Abstract
High Arctic polynyas are predictable areas of open water, which offer long-distance migrant seabirds a reliable source of food during a period when they have to replenish and accumulate energy for reproduction. Investigating the interaction between species nesting sympatrically in the vicinity of polynyas should provide insights into the role that such oceanographic features play for pre-breeding seabirds. We used stable isotopes (δ13C and δ15N) to compare the diet of two ground-nesting seabirds, Sabine's gull (Xema sabini) and Arctic tern (Sterna paradisaea), nesting on an island adjacent to a recurring polynya in the Canadian high Arctic in 2008 and 2009. We show that, unlike Arctic terns, the diet of Sabine's gulls appears to include a non-negligible amount of terrestrially derived prey during early incubation, and that overall both species segregate their dietary niche during pre-laying and early incubation.
Keywords: niche segregation, stable isotopes, Sabine's gull, Arctic tern, incubation
1. Introduction
For long-distance migrants, the optimal timing of arrival at breeding sites must closely match favourable conditions for initiating reproduction [1]; however, environmental cues influencing migration may not accurately predict conditions at the nesting location [2]. Such a climatic and/or resource mismatch could constrain individuals upon their arrival and delay the onset of reproductive activities [3]. Thus, the selection of breeding sites near areas with predictable resources could confer an advantage to individuals, especially those breeding in generally barren habitats such as the high Arctic, a region in which the time available for breeding is extremely constrained, and where spring snow cover has a marked effect on annual variation in peak food abundance and timing [4,5] as well as nest site availability [6].
Polynyas in the Canadian Arctic Archipelago are kept free of ice by a combination of tidal currents, wind and upwelling, factors which also contribute to making these areas highly productive [7,8]. For Arctic seabirds, polynyas near suitable breeding sites can provide predictable resources during the pre-breeding and breeding season as they provide reliable access to open water within regions otherwise still covered by pack ice [7–9]. Given the persistence of sea ice throughout the summer in the Canadian high Arctic [10], polynyas are probably key features which led to the colonization of this region by seabirds [11,12]. However, high inter-annual variation in the extent of open water early in the season at some polynyas could limit access to marine resources, and ultimately cause or enhance intra- and interspecific competition among seabirds nesting near these sites.
Many Arctic seabirds are primarily income breeders (e.g. Fulmarus glacialis [13]; Sterna paradisaea [14]) relying on exogenous nutrients for egg formation. These species replenish energetic and nutrient reserves on their breeding grounds after their northward migration, and as such rely on local foraging opportunities. The availability of resources upon arrival at breeding sites will thus have a major influence on an individual's capacity to reproduce, the timing of breeding and on their relative investment [15–17]. Investigating the interaction between species nesting sympatrically near polynyas would thus be expected to provide insights into the role of these oceanographic features in attracting and sustaining colonies of seabirds in the high Arctic.
We compared the diet of two ground-nesting seabirds, Sabine's gulls (Xema sabini) and Arctic terns (Sterna paradisaea), on a small island adjacent to a recurring polynya in the Canadian high Arctic Archipelago. Both species are highly pelagic, trans-equatorial migrants and are approaching the northern extent of their range at this site. A previous study of these species at lower latitudes [18] suggested that they overlapped in diet during the pre-breeding season, but shifted towards foraging in different areas and presumably on different prey as the season progressed. The study area used in that project [18] represented typical breeding habitat for both Sabine's gulls and Arctic terns across most of their range in the Canadian Arctic: coastal low Arctic tundra with extensive freshwater wetlands and ponds in which birds forage almost exclusively terrestrially during the breeding season. At our study site, 1500 km to the north, birds nest on a small gravel island which is typically surrounded by extensive sea ice for hundreds of kilometres in all directions, except for a small polynya of about 10 km2 which occurs immediately adjacent to the island [12]. Consequently, we expected that marine resources available to these species would be limited to those available in the polynya. We predicted: (i) both species would rely heavily on marine prey accessible within the polynya, and (ii) that the species would select different prey to minimize interspecific competition. To test these predictions, we examined stable isotope values (δ13C and δ15N) in blood plasma samples from both Sabine's gull and the Arctic tern to determine if they differed in their isotopic niche at this high Arctic colony.
2. Material and methods
Nasaruvaalik Island, Nunavut is located in the Canadian high Arctic (75°49′ N, 96°18′ W) and supports the largest and most diverse colony of ground-nesting seabirds in the region [12]. Arctic terns are the most numerous seabirds at this site (approx. 500 nests) and Sabine's gulls (approx. 30 nests) breed within the tern colony extent. Both species winter in the Southern Hemisphere [19–22] and are income breeders [14], relying on resources obtained upon arrival at the breeding site to perform breeding and pair-bonding displays and initiate egg-laying.
To investigate adult diet during the pre-laying and incubation periods, we used two approaches. First, we sampled blood of individual birds during incubation, and centrifuged each sample to separate the blood cells from the plasma, keeping the latter since this tissue reflects diet in the previous 2–7 days [23,24]. In 2008, sampling was done on the 7–15 July for Sabine's gull (n = 18) and 15–28 July for the Arctic tern (n = 10); while in 2009 both species were sampled on the 11–18 July (Sabine's gull, n = 20; Arctic tern, n = 24). Birds were captured during incubation using a bow-net trap, and their eggs were removed and kept warm while dummy eggs were placed in the nest. If a bird took more than 30 min to resettle on its nest, we abandoned the capture attempt, so the bird could resume incubation. Second, during 2008–2011 we collected prey brought back to the nest site by breeding birds. In July 2011, we also sampled other putative prey items in the marine and terrestrial environments using aquatic D-frame nets to sweep through the water column, or by picking up individuals on the land (table 1; see also [26]). We used prey samples as references for further interpretation of the bird isotopic values, although we were unable to collect all prey known to be consumed by the two species. In 2008, the first egg was found on 25 June for Sabine's gull and 28 June for the Arctic tern, and 30 June for both species in 2009. Sabine's gull and the Arctic tern were feeding in the polynya when researchers arrived each year (16 June 2008 and 17 June 2009). Note that this work was a subset of a larger project on the breeding biology of both species at high latitudes [27,28].
Table 1.
Mean ± s.d. of plasma δ13C and δ15N values of Sabine's gull and Arctic terns in 2008 and 2009, as well as of different food items from the marine and terrestrial food webs collected at Nasaruvaalik Island, Nunavut. Not all possible food items present in the diet of the species were collected.
| n | δ13C (‰)a | δ15N (‰) | ||
|---|---|---|---|---|
| 2008 | Sabine's gull | 18 | −17.53 ± 2.04 | 13.52 ± 1.76 |
| Arctic tern | 10 | −17.03 ± 0.64 | 14.71 ± 0.38 | |
| 2009 | Sabine's gull | 20 | −23.22 ± 0.93 | 8.84 ± 0.71 |
| Arctic tern | 22 | −15.89 ± 0.89 | 13.72 ± 1.04 | |
| marine food webb | ||||
| harpacticoid copepod | 8 | −17.23 ± 1.56 | 5.97 ± 0.46 | |
| calanoid copepod | 8 | −15.34 ± 3.66 | 9.92 ± 1.08 | |
| amphipodc | 19 | −16.33 ± 2.85 | 10.41 ± 2.09 | |
| Arctic cod (Boreogadus saida) | 16 | −20.17 ± 1.36 | 13.51 ± 0.61 | |
| terrestrial food webd | ||||
| saxifrage | 3 | −29.73 ± 1.08 | 1.55 ± 0.29 | |
| lichen | 10 | −25.15 ± 1.94 | −0.25 ± 2.48 | |
| terrestrial invertebrated | 22 | −26.73 ± 1.08 | 6.12 ± 2.15 | |
| moth | 4 | −28.75 ± 0.71 | 13.40 ± 2.06 | |
aThe bird and marine food web δ13C values were normalized for lipid content [25] except for Arctic cod samples, which were lipid-extracted.
bItems were collected in 2008, 2009 and 2011 at Nasaruvaalik Island.
cAmphipod includes species from the genus Gammarus, Gammaracanthus and Themisto.
dTerrestrial invertebrate includes chironomids, collembolla and other flies; moths were separated from that group because of their surprisingly high δ15N values.
Blood was collected by pricking the brachial vein with a 27-gauge needle and collecting approximately 150 µl of blood in heparinized capillary tubes [29]. Within 5 h, the blood was spun in a microhaematocrit centrifuge for 5 min to separate the plasma from the blood cells [25], and the plasma was then frozen at −20°C until analysis. Tissue samples (plasma and prey) were analysed by the Stable Isotopes in Nature Laboratory (SINLAB) at the University of New Brunswick using well-established procedures. Briefly, samples were combusted in an elemental analyser (NC2500), and gases were sent to the isotope-ratio mass spectrometer (Delta Plus/Conflo II) using a continuous flow interface. Data are reported as differences in isotopic ratios, for which the units are parts per mil (‰) compared with Vienna-Pee Dee Belemnite (V-PDB) for carbon, and atmospheric nitrogen (AIR) for nitrogen. Procedural details are outlined in English et al. [30]. During analyses, three secondary standards were run by SINLAB: nicotinamide (δ15N: −1.78‰, s.d. ± 0.01; δ13C: −34.22 ± 0.074‰, n = 3), bone liver standard (δ15N: 7.25 ± 0.18‰; δ13C: −18.68 ± 0.03‰, n = 3) and muskellunge (Esox masquinongy) muscle standard (δ15N: 12.43 ± 0.07‰; δ13C: −23.30 ± 0.01‰, n = 3). Check standards were also run to assess analytical accuracy: acetanilide (δ15N: −2.06 ± 0.11‰; δ13C: −27.65 ± 0.05‰, n = 5), N2 (δ15N: 20.58‰, n = 1) and CH7 (δ13C −31.84‰, n = 1). Arctic cod (Boreogadus saida) muscles were lipid-extracted; however, no lipids were extracted from our plasma samples, and thus we used the method suggested by Post et al. [31] to normalize δ13C for lipid content of the birds and other marine prey sources:
We used multivariate analysis of variance (MANOVA) to test the effect of the factors ‘species’ and ‘years’ as well as the linear covariate ‘date sampled’ (days in July) and their interactions on both normalized δ13C (δ13Cn) and δ15N. Individuals were sampled early in the breeding season but on different days, and their isotopic value could have represented food ingested at their arrival at the breeding colony. Accordingly, we incorporated sampling date in our models. Half-life of carbon and nitrogen in blood plasma for small seabirds is less than 7 days [23], although it takes more than twice that long to reach isotopic equilibrium after a switch in diet [32]. If differences were found following the MANOVA model (p ≤ 0.05), we used a generalized linear model (GLM, family = Gaussian) approach to test, with F-statistic, the effect of the explanatory variables mentioned above and their interaction on each response variable (δ13Cn and δ15N). We selected the best model using Akaike's information criterion for small sample size (AICc). Based on AICc model selection (electronic supplementary material, S1), the top three models were equivalent (ΔAIC < 2.0) at explaining variation in δ13Cn, but we chose to present the third model that tested for the effect of ‘species’, ‘year’ and ‘date sampled’ along with the interactions between ‘year’ and ‘species’, and between ‘date sampled’ and ‘species’. For δ15N, we kept the model that included all of the fixed effects and their interaction (electronic supplementary material, S1). To represent the influence of date sampled on δ13Cn and δ15N, we used the residuals of the GLM model without the effect of date sampled, and plotted those residuals against date (figure 1). We used stable isotopes Bayesian ellipses in R (SIBER) from the package ‘SIBER’ [33] and used a probabilistic method [33] to assess if differences in isotopic niche width (‰2) between species each year and within species between years were significant following the estimated a posteriori distribution of Bayesian-simulated standard ellipse areas (SEAb).
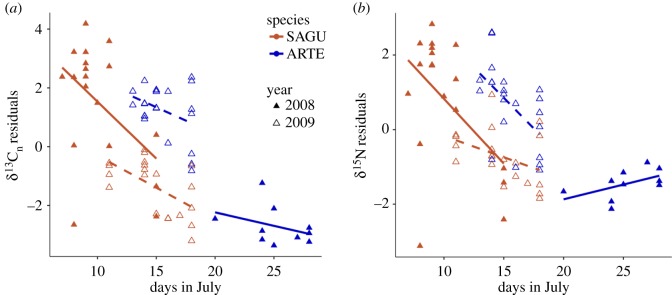
Figure 1.
Relationship between date sampled and the residuals extracted from GLM models with only species and year as fixed effects to show the significant influence of date sampled on the variation in (a) δ13Cn and (b) δ15N for Sabine's gull (SAGU; orange) and Arctic tern (ARTE; blue) in 2008 (filled) and 2009 (open) at Nasaruvaalik Island. Trend lines represent the best linear fit to visually display the direction of the relationship for δ13Cn and δ15N for each year and species.
3. Results
Bird species, year, date sampled and their interaction were significant in explaining variation in δ13Cn and δ15N values in breeding gulls and terns (MANOVA; F7,62 > 5.72, p < 0.005). Only in 2009, Sabine's gulls had lower δ13Cn than Arctic terns (table 1; GLM; F5,69 = 9.96, p = 0.002; figure 2a), and gull δ13Cn values overlapped with that of the values in terrestrial-based prey (table 1). For both species, individual birds sampled later generally had lower δ13Cn (GLM; F5,69 = 146.08, p < 0.001; figure 1), which indicated a rapid turnover rate of carbon following arrival on the breeding ground and a switch in diet upon arrival at the breeding colony (figure 1). The interaction between ‘date sampled’ and ‘species’ was not significant at explaining variation in δ13Cn (F5,69 = 1.58, p = 0.22). Arctic terns had a diet more enriched in nitrogen than Sabine's gull, and in 2008, both species foraged at a higher trophic level than in 2009 (table 1), a tendency that seemed mostly driven by the low δ15N values of Sabine's gull in 2009, which could be the result of different sampling dates between years, resulting in the significance of the three-way interaction terms (figures 1b and 2a; GLM; F7,69 = 7.99, p = 0.006).
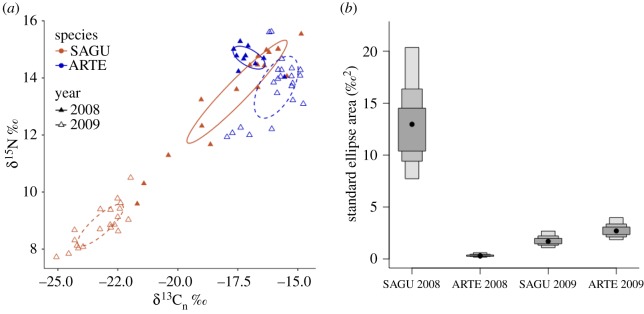
Figure 2.
Isotopic niche area based on δ13Cn and δ15N values in the plasma of adult Sabine's gulls (SAGU) and Arctic terns (ARTE) sampled during incubation at Nasaruvaalik Island, Nunavut in 2008 and 2009. (a) Standard ellipses (40% credible interval, [30]) of the individuals are represented. (b) Density plots showing the mean ellipse areas (black dot) and their credible intervals (50%, 75% and 95%) obtained following a Bayesian approach of posterior estimate of simulated standard ellipse areas (SEAb; [30]).
Based on the probabilistic method of the Bayesian-simulated ellipses [33], the isotopic niche width varied across species and years. Sabine's gulls had a much larger niche width (SEAb 12.07‰2) than Arctic terns (0.38‰2, p < 0.001) in 2008 (figure 2b). Both species had similar niche width in 2009, but were clearly segregated (SEAb of 1.75‰2 and 2.49‰2, respectively; figure 2a,b). Sabine's gull niche width was larger in 2008 than 2009 (p < 0.001), while the opposite was observed for Arctic terns (p < 0.001; figure 2b).
4. Discussion
As in other polynyas in the Canadian Arctic, the recurrent open water surrounding Nasaruvaalik Island appears to provide a predictable foraging environment for long-distance migratory seabirds upon their arrival at their breeding site. However, the total area of open water of the polynya is quite restricted in late June when the birds arrive (estimated 5 × 2 km; sufficiently small to not be mapped in [8]). Although we regularly observed both bird species foraging in the polynya, stable isotope values (δ13Cn and δ15N) in the plasma of Sabine's gulls and Arctic terns over two breeding seasons revealed that Sabine's gulls have a broader isotopic niche than Arctic terns, and that gulls incorporate terrestrially derived prey into their diet, especially in 2009.
Our prediction that these two species would feed on different prey in the marine environment to reduce competition with each other was partially supported, but with the surprising result that Sabine's gulls were also exploiting non-marine prey, especially in 2009, while the elongated ellipse area suggested this also happened in 2008. Indeed, during several years of detailed study [27], we observed Sabine's gulls foraging in both marine and terrestrial environments annually, although we did not quantify observations nor time spent foraging in each environment. Nonetheless, based on those observations, we did not expect such a dramatic isotopic shift as we found. While higher δ13Cn and δ15N values in Arctic terns suggest that individuals were feeding at a high trophic level within the marine environment during the pre-laying and incubation periods, Sabine's gulls appeared to rely on different prey once they arrived at the colony; their δ13Cn and δ15N values were relatively similar to those of terns early in the breeding season (presumably reflecting the diet during migration or at arrival), but exhibited markedly lower values of both δ13Cn and δ15N once they were foraging locally. In fact, the low δ13Cn values of the gulls were consistent with the measured δ13C values of terrestrial prey at Nasaruvaalik Island (table 1), supporting observations of terrestrial invertebrates as important food sources for low Arctic Sabine's gull during the breeding season ([19,34–36]), and suggesting that even in the high Arctic, Sabine's gulls clearly exploit terrestrial prey resources if and when available.
For both species, we noted that δ13Cn values were strongly influenced by the date sampled. For Sabine's gull, this relationship suggests that conditions at arrival (snow cover, low abundance of insects) are not favourable to terrestrial feeding, thus that species might rely heavily on the polynya at that time. The decreasing trend in the δ13Cn values following date sampled, and the negative effect of date sampled by year on the δ15N values might also suggest a diet depleted in carbon and nitrogen at this high Arctic colony for Sabine's gull compared to the food consumed during recent migration, and that isotopic equilibrium was not yet attained upon the switch in diet after arrival at the breeding ground [37,38], especially in 2008 when Sabine's gull were sampled earlier. Sabine's gulls nesting at Nasaruvaalik Island spend the winter off the coast of Peru [21] and were recorded in the Beaufort Sea typically by 6 June on their spring migration north, reaching Nasaruvaalik Island by 9–25 June [39]. The isotopic values of marine invertebrates (i.e. euphausiids, Neocalanus sp., Thysanoessa sp.; credible food sources for migrating Sabine's gulls; [34]) in their staging and migration areas are around δ13C −19‰ and δ15N 11‰ [35–40], values that contrast with the high Arctic terrestrial and marine food webs of Nasaruvaalik Island (table 1).
Arctic terns in 2008 had a highly constrained isotopic niche, but were also sampled later in incubation, which suggested that they had reached the isotopic equilibrium associated with their breeding ground diet. In 2009, both species were sampled during the same period and their isotopic niche widths were clearly distinct and of similar size. We noted that 2009 followed a peak in abundance of lemmings (Dicrostonyx sp.), and large numbers of chironomids and other unidentified flies were observed coming out of the lemming burrows early in the season, which Sabine's gulls were observed eating. While terrestrial invertebrates are present every season, particularly high numbers associated with stochastic events such as peak lemming cycles are probably uncommon. Large numbers of lemmings have only been recorded in two of the last 10 years on Nasaruvaalik Island, but the appearance of this additional supplement to their diet during the pre-breeding season was clearly exploited by the gulls and not by the terns. This high terrestrial prey abundance could also have influenced the difference observed in δ15N and δ13Cn values of Sabine's gulls between 2008 and 2009, suggesting prey differences between years.
5. Conclusion
Together, our results corroborate the inclusion of terrestrial food sources in the diet of Sabine's gull, even at the northern limit of their known breeding range in a distinctly marine environment. Non-systematic observations in other years of data confirm that Sabine's gulls use terrestrial food sources each year at this latitude. We are unsure if invertebrates comprise as much of the diet as in 2009, a year of high terrestrial invertebrate productivity, although the pattern from 2008 suggested that a shift to predominantly terrestrial prey was underway during our sampling. While the proximity of a highly productive polynya makes Nasaruvaalik Island a particularly suitable nesting site for ground-nesting seabirds like Sabine's gulls and Arctic terns, the role of the numerically far more abundant Arctic terns as biovectors of nutrients [41] could also render the habitat within the tern colony particularly favourable for Sabine's gulls. Like many other seabirds, Arctic terns are important instruments of nutrient transport from the ocean to their colony, enhancing productivity of the terrestrial food web where they breed [41–45]. This nutrient enrichment is clearly visible in the high Arctic where tern colonies are much more vegetated than the barren surrounding environment [46] and thus potentially more attractive to Sabine's gulls [47]. We suggest that our results provide a novel interpretation of one of the potential factors leading to the well-noted but inconclusively explained nesting association between these two species in the high Arctic, which almost invariably occur together at higher latitudes [34].
Supplementary Material
Acknowledgements
The authors are grateful for assistance from the Nasaruvaalik Island field teams of 2008, 2009 and 2011, in particular Josh Boadway, and Dominic Cormier for analytical insights. We also value the comments and advice of Morten Frederiksen and Felipe Ceia who reviewed this manuscript.
Ethics
All research involving capture, handling and blood collection of Sabine's gulls (Xema sabini) and Arctic terns (Sterna paradisaea) was made following the ethical guidelines of the Canadian Council on Animal Care (permits EC-PN-12-020) and was conducted under valid and appropriate territorial and federal wildlife permits each year (e.g. WL2008-1035, WL2011-045, NUN-SCI-09-01, NUN-SCI-12-04).
Data accessibility
The data used for this study have been deposited in Dryad Digital Repository: (http://dx.doi.org/10.5061/dryad.n2d15) [48].
Authors' contributions
I.P. carried out statistical analyses and wrote the manuscript. M.L.M. conceived and designed the experiment, collected data and wrote the manuscript. K.A.B. conceived and designed the experiment, collected data, processed the samples and edited the manuscript. S.E.D. and M.M. collected data and edited the manuscript. All authors gave their final approval for publication.
Competing interests
Authors of this manuscript have no competing interests.
Funding
Funding for this research was provided by federal in-kind grants from Natural Resources Canada, Environment Canada (W774-L033-8B013-101) and the Nunavut General Monitoring Program (58-0-205569).
References
- 1.Smith RJ, Moore FR. 2005. Arrival timing and seasonal reproductive performance in a long-distance migratory landbird. Behav. Ecol. Sociobiol. 57, 231–239. (doi:10.1007/s00265-004-0855-9) [Google Scholar]
- 2.Both C, Bijlsma R, Visser M. 2005. Climatic effects on timing of spring migration and breeding in a long-distance migrant, the pied flycatcher Ficedula hypoleuca. J. Avian Biol. 36, 368–373. (doi:10.1111/j.0908-8857.2005.03484.x) [Google Scholar]
- 3.Durant JM, Hjermann D, Ottersen G, Stenseth NC. 2007. Climate and the match or mismatch between predator requirements and resource availability. Clim. Res. 33, 271–283. (doi:10.3354/cr033271) [Google Scholar]
- 4.McKinnon L, Picotin M, Bolduc E, Juillet C, Bêty J. 2012. Timing of breeding, peak food availability, and effects of mismatch on chick growth in birds nesting in the high Arctic. Can. J. Zool. 90, 961–971. (doi:10.1139/z2012-064) [Google Scholar]
- 5.Bolduc E, et al. 2013. Terrestrial arthropod abundance and phenology in the Canadian Arctic: modeling resource availability for arctic-nesting insectivorous birds. Can. Entomol. 145, 155–170. (doi:10.4039/tce.2013.4) [Google Scholar]
- 6.Meltofte H, Høye TT, Schmidt NM. 2008. Effects of food availability, snow and predation on breeding performance of waders at Zackenberg. Adv. Ecol. Res. 40, 325–343. (doi:10.1016/S0065-2504(07)00014-1) [Google Scholar]
- 7.Stirling I, Cleator H. 1981. Polynyas in the Canadian Arctic. Ottawa, Canada: Canadian Wildlife Service; Occasional Paper No. 45. [Google Scholar]
- 8.Hannah CG, Dupont F, Dunphy M. 2009. Polynyas and tidal currents in the Canadian Arctic Archipelago. Arctic Inst. North Am. 62, 83–95. [Google Scholar]
- 9.Stirling I. 1997. The importance of polynyas, ice edges, and leads to marine mammals and birds. J. Mar. Syst. 10, 9–21. (doi:10.1016/S0924-7963(96)00054-1) [Google Scholar]
- 10.Tivy A, Howell SEL, Alt B, McCourt S, Chagnon R, Crocker G, Carrieres T, Yackel JJ. 2011. Trends and variability in summer sea ice cover in the Canadian Arctic based on the Canadian Ice Service Digital Archive, 1960-2008 and 1968-2008. J. Geophys. Res. Ocean 116, C03007 (doi:10.1029/2009JC005855) [Google Scholar]
- 11.Mallory M, Gilchrist H. 2003. Marine birds breeding in Penny Strait and Queens Channel, Nunavut, Canada. Polar Res. 22, 399–403. (doi:10.3402/polar.v22i2.6469) [Google Scholar]
- 12.Maftei M, Davis SE, Mallory ML. 2015. Assessing regional populations of ground-nesting marine birds in the Canadian high Arctic. Polar Res. 34, 25505 (doi:10.3402/polar.v34.25055) [Google Scholar]
- 13.Mallory ML, Forbes MR, Ankney CD, Alisauskas RT. 2008. Nutrient dynamics and constraints on the pre-laying exodus of high Arctic northern fulmars. Aquat. Biol. 4, 211–223. (doi:10.3354/ab00113) [Google Scholar]
- 14.Bond AL, Diamond AW. 2010. Nutrient allocation for egg production in six Atlantic seabirds. Can. J. Zool. 88, 1095–1102. (doi:10.1139/Z10-082) [Google Scholar]
- 15.Moe B, et al. 2009. Climate change and phenological responses of two seabird species breeding in the high-Arctic. Mar. Ecol. Prog. Ser. 393, 235–246. (doi:10.3354/meps08222) [Google Scholar]
- 16.Gaston AJ, Gilchrist HG, Mallory ML. 2005. Variation in ice conditions has strong effects on the breeding of marine birds at Prince Leopold Island, Nunavut. Ecography 28, 331–344. (doi:10.1111/j.0906-7590.2005.04179.x) [Google Scholar]
- 17.Love OP, Gilchrist HG, Descamps S, Semeniuk CAD, Bêty J. 2010. Pre-laying climatic cues can time reproduction to optimally match offspring hatching and ice conditions in an Arctic marine bird. Oecologia 164, 277–286. (doi:10.1007/s00442-010-1678-1) [DOI] [PubMed] [Google Scholar]
- 18.Abraham DM, Ankney CD. 1984. Partitioning of foraging habitat by breeding Sabine's gulls and Arctic terns. Wilson Bull. 96, 161–172. [Google Scholar]
- 19.Fijn RC, Hiemstra D, Phillips RA, Winden J. 2013. Arctic terns Sterna paradisaea from the Netherlands migrate record distances across three oceans to Wilkes Land, east Antarctica. Ardea 101, 3–12. (doi:10.5253/078.101.0102) [Google Scholar]
- 20.Egevang C, Stenhouse IJ, Phillips RA, Petersen A, Fox JW, Silk JRD. 2010. Tracking of Arctic terns Sterna paradisaea reveals longest animal migration. Proc. Natl Acad. Sci. USA 107, 2078–2081. (doi:10.1073/pnas.0909493107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis SE, Maftei M, Jones IL, Mallory ML. 2017. Tracking the trans-equatorial migration of Sabine's gulls (Xema sabini) from a breeding site in the Central Canadian Arctic reveals staging and wintering areas in the Pacific Ocean. PLoS ONE 11, e0166043. [Google Scholar]
- 22.Stenhouse IJ, Egevang C, Phillips RA. 2012. Trans-equatorial migration, staging sites and wintering area of Sabine's gulls Larus sabini in the Atlantic Ocean. Ibis 154, 42–51. (doi:10.1111/j.1474-919X.2011.01180.x) [Google Scholar]
- 23.Vander Zanden MJ, Clayton MK, Moody EK, Solomon CT, Weidel BC. 2015. Stable isotope turnover and half-life in animal tissues: a literature synthesis. PLoS ONE 10, e0116182 (doi:10.1371/journal.pone.0116182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hobson KA, Clark RG. 1993. Turnover of 13C in cellular and plasma fractions of blood: implications for nondestructive sampling in avian dietary studies. Auk 110, 638–641. (doi:10.2307/4088430) [Google Scholar]
- 25.Howlett JC. 2000. Clinical and diagnostic procedures. In Avian medicine, 1st edn (ed Samour J.). London, UK: Butterworth-Heinemann. [Google Scholar]
- 26.Clayden M, Arsenault L, Kidd K, O'Driscoll NJ, Mallory ML. 2015. Mercury bioaccumulation and biomagnification in a small Arctic polynya ecosystem. Sci. Total Environ. 509--510, 206–215. (doi:10.1016/j.scitotenv.2014.07.087) [DOI] [PubMed] [Google Scholar]
- 27.Mallory ML, Boadway KA, Davis SE, Maftei M, Diamond AW. In press Breeding biology of Arctic terns (Sterna paradisaea) in the Canadian high Arctic. Polar Biol. (doi:10.1007/s00300-016-2072-1) [Google Scholar]
- 28.Mallory ML, Boadway KA, Davis SE, Maftei MT. 2012. Breeding biology of Sabine's gull (Xema Sabini) in the Canadian high Arctic. Polar Biol. 35, 335–344. [Google Scholar]
- 29.Gaunt AS, Oring LW, Able KP, Anderson DW, Baptista LF, Barlow JC, Wingfield JC. 1999. Guidelines to the use of wild birds in research. Washington, DC: The Ornithological Council. [Google Scholar]
- 30.English M, Robertson GJ, Mallory ML. 2015. Trace element and stable isotope analysis of fourteen species of marine invertebrates from the Bay of Fundy, Canada. Mar. Pollut. Bull. 101, 466–472. (doi:10.1016/j.marpolbul.2015.09.046) [DOI] [PubMed] [Google Scholar]
- 31.Post DM, Layman C, Arrington DA, Takimoto G, Quattrochi J, Montaña CG. 2007. Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152, 179–189. (doi:10.1007/s00442-006-0630-x) [DOI] [PubMed] [Google Scholar]
- 32.Bearhop S, Waldron S, Votier SC, Furness RW. 2002. Factors that influence assimilation rates and fractionation of nitrogen and carbon stable isotopes in avian blood and feathers. Physiol. Biochem. Zool. 75, 451–458. (doi:10.1086/342800) [DOI] [PubMed] [Google Scholar]
- 33.Jackson AL, Inger R, Parnell AC, Bearhip S. 2011. Comparing isotopic niche widths among and within communities: SIBER—stable isotope Bayesian ellipses in R. J. Anim. Ecol. 80, 595–602. (doi:10.1111/j.1365-2656.2011.01806.x) [DOI] [PubMed] [Google Scholar]
- 34.Day RH, Stenhouse IJ, Gilchrist HG. 2001. Sabine's gull (Xema sabini). In The birds of North America online (ed. Poole A.). Ithaca, NY: Cornell Laboratory of Ornothology. [Google Scholar]
- 35.Boadway KA. 2012. Finding the ‘Arctic’ in the Arctic tern: breeding biology and diet across the latitudinal range of an iconic seabird. Thesis, University of New Brunswick, Fredericton, New Brunswick, Canada.
- 36.Stenhouse IJ, Gilchrist HG, Montevecchi WA. 2001. Reproductive biology of the Sabine's gull in the Canadian Arctic. Condor 103, 98–107. (doi:10.1650/0010-5422(2001)103[0098:RBOSSG]2.0.CO;2) [Google Scholar]
- 37.Hobson KA, Piatt JF, Pitocchelli J. 1994. Using stable isotopes to determine seabird trophic relationships. J. Anim. Ecol. 63, 786–798. (doi:10.2307/5256) [Google Scholar]
- 38.Oppel S, Powell AN. 2010. Carbon isotope turnover in blood as a measure of arrival time in migratory birds using isotopically distinct environments. J. Ornithol. 151, 123–131. (doi:10.1007/s10336-009-0434-y) [Google Scholar]
- 39.Davis SE. 2015. Migration ecology of Sabine's gulls (Xema sabini) from the Canadian high Arctic. Thesis, Memorial University, St John's, Newfoundland and Labrador, Canada.
- 40.Davies WE, Hipfner JM, Hobson KA, Ydenberg RC. 2009. Seabird seasonal trophodynamics: isotopic patterns in a community of Pacific alcids. Mar. Ecol. Prog. Ser. 382, 211–219. (doi:10.3354/meps07997) [Google Scholar]
- 41.Michelutti N, Blais JM, Mallory ML, Brash J, Thienpont J, Kimpe LE, Douglas MSV, Smol JP. 2010. Trophic position influences the efficacy of seabirds as metal biovectors. Proc. Natl Acad. Sci. USA 107, 10 543–10 548. (doi:10.1073/pnas.1001333107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michelutti N, Keatley BE, Brimble S, Blais JM, Liu H, Douglas MSV, Mallory ML, Macdonald RW, Smol JP. 2009. Seabird-driven shifts in Arctic pond ecosystems. Proc. R. Soc. B 276, 591–596. (doi:10.1098/rspb.2008.1103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zwolicki A, Zmudczyńska-Skarbek KM, Iliszko L, Stempniewicz L. 2013. Guano deposition and nutrient enrichment in the vicinity of planktivorous and piscivorous seabird colonies in Spitsbergen. Polar Biol. 36, 363–372. (doi:10.1007/s00300-012-1265-5) [Google Scholar]
- 44.Sekercioglu CH. 2006. Increasing awareness of avian ecological function. Trends Ecol. Evol. 21, 464–471. (doi:10.1016/j.tree.2006.05.007) [DOI] [PubMed] [Google Scholar]
- 45.Hahn S, Bauer S, Klaassen M. 2007. Estimating the contribution of carnivorous waterbirds to nutrient loading in freshwater habitats. Freshw. Biol. 52, 2421–2433. (doi:10.1111/j.1365-2427.2007.01838.x) [Google Scholar]
- 46.Blais JM, Macdonald RW, Mackay D, Webster E, Harvey C, Smol JP. 2007. Biologically mediated transport of contaminants to aquatic systems. Environ. Sci. Technol. 41, 1075–1084. (doi:10.1021/es061314a) [DOI] [PubMed] [Google Scholar]
- 47.Stenhouse IJ, Gilchrist HG, Montevecchi WA. 2005. Factors affecting nest-site selection of Sabine's gulls in the eastern Canadian Arctic. Can. J. Zool. 83, 1240–1245. (doi:10.1139/z05-107) [Google Scholar]
- 48.Pratte I, Boadway KA, Davis SE, Maftei M, Mallory ML. 2017. Data from: Diet dichotomy between two migrant seabirds breeding near a high Arctic polynya. Dryad Digital Repository. (http://dx.doi.org/10.5061/dryad.n2d15) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Pratte I, Boadway KA, Davis SE, Maftei M, Mallory ML. 2017. Data from: Diet dichotomy between two migrant seabirds breeding near a high Arctic polynya. Dryad Digital Repository. (http://dx.doi.org/10.5061/dryad.n2d15) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data used for this study have been deposited in Dryad Digital Repository: (http://dx.doi.org/10.5061/dryad.n2d15) [48].