Abstract
Objectives:
This paper sought to provide normative values for grip strength among older adults across different age groups in northwest Russia and to investigate their predictive value for adverse events.
Methods:
A population-based prospective cohort study of 611 community-dwelling individuals 65+. Grip strength was measured using the standard protocol applied in the Groningen Elderly Tests. The cut-off thresholds for grip strength were defined separately for men and women of different ages using a weighted polynomial regression. A Cox regression analysis, the c-statistic, a risk reclassification analysis, and bootstrapping techniques were used to analyze the data. The outcomes were the 5-year mortality rate, the loss of autonomy and mental decline.
Results:
We determined the age-related reference intervals of grip strength for older adults. The 5th and 10th percentiles of grip strength were associated with a higher risk for malnutrition, low autonomy, physical and mental functioning and 5-year mortality. The 5th percentile of grip strength was associated with a decline in autonomy.
Conclusions:
This study presents age- and sex-specific reference values for grip strength in the 65+ Russian population derived from a prospective cohort study. The norms can be used in clinical practice to identify patients at increased risk for adverse outcomes.
Keywords: Grip Strength, Age-related Reference Intervals, Older Adults
Introduction
Recent clinical and epidemiological studies have shown that the grip strength of middle aged and older adults is an important marker of current health, and it is essential to follow people during aging, injury and rehabilitation[1-7]. Different studies have confirmed that low grip strength is correlated with sarcopenia, frailty, malnutrition and a loss of bone mineral density, suggesting that this measure can be used to screen people at risk for osteoporosis, a loss of physical functionality, and negative effects following health recovery after illness and surgery[1-7]. Low grip strength also predicts the onset of dependency regarding the activities of daily living and cognitive impairment as well as the all-cause and cardiovascular mortality rates[3].
Grip strength is measured quantitatively using a hand dynamometer. This method is simple and effective, and it can be used during a general examination of the patient at any medical center. However, the use of grip strength to predict the risk of an adverse event, the outcomes of care, or both for individuals requires cut-off values that are appropriate to a given population. Unfortunately, the existing normative data in Russia have focused primarily on children, adolescents, and young adults[8]. Some population-based normative data for older adults are available from other research areas[9-16]. However, populations of older people in different countries are not similar because of difference in lifespan, socioeconomic status, the prevalence of chronic diseases, disability and the use of healthcare. In addition, concerns exist that the reference ranges derived from one population of community-dwelling older adults might not be representative of another population, and country-specific norms might be needed[16].
Several studies have reported that low socio-economic status, high alcohol consumption are independent risk factors for decreased grip strength among older adults[17-19]. Additionally, low grip strength has been associated with increased all-cause and cardiovascular mortality rates[18,20,21].
Compared with other countries, the Russian population 65+ exhibits its own characteristics that distinguish it from other countries. Russia combines features of developed and developing countries, with a high mortality rate from non-communicable diseases, which resembles developed countries, and a low social- economic status, a lack of necessary medical and surgical treatments and a low overall life expectancy as in developing countries[22-25]. For example, men aged 50-54 years old in Russia have even a higher risk of dying from ischemic heart diseases than 75-79-year-old men in France. Socio-economic factors are also important. The rate of poverty in the entire population varies between 18% and 50%, some authors even claim that up to 70% of aging couples can be considered as poor[26].
Because no studies have measured the grip strength of older adults in Russia, it is necessary to conduct this type of study. Thus, this paper aimed to provide normative values for grip strength of older adults across different age groups in northwest Russia and to investigate grip strength’s usefulness as a predictor of mortality and both cognitive and physical decline in older adults in northwest Russia.
Methods
Study design and population
The data for the current study were extracted from the first population-based prospective cohort study of community-dwelling individuals above 65 years old in northwest Russia. The primary care clinic (Policlinic no. 95) serves a population of 58,000 inhabitants based on a territorial concept of administration. Of this population, 10,986 people are above 65 years old. The initial cohort was composed of a random sample of 611 people. All of the initial data were collected between March and December 2009 (T0). A second assessment (T1) was performed an average of 33.4±3 months after the first data collection between February and August 2012. The total follow-up period was 5 years (T2).
Major study parameters
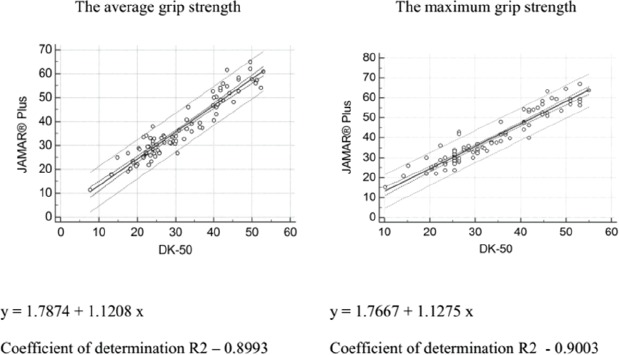
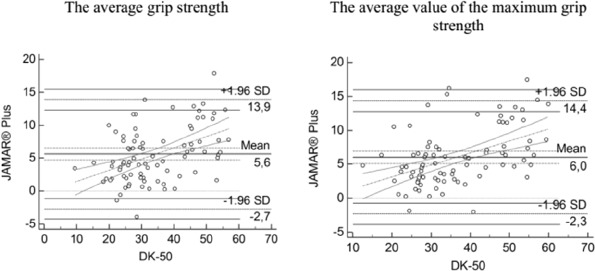
- Grip strength was measured using a carpal dynamometer (DK-50, Nizhni Tagil, Russian Federation) according to the standard protocol applied in the Groningen Elderly Test[27]. The maximum reading (daN) of three attempts for each hand was recorded. The highest and average values of the stronger hand were used in the analyses. The stronger hand was defined as the dominant hand. The validity of the measurements obtained using the DK50 dynamometer was investigated in a dedicated study in which the JAMAR® Plus digital hand dynamometer was used as a gold standard (supplementary file).
- Anthropometric measurements, including height, weight, mid-arm circumference (MAC), and triceps skinfold thickness (TSF) were measured. Mid-arm muscle area (MAMA) was calculated as follows: MAMA (cm) = MAC (cm)-3.142 x TSF (cm). The cutoff values for MAMA were ≥23 cm in men and ≥21 cm in women[28].
- The Short Physical Performance Battery (SPPB) consists of timed measurements of the following activities: quickly walking, rising from a chair, putting on and taking off a cardigan and maintaining balance in a tandem stand. An overall performance scale (ranging from 0 to 14) was created by summing the scores from the individual tests. The cutoff value for poor physical performance was defined as an SPPB score <8[28,29].
- The 15-item Geriatric Depression Scale (GDS-15) was used to screen for depressive symptoms. The cutoff for depression was defined as a score of more than 5[30].
- The Mini-Mental State Examination (MMSE) was used to determine cognitive impairment. The cutoff value was 24[31].
- The Barthel Index (BI) of activities of daily living was used to determine the baseline level of functioning and the consequent degree of dependence. The cutoff for dependency was defined as a score of less than 95[32].
- Nutritional status was evaluated using the Mini Nutritional Assessment (MNA) questionnaire. An MNA score between 17.0 and 23.5 was interpreted as at risk for malnutrition, and a score of less than 17.0 was an indicator of malnutrition[33].
- Spirometry. Two portable microspirometers (MIR Spirobank, Rome, Italy) were used for this procedure. Poor lung function was expressed as the lowest quintile of a forced expiratory volume in 1 second divided by height cubed (FEV1/Ht3) adjusted for age[28].
- Laboratory tests. Hemoglobin was determined using the cyanide-free hemoglobinometry method with an Abbott Cell-Dyn 3700 hematology analyzer. C-reactive protein, serum creatinine, total protein, and albumin were determined using the immunoturbidimetric method with a Roche Cobas Integra 400 analyzer. The B-type natriuretic peptide, thyrotropin, testosterone, and vitamin D3 were determined using a chemiluminescent microparticle immunoassay for the quantitative determination of human BNP with the Architect i1000 System. The following normal reference ranges for each laboratory test were used: CRP, 0-5 mg/L; total protein, 64-87 g/L; albumin, 35-50 g/L; thyrotropin, 0.23.2 uU/ml; vitamin D3 (25-OH), 30-100 ng/ml; creatinine, 53-106 mmol/L for men and 44-88 mmol/L for women; hemoglobin (venous blood), 130-170 g/L for men and 120-150 g/L for women. Renal function was estimated using the Modification of Diet in Renal Disease formula (MDRD). A glomerular filtration rate lower than 60 mL/min indicates a decline of renal function.
- Details concerning past and current medical problems were collected based on patients’ medical histories, medical records, or both. Categories of multimorbidity were defined based on the distribution of the disease count (DC): Level 1: DC <3; level 2: DC 3-4; and level 3: DC >5[34].
Outcome measures
Mortality
The mortality data were determined using the National Death Registry.
Mental decline
Relevant declines in the MMSE were determined using the Edwards-Nunnally index[35]. This index is used to determine the probability of a substantial individual change and avoids the problem of regression to the mean. Based on the scale reliability and the 95% confidence intervals (CIs) of the mean score at T0, the index was used to assess whether a significant change had occurred between T0 and T1.
Decline of autonomy
A significant decline in the BI, ranging from 0 to 100, was calculated using the formula (T0-T1)*100/T0. To account for temporal variability in the BI score, only participants with a more than 20% change in their scores were defined as having a significant decline.
The local ethics committee of The North-Western State medical University named after I.I. Mechnikov approved this research for Postgraduate Studies and informed consent was obtained from all participants.
Statistical analyses
Means (±SD) were calculated for the grip strength measurements of the dominant hand. A repeated-measures analysis of variances (ANOVA) was used to estimate the difference in grip strength across three attempts. Differences among the participants with different grip strength scores were compared using the Mann-Whitney U test for continuous variables or the chi-square test for categorical variables.
Age-related reference intervals of grip strength for men and women were modeled with regard to age using a weighted polynomial regression[36]. The coefficients for skewness and kurtosis were used to measure the degree of symmetry and peakedness/flatness in the grip strength distribution separately for men and women. The Shapiro-Wilk test was used to test for a normal distribution. The distribution of the grip strength scores was normal for men. The distribution of the grip strength scores for women was positively skewed and showed a leptokurtic distribution; thus, we performed a Box-Cox power transformation for this variable prior to calculation. The Tukey test was used to inspect outliers. The resulting grip strength values for women were back-transformed to their original scale. Z-scores were used to evaluate how well the model fit the data.
The classification correspondence between the 5th (P5) and 10th (P10) percentiles of the average grip strength (AGS) and the maximum grip strength (MGS) values were tested using kappa statistics and considered as excellent for values ranging from 0.81-1; good for values ranging from 0.61-0.80; moderate for values ranging from 0.41-0.60; fair for values ranging from 0.21-0.40; and poor for values less than 0.21. Kaplan-Meier curves were used to assess the relationship with the mortality rate, and the log rank test was used to compare different strata. Cox proportional hazard regression models adjusted for age, gender and comorbidity levels as well as lung function, nutritional status, anemia and inflammation were used to estimate the hazard ratios (HRs) for mortality. The robust group was used as a reference category. Harrell’s C was used to estimate the probability of the concordance between predicted risk and the observed order of events for a randomly selected pair of participants. A zone of uncertainty for Harrell’s C index was also calculated using a jackknife procedure. The net reclassification improvement (NRI) and the integrated discrimination improvement (IDI) analysis in the context of censored survival outcomes was performed to measure the benefit of the average or maximum measurements of grip strength to predict mortality. The effect of impaired handgrip strength on the likelihood of autonomy and mental limitation was examined using an age and sex multimorbidity-adjusted logistic regression model using loss of autonomy and mental decline as the dependent variables. The variables were first checked for multicollinearity.
All statistical calculations were performed using SPSS 20.0 (IBM, Armonk, NY, USA), Stata 13.0 (StataCorp, College Station, TX), MedCalc 11.5.00 (MedCalc Software, Oostende) and SAS, University edition (SAS Institute, Inc., Cary, NC). The level of significance adopted was p<0.05.
Results
Sample
Table 1 summarizes the demographic and health characteristics of the sample. The present study assessed 611 participants. Nine participants had missing grip strength data and were excluded from the analysis. Thus, the total sample of the first assessment was 602 participants (166 man and 436 women; Table 1). The age range was 65-91 years.
Table 1.
Health characteristics of the Crystal populations.
| 1st assessment (n=601) | 2nd assessment (n=378) | |||
|---|---|---|---|---|
| Characteristics | Men (n=166) | Women (n=436) | Men (n=94) | Women (n=284) |
| Age group, n (%) | ||||
| 65-69 | 45 (27.1) | 85 (19.5) | 12 (7.2) | 13 (3.0) |
| 70-74 | 51 (30.7) | 123 (28.2) | 64 (38.6) | 136(31.2) |
| 75-79 | 46 (27.7) | 107 (24.5) | 43 (25.9) | 110 (25.2) |
| 80-84 | 20 (12.0) | 81 (18.6) | 36 (21.7) | 101 (23.2) |
| 85-89 | 3 (1.8) | 36 (8.3) | 9 (5.4) | 58 (13.3) |
| 90+ | 1 (0.6) | 4 (0.9) | 2 (1.2) | 18 (4.1) |
| AGS by age group, mean (±SD) | ||||
| 65-69 | 31.2(±8.3) | 17.2(±5.3) | 25.3(±13.1) | 15.9(±3.4) |
| 70-74 | 27.2(±7.7) | 15.6(±4.7) | 26.7(±10.1) | 15.2(±4.7) |
| 75-79 | 23.9(±7.6) | 14.5(±5.0) | 20.4(±6.7) | 12.0(±3.9) |
| 80-84 | 21.9(±7.9) | 11.7(±4.6) | 16.0(±8.0) | 11.2(±4.0) |
| 85-89 | 19.7(±6.2) | 11.6(±4.4) | 12.2(±1.8) | 9.9(±3.8) |
| 90+ | 7.8 | 11.4(±3.7) | - | 8.9(±5.3) |
| MGS by age group, mean(±SD) | ||||
| 65-69 | 32.2(±8.5) | 18.2(±5.3) | 26.5(±13.9) | 16.8(±3.4) |
| 70-74 | 28.5(±8.2) | 16.6(±4.7) | 27.9(±10.4) | 16.1(±4.9) |
| 75-79 | 24.9(±7.5) | 15.6(±5.2) | 21.7(±6.7) | 13.2(±4.0) |
| 80-84 | 22.9(±8.1) | 12.6(±4.7) | 17.0(±8.1) | 12.3(±4.1) |
| 85-89 | 20.7(±6.5) | 12.5(±4.3) | 13.3(±2.5) | 10.9(±4.1) |
| 90+ | 10.2 | 12.5(±4.1) | - | 9.5(±5.5) |
| Coronary artery diseases. n (%) | 127 (74.7) | 357 (81.0) | 83 (88.3) | 258 (90.8) |
| Myocardial infarction. n (%) | 31 (18.7) | 45 (10.3) | 22 (23.4) | 22 (7.7) |
| Episode or chronic atrial fibrillation. n (%) | 51 (30.7) 133 (30.5) | 18 (19.1) | 52 (18.3) | |
| Stroke, n (%) | 28 (16.9) | 61(14.0) | 14 (14.9) | 42 (14.8) |
| Diabetes mellitus, n (%) | 20 (12.0) | 66 (15.1) | 14 (14.9) | 51 (18.0) |
| COPD, n (%) | 38 (22.9) | 103 (23.6) | 24 (25.5) | 43 (15.1) |
| Asthma, n (%) | 3 (1.8) | 24 (5.5) | 3 (3.2) | 19 (6.7) |
| Peripheral arterial disease, n (%) | 37 (22.3) | 98 (22.5) | 19 (20.2) | 74 (26.1) |
| Physical Performance Tests score <8, n (%) | 9 [7-12] | 9 [6-11] | 8 [6-10] | 8 [5-10] |
| Barthel Index < 95, n (%) | 32 (19.3) | 109 (25.0) | 13 (13.8) | 53 (18.7) |
| Geriatric Depression Scale score > 5, n (%) | 126 (75.9) | 274 (62.8) | 73 (77.7) | 198 (69.7) |
| Mini-Mental State Examination score | ||||
| 25-30 Normal, n (%) | 118 (71.1) | 278 (63.8) | 58 (61.7) | 165 (58.1) |
| 21-24 Mild, n (%)** | 28 (16.9) | 95 (21.8) | 15 (16.0) | 61 (21.5) |
| 10-20 Moderate, n (%) | 19 (11.4) | 57 (13.1) | 17 (18.1) | 50 (17.6) |
| 0-9 Severe, n (%) | 1 (0.6) | 6 (1.4) | 4 (4.3) | 8 (2.8) |
| Mini-Nutritional Assessment | ||||
| <17 “Malnourished”, n (%) | 137 (82.5) | 354 (81.2) | 61 (64.9) | 176 (63.9) |
| 17-23.5 “At risk of malnutrition”, n (%) | 26 (15.7) | 77 (17.7) | 32 (34.0) | 94 (33.6) |
| >23.5 “Normal nutritional status”, n (%) | 3 (1.8) | 5 (1.1) | 1 (1.1) | 7 (2.5) |
| BMI, kg/m2, mean (±SD) | 27.2 (±4.4) | 28.8 (±5.0) | 27.3 (±4.1) | 29.1 (±5.2) |
| FEV1, ml, mean (±SD) | 2.6 (±0.7) | 1.9 (±0.5) | 2.6 (0.6) | 1.9 (±0.6) |
AGS, average grip strength; MGS, maximum grip strength; SD, Standard deviation; BMI, Body mass index; FEV1, forced expiratory volume in 1 second.
A total of 379 participants were available for the second assessment (102 participants died before the second assessment, and 130 patients refused to participate). No significant differences were found with regard to the baseline characteristics of the patients who participated and those who refused to participate in the second assessment[28]. One participant had missing grip strength data and was excluded from the analysis. The total number of participants was 378 (94 men and 285 women). The age range was 68-94 years (Table 1).
We observed high rates of cardiovascular disease (86.7%) and depression (34.2%) as well as different degrees of cognitive (34.6%) and vision (89.5%) impairments, and urinary incontinence (41.1%) at baseline. The disease prevalence was similar between men and women. However, rates of depression and asthma were higher in women (p<0.05), and the prevalence of past myocardial infarction was higher in men (p<0.05).
A repeated-measures analysis of variance revealed a slight difference across three grip strength measurement attempts. The third grip strength attempt was a slightly weaker than the first and second attempts (from 0.01(±0.09) kg to 0.23(±0.06) kg). This effect was observed in both genders. AGS and MGS were higher in men than women, slightly correlated with body mass index (BMI; the correlation coefficients were 0.173 in women and 0.267 men) and decreased with age. The differences in the mean AGS and MGS between the dominant and nondominant hands were 3.4(±2.9) and 3.6(±3.2), respectively. The mean (±SD) AGS and MGS values at the first and the second assessments are listed in Table 1. After applying a correction factor to make the values comparable with those generated by the JAMAR® Plus dynamometer that we considered as a reference standard, slightly higher values were obtained: +3.9(±1.0) kg for AGS and +4.2(±1.1) kg for MGS (supplementary file).
The development of age-related grip strength reference intervals
To create age-related grip strength reference intervals for healthy older people, we chose participants from the first (84 men and 185 women) and second (37 men and 87 women) assessments with MMSE scores >=23, BI scores >95 and SPPB scores >=8. The grip strength data taken at the second and first assessments were mixed to increase the amount of older people for the calculation of the age-related reference interval. The results are presented in Figures 1 and 2.
Figure 1.

Age-related declines in average grip strength.
Figure 2.
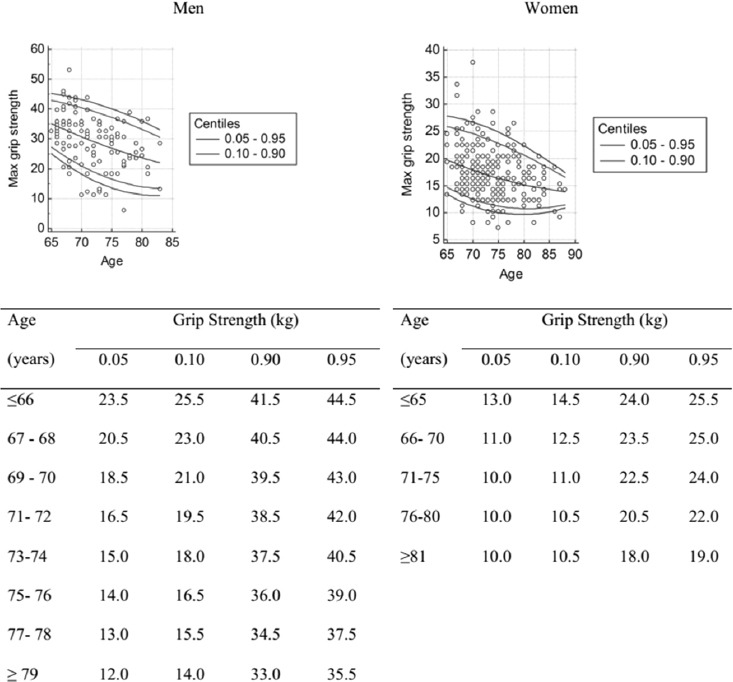
Age-related declines in maximum value grip strength.
The men were stronger than the women. Figure 1 shows an almost linear course of age-related grip strength decline, with a tendency to level off among the oldest women (Figures 1 and 2). The fastest rate of grip strength decline occurred between ages 70 and 75 in men (1 kg/year) and slowed down significantly after age 75 (0.5 kg/year). The mean annual loss in grip strength in women was 0.3-0.4 kg.
The agreement between the P5 of the AGS and MGS was excellent with, a kappa coefficient of 0.97 (0.94 to 0.99). The kappa coefficients for the P10 of the AGS and MGS were slight lower (0.81 (0.77 to 0.89)). Participants with low AGS and MGS (P5/P10) were older and showed higher rates of pulmonary disease, stroke, peripheral arterial disease, past fractures, DC, mental illness, low autonomy and physical function compared with participants with higher values of AGS and MGS (Table 2). The decreases in AGS and MGS were associated with an increased prevalence of these pathology conditions. The prevalence of malnutrition risk according the MNA and an unintentional weight loss of 6 kg over the past 6 months (or 3 kg over the past 3 months) were higher in participants with low grip strength; nevertheless, neither MAC nor MAMA significantly differed between groups. We did not find differences with regard to the levels of vitamin D, total protein, thyrotropin, testosterone, or B-type natriuretic peptide in the analyzed groups; however, the albumin levels of participants with low grip strength were lower.
Table 2.
Health characteristics of the participants with different average and maximum grip strength values.
| Characteristics | P5 of AGS/MGS | >P5 of AGS/MGS | P10 of AGS/MGS | >P10 of AGS/MGS |
|---|---|---|---|---|
| Demographic | ||||
| Age, median [IQR] | 77 [72 - 87] | 74 [70 - 79]* | 77 [72 - 83] | 70 [73 - 78]* |
| age group 65-70 years | 14 (17.7) | 158 (30.3) | 20 (16.8) | 152 (31.5) |
| age group 71-75 years | 17 (21.5) | 140 (26.8) | 27 (22.7) | 130 (27.0) |
| age group 76-80 years | 26 (32.9) | 128 (24.5) | 32 (26.9) | 122 (25.3) |
| age group 81-85 years | 13 (16.5) | 71 (13.6) | 24 (20.2) | 60 (12.4) |
| age group 86 -90 years | 8 (10.1) | 24 (4.6) | 14 (11.8) | 18 (3.7) |
| age group > 90 years | 1 (1.3) | 1 (0.2) | 2 (1.7) | - |
| Gender | ||||
| Women, n (%) | 65 (82.3) | 371 (71.1)* | 95 (79.8) | 341 (70.7)* |
| Death, n (%) | 37 (46.8) | 136 (26.1)* | 52 (43.7) | 121 (25.1)* |
| Details of medical problem | ||||
| Cardiovascular disease: | ||||
| Coronary artery diseases, n (%) | 68 (86.1) | 409(78.4) | 99 (83.2) | 378 (78.4) |
| Myocardial infarction, n (%) | 13 (16.5) | 63 (12.1) | 18 (15.1) | 58 (12.0) |
| Episode or chronic atrial fibrillation. n (%) | 33 (41.8) | 107 (20.5) | 41 (34.5) | 143 (29.7) |
| Stroke, n (%) | 18 (22.8) | 71 (13.6)* | 25 (21.0) | 64 (13.3)* |
| Diabetes mellitus, n (%) | 15 (19.0) | 71 (13.6) | 17 (14.3) | 69 (14.3) |
| COPD, n (%) | 33 (41.8) | 107 (20.5)* | 43 (36.1) | 97 (20.1)* |
| Asthma, n (%) | 8 (10.1) | 19 (3.6)* | 9 (7.6) | 18 (3.7) |
| Peripheral arterial disease, n (%) | 29 (36.7) | 106 (20.3)* | 36 (30.3) | 99 (20.5)* |
| Cancer, n (%) | 5 (6.3) | 16 (3.1) | 6 (5.0) | 15 (3.1) |
| Parkinson, n (%) | 1 (1.3) | 6 (1.1) | 2 (1.7) | 5 (1.0) |
| Fracture in anamnesis, n (%) | 43 (54.4 | 203 (38.9)* | 61 (51.3) | 185 (38.4)* |
| Incontinence, n (%) | 45 (57.0) | 200 (38.3)* | 67 (56.3) | 178 (36.9)* |
| Vision problems | 70 (88.6) | 469 (89.8) | 109 (91.6) | 430 (89.2) |
| Hearing problems | 48 (60.8 | 316 (60.5) | 78(65.5) | 286 (59.3) |
| Problems with grocery shopping | 33 (41.8) | 67 (12.8)* | 46(38.7) | 54 (11.2)* |
| Problems with walking outside house (around the house or to a neighbor) | 25 (31.6) | 39 (7.5)* | 35 (29.4) | 29(6.0)* |
| Problems with getting (un)dressed | 39 (7.5) | 10 (1.9)* | 7 (5.9) | 8 (1.7)* |
| Problems with visiting the restroom | 4 (5.1) | 11 (2.1) | 6 (5.0) | 9 (1.9) |
| Disease count (DC) | ||||
| DC <3, n (%) | 56 (70.9) | 461(88.3)* | 91 (76.5) | 426 (88.4)* |
| DC 3-4, n (%) | 20 (25.3) | 59 (11.3)* | 25 (21.0) | 54 (11.2)* |
| DC >5, n (%) | 3 (3.8) | 2 (0.4)* | 3 (2.5) | 2 (0.4)* |
| Physical Performance Tests score <8, n (%) | 54 (68.4) | 179 (34.3)* | 77 (64.7) | 156 (32.4)* |
| Barthel Index < 95, n (%) | 41 (51.9) | 100 (19.2)* | 55 (46.2) | 86 (17.8)* |
| Geriatric Depression Scale score > 5, n (%) | 50 (63.3) | 151 (28.9)* | 71 (59.7) | 130 (27.0)* |
| Mini-Mental State Examination score | ||||
| 25-30 Normal, n (%) | 27 (34.2) | 368 (70.5)* | 44 (37.0) | 351 (72.8)* |
| 21-24 Mild, n (%)** | 21 (26.6) | 102 (19.5)* | 34 (28.6) | 89 (18.5)* |
| 10-20 Moderate, n (%) | 25 (31.6) | 51 (9.8)* | 35 (29.4) | 41 (8.5)* |
| 0-9 Severe, n (%) | 6 (7.6) | 1 (0.2)* | 6 (5.0) | 1 (0.2)* |
| Mini-Nutritional Assessment: | ||||
| <17 “Malnourished”, n (%) | 4 (5.1) | 4 (0.8)* | 4 (3.4) | 4 (0.8)* |
| 17-23.5 “At risk of malnutrition”, n (%) | 26 (32.9) | 76 (14.6)* | 35 (29.4) | 67 (13.9)* |
| >23.5 “Normal nutritional status”, n (%) | 49 (62.0) | 442 (84.7)* | 80 (67.2) | 411 (85.3)* |
| Unintentional weight loss of 6 kg in the past 6 months or 3 kg in 3 month | 19 (24.1) | 65 (12.5)* | 25(21.0) | 59 (12.2)* |
| Body mass index, mean (±SD) | 27.4(±4.9) | 28.8(±4.9)* | 26.9(±4.8) | 29.1(±4.9)* |
| Mid-arm circumference, mean (±SD) | 24.3(±5.7) | 24.4(±4.7) | 24.4(±6.9) | 24.4(±4.2) |
| Mid- arm circumference < 23 in men and <21 in women, n (%) | 21 (26.9) | 125 (24.0) | 31(26.3) | 83 (17.2) |
| FEV1 divided by height cubed, mean (±SD) | 0.4±0.1 | 0.5±0.13 | 0.4±0.1 | 0.5±0.1* |
| Anemia, n (%) | 22 (27.8) | 92 (17.6)* | 33 (28.5) | 113 (23.2)* |
| Vitamin D, median [IQR] | 18.5[16.0 -21.4] | 17.8[14.1-22.1] | 18.5[16.0 -21.8] | 17.6 [14.0-21.8] |
| Albumin < 35 g/L, n (%) | 9 (12.3) | 5 (1.6)* | 9 (8.8) | 5 (1.8)* |
| Total protein < 64 g/L, n (%) | 2 (2.7) | 11 (3.6) | 4 (3.9) | 9 (3.2) |
| Thyrotropin > 3.2 uU/ml, n (%) | 12 (16.4) | 73 (23.7) | 19 (18.4) | 66 (23.7) |
| Testosterone < 9.9 nmol/l, n (%) | 3 (17.6) | 13 (16.9) | 6 (20.7) | 10 (15.4) |
| B-type natriuretic peptide > 100 pg/ml, median [IQR] | 22 (30.1) | 82 (26.6) | 33 (32.0) | 71 (25.5) |
| Decrease in glomerular filtration rate (MDRD <60), n (%) | 16 (20.3) | 97 (18.6) | 26 (21.8) | 87 (18.0) |
AGS, average grip strength; MGS, maximum grip strength; P5, 5th percentile of grip strength; P10, 10th percentile of grip strength; SD, standard deviation; IQR, interquartile range; MDRD, Modification of Diet in Renal Disease formula; DC, disease count; FEV1, forced expiratory volume in 1 second;
p<0.05.
Outcomes
A total of 180 (29.5%) patients died during the follow-up period. The Kaplan-Meier curves showed a lower cumulative survival rate for all-cause mortality for participants in the P10 and the P5 of AGS and MGS (log-rank test, p<0.001; Figure 3). After adjusting for potential confounders including age, sex, comorbidity levels, nutritional status, poor lung function, anemia and inflammation, only the P5 of AGS was associated with the 5-year mortality rate (HR [95% CIs]= 1.56 [1.01-2.43]) (Table 3). Additionally, the P5 of AGS was better at improving the reclassification of those who were in the lower risk group for all-cause mortality compared with the P5 of MGS. However, an IDI analysis did not a reveal difference between the P5 of AGS and that of MGS (Table 3). The negative predictive value (NPV) ranged from 84.58 (80.87-87.87) for the P5 of AGS to 89.42 (85.87-92.33) for the P10 of MGS.
Figure 3.
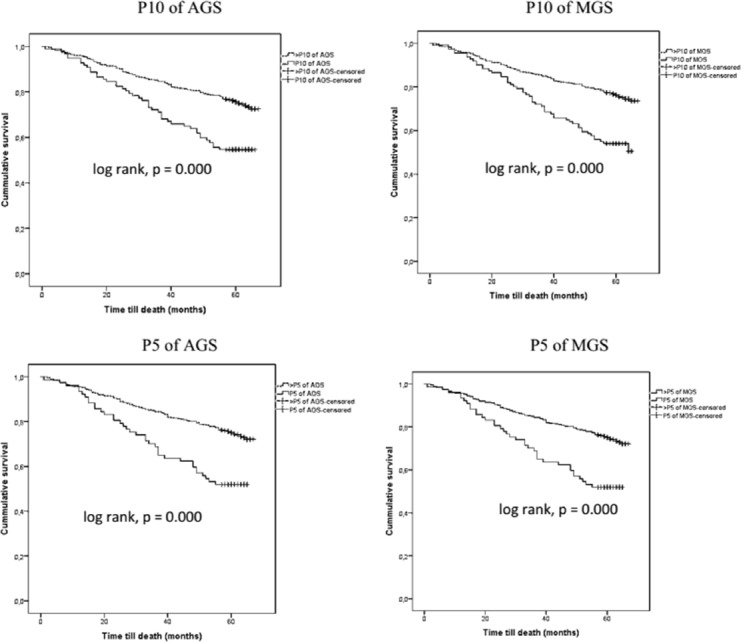
Kaplan-Meier curves for all-cause 5-year mortality based on the P10 and P5 the values of the average and maximum grip strength.
Table 3.
The predictive values of AGS and MGS for mortality at a 5-year follow-up assessment.
| Predictors | AGS | MGS | ||||
|---|---|---|---|---|---|---|
| HR (95% CIs) | HR (95% CIs) | HR (95% CIs) | HR (95% CIs) | HR (95% CIs) | HR (95% CIs) | |
| P5 | 2.22(1.54 -3.19)* | 2.04(1.97- 2.98)* | 1.56(1.01-2.43)* | 2.22 (1.54-3.19)* | 2.03 (1.39 - 2.96)* | 1.48(0.96-2.29) |
| Sex | 1.98(1.42-2.75)* | 1.96(1.35-2.85)* | 1.98 (1.42 - 2.75)* | 1.92(1.33-2.79) | ||
| Age | 1.12(1.09-1.15)* | 1.11(1.08-1.14)* | 1.12 (1.09 - 1.15)* | 1.11(1.08-1.14) | ||
| Comorbidity | 0.86(0.57-1.28) | 0.79(0.49-1.27) | 0.86 (0.57 - 1.28) | 0.79(0.49-1.27) | ||
| FEV1/Ht3 | 1.47(1.02-2.12)* | 1.47(1.02-2.12) | ||||
| Anemia | 1.52(1.04-2.22)* | 1.54(1.06-2.24) | ||||
| CRP>5 g/L | 1.35(0.87-2.08) | 0.74(0.48-1.14) | ||||
| MNA 17-23 | 1.31(0.86-2.01) | 1.32(0.86-2.02) | ||||
| MNA <17 | 3.57(1.46-8.72)* | 3.66(1.50-8.92) | ||||
| Harrell’s C | 0.55 (0.52-0.58) | 0.57 (0.53 - 0.60) | ||||
| Sensitivity, % | 48.05 (36.52-59.74) | 47.74(36.01-59.07) | ||||
| Specificity, % | 71.16 (66.86 - 76.19) | 71.10(66.79-75.89) | ||||
| AUC | 0.60 (0.55 - 0.64) | 0.59(0.55-0.63) | ||||
| PPV, % | 21.26(15.44-28.10) | 21.26(15.44-28.10) | ||||
| NPV, % | 89.42(85.87-92.33) | 89.15(85.57-92.10) | ||||
| NRI | 0.257 (0.1284, 0.3966) | |||||
| NRI events | -0.5589 | |||||
| NRI non-events | 0.8159 | |||||
| IDI | 0.0006(-0.0008, 0.002) | |||||
| P10 | 2.05(1.49-2.86)* | 1.66(1.18-2.35)* | 1.35(0.92-2.00) | 2.10(1.52-2.89)* | 1.60(1.14-2.24)* | 1.28(0.87-1.88) |
| Sex | 1.86(1.34-2.58)* | 1.86(1.29-2.68)* | 1.90(1.37-2.62) | 1.88(1.30-2.71)* | ||
| Age | 1.12(1.09-1.15)* | 1.11(1.08-1.14) | 1.12(1.09-1.14) | 1.11(1.08-1.14)* | ||
| Comorbidity | 0.89(0.59-1.32) | 0.81(0.50-1.30) | 0.91(0.61-1.35) | 0.83(0.52-1.33) | ||
| FEV1/Ht3 | 1.47(1.02-2.12)* | 1.45(1.01-2.09)* | ||||
| Anemia | 1.55(1.06-2.26)* | 1.55(1.08-2.26)* | ||||
| CRP>5 g/L | 0.72(0.47-1.12) | 0.73(0.47-1.12) | ||||
| MNA 17-23 | 1.30(0.84-1.99) | 1.31(0.86-2.02) | ||||
| MNA <17 | 3.87(1.60-9.34)* | 4.00(1.67-9.62) | ||||
| Harrell’s C | 0.55 (0.52-0.58) | 0.55 (0.52-0.58) | ||||
| Specificity, % | 44.86 (35.23-54.78) | 45.00(35.91-54.35) | ||||
| Specificity, % | 74.55 (70.47 -78.33) | 75.10(70.99 - 78.90) | ||||
| AUC | 0.60 (0.56 - 0.64) | 0.60 (0.56 - 0.64) | ||||
| PPV, % | 27.59(21.09-34.86) | 31.03(24.25-38.48) | ||||
| NPV, % | 86.21 (82.58-89.34) | 84.58(80.87-87.87) | ||||
AGS, average grip strength; MGS, maximum grip strength; P5, 5th percentile of grip strength; P10, 10th percentile of grip strength; MNA, the Mini nutritional assessment; FEV1/Ht3, the FEV1 divided by height cubed; CRP, C-reactive protein; HR, Hazard ratio; AUC, area under the curve; NPV, negative predictive value; PPV, positive predictive value;
p<0.05.
Table 4.
The association between AGS/MGS and a decline in the autonomy of the Crystal population.
| Predictors | AGS | MGS | ||
|---|---|---|---|---|
| OR (95% CIs) | OR (95% CIs) | OR (95% CIs) | OR (95% CIs) | |
| P5 | 5.14(1.81-14.62)* | 4.09(1.27-13.15)* | 4.97(1.75-14.11)* | 3.93(1.23-12.58)* |
| Sex | 1.27(0.33-4.96) | 1.28(0.33-4.98) | ||
| Age | 1.22(1.11-1.34)* | 1.22(1.11-1.34)* | ||
| Comorbidity | 0.85(0.21-3.42) | 0.87(0.22-3.48) | ||
| Sensitivity, % | 33.33 (13.34 - 59.01) | 33.33 (13.34 - 59.01) | ||
| Specificity, % | 91.14 (87.72 - 93.86) | 91.11 (87.68 - 93.84) | ||
| AUC | 0.62 (0.57 - 0.67) | 0.62 (0.57 - 0.67) | ||
| PPV, % | 15.79 (6.02 - 31.25) | 15.79 (6.02 - 31.25%) | ||
| NPV, % | 96.48 (93.93 - 98.17) | 96.47 (93.92 - 98.16) | ||
AGS, average grip strength; MGS, maximum grip strength; P5, 5th percentile of grip strength; OR, odds ratio; AUC, area under the curve; NPV, negative predictive value; PPV, positive predictive value;
p<0.05.
After 2.5 years of follow up, mental decline was detected in 41.0 % of participants, and autonomy decline was detected in 6.9% of participants. We found an association between the P10 of AGS and MGS and autonomy decline. Nevertheless, this association remained significant only for the P5 of AGS and MGS after adjusting for age, sex and comorbidity. The AUC was 0.62 (0.57-0.67), and the NPV was 96.47 (93.92-98.16) for the P5 of AGS and 96.47 (93.92-98.16) for the P5 of MGS. Mental decline in the younger age group was only associated with the P5 of AGS; however, this difference was not significant after adjusting for age and sex.
Discussion
Major findings
Based on a prospective cohort study, we determined the age-related reference intervals of grip strength for older adults. The use of the P5 and P10 of grip strength data that were obtained by our study from clinical practice can help to identify the patients who are at higher risk of malnutrition, low autonomy, low physical and mental functioning and all-cause 5-year mortality. Only the P5 of AGS and MGS were associated with autonomy decline after adjusting for age, sex and comorbidity levels.
Interpretation of findings in relationship to previously published studies
Cross-sectional and longitudinal studies have previously reported an age-related decline in grip strength[10,11,16,37]. These trends can be observed in the current study in which the mean annual loss in grip strength was 1 kg for men younger than 75 years old and 0.5 kg older than 75 years old. The mean annual grip strength loss in women was 0.3-0.4 kg. Mean (SE) losses of 0.65 (0.02) kg/year for men and 0.34 (0.01) kg/year for women were observed in three nationwide population-based surveys in Denmark[16]. The Women’s Health and Aging Study II found high annual grip strength losses in women (1.10-1.31 kg, age 70-75; 0.50-0.39 kg, age 76+)[37].
The grip strength values obtained in the current study were lower than those reported in other population-based studies[10,11]. The use of another dynamometer might explain the lower results of our study. The Jamar hand dynamometer (Lafayette Instrument Company, USA) is the most widely cited in the literature, and it is accepted as the gold standard[38]. However, the grip strength of our population was within the lower limit of normal of the other studies, even after correcting for the JAMAR® Plus dynamometer measurements standard (Table 5)[10,11]. The reasons for the low grip strength value in our population should be investigated in the future.
Table 5.
AGS and MGS after correction for JAMAR® Plus dynamometer.
| 1st assessment (n = 601) | 2nd assessment (n=378) | |||
|---|---|---|---|---|
| Men ( n=166) | Women (n = 436) | Men ( n=166) | Women (n = 436) | |
| AGS by age group, mean(±SD) | ||||
| 65-69 | 36.7(±9.3) | 21.1(±5.9) | 30.0(±14.7) | 19.6(±3.8) |
| 70-74 | 32.3(±8.7) | 19.3(±5.2) | 31.7(±11.4) | 18.8(±5.3) |
| 75-79 | 28.6(±8.5) | 18.0(±5.6) | 24.7(±7.5) | 15.3(±4.3) |
| 80-84 | 26.3(±8.8) | 14.9(±5.2) | 19.8(±8.9) | 14.4(±4.5) |
| 85-89 | 23.9(±6.9) | 14.7(±4.9) | 15.5(±2.0) | 12.9(±4.2) |
| 90+ | 10.5 | 14.5(±4.1) | - | 11.8(±5.9) |
| MGS by age group, mean(±SD) | ||||
| 65-69 | 38.1(±9.6) | 22.2(±5.9) | 31.7(±15.7) | 20.7(±3.8) |
| 70-74 | 33.8(±9.2) | 20.5(±5.3) | 33.2(±11.8) | 19.9(±5.5) |
| 75-79 | 29.8(±8.5) | 19.4(±5.8) | 26.2(±7.6) | 16.6(±4.5) |
| 80-84 | 27.6(±9.2) | 16.0(±5.3) | 20.9(±9.2) | 15.6(±4.6) |
| 85-89 | 25.1(±7.3) | 15.8(±4.9) | 16.7(±2.8) | 14.1(±4.6) |
| 90+ | 13.3 | 15.8(±4.6) | - | 12.5(±6.2) |
AGS – average grip strength; MGS – maximum grip strength.
To our knowledge, our study is the first to model grip strength reference intervals by age using a weighted polynomial regression. Previous research has presented reference values for older adults using quintiles/quartiles/tertiles, mean (±SD) or percentiles that encompass a broad age range but are divided into restricted 5-year age groups of men and women[9-16,37-39]. The benefit of the present approach is that it accounts for the age heterogeneity of the population, possible outliers, and the different rates of grip strength decline in men and women to calculate reference intervals, leading to the creation of two-year intervals for men and five-year intervals for women. In addition, the normal distribution test and the Box-Cox power transformation were performed to increase the accuracy of the calculations.
In healthy people, age and sex are the strongest predictors of grip strength. Various other factors such as disease severity, co-morbidities, medical treatments, immobilization, systemic inflammation, infection, and electrolyte imbalances also contribute to muscle weakness[4]. Thus, grip strength is an indicator of overall health and a marker of disease severity, which is associated with mortality. Recent studies have proposed using low grip strength as a predictor of cardiovascular diseases and mental and autonomy decline as well as the all-cause, cardiovascular and respiratory mortality rates[3,4,7,40,41]. However, the above pathophysiological processes related to diseases commonly underlying death and associated with strength declines cannot fully explain the association between grip strength and mortality[7].
Our analysis revealed a strong association between low grip strength and malnutrition, risk of malnutrition according the MNA and an unintentional weight loss of 6 kg over the past 6 months (or 3 kg over the past 3 months). At the same time, we did not find a correlation between low grip strength and either MAC or MAMA. Reduced grip strength due to malnutrition is associated with morphological changes of selective type II fiber (anaerobic, glycolytic, and fast twitch) atrophy, loss of contractile elements, calcium content, decreases in muscle enzymes (phosphofructokinase, and succinate dehydrogenase), some muscle amino acid levels (glutamine, glycine, and alanine) and electrolyte changes[4,42,43]. However, previous studies have also failed to find a correlation between malnutrition and muscle weight, size or function[3,43].
A weak positive relationship was found between BMI and grip strength in this sample.
Past research exploring the relationship between BMI and grip strength has provided contradictory findings. Numerous researchers have reported that higher BMI is positively correlated with higher grip strength and proposed a mandatory adjustment of grip strength for BMI[5,44]. Several studies have found no association between grip strength and BMI[4,9,15]. Several reasons might explain why muscle strength is affected in obesity. Obese participants have greater muscle mass as well as more type IIb but fewer type I muscle fibers, which can lead to increased grip strength[4]. On the other hand, high body weight is associated with a sedentary lifestyle, reduced physical activity and mobility, insulin resistance, and chronic inflammation, which in turn can lead to muscle mass catabolism and grip strength decline[4,5,45]. Therefore, despite the fact that the muscle function of the lower limbs in obese people is usually higher, the upper limbs remain similar to those of individuals of normal or low body weight. In addition, although previously reports have shown that an increase in BMI is associated with higher grip strength, an increase in waist circumference is associated with lower grip strength[46]. Furthermore, long-term obesity was associated with poor grip strength later in life according to the Health 2000 Survey[47].
In contrast to other studies[6,48], we did not find an association between low grip strength and mental decline. This lack of association might reflect the relatively brief follow-up period or the method of calculation of mental decline in our study. For example, a significant association between MGS and mental decline was reported during the 7-year follow-up period within the HEPESE study. The predicted change in MMSE score over time was 0.87 points (SE=0.16, p<0.0001) for the 1st quartile of grip strength, 0.29 points (SE=0.15, p=0.05) for the 2nd quartile, and 0.25 points (SE=0.14, p=0.07) for the 3rd quartile[48]. The Edwards-Nunnally index accounts for the scale reliability and the 95% CIs of the mean score at baseline, and it might not detect these small changes in MMSE score[35].
As in previous studies, low grip strength was associated with a low Barthel Index and a decline in autonomy during the 2.5-year follow-up period[6,39,49]. The association between autonomy decline and low grip strength might be one of direct effects of grip strength on mortality[7]. Difficulties in performing daily activities are correlated with the reduced frequency of actually performing these activities. For example, low grip strength in our study was associated with problems with grocery shopping, walking outside the house (around the house or to a neighbor), getting (un)dressed, and visiting the restroom. Accordingly, declines in activities of daily living can predict declines in levels of physical activity and muscle strength[7]. Consequently, people with low muscle strength are often physically inactive and disabled, which makes them more vulnerable to develop malnutrition and have accidents such as injurious falls or other adverse outcomes[7].
Several methods are used to determine grip strength with a dynamometer: the calculation of the average value of the two or three attempts of dominant, non-dominant, or both hands; the calculation of the average value of three attempts produced each hand; and the estimation of the maximum grip strength of the dominant hand[6,11,15,50,51]. Our study compared two approaches: the calculation of AGS and MGS. Previous studies have not found a significant difference between these approaches[51,52]. In our study, the use of AGS after adjusting for sex, age, and other potential confounds was slightly better able to identify participants with a lower risk of death compared with MGS (based on the NRI for events and non-events). Thus, although AGS was slightly more sensitive, both approaches can be used in clinical practice.
Strengths and limitations
Our study has certain limitations. We did not collect information concerning the exact causes of death. The brief period between the first and second assessments might have influenced the lack of association between low grip strength and mental decline.
One major limitation is the small sample sizes across the different age groups that yielded large confidence intervals. Therefore, our calculations should be replicated on a large population-based samples. The strengths of our study are its prospective design, the comprehensive assessment performed, the 5-year follow-up assessment regarding mortality data without a loss of mortality data, and the innovative new approach to develop age-related reference intervals.
Conclusions
This study presented age- and sex-specific reference values for grip strength in the 65+ Russian population derived from prospective cohort study. These norms can be used in clinical practice to identify patients at increased risk of adverse outcomes.
Footnotes
The President of the Russian Federation (Grant 192-RP) and the Foundation Louvain supported this work. The authors have no financial or personal conflicts of interest concerning this paper.
Edited by: A. Ireland
References
- 1.Hogrel JY. Grip strength measured by high precision dynamometry in healthy subjects from 5 to 80 years. BMC Musculoskelet Disord. 2015;16:139. doi: 10.1186/s12891-015-0612-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Monaco M, Di Monaco R, Manca M, Cavanna A. Handgrip strength is an independent predictor of distal radius bone mineral density in postmenopausal women. Clin Rheumatol. 2000;19(6):473–6. doi: 10.1007/s100670070009. [DOI] [PubMed] [Google Scholar]
- 3.Gale CR, Martyn CN, Cooper C, Sayer AA. Grip strength, body composition, and mortality. Int J Epidemiol. 2007;36(1):228–35. doi: 10.1093/ije/dyl224. [DOI] [PubMed] [Google Scholar]
- 4.Norman K, Stobaus N, Gonzalez MC, Schulzke JD, Pirlich M. Hand grip strength: outcome predictor and marker of nutritional status. Clin Nutr. 2011;30(2):135–42. doi: 10.1016/j.clnu.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Sallinen J, Stenholm S, Rantanen T, Heliovaara M, Sainio P, Koskinen S. Hand-grip strength cut points to screen older persons at risk for mobility limitation. J Am Geriatr Soc. 2010;58(9):1721–6. doi: 10.1111/j.1532-5415.2010.03035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taekema DG, Gussekloo J, Maier AB, Westendorp RG, de Craen AJ. Handgrip strength as a predictor of functional, psychological and social health. A prospective populationbased study among the oldest old. Age Ageing. 2010;39(3):331–7. doi: 10.1093/ageing/afq022. [DOI] [PubMed] [Google Scholar]
- 7.Rantanen T, Volpato S, Ferrucci L, Heikkinen E, Fried LP, Guralnik JM. Handgrip strength and cause-specific and total mortality in older disabled women: exploring the mechanism. J Am Geriatr Soc. 2003;51(5):636–41. doi: 10.1034/j.1600-0579.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- 8.Баранов AA, Кучма BP, Скоблина НА. Физиче ское развитиедетей и подростков на рубе жетысячелетий. Москва: ИздательНаучныйцентрздоровьядетейРАМН; 2008. [Google Scholar]
- 9.Peters MJ, van Nes SI, Vanhoutte EK, Bakkers M, van Doorn PA, Merkies IS, et al. Revised normative values for grip strength with the Jamar dynamometer. J Peripher Nerv Syst. 2011;16(1):47–50. doi: 10.1111/j.1529-8027.2011.00318.x. [DOI] [PubMed] [Google Scholar]
- 10.Bohannon RW, Peolsson A, Massy-Westropp N, Desrosiers J, Bear-Lehman J. Reference values for adult grip strength measured with a Jamar dynamometer: a descriptive metaanalysis. Physiotherapy. 2006;92(1):11–5. [Google Scholar]
- 11.Bohannon RW, Bear-Lehman J, Desrosiers J, Massy-Westropp N, Mathiowetz V. Average grip strength: a meta-analysis of data obtained with a Jamar dynamometer from individuals 75 years or more of age. J Geriatr Phys Ther. 2007;30(1):28–30. [PubMed] [Google Scholar]
- 12.Cuesta-Vargas A, Hilgenkamp T. Values of Grip Strength Measured with a Jamar Dynamometer in 1526 Adults with Intellectual Disabilities and Compared to Adults without Intellectual Disability. PLoS ONE. 2015;10(6):e0129585. doi: 10.1371/journal.pone.0129585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Werle S, Goldhahn J, Drerup S, Simmen BR, Sprott H, Herren DB. Age- and genderspecific normative data of grip and pinch strength in a healthy adult Swiss population. J Hand Surg Eur Vol. 2009;34(1):76–84. doi: 10.1177/1753193408096763. [DOI] [PubMed] [Google Scholar]
- 14.Mohammadian M, Choobineh A, Haghdoost A, Hasheminejad N. Normative Data of Grip and Pinch Strengths in Healthy Adults of Iranian Population. Iran J Public Health. 2014;43(8):1113–22. [PMC free article] [PubMed] [Google Scholar]
- 15.Luna-Heredia E, Martin-Pena G, Ruiz-Galiana J. Handgrip dynamometry in healthy adults. Clin Nutr. 2005;24(2):250–8. doi: 10.1016/j.clnu.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Frederiksen H, Hjelmborg J, Mortensen J, McGue M, Vaupel JW, Christensen K. Age trajectories of grip strength: cross-sectional and longitudinal data among 8,342 Danes aged 46 to 102. Ann Epidemiol. 2006;16(7):554–62. doi: 10.1016/j.annepidem.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Mohd Hairi F, Mackenbach JP, Andersen-Ranberg K, Avendano M. Does socioeconomic status predict grip strength in older Europeans?Results from the SHARE study in non-institutionalised men and women aged 50+ J Epidemiol Community Health. 2010;64(9):829–37. doi: 10.1136/jech.2009.088476. [DOI] [PubMed] [Google Scholar]
- 18.Bowman OJ, Wallace BA. The Effects of Socioeconomic Status on Hand Size and Strength, Vestibular Function, Visuomotor Integration, and Praxis in Preschool Children. Am J Occup Ther. 1990;44(7):610–21. doi: 10.5014/ajot.44.7.610. [DOI] [PubMed] [Google Scholar]
- 19.Quan S, Jeong JY, Kim DH. The Relationship between Smoking, Socioeconomic Status and Grip Strength among Community-dwelling Elderly Men in Korea: Hallym Aging Study. Epidemiol Health. 2013;35:e2013001. doi: 10.4178/epih/e2013001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strand BH, Cooper R. The association of grip strength from midlife onwards with all-cause and cause-specific mortality over 17 years of follow-up in the Tromsø Study. J Epidemiol Community Health. 2016 doi: 10.1136/jech-2015-206776. doi: 10.1136/jech-2015-206776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheung CL, Nguyen US, Au E, Tan KC, Kung AW. Association of handgrip strength with chronic diseases and multimorbidity: A cross-sectional study. Age (Dordr) 2013;35(3):929–41. doi: 10.1007/s11357-012-9385-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Townsend N, Nichols M, Scarborough P, Rayner M. Cardiovascular disease in Europe-epidemiological update 2015. Eur Heart J. 2015;36(40):2696–705. doi: 10.1093/eurheartj/ehv428. [DOI] [PubMed] [Google Scholar]
- 23.Organization WH. World Health Statistics 2016: Monitoring health for the SDGs. Switzerland, Geneva: WHO Press; 2016. [Google Scholar]
- 24.Maximova TM, Belov VB, Lushkina NP, Karpova VM Study on global AGEing and adult health (SAGE), Wave 1. Russian Federation National Report. Design & Editorial Services. London (United Kingdom): World Health Organization; 2014. [Google Scholar]
- 25.Banegas JR, López-García E, Dallongeville J, Guallar E, Halcox JP, Borghi C, et al. Achievement of treatment goals for primary prevention of cardiovascular disease in clinical practice across Europe: the EURIKA study. Eur Heart J. 2011;32(17):2143–52. doi: 10.1093/eurheartj/ehr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strizhitskaya O. Aging in Russia. Gerontologist. 2016;56(5):795–9. doi: 10.1093/geront/gnw007. [DOI] [PubMed] [Google Scholar]
- 27.Lemmink K, Han K, de Greef MH, Rispens P, Stevens M. Reliability of the Groningen fitness test for the elderly. J Aging Phys Act. 2001;9(2):194–212. [Google Scholar]
- 28.Turusheva A, Frolova E, Hegendoerfer E, Degryse JM. Predictors of short-term mortality, cognitive and physical decline in older adults in northwest Russia: a population-based prospective cohort study. Aging Clin Exp Res. 2016 doi: 10.1007/s40520-016-0613-7. [DOI] [PubMed] [Google Scholar]
- 29.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 30.de Craen AJ, Heeren T, Gussekloo J. Accuracy of the 15-item geriatric depression scale (GDS-15) in a community sample of the oldest old. Int J Geriatr Psychiatry. 2003;18(1):63–6. doi: 10.1002/gps.773. [DOI] [PubMed] [Google Scholar]
- 31.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 32.Mahoney FI. Functional evaluation: the Barthel index. Md State Med J. 1965;14:61–5. [PubMed] [Google Scholar]
- 33.Vellas B, Guigoz Y, Garry PJ, Nourhashemi F, Bennahum D, Lauque S, et al. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition. 1999;15(2):116–22. doi: 10.1016/s0899-9007(98)00171-3. [DOI] [PubMed] [Google Scholar]
- 34.Boeckxstaens P, Vaes B, Van Pottelbergh G, De Sutter A, Legrand D, Adriaensen W, et al. Multimorbidity measures were poor predictors of adverse events in patients aged >/=80 years: a prospective cohort study. J Clin Epidemiol. 2015;68(2):220–7. doi: 10.1016/j.jclinepi.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Speer DC, Greenbaum PE. Five methods for computing significant individual client change and improvement rates: support for an individual growth curve approach. J Consult Clin Psychol. 1995;63(6):1044–8. doi: 10.1037//0022-006x.63.6.1044. [DOI] [PubMed] [Google Scholar]
- 36.Altman DG. Construction of age-related centiles using absolute residuals. Stat Med. 1993;12(10):917–24. doi: 10.1002/sim.4780121003. [DOI] [PubMed] [Google Scholar]
- 37.Xue QL, Beamer BA, Chaves PH, Guralnik JM, Fried LP. Heterogeneity in Rate of Decline in Grip, Hip, and Knee Strength and the Risk of All-Cause Mortality: The Women’s Health and Aging Study II. J Am Geriatr Soc. 2010;58(11):2076–84. doi: 10.1111/j.1532-5415.2010.03154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40(4):423–9. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 39.Wennie Huang WN, Perera S, VanSwearingen J, Studenski S. Performance measures predict onset of activity of daily living difficulty in community-dwelling older adults. J Am Geriatr Soc. 2010;58(5):844–52. doi: 10.1111/j.1532-5415.2010.02820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hasheminejad N, Namdari M, Mahmoodi MR, Bahrampour A, Azmandian J. Association of Handgrip Strength With Malnutrition-Inflammation Score as an Assessment of Nutritional Status in Hemodialysis Patients. Iran J Kidney Dis. 2016;10(1):30–5. [PubMed] [Google Scholar]
- 41.Leong DP, Teo KK, Rangarajan S, Lopez-Jaramillo P, Avezum A, Jr, Orlandini A, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386(9990):266–73. doi: 10.1016/S0140-6736(14)62000-6. [DOI] [PubMed] [Google Scholar]
- 42.Russell DM, Walker PM, Leiter LA, Sima AA, Tanner WK, Mickle DA, et al. Metabolic and structural changes in skeletal muscle during hypocaloric dieting. Am J Clin Nutr. 1984;39(4):503–13. doi: 10.1093/ajcn/39.4.503. [DOI] [PubMed] [Google Scholar]
- 43.Bissonnette D, Madapallimatam A, Jeejeebhoy K. Effect of hypoenergetic feeding and high-carbohydrate refeeding on muscle tetanic tension, relaxation rate, and fatigue in slow-and fast-twitch muscles in rats. Am J Clin Nutr. 1997;66(2):293–303. doi: 10.1093/ajcn/66.2.293. [DOI] [PubMed] [Google Scholar]
- 44.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 45.Kriketos AD, Pan DA, Lillioja S, Cooney GJ, Baur LA, Milner MR, et al. Interrelationships between muscle morphology, insulin action, and adiposity. A Am J Physiol. 1996;270(6):R1332–R9. doi: 10.1152/ajpregu.1996.270.6.R1332. [DOI] [PubMed] [Google Scholar]
- 46.Keevil VL, Luben R, Dalzell N, Hayat S, Sayer AA, Wareham NJ, et al. Cross-sectional associations between different measures of obesity and muscle strength in men and women in a British cohort study. J Nutr Health Aging. 2015;19(1):3–11. doi: 10.1007/s12603-014-0492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stenholm S, Sallinen J, Koster A, et al. Association between obesity history and hand grip strength in older adults-exploring the roles of inflammation and insulin resistance as mediating factors. J Gerontol A Biol Sci Med Sci. 2011;66(3):341–8. doi: 10.1093/gerona/glq226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alfaro-Acha A, Al Snih S, Raji MA, Kuo YF, Markides KS, Ottenbacher KJ. Handgrip strength and cognitive decline in older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2006;61(8):859–65. doi: 10.1093/gerona/61.8.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rantanen T, Avlund K, Suominen H, Schroll M, Frändin K, Pertti E. Muscle strength as a predictor of onset of ADL dependence in people aged 75 years. Aging Clin Exp Res. 2002;14(3 Suppl):10–5. [PubMed] [Google Scholar]
- 50.Roberts HC, Syddall HE, Sparkes J, Ritchie J, Butchart J, Kerr A, et al. Grip strength and its determinants among older people in different healthcare settings. Age Ageing. 2013;43(2):241–6. doi: 10.1093/ageing/aft118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coldham F, Lewis J, Lee H. The reliability of one vs. three grip trials in symptomatic and asymptomatic subjects. J Hand Ther. 2006;19(3):318–27. doi: 10.1197/j.jht.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Haidar S, Kumar D, Bassi R, Deshmukh S. Average versus maximum grip strength: which is more consistent? J Hand Surg Br. 2004;29(1):82–4. doi: 10.1016/j.jhsb.2003.09.012. [DOI] [PubMed] [Google Scholar]