Figure S2C). Overall, responses to consensus peptides occurred in epitopes that were highly divergent from the corresponding mouse sequence, with multiple point mutations per epitope or large insertions or deletions (Figure 2D; Figure S2C).
Figure 2.
Characterization of Consensus WT1-Specific IFN-γ Responses and Immunodominant Epitopes for pSynConWT1-S Vaccine
(A) DNA vaccine immunization schedule showing the dosage of vaccine. C57BL/6 (B6) mice (n = 5 per group) were immunized at weeks 0, 2, 4, and 6 with pSynConWT1-S, pMuWT1-opt, and pMuWT1-nonopt via i.m. injection or EP. (B) Frequency of WT1-specific IFN-γ spot-forming units (SFUs) per million splenocytes isolated from vaccinated mice, determined by IFN-γ ELISpot assay using consensus peptides. (C) Matrix mapping to determine the WT1 consensus-specific immunodominant epitopes, comparing naive and pSynConWT1-S vaccinated mice. (D) List of immunodominant epitopes identified in the matrix mapping in (C), and comparison to the corresponding native mouse sequence. Error bars represent the average ± SEM.
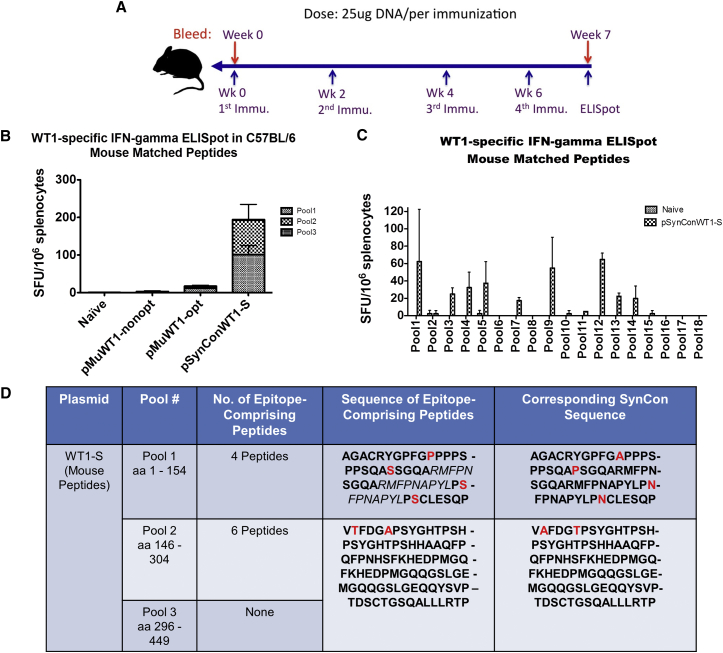
pSynConWT1-S and pSynConWT1-L DNA Vaccines Can Break Tolerance to Native Mouse WT1
To assess whether our SynConWT1-S DNA vaccine can break tolerance against the germline antigen WT1, we vaccinated C57BL/6 mice intramuscularly, followed by EP with pMuWT1-nonopt, pMuWT1-opt, pSynConWT1-L, or pSynConWT1-S (Figure 3A; Figure S3A). We performed IFN-γ ELISpots using peptides generated from the native mouse WT1 sequence (mouse-matched peptides). The results reveal that vaccination with pSynConWT1-S induces strong cellular immune responses against mouse-matched peptides (∼200 SFUs per 106 splenocytes), whereas pMuWT1-nonopt or pMuWT1-opt do not (Figure 3B). This indicates that our SynCon-designed antigens are able to induce a neo-antigen-like response that can break tolerance against germline antigens.
Figure 3.
Characterization of Mouse WT1-Specific IFN-γ Responses and Immunodominant Epitopes for pSynConWT1-S Vaccine
(A) DNA vaccine immunization schedule showing the dosage of vaccine. C57BL/6 (B6) mice (n = 5 per group) were immunized at weeks 0, 2, 4, and 6 with pSynConWT1-S, pMuWT1-opt, and pMuWT1-nonopt via i.m. injection or EP. (B) Frequency of WT1-specific IFN-γ spot-forming units (SFUs) per million splenocytes isolated from vaccinated mice, determined by IFN-γ ELISpot assay using mouse peptides. (C) Matrix mapping to determine the WT1 mouse-specific immunodominant epitopes, comparing naive and pSynConWT1-S vaccinated mice. (D) List of immunodominant epitopes identified in the matrix mapping in (C), and comparison to the corresponding SynCon sequence. Error bars represent the average ± SEM.
We next determined the specific epitopes in the native mouse WT1 that elicited cellular immune responses after DNA vaccination with pSynConWT1-S or pSynConWT1-L by performing matrix mapping. We performed IFN-γ ELISpots using 18 matrix pools from the library of mouse-matched peptides. We found that 7 of 18 mouse-matched matrix pools showed greater than 20 SFUs per 106 splenocytes for pSynConWT1-S and 9 of 18 matrix pools showed this for SynConWT1-L (Figure 3C), indicating that these vaccines can elicit immune responses to a range of epitopes. From these pSynConWT1-S matrix pools, we identified ten epitope-comprising peptides (Figure 3D). These epitope-comprising peptides include the WT1126–134 epitope identified in patient samples and new immunodominant epitope-containing peptides that have not been previously described.8, 26, 32, 33 The epitope-comprising peptides for both pSynConWT1-S and pSynConWT1-L within the native mouse sequence are highly homologous to the SynCon sequence, with zero to two point mutations per peptide. These results highlight the versatility of the synthetic constructs to break tolerance and induce a strong immune response.
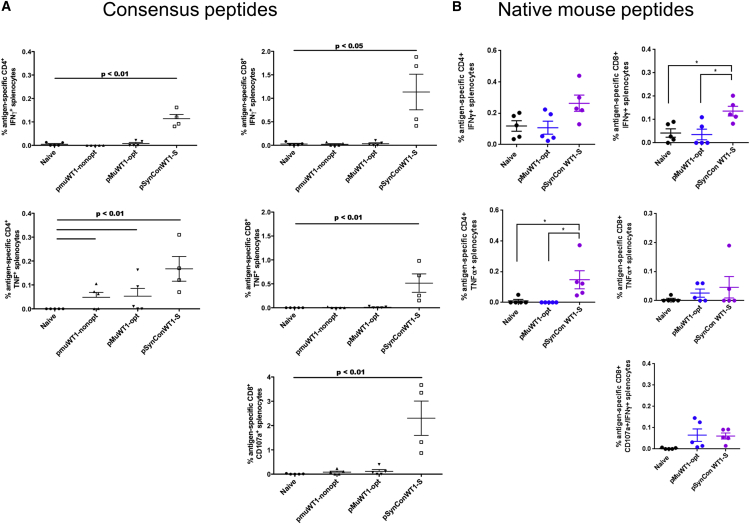
Vaccination with pSynConWT1-S Enhances the Cytokine Production in CD8 T Cells
To further characterize the responses induced by pSynConWT1-S, we performed intracellular cytokine staining to measure the expression or secretion of IFN-γ, CD107a, and tumor necrosis factor alpha (TNF-α) in both CD4+ and CD8+ T cells. Developing the CD4 immune response is highly relevant to the generation of long-term memory response. The average frequency of CD8+IFN-γ+ and CD8+TNF-α+ cells in response to consensus peptide stimulation in pSynConWT1-S immunized mice was significantly higher than that of the pMuWT1-nonopt group or the pMuWT1-opt group (Figure 4A). The average frequency of CD8+IFN-γ+ cells in response to native peptides was also significantly higher in pSynConWT1-S immunized mice compared to naive or pMuWT1-opt groups (Figure 4B). We next assessed cytotoxic potential in vaccinated versus naive mice by quantifying the transient surface expression of CD107a, a marker of cytolytic degranulation on CD8+ T cells. In response to consensus peptide stimulation, we detected significantly higher percentages of CD8+CD107a+ T cells in mice vaccinated with pSynConWT1-S compared to those vaccinated with pMuWT1-nonopt or pMuWT1-opt (Figure 4A). We also observed a trend toward higher CD8+CD107a+IFN-γ+ T cells in mice vaccinated with pSynConWT1-S in response to native peptides compared to naive mice, although this trend was not statistically significant (Figure 4B).
Figure 4.
CD4 and CD8 Cytokine Responses to Consensus and Mouse WT1 Peptides for pSynConWT1-S Vaccine
(A and B) Expression of CD107a, IFN-γ, and TNF-α was assessed by intracellular staining of splenocytes stimulated with consensus WT1 peptides (A) or native mouse peptides (B) for 5 hr. Background-subtracted percentages of WT1-specific CD4+ or CD8+ T cells producing CD107a, IFN-γ, and TNF-α were calculated. Error bars represent the average ± SEM.
We further evaluated cytokine production in CD4+ cells from the immunized mice. We observed an increase in the production of IFN-γ and TNF-α in CD4+ cells in the immunized mice, which was highest in the pSynConWT1-S group in response to consensus peptides (Figure 4A). We also observed an increase in CD4+TNF-α+ responses to native peptides in pSynConWT1-S splenocytes compared to naive or pMuWT1-opt splenocytes (Figure 4B). Overall, the responses were much higher in the CD8+ compartment, suggesting that immune responses elicited by pSynConWT1-S are skewed toward CD8+ cytotoxic lymphocytes.
Vaccination with pSynConWT1-S Induces Robust Antibody Responses
The induction of antibody responses is dependent on CD4 T cell immunity. We next examined these responses as a measure of breaking tolerance in the CD4 and B cell compartment. To detect vaccine-induced WT1-specific antibodies, we performed ELISA on mouse sera collected 1 week following the last vaccination. As shown in Figure 5A, only vaccination with pSynConWT1-S, but not pMuWT1-nonopt or pMuWT1-opt, was capable of generating WT1-specific antibodies, consistent with a helper T cell response. Furthermore, sera from mice vaccinated with pSynConWT1-S or, to a lesser extent, pMuWT1-opt were able to recognize WT1 protein expressed naturally in HEK293T cell lysates, confirmed using western blot (Figure 5B).
Figure 5.
pSynConWT1-S Induces Robust Antibody Responses
(A) WT1-specific IgG endpoint binding titers, performed using ELISA. (B) Immunoblot assay of HEK293T cells with sera from vaccinated mice. (C) Immunohistochemical analysis of WT1 expression in human renal, lung, skin, ovarian, and cervical cancer using naive sera, a commercial WT1 antibody, or pSynConWT1-S mouse sera.
To determine whether these vaccine-induced antibodies could recognize native WT1 expressed on human tumors, we performed immunohistochemical analysis on clinical samples of human renal, ovarian, cervical, lung, and skin cancers (Figure 5C). We compared staining performed with sera from naive mice, sera from pSynConWT1-S vaccinated mice, and a commercial WT1 antibody (Figure 5C). We observed strong staining of the clinical samples with the WT1 commercial antibody and the pSynConWT1-S vaccinated mouse sera, but not with the naive mouse sera (Figure 5C). Thus, WT1-specific antibodies induced by pSynConWT1-S vaccination react with human tumor samples.
Vaccination with pSynConWT1-S or pSynConWT1-L Elicits Therapeutic Antitumor Immunity
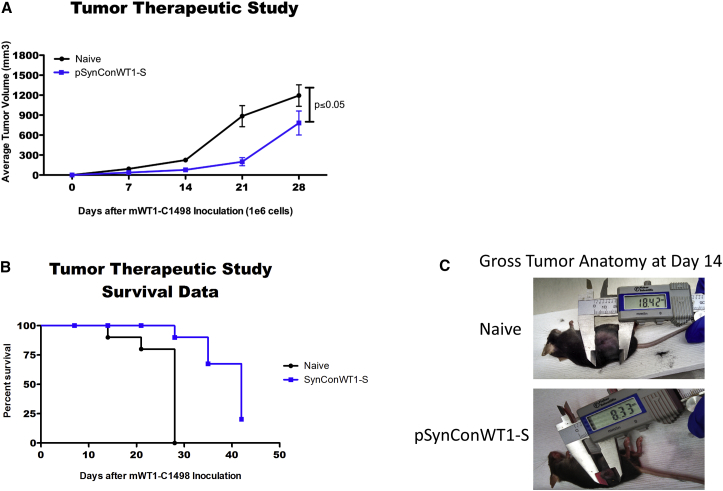
To determine whether pSynConWT1-S or pSynConWT1-L could elicit an immune response against WT1-expressing tumors, we conducted an in vivo therapeutic study. We first challenged mice with 1 × 106 acute myeloid leukemia cells (mWT1-C1498). Three days after tumor implantation, mice were immunized with pSynConWT1-S or pSynConWT1-L at 1-week intervals (Figure 6A; Figure S4A). Every mouse exhibited tumor growth, but the tumors in the pSynConWT1-S or pSynConWT1-L immunized mice were significantly smaller than those of naive from day 14 to day 28 (Figures 6A and 6C; Figure S4A). Furthermore, although all naive mice were euthanized due to tumor burden on or before day 28, 70% of the pSynConWT1-S and pSynConWT1-L mice survived 28 days after tumor implantation (Figure 6B; Figure S4B). We next examined whether our pSynConWT1-S vaccine could induce superior anti-tumor responses compared to the pMuWT1-opt vaccine. We performed a similar in vivo therapeutic tumor study, in which we immunized mice 3 days after tumor implantation at 1-week intervals. Although the pSynConWT1-S vaccine significantly attenuated tumor growth and prolonged survival of the mice, the pMuWT1-opt vaccine did not have any significant anti-tumor effect compared to control mice (Figures S5A and S5B). Overall, vaccination with the synthetic constructs pSynConWT1-S and pSynConWT1-L slowed tumor growth and improved survival of tumor-bearing mice.
Figure 6.
Anti-tumor Immunity Elicited by pSynConWT1-S
(A) Mice were challenged with 106 mWT1-C1498 tumor cells injected subcutaneously and were vaccinated weekly starting 3 days post-tumor implant. Tumor measurements are reported in terms of tumor volume only for surviving mice until day 28. (B) Survival data from the tumor therapeutic challenge in (A). Vaccination with pSynConWT1-S extended survival in tumor-bearing mice. (C) Representative image of tumor size in naive or pSynConWT1-S vaccinated groups at day 14 post-mWT1-C1498 implantation. Error bars represent the average ± SEM.
Vaccine-Induced WT1-Specific Immune Responses Break Tolerance in Rhesus Macaques
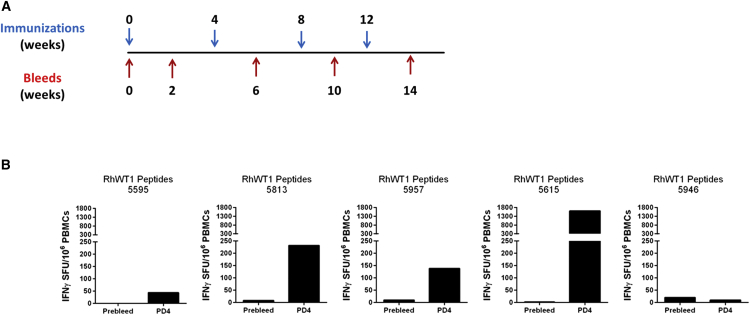
The matrix mapping assays in Figures 2 and Figure S2C revealed that the pSynConWT1-L construct showed increased breadth of responses compared to the pSynConWT1-S construct. Therefore, we sought to determine the level of immune responses induced by pSynConWT1-L in NHPs, rhesus macaques. Human native WT1 shares 99.5% sequence identity with NHP native WT1; thus, this model is ideal for assessing whether our pSynConWT1-L construct is capable of breaking tolerance. We vaccinated NHPs with our full-length construct, pSynConWT1-L, four times at 4-week intervals (Figure 7A). We performed the IFN-γ ELISpot assay using the pre-bleed samples to establish the background level of immune response for individual animal in the study (Figure 7A). To determine whether these NHPs induced a response against native rhesus WT1 protein, we stimulated the week 14 NHP peripheral blood mononuclear cells (PBMCs) with native rhesus peptides and performed an IFN-γ ELISpot assay. As shown in Figure 7B, four of five NHPs showed responses in non-cultured ELISpot assays to the native peptides, ranging from ∼50 to 1,500 SFUs per 106 PBMCs (Figure 7B). No apparent signs of toxicity or adverse effects were observed in the NHPs, as assessed by attending animal care staff (Table S1). These results further illustrate that the SynCon DNA vaccination approach is capable of breaking tolerance and inducing a neo-antigen-like response in a species that closely resembles humans.
Figure 7.
Vaccination with pSynConWT1-L-Induced WT1-Specific Immune Responses in Rhesus Macaques
(A) DNA vaccine immunization schedule showing the number of vaccinations and bleeds. Rhesus macaques (n = 5 per group) were immunized at weeks 0, 4, 8, and 12 with 2 mg of pSynConWT1-L intramuscularly, followed by EP. (B) PBMCs were isolated and stimulated with native rhesus WT1 peptides for 24 hr. Shown is the frequency of WT1-specific IFN-γ secreting cells per million PBMCs, determined by IFN-γ ELISpot assay at week 14.
Discussion
A surge in interest to develop effective therapeutic cancer vaccines sparked an effort by the National Cancer Institute to compile a list of cancer antigens with high priority for therapeutic development.4 Criteria for these antigens included immunogenicity, oncogenicity, specificity, and expression level in tumor cells. Because these tumor antigens are self-antigens, immunogenicity remains a limiting factor for immune therapy development. There has thus been recent interest in identifying tumor-specific somatic mutations that lead to presentation of new epitopes on MHC class I or MHC class II that are recognized as foreign by the host immune system. These neo-antigens are patient specific, and CD4+ T cells can respond to these mutated epitopes to elicit tumor regression.34, 35 Furthermore, adoptive transfer of tumor-infiltrating T lymphocytes (TILs) and successful checkpoint blockade cancer immunotherapy are both thought to elicit tumor rejection that is mediated by recognition of tumor-specific neo-antigens.36, 37 Peptide- and RNA-based vaccines against these tumor-specific neo-antigens have shown anti-tumor immunity in mouse models.34, 36 However, these vaccination approaches are highly patient specific and typically target passenger mutations—mutations that do not contribute to the fitness of a clone—that are not necessary for survival of the tumor cells. Furthermore, not all patients have somatic mutations that are capable of eliciting an immune response.38
Here, we describe a novel approach for targeting the germline tumor antigen WT1 using a SynCon DNA vaccine platform that is capable of eliciting a neo-antigen-like immune response jointly against MHC class I and class II epitopes carried in tumor antigens of relevance. Previous efforts to develop a peptide- or DNA-based vaccine for WT1 yielded mixed results, with MHC restriction and limited immunogenicity.8, 25, 27 Our laboratory has reported that DNA vaccines delivered by EP are effective in generating strong cytotoxic T lymphocyte responses in animals and humans.22, 23, 24 However, this is our first report of novel SynCon sequences in the context of self-tumor antigens that are able to break tolerance to both mouse and rhesus WT1 native antigens.
It has been appreciated for decades that tumor cells exposed to mutagens are more immunogenic and that immunization with these mutagenized tumor cells can induce protection against the parental tumor cells.39 One study showed that mutagenesis of a DNA encoding a self-antigen was sufficient to induce auto-immune responses, but only one self-reactive DNA clone was identified per 239 randomly generated mutations.28 In addition, this response was dependent on both MHC class I and MHC class II presentation, suggesting CD4 T cell help is required for the full self-antigen response. Conversely, another study reported that immunization with xenogeneic DNA was sufficient to induce auto-immunity even in the absence of MHC class II molecules because of the formation of a dominant heteroclitic epitope.40 This is also in contrast to rationally designed DNA vaccines intended to specifically enhance antigen processing and presentation, which depend only on CD8 cells, not CD4 help.29 This dependence on CD4 help may depend on the specific antigen or epitope and on the strength of the existing interaction between the TCR and MHC-peptide complex. It is likely that responses against MHC peptides with lower presentation frequency or lower affinity for the TCR will be more likely to require MHC class II presentation and CD4 T cell help, consistent with the notion that most auto-immune disorders result from aberrant MHC class II presentation.41 Our intracellular cytokine staining illustrated the induction of WT1-specific CD4+ T cells and to a greater extent CD8+ T cells upon vaccination with our SynCon WT1 DNA vaccine.
In our vaccine design approach, we introduced 23 amino acid changes into the 312 amino acid sequence of WT1-S. We were able to generate auto-immune responses to 10 of the 51 epitope-comprising peptides, which typically occurred for peptides that were minimally altered. We also generated responses against self-peptides that were not mutated in our consensus vaccine design. These responses were not observed upon vaccination with the native antigen, indicating that mutations in self can induce epitope spreading to non-mutated epitopes in the same protein.
Previous studies and WT1 clinical trials have focused specifically on two human WT1 epitopes: WT1126–134 and WT1235–243.33, 42, 43 Our peptide mapping studies revealed that our SynCon WT1 DNA vaccines were able to elicit some previously identified human immunodominant epitopes in mice, as well as some new immunodominant WT1 epitopes, which contributed to the robust immune response seen in mice and suggests that delivery of multiple epitopes could expand the diversity of T cell responses and improve clinical efficacy. Matrix mapping studies also revealed that synthetic sequences that were highly divergent from the native mouse sequence were able to elicit an immune response; however, these sequences were less effective at breaking tolerance and inducing auto-immunity. Sequences that were highly homologous, with only one or two point mutations per 15-mer, were able to elicit the best response against native WT1 peptides. Thus, this novel SynCon technology using consensus sequences that share 92%–95% homology with the native antigen is able to induce a neo-antigen-like response against the germline antigen WT1 to enhance T cell recognition of WT1 epitopes and elicit an anti-tumor response. Further studies to potentially improve these immune responses will include combination therapy with immune plasmid adjuvants such as interleukin-12 (IL-12) and combination therapies with immune checkpoint blockade. In addition, future studies expanding on this consensus vaccine design concept will focus on determination of additional details for the optimal levels of divergence and sequence elements required for robust immune responses. These data have implications for antigen development targeting diverse germline cancer immune therapy targets.
Materials and Methods
WT1 DNA Immunogen Design and Construction
The consensus WT1 immunogen was designed by generating a consensus sequence using 16 GenBank WT1 sequences collected from human and lower animals, such as rhesus macaque, mouse, and rat. The consensus sequence was obtained after performing multiple alignment by ClustalW. No additional mutations were added to the sequence, with the exception of mutations introduced to inactivate the function of WT1. The consensus sequence was sub-cloned into the pGX0001 expression vector and designated pSynConWT1-S (lacking terminal zinc fingers) or pSynConWT1-L (with mutated terminal zinc fingers). WT1 constructs encoding the native mouse WT1 protein (NP_659032) were also made. All plasmids were codon and RNA optimized with an IgE leader sequence and a Kozak sequence at the N terminus, with the exception of the pMuWT1-nonopt plasmid, which only contains a full-length non-optimized mouse WT1 gene.
Immunoblot and Immunofluorescence
Human RD cells were cultured in complete DMEM with 10% fetal bovine serum (FBS; Gibco) and transfected using TurboFectin 8.0 (OriGene) transfection reagent following the manufacturer’s protocol. 48 hr later, cells were lysed using radioimmunoprecipitation assay (RIPA) lysis buffer. Western blot analysis was performed with an anti-WT1 monoclonal antibody (ab89901) and visualized with horseradish peroxidase (HRP)-conjugated anti-rabbit immunoglobulin G (IgG) using an enhanced chemiluminescence (ECL) western blot analysis system (GE Amersham).
For indirect immunofluorescence, RD cells were plated on two-well chamber slides (BD Biosciences) and transfected (1 μg/well) using TurboFectin 8.0 (OriGene) transfection reagent. 48 hr after transfection, the cells were washed with PBS, fixed using paraformaldehyde and permeabilized for 10 min. The cells were then incubated with anti-WT1 monoclonal antibody (Abcam, Cat. No. ab89901) at a 1:500 dilution for 60 min at room temperature. The slides were then incubated with the Alexa 555-(phalloidin)-conjugated anti-rabbit secondary antibody (Cell Signaling Technology) for 60 min and analyzed by fluorescence microscopy (Leica DM4000B) using the SPOT Advanced Software program.
Mice and Immunization
Female 8-week-old C57BL/6 mice were purchased from Jackson Laboratory. Their care was in accordance with the guidelines of the NIH, the University of Pennsylvania Institutional Animal Care and Use Committee (IACUC), and the Wistar Institute IACUC. Mice were divided into groups and immunized with 25 μg of each plasmid by intramuscular (i.m.) injection into the tibialis interior (TA) muscle, followed by EP using the CELLECTRA adaptive constant current EP device (Inovio Pharmaceuticals). Two 0.1-A constant current square-wave pulses were delivered through a triangular three-electrode array consisting of 26-gauge solid stainless steel electrodes. Each pulse was 52 ms in length with a 1-s delay between pulses. The mice received a total of four immunizations given 2 weeks apart.
ELISpot Assay
Two sets of peptides, the first spanning the entire mouse native WT1 protein sequence and the second spanning the consensus WT1 protein sequence, each containing 15 amino acid residues overlapping by 8 amino acids, were synthesized from GenScript. Mouse IFN-γ ELISpot assay (R&D Systems) was performed according to the manufacturer’s instructions. Splenocytes were stimulated with peptides (at a concentration of 2 μg/mL/peptide), complete culture medium (negative control), or concanavalin A (5 μg/mL, positive control) for 24 hr. The average number of spot-forming cells (SFCs) was adjusted to 1 × 106 splenocytes. The samples were analyzed in triplicate from two independent experiments.
For epitope mapping studies, all WT1 peptides were pooled into matrix pools (17 for consensus and 18 for mouse native WT1), and IFN-γ ELISpot assay was performed as described earlier.
Intracellular Cytokine Staining
Splenocytes were stimulated with peptides for 5 hr at 37°C, 5% CO2. Phorbol myristate acetate (PMA) and complete medium were used as controls. Cells were stained with fluorescein isothiocyanate (FITC) anti-mouse CD107a as a marker for degranulation. Following incubation, the cells were first stained with ViViD Dye (LIVE/DEAD Fixable Violet Dead Cell Stain Kit; Invitrogen, L34955), and then washed and stained with the following extracellular antibodies: APC-Cy7 anti-mouse CD3e, PerCP-Cy5.5 anti-mouse CD4, and APC anti-mouse CD8a (BD Biosciences). Cells were then permeabilized and washed with the Cytofix/Cytoperm kit (BD Biosciences). Intracellular cytokines were subsequently stained with the following antibodies: Alexa Fluor 700 anti-mouse IFN-γ and PE-Cy7 anti-mouse TNF (BD Biosciences). Cells were then washed, fixed, and acquired using an LSR II flow cytometer with BD FACSDiva software (BD Biosciences), and analyzed with FlowJo software.
WT1-Specific Antibody Determination
The measurement of IgG antibodies specific for native mouse WT1 was performed by ELISA in both immunized and control mice. The plates were coated with 1.0 μg/mL of WT1 protein (MyBiosource), and standard ELISA was carried out. The mathematical formula used to calculate the endpoint titer cutoffs is expressed as the standard deviation multiplied by a factor that was based on the number of negative controls (naive sera) (n = 5) and the confidence level (95%). The endpoint titer is reported as the reciprocal of the last dilution that remained above the endpoint cutoff.
Immunohistochemistry
Human cancer tissue sections—cervical, ovarian, melanoma, renal, and lung—were obtained from the Tumor Tissue and Biospecimen Bank at the University of Pennsylvania and were previously reviewed by a pathologist. Tissue sections were de-paraffinized with xylene and then rehydrated through graded alcohols to water. Endogenous peroxidase was blocked using BLOXALL (Vector Laboratories), and for antigen retrieval, slides were microwaved in 10 mM sodium citrate (pH 6.0) for 10 min and then allowed to cool at room temperature for 20 min. Slides were then treated with horse serum albumin to block nonspecific staining and immunostained using anti-WT1 polyclonal antibodies (Abcam Cat. No. ab89901) and sera obtained from WT1 vaccinated mice by the avidin-biotin horseradish peroxidase method (Vectastain Elite ABC Kit). A slide incubated with naive mouse sera was used as a negative control. The incubation with the primary antibody was performed at 4°C overnight in a moisture chamber. After the treatment with biotinylated secondary antibody, the slide-mounted sections were stained with diaminobenzidine with hydrogen peroxide and counterstained with hematoxylin.
Tumor Challenge
mWT1-C1498, a murine leukemia cell line expressing native mouse WT1 and syngeneic to C57BL/6 mice, was provided by Dr. H. Sugiyama (Osaka University Graduate School of Medicine).19 mWT1-C1498 cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin (Mediatech), 0.5 mg/mL G418, and 50 μM 2-mercaptoethanol (Invitrogen) in a 37°C incubator with 5% CO2.
Ten male 8-week-old C57BL/6 mice were injected with 1 × 106 mWT1-C1498 cells subcutaneously into the right flank. Mice were immunized weekly beginning 3 days post-implantation for a total of three vaccinations. Tumors were measured once a week thereafter, with digital calipers spanning the shortest (width) and longest (length) surface diameters as previously described. Tumor volumes were calculated according to the following formula: V = length × width 2 × π/6. Mice were sacrificed when the tumor diameter reached 20 mm.
NHP Studies
Five rhesus macaques were vaccinated with pSynConWT1-L, four times intramuscularly, followed by EP using the CELLECTRA adaptive constant current EP device (Inovio Pharmaceuticals), 4 weeks apart, at 2 mg DNA per immunization at a 0.5-A current and three 52-ms pulses. Blood was collected 2 weeks after each immunization, and PBMCs were isolated by standard Ficoll-Hypaque density gradient centrifugation. Monkey IFN-γ ELISpots were conducted on isolated PBMCs. Antigen-specific responses were determined by subtracting the number of spots in the negative control wells from the wells containing peptides.
Statistical Analysis
Statistical significance was determined using a standard, two-tailed Student’s t test for experiments in which two experimental groups were compared. For experiments containing more than two experimental groups, one-way ANOVA was performed, followed by multiple comparison using Tukey’s honest SD (HSD) test. Differences were considered statistically significant if the p value was less than 0.05. All statistical analyses were performed using GraphPad Prism software.
Author Contributions
J.N.W., A.S.K., N.Y.S., and D.B.W. were involved in study conception and design. J.N.W., B.F., E.K.D., K.A.K., J.C., A.S.-F., J.Y., and H.L. were involved in acquisition, analysis, and interpretation of data. J.N.W., E.K.D., and D.B.W. drafted the manuscript. All authors were involved in critical revision of the manuscript.
Conflicts of Interest
J.N.W., B.F., K.A.K., J.Y., A.S.K., and N.Y.S. are employees of Inovio Pharmaceuticals and as such receive salary and benefits, including ownership of stock and stock options. D.B.W. discloses grant funding, industry collaborations, speaking honoraria, and fees for consulting. His service includes serving on scientific review committees and advisory boards. Remuneration includes direct payments, stock, or stock options. He notes potential conflicts associated with his work with Pfizer, Bristol Myers Squibb, Inovio Pharmaceuticals, Touchlight, Oncosec, Merck, VGXI, and potentially others. Licensing of technology from his laboratory has created more than 150 jobs in the private sector of the biotechnology and pharmaceutical industry. The other authors declare no competing financial interests.
Acknowledgments
This work was supported in part by the W.W. Smith Family Trust, funding from the Basser Foundation, and a grant from Inovio Pharmaceuticals to D.B.W.
Footnotes
Supplemental Information includes five figures and one table and can be found with this article online at http://dx.doi.org/10.1016/j.ymthe.2017.01.022.
Supplemental Information
References
- 1.Cheever M.A., Higano C.S. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin. Cancer Res. 2011;17:3520–3526. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 2.Lee D.W., Kochenderfer J.N., Stetler-Stevenson M., Cui Y.K., Delbrook C., Feldman S.A., Fry T.J., Orentas R., Sabatino M., Shah N.N. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolchok J.D., Kluger H., Callahan M.K., Postow M.A., Rizvi N.A., Lesokhin A.M., Segal N.H., Ariyan C.E., Gordon R.A., Reed K. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheever M.A., Allison J.P., Ferris A.S., Finn O.J., Hastings B.M., Hecht T.T., Mellman I., Prindiville S.A., Viner J.L., Weiner L.M., Matrisian L.M. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin. Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pritchard-Jones K., Fleming S., Davidson D., Bickmore W., Porteous D., Gosden C., Bard J., Buckler A., Pelletier J., Housman D. The candidate Wilms’ tumour gene is involved in genitourinary development. Nature. 1990;346:194–197. doi: 10.1038/346194a0. [DOI] [PubMed] [Google Scholar]
- 6.Wagner K.-D., Wagner N., Schedl A. The complex life of WT1. J. Cell Sci. 2003;116:1653–1658. doi: 10.1242/jcs.00405. [DOI] [PubMed] [Google Scholar]
- 7.Inoue K., Sugiyama H., Ogawa H., Nakagawa M., Yamagami T., Miwa H., Kita K., Hiraoka A., Masaoka T., Nasu K. WT1 as a new prognostic factor and a new marker for the detection of minimal residual disease in acute leukemia. Blood. 1994;84:3071–3079. [PubMed] [Google Scholar]
- 8.Van Driessche A., Berneman Z.N., Van Tendeloo V.F.I. Active specific immunotherapy targeting the Wilms’ tumor protein 1 (WT1) for patients with hematological malignancies and solid tumors: lessons from early clinical trials. Oncologist. 2012;17:250–259. doi: 10.1634/theoncologist.2011-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohminami H., Yasukawa M., Fujita S. HLA class I-restricted lysis of leukemia cells by a CD8(+) cytotoxic T-lymphocyte clone specific for WT1 peptide. Blood. 2000;95:286–293. [PubMed] [Google Scholar]
- 10.Gao L., Bellantuono I., Elsässer A., Marley S.B., Gordon M.Y., Goldman J.M., Stauss H.J. Selective elimination of leukemic CD34(+) progenitor cells by cytotoxic T lymphocytes specific for WT1. Blood. 2000;95:2198–2203. [PubMed] [Google Scholar]
- 11.Rezvani K., Brenchley J.M., Price D.A., Kilical Y., Gostick E., Sewell A.K., Li J., Mielke S., Douek D.C., Barrett A.J. T-cell responses directed against multiple HLA-A*0201-restricted epitopes derived from Wilms’ tumor 1 protein in patients with leukemia and healthy donors: identification, quantification, and characterization. Clin. Cancer Res. 2005;11:8799–8807. doi: 10.1158/1078-0432.CCR-05-1314. [DOI] [PubMed] [Google Scholar]
- 12.Scheibenbogen C., Letsch A., Thiel E., Schmittel A., Mailaender V., Baerwolf S., Nagorsen D., Keilholz U. CD8 T-cell responses to Wilms tumor gene product WT1 and proteinase 3 in patients with acute myeloid leukemia. Blood. 2002;100:2132–2137. doi: 10.1182/blood-2002-01-0163. [DOI] [PubMed] [Google Scholar]
- 13.Di Stasi A., Jimenez A.M., Minagawa K., Al-Obaidi M., Rezvani K. Review of the results of WT1 peptide vaccination strategies for myelodysplastic syndromes and acute myeloid leukemia from nine different studies. Front. Immunol. 2015;6:36. doi: 10.3389/fimmu.2015.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morita S., Oka Y., Tsuboi A., Kawakami M., Maruno M., Izumoto S., Osaki T., Taguchi T., Ueda T., Myoui A. A phase I/II trial of a WT1 (Wilms’ tumor gene) peptide vaccine in patients with solid malignancy: safety assessment based on the phase I data. Jpn. J. Clin. Oncol. 2006;36:231–236. doi: 10.1093/jjco/hyl005. [DOI] [PubMed] [Google Scholar]
- 15.Yasukawa M., Fujiwara H., Ochi T., Suemori K., Narumi H., Azuma T., Kuzushima K. Clinical efficacy of WT1 peptide vaccination in patients with acute myelogenous leukemia and myelodysplastic syndrome. Am. J. Hematol. 2009;84:314–315. doi: 10.1002/ajh.21387. [DOI] [PubMed] [Google Scholar]
- 16.Ohta H., Hashii Y., Yoneda A., Takizawa S., Kusuki S., Tokimasa S., Fukuzawa M., Tsuboi A., Murao A., Oka Y. WT1 (Wilms tumor 1) peptide immunotherapy for childhood rhabdomyosarcoma: a case report. Pediatr. Hematol. Oncol. 2009;26:74–83. doi: 10.1080/08880010802435500. [DOI] [PubMed] [Google Scholar]
- 17.Rezvani K., Yong A.S., Mielke S., Jafarpour B., Savani B.N., Le R.Q., Eniafe R., Musse L., Boss C., Kurlander R., Barrett A.J. Repeated PR1 and WT1 peptide vaccination in Montanide-adjuvant fails to induce sustained high-avidity, epitope-specific CD8+ T cells in myeloid malignancies. Haematologica. 2011;96:432–440. doi: 10.3324/haematol.2010.031674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuball J., de Boer K., Wagner E., Wattad M., Antunes E., Weeratna R.D., Vicari A.P., Lotz C., van Dorp S., Hol S. Pitfalls of vaccinations with WT1-, Proteinase3- and MUC1-derived peptides in combination with MontanideISA51 and CpG7909. Cancer Immunol. Immunother. 2011;60:161–171. doi: 10.1007/s00262-010-0929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oka Y., Udaka K., Tsuboi A., Elisseeva O.A., Ogawa H., Aozasa K., Kishimoto T., Sugiyama H. Cancer immunotherapy targeting Wilms’ tumor gene WT1 product. J. Immunol. 2000;164:1873–1880. doi: 10.4049/jimmunol.164.4.1873. [DOI] [PubMed] [Google Scholar]
- 20.Oka Y., Tsuboi A., Taguchi T., Osaki T., Kyo T., Nakajima H., Elisseeva O.A., Oji Y., Kawakami M., Ikegame K. Induction of WT1 (Wilms’ tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc. Natl. Acad. Sci. USA. 2004;101:13885–13890. doi: 10.1073/pnas.0405884101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kutzler M.A., Weiner D.B. DNA vaccines: ready for prime time? Nat. Rev. Genet. 2008;9:776–788. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trimble C.L., Morrow M.P., Kraynyak K.A., Shen X., Dallas M., Yan J., Edwards L., Parker R.L., Denny L., Giffear M. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet. 2015;386:2078–2088. doi: 10.1016/S0140-6736(15)00239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bagarazzi M.L., Yan J., Morrow M.P., Shen X., Parker R.L., Lee J.C., Giffear M., Pankhong P., Khan A.S., Broderick K.E. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci. Transl. Med. 2012;4:155ra138. doi: 10.1126/scitranslmed.3004414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan J., Pankhong P., Shin T.H., Obeng-Adjei N., Morrow M.P., Walters J.N., Khan A.S., Sardesai N.Y., Weiner D.B. Highly optimized DNA vaccine targeting human telomerase reverse transcriptase stimulates potent antitumor immunity. Cancer Immunol. Res. 2013;1:179–189. doi: 10.1158/2326-6066.CIR-13-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuboi A., Oka Y., Ogawa H., Elisseeva O.A., Li H., Kawasaki K., Aozasa K., Kishimoto T., Udaka K., Sugiyama H. Cytotoxic T-lymphocyte responses elicited to Wilms’ tumor gene WT1 product by DNA vaccination. J. Clin. Immunol. 2000;20:195–202. doi: 10.1023/a:1006637529995. [DOI] [PubMed] [Google Scholar]
- 26.Chaise C., Buchan S.L., Rice J., Marquet J., Rouard H., Kuentz M., Vittes G.E., Molinier-Frenkel V., Farcet J.P., Stauss H.J. DNA vaccination induces WT1-specific T-cell responses with potential clinical relevance. Blood. 2008;112:2956–2964. doi: 10.1182/blood-2008-02-137695. [DOI] [PubMed] [Google Scholar]
- 27.Tan Z., Zhou J., Cheung A.K.L., Yu Z., Cheung K.-W., Liang J., Wang H., Lee B.K., Man K., Liu L. Vaccine-elicited CD8+ T cells cure mesothelioma by overcoming tumor-induced immunosuppressive environment. Cancer Res. 2014;74:6010–6021. doi: 10.1158/0008-5472.CAN-14-0473. [DOI] [PubMed] [Google Scholar]
- 28.Engelhorn M.E., Guevara-Patiño J.A., Noffz G., Hooper A.T., Lou O., Gold J.S., Kappel B.J., Houghton A.N. Autoimmunity and tumor immunity induced by immune responses to mutations in self. Nat. Med. 2006;12:198–206. doi: 10.1038/nm1363. [DOI] [PubMed] [Google Scholar]
- 29.Guevara-Patiño J.A., Engelhorn M.E., Turk M.J., Liu C., Duan F., Rizzuto G., Cohen A.D., Merghoub T., Wolchok J.D., Houghton A.N. Optimization of a self antigen for presentation of multiple epitopes in cancer immunity. J. Clin. Invest. 2006;116:1382–1390. doi: 10.1172/JCI25591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoll R., Lee B.M., Debler E.W., Laity J.H., Wilson I.A., Dyson H.J., Wright P.E. Structure of the Wilms tumor suppressor protein zinc finger domain bound to DNA. J. Mol. Biol. 2007;372:1227–1245. doi: 10.1016/j.jmb.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 31.Yang J.S., Kim J.J., Hwang D., Choo A.Y., Dang K., Maguire H., Kudchodkar S., Ramanathan M.P., Weiner D.B. Induction of potent Th1-type immune responses from a novel DNA vaccine for West Nile virus New York isolate (WNV-NY1999) J. Infect. Dis. 2001;184:809–816. doi: 10.1086/323395. [DOI] [PubMed] [Google Scholar]
- 32.Gaiger A., Reese V., Disis M.L., Cheever M.A. Immunity to WT1 in the animal model and in patients with acute myeloid leukemia. Blood. 2000;96:1480–1489. [PubMed] [Google Scholar]
- 33.Eslami N.S., Shokrgozar M.A., Mousavi A., Azadmanesh K., Nomani A., Apostolopoulos V., Day S., Amanzadeh A., Alimohammadian M.H. Simultaneous immunisation with a Wilms’ tumour 1 epitope and its ubiquitin fusions results in enhanced cell mediated immunity and tumour rejection in C57BL/6 mice. Mol. Immunol. 2012;51:325–331. doi: 10.1016/j.molimm.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 34.Kreiter S., Vormehr M., van de Roemer N., Diken M., Löwer M., Diekmann J., Boegel S., Schrörs B., Vascotto F., Castle J.C. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015;520:692–696. doi: 10.1038/nature14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linnemann C., van Buuren M.M., Bies L., Verdegaal E.M.E., Schotte R., Calis J.J.A., Behjati S., Velds A., Hilkmann H., Atmioui D.E. High-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nat. Med. 2015;21:81–85. doi: 10.1038/nm.3773. [DOI] [PubMed] [Google Scholar]
- 36.Gubin M.M., Zhang X., Schuster H., Caron E., Ward J.P., Noguchi T., Ivanova Y., Hundal J., Arthur C.D., Krebber W.J. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snyder A., Makarov V., Merghoub T., Yuan J., Zaretsky J.M., Desrichard A., Walsh L.A., Postow M.A., Wong P., Ho T.S. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bai X., Zhang Q., Wu S., Zhang X., Wang M., He F., Wei T., Yang J., Lou Y., Cai Z., Liang T. Characteristics of tumor infiltrating lymphocyte and circulating lymphocyte repertoires in pancreatic cancer by the sequencing of T cell receptors. Sci. Rep. 2015;5:13664. doi: 10.1038/srep13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boon T., Van Pel A. Teratocarcinoma cell variants rejected by syngeneic mice: protection of mice immunized with these variants against other variants and against the original malignant cell line. Proc. Natl. Acad. Sci. USA. 1978;75:1519–1523. doi: 10.1073/pnas.75.3.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gold J.S., Ferrone C.R., Guevara-Patiño J.A., Hawkins W.G., Dyall R., Engelhorn M.E., Wolchok J.D., Lewis J.J., Houghton A.N. A single heteroclitic epitope determines cancer immunity after xenogeneic DNA immunization against a tumor differentiation antigen. J. Immunol. 2003;170:5188–5194. doi: 10.4049/jimmunol.170.10.5188. [DOI] [PubMed] [Google Scholar]
- 41.Marrack P., Kappler J.W. Do MHCII-presented neoantigens drive type 1 diabetes and other autoimmune diseases? Cold Spring Harb. Perspect. Med. 2012;2:a007765. doi: 10.1101/cshperspect.a007765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsuboi A., Oka Y., Udaka K., Murakami M., Masuda T., Nakano A., Nakajima H., Yasukawa M., Hiraki A., Oji Y. Enhanced induction of human WT1-specific cytotoxic T lymphocytes with a 9-mer WT1 peptide modified at HLA-A*2402-binding residues. Cancer Immunol. Immunother. 2002;51:614–620. doi: 10.1007/s00262-002-0328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oka Y., Tsuboi A., Fujiki F., Shirakata T., Nishida S., Hosen N., Nakajima H., Li Z., Kawase I., Oji Y., Sugiyama H. “Cancer antigen WT1 protein-derived peptide”-based treatment of cancer—toward the further development. Curr. Med. Chem. 2008;15:3052–3061. doi: 10.2174/092986708786848631. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.