Abstract
Breast cancer metastasis is a complex and still weakly understood process that involves diverse cellular pathways. It accounts for the majority of deaths from breast cancer. Recently, microRNAs (miRNAs), small non-coding RNAs that regulate gene expression post-transcriptionally, have been shown to be involved in breast cancer metastasis. In particular, in a recent work it has been found that miR-429 may have a role in the inhibition of migration and invasion of breast cancer cells. Its target gene CRKL has been identified as a potential candidate. In this paper, by using systems biology tools we have shown that CRKL is involved in positive regulation of ERK1/2 signaling pathway and contribute to the regulation of LYN through a topological generalization of feed forward loop.
Keywords: Breast cancer, Metastasis, MicroRNA, CRKL
Introduction
Breast cancer is the most common type of cancer among women. However, the majority of deths from breast cancer are not due to the primary tumour itself, but are the result of metastasis. Breast cancer metastasis is a very complex process that comprises a series of sequential steps and involves several processes (Olivia et al. 2012). Recently an involvement of microRNAs has been observed in almost every type of cancer including breast cancer.
MicroRNAs are an evolutionarily conserved class of small (about 22 nucleotides) non-coding RNAs that negatively regulate gene expression post transcriptionally by base-pairing to the 3′ UTR of target mRNAs leading to repression of protein production or mRNA degradation. Each miRNA can regulate the expression of hundreds of genes and a single transcript can be targeted by multiple miRNAs. [For a recent introduction see for example (Malik and Jens 2014)].
Since their discovery in 1993 (Lee et al. 1993) there have been increasing evidences that they are involved in important biological processes. Indeed, in normal cells, miRNAs control normal rates of cellular growth, proliferation, differentiation and apoptosis. Involvement of miRNAs in diverse cellular events indicates that their dysregulated expression and function may lead to development of different diseases including cancer. A potential role of miRNAs in the development and progression of cancer has been suggested by some observations early in the history of miRNAs. One of these observations is that tumor cell lines and malignant tumours were found to have widespread deregulation of miRNA expression compared to normal tissues (Stefanie et al. 2008). Thus, tumour formation may be a consequence of a reduction of a tumour suppressor miRNA or overexpression of an oncogene miRNA. Examples of tumor suppressor miRNAs are let-7 family, miR-29, miR-34 and miR -15, and of oncogene miRNAs are miR-17–92, miR-155 and miR-221.
miRNA involvement has been observed in almost every type of cancer including breast cancer (Masood et al. 2011; Victoria 2010). Several miRNAs have been found to be involved into breast cancer metastasis. For instance, miR-720 suppresses invasion and migration in breast cancer cells by targeting TWIST1 (Li et al. 2014). miR-29b regulates migration of human breast cancer cells by modulating PTEN expression (Wang et al. 2011).
One miRNA that have been shown to play a role in breast cancer metastasis by inhibiting migration and invasion of breast cancer cells is miR-429 which belongs to miR-200 family that contains also miR-200a, miR-200b, miR-200c and miR-141. These five members are found in two clusters. In human, miR-200a, miR-200b and miR-429 are located on chromosome 1, while miR-200c and miR-141 are on chromosome 12. They are highly enriched in epithelial tissues (Jun et al. 2005).
There are growing evidences that miR-200 microRNAs are involved in cancer metastasis. Indeed, they have been shown to inhibit epithelial-mesenchymal transition (EMT) which is the initiating step of metastasis by maintaining the epithelial phenotype through direct targeting of transcriptional repressors of E-cadherin, ZEB1 and ZEB2 (Jun et al. 2005; Sun-Mi et al. 2008; Manav et al. 2008). There is also evidence that the miR-200 family is up-regulated in distal breast metastasis indicating that these miRNAs are important for colonization of metastatic breast cancer cells through induction of mesenchymal to epithelial transition (Bylgja et al. 2014).
Recently a study carried out by Ye and co-workers revealed that decreased expression of miR-429 was probably involved in negatively regulating bone metastasis of breast cancer cells (Ye et al. 2015). On the other hand, its overexpression remarkably suppressed invasion in vitro. By combining the in silico analysis of miR-429 targets and global transcriptional profile they have identified CRKL as a potential target that was probably a fundamental node in controlling bone metastasis of breast cancer.
The aim of this paper is to shed more light on the role of CRKL in breast cancer metastasis by studying the most involved pathways through which it can play this role. To achieve this goal we use systems biology tools since such study can be successfully carried out in a global picture taking into account the different interactions between genes and proteins. Such a picture can only be drawn in the framework of systems biology.
Materials and methods
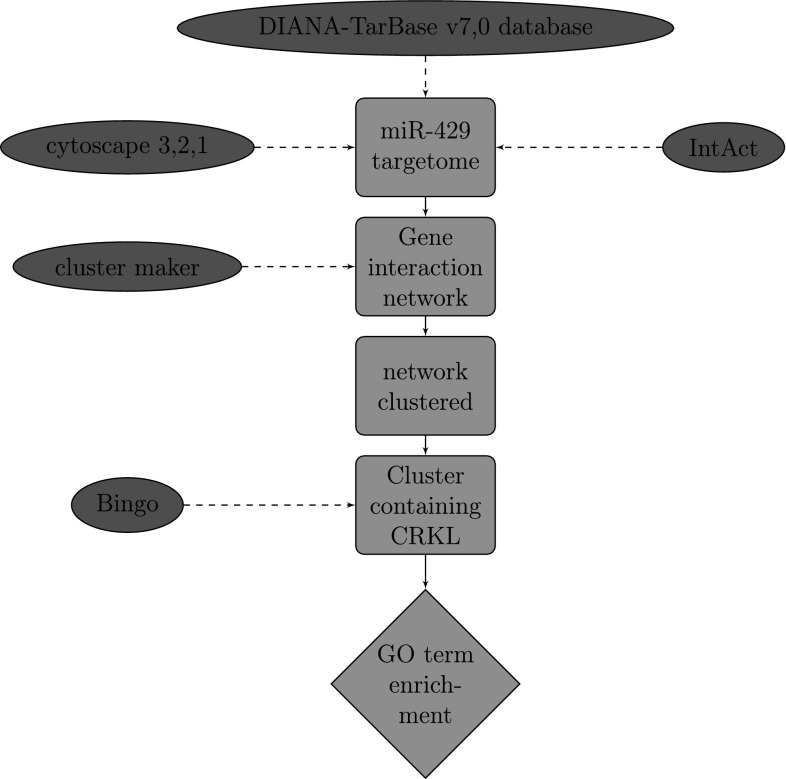
We begin by downloading the list of target genes of miR-429 from the DIANA-TarBase v7,0 database that catalogues published experimentally validated miRNA gene interactions (Vlachos et al. 2015). With the list of genes we have just obtained we use IntAct to find curated interactions.1
To visualize the resulting network we use the cytoscape 3.2.1, an open source software platform for visualizing complex networks and integrating these with any type of attribute data (Shannon et al. 2003). In order to identify groups of genes that share a similar function we use the clusterMaker, a Cytoscape application developed at UCSF that allows the user to easily create and visualize topological clusters using a great variety of methodologies, here we use the GLay Community Clustering algorithm.
In order to determine which Gene Ontology (GO) terms are significantly overrepresented in this set of genes we use the BiNGO Plugin of Cytoscape (Maere et al. 2005) (Fig. 1).
Fig. 1.
The flowchart of the overall work
Results and discussion
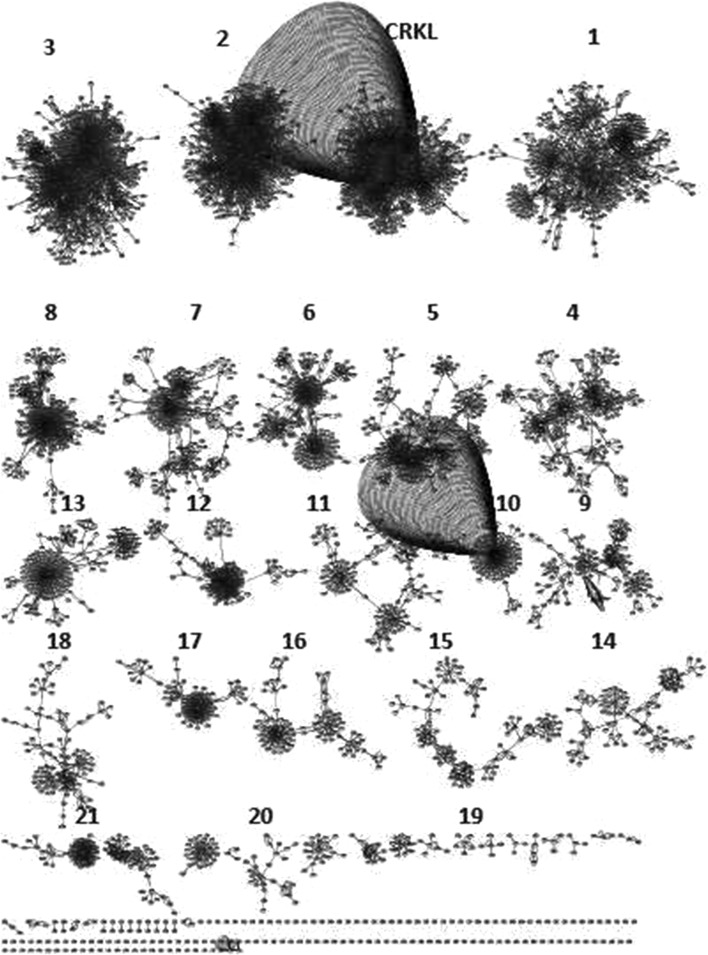
By uploading the list of target genes onto cytoscape and after filtering by taxonomy identifier and choosing the human case the resulting network consists of 5004 nodes and 13108 edges. Then, by applying the clusterMaker cytoscape plugin we obtain the result shown in Fig. 2.
Fig. 2.
The result of the Glay Community Clustering Algorithm applied to the targetome of miR-429
In our study we focus on the signaling process because of its important role in mediating each stage of tumour metastasis (Emily and Morag 2012). By applying the BINGO plug-in to the different clusters we find that only the one containing CRKL presents a cross-talk with the ERK1/2 cascade. From the network view of the result of GO analysis by BINGO we see that all the branches going out from the regulation of signaling process term converge on the positive regulation of ERK 1 and ERK 2 cascade term which has a p-value equal to 1.5 × 10−9. The parent term is the positive regulation of MAPK cascade with a p-value equal to 1.47 × 10−32. As it is clear from Table 1 only the cluster containing CRKL presents an enrichment of the ERK1/2 pathway. This shows the involvement of this gene in the process of breast cancer metastasis regulated by this pathway.
Table 1.
Results of the enrichment analysis for all the clusters with respect to the positive regulation of ERK1/2 pathway
| Clusters | Nodes | Edges | Positive regulation of ERK1/2 p value |
|---|---|---|---|
| 1 | 485 | 1000 | – |
| CRKL | 651 | 2784 | 1.5 × 10−9 |
| 2 | 664 | 2448 | – |
| 3 | 733 | 1580 | – |
| 4 | 203 | 434 | – |
| 5 | 209 | 434 | – |
| 6 | 211 | 511 | – |
| 7 | 222 | 416 | – |
| 8 | 245 | 637 | – |
| 9 | 105 | 245 | – |
| 10 | 113 | 273 | – |
| 11 | 133 | 202 | – |
| 12 | 135 | 534 | – |
| 13 | 185 | 247 | – |
| 14 | 85 | 152 | – |
| 15 | 93 | 195 | – |
| 16 | 97 | 158 | – |
| 17 | 100 | 229 | – |
| 18 | 99 | 157 | – |
| 19 | 60 | 107 | – |
| 20 | 81 | 116 | – |
| 21 | 95 | 249 | – |
The extracellular signal-regulated kinase ERK 1/2 mitogen activated protein MAP kinase module is known as a conserved signaling pathway that plays a major role in the control of cell proliferation, survival and differentiation. Its involvement in cancer has been proved by convincing evidences. In vitro studies of cancer cell lines and in vivo findings from animal models have highlighted the role of ERK 1/2 signaling in cell survival, motility, invasiveness and angiogenesis. Accumulating evidences suggest that the ERK1/2 signaling pathway also contributes to the increased motility, invasiveness and dissemination of tumor cells. ERK 1/2 may then contribute to every step of the metastatic process [For a review see (Wen-Sheng and Chi-Tan 2010)].
Crk adaptor proteins (CrkI, CrkII and CrkL) play an important role during cellular signaling through their SH2 and SH3 domains by mediating the formation of protein complexes. Increased levels of Crk proteins are observed in several human cancers and the expression levels correlate with aggressive and malignant behaviour of cancer cells (Ganapathy and Raymond 2010; Emily and Morag 2012). The Crk family of adaptors is also implicated in cancer metastasis (Tsuda and Tanaka 2012). In case of breast cancer the importance of Crk proteins in regulating growth of aggressive basal breast cancer cells has been shown in Fathers et al. (2012).
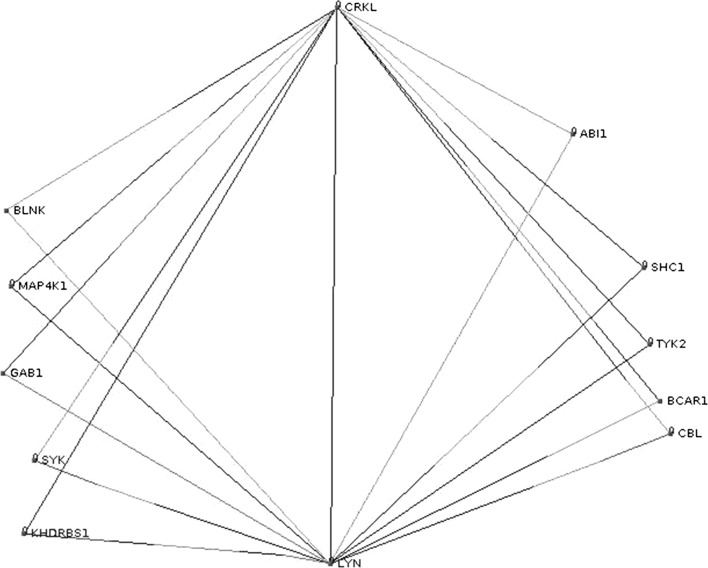
On another side, by performing a network motif search using visant, the analysis tool for biological networks and pathways2 we found that CRKL is involved in many feed forward loops, some of them contain LYN a member of Src family kinases (SFKs) composed of nine family members, Src, Yes, Fyn, Lyn, Hck, Fgr, Blk, Lck, and Frk, that share similar structure and function. SFKs interact with receptor tyrosine kinases, including epidermal growth factor receptor (EGFR) and VEGF receptor, regulating cell proliferation and gene expression via the Ras/ERK/MAPK pathway. The involvement of SFKs in signaling pathways and its effect on cell adhesion and migration via interaction with other molecules including scaffold proteins such as p 130Cas have been detailed in reference (Tsuda and Tanaka 2012). In particular, Lyn was found to play a role in inhibiting tumor growth and metastasis in Ewing’s sarcoma (Hui et al. 2008). It was also shown to be a mediator of epithelial-mesenchymal transition and target of dasatinib in breast cancer (Yoon-La et al. 2010). The functions of the Lyn tyrosine kinase in health and disease have been reviewed in Ingley (2012).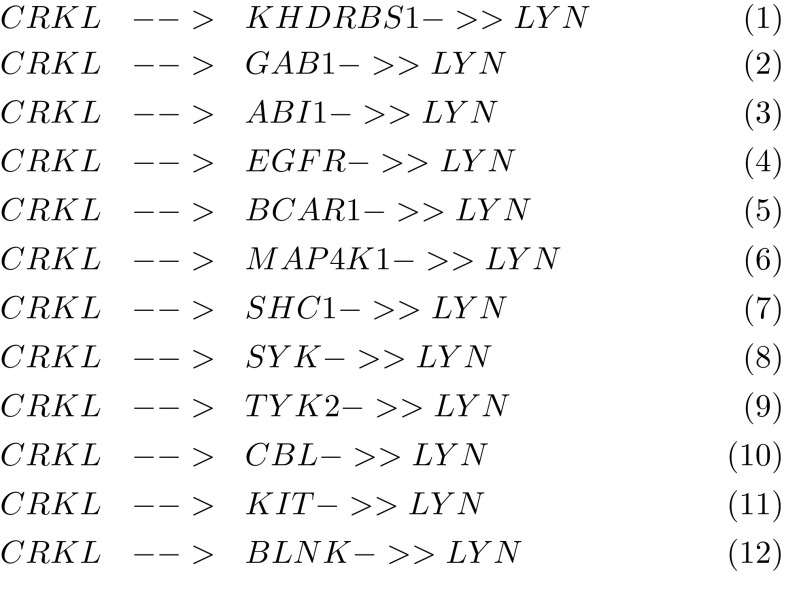
According to Fig. 3 CRKL and LYN are involved in a topological generalization of the feed forward loop (FFL) which consists in its basic form of only three nodes: an input (X), moderator (Y) and output (Z) node. It is encountered with some of its topological generalizations as a key functional component in gene transcription, signal transduction and neural networks (Alon 2007). However, the present case represents a multi-y generalization of the FFL which needs further study, in particular the determination of the sign of the different edges (activation or repression). We can suspect a combinatorial control of LYN by CRKL together with the other genes. These results suggest that CRKL regulates migration and invasion of breast cancer cells by regulating ERK/MAPK pathway and via its interaction with LYN through a feed forward loop.
Fig. 3.
A multi-y generalization of feed forward network motif where CRKL is the input and LYN is the output
Footnotes
IntAct is one of the largest available repositories for curated molecular interactions data, storing PPIs as well as interactions involving other molecules. It is hosted by the European Bioinformatics Institute. IntAct has evolved into a multisource curation platform and many other databases curate into IntAct and make their data available through it (Sandra et al. 2014).
References
- Alon U. An introduction to systems biology design principles of biological circuits. London: Chapman & Hall/CRC; 2007. [Google Scholar]
- Bylgja H, Eirikur B, Jon Thor B, Magnus Karl M, Thorarinn G. Functional role of the microRNA-200 family in breast morphogenesis and neoplasia. Genes. 2014;5:804–820. doi: 10.3390/genes5030804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emily SB, Morag P. Models of crk adaptor proteins in cancer. Genes Cancer. 2012;3(5–6):341–352. doi: 10.1177/1947601912459951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathers K, Bell E, Rajadurai C, Cory S, Zhao H, Mourskaia A, Zuo D, Madore J, Monast A, Mes-Masson A, Grosset A, Gaboury L, Hallet M, Siegel P, Park M. Crk adaptor proteins act as key signaling integrators for breast tumorigenesis. Breast Cancer Res. 2012;14(3):R74. doi: 10.1186/bcr3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathy S, Raymond BB. Emerging roles for crk in human cancer. Genes Cancer. 2010;1(11):1132–1139. doi: 10.1177/1947601910397188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui G, Zhichao Z, Gary EG, Shu-Fang J, Jaime M, Anil KS, Seth JC, Eugenie SK. Targeting LYN inhibits tumor growth and metastasis in Ewing’s sarcoma. Mol Cancer Ther. 2008;7(7):1807–1816. doi: 10.1158/1535-7163.MCT-08-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingley E. Functions of the LYN tyrosine kinase in health and disease. Cell Commun Signal. 2012;10(1):21. doi: 10.1186/1478-811X-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun L, Gad G, Eric AM, Ezequiel A-S, Justin L, David P, Alejandro S-C, Benjamin LE, Raymond HM, Adolfo AF, James RD, Tyler J. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- Lee R, Feinbaum R, Ambros V. The c. elegans heterochronic gene lin-4 encodes small rnas with antisense complementarity to lin-14. Cell. 1993;75:843. doi: 10.1016/0092-8674(93)90529-Y. [DOI] [PubMed] [Google Scholar]
- Li L, Zhang C, Liu L, Yi C, Lu S, Zhou X, Zhang Z, Peng Y, Yang Y, Yun J. mir-720 inhibits tumor invasion and migration in breast cancer by targeting twist1. Carcinogenesis. 2014;35(2):469–78. doi: 10.1093/carcin/bgt330. [DOI] [PubMed] [Google Scholar]
- Maere S, Heymans K, Kuiper M. BiNGO: a cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics. 2005;21:3448–3449. doi: 10.1093/bioinformatics/bti551. [DOI] [PubMed] [Google Scholar]
- Malik Y, Jens A, editors. miRNomics: microRNA biology and computational analysis. London: Springer; 2014. [Google Scholar]
- Manav K, sther SL, Guohong H, Yibin K. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem. 2008;283(22):14910–14914. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masood u R K, Mahmood AK, Faraz AM, Faryal R. Role of miRNAs in breast cancer. Asian Pacific J Cancer Prev. 2011;12:3175–3180. [PubMed] [Google Scholar]
- Olivia JS, Boon-Huat B, George Y, Yingnan Y. Breast cancer metastasis. Cancer Genomics Proteomics. 2012;9:311–320. [PubMed] [Google Scholar]
- Sandra O, Mais A, Bruno A, Lionel B, Leonardo B, Fiona B-C, Nancy HC, Gayatri C, Carol C, Noemi d-T, Margaret D, Marine D, Eugenia G, Ursula H, Marta I, Sruthi J, Rafael J, Jyoti K, Astrid L, Luana L, Ruth C,L, Birgit M, Anna N,M, Mila M, Daniele P, Livia P, Pablo P, Arathi R, Sylvie R-B, Bernd R, Andre S, Michael T, Kim v R, Gianni C, Henning H. The MIntAct project-IntAct as a common curation platform for 11 molecular interaction databases. Nucleic Acids Res. 2014;42:D358–D363. doi: 10.1093/nar/gkt1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P, Markiel A, Ozier O, Baliga N, Wang J, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanie S, Eric AM, Carlos C. MicroRNA—implications for cancer. Virchows Archiv. 2008;452:1–10. doi: 10.1007/s00428-007-0532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun-Mi P, Arti BG, Ernst L, Marcus EP. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Tanaka S. Roles for crk in cancer metastasis and invasion. Genes Cancer. 2012;3(5–6):334–340. doi: 10.1177/1947601912458687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoria JF. MicroRNAs and breast cancer. Open Cancer J. 2010;3:55–61. [Google Scholar]
- Vlachos IS, Paraskevopoulou MD, Karagkouni D, Georgakilas G, Vergoulis T, Kanellos I, Anastasopoulos IL, Maniou S, Karathanou K, Kalfakakou D, Fevgas A, Dalamagas T, Hatzigeorgiou AG. DIANA-TarBase v7.0: indexing more than half a million experimentally supported miRNA:mRNA interactions. Nucleic Acids Res. 2015;43:D153–D159. doi: 10.1093/nar/gku1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Bian Z, Wei D, JG Z. mir-29b regulates migration of human breast cancer cells. Mol Cell Biochem. 2011;352:197–207. doi: 10.1007/s11010-011-0755-z. [DOI] [PubMed] [Google Scholar]
- Wen-Sheng W, Chi-Tan H, editors. Signal transduction in cancer metastasis. London: Springer; 2010. [Google Scholar]
- Ye Z, Ma G, Zhao Y, Xiao Y, Zhan Y, Jing C, Gao K, Liu Z, SJ Y. mir-429 inhibits migration and invasion of breast cancer cells in vitro. Int J Oncol. 2015;46(2):531–538. doi: 10.3892/ijo.2014.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon-La C, Melanie B, Mi JK, Young KS, Seok JN, Jung-Hyun Y, Jessica K, Andrew KG, Jonathan RP. LYN is a mediator of epithelial-mesenchymal transition and target of dasatinib in breast cancer. Cancer Res. 2010;70(6):2296–2306. doi: 10.1158/0008-5472.CAN-09-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]