Abstract
Lungs are specialized organs originated from the posterior pharyngeal cavity and considered as plesiomorphic for osteichthyans, as they are found in extant basal actinopterygians (i.e. Polypterus) and in all major groups of extant sarcopterygians. The presence of a vestigial lung in adult stages of the extant coelacanth Latimeria chalumnae is the result of allometric growth during ontogeny, in relation with long-time adaptation to deep water. Here, we present the first detailed histological and anatomical description of the lung of Latimeria chalumnae, providing new insights into its arrested differentiation in an air-breathing complex, mainly represented by the absence of pneumocytes and of compartmentalization in the latest ontogenetic stages.
Keywords: Actinistia, Latimeria chalumnae, air-breathing organ, lung, histology, ontogeny
1. Introduction
The specialization for air-breathing may have arisen in fishes during the Silurian (from 438 million to 408 million years ago) [1–3], some millions of years before the origin and terrestrialization of the first tetrapods [4–11]. This specialization has probably emerged independently, as many structures (such as lung, skin and gills) named as air-breathing organs (ABOs) [1] can be involved in this process.
Osteichthyans present specialized organs that are originated from the posterior pharyngeal cavity [3,12], such as lungs and gas bladders, to which the homology is still debatable. Lungs, as a ventral derivate, are considered as plesiomorphic for osteichthyans [11,13], as they are found in the extant basal actinopterygian polypterids [14–16], and in all major groups of extant sarcopterygians: coelacanths [17,18], lungfishes [1,19–21] and tetrapods.
A pulmonary apparatus was described for fossil coelacanths [17], and the presence of a vestigial lung in the extant coelacanth Latimeria chalumnae Smith, 1939 [22,23], has been recently confirmed [18]. However, the histological anatomy of the extant coelacanth lung still needs to be fully described, as few studies about the anatomy and histology of this organ have been made in this lobe-finned sarcopterygian. Here, we present a detailed structural study with a histological and anatomical description of the vestigial lung of L. chalumnae, providing new insights into the arrested differentiation of this organ into a functional ABO.
2. Material and methods
Anatomical observations were made from new dissections of pre-dissected adult specimens CCC 3 (MNHN C3, isolated viscus from an adult male of 129 cm total length (TL), caught in Comoro Islands in 1953), CCC 24 (MNHN C22, isolated viscus from a female of 145 cm TL, caught in Comoro Islands in 1960), CCC 28 (MNHN C25, isolated viscus from a male of 130 cm TL, caught in Comoro Islands in 1961) and CCC 79 (MNHN C67, isolated viscus from a female of 163 cm TL, caught in Comoro Islands in 1972) deposited in the Collection of Comparative Anatomy of the Muséum national d'Histoire naturelle (MNHN). Further details of these specimens are provided elsewhere [24,25]. Since their capture, all of these specimens were stored in formalin solution (10%), except CCC 79, which is stored in alcohol after a short fixation in formalin.
CCC 3 and CCC 28 were also scanned by X-ray tomography at the Platform AST-RX of the MNHN, Paris (CAT scan), using a voltage of 245 kV and current of 430 mA for both specimens. A voxel size of 58.83 µm and 2200 views were acquired for CCC 3; and a voxel size of 54.24 µm and 2550 views were acquired for CCC 28.
CCC 202.1 (SAIAB 76199, early embryo of 4 cm TL found inside the female CCC 202, caught in Tanzania in 2005) and CCC 162.21 (ZSM 28409, late embryo of 35.6 cm TL found inside the female CCC 162, caught in Mozambique in 1991) were scanned using long-propagation phase-contrast synchrotron X-ray microtomography at the ID19 beamline of the European Synchrotron Radiation Facility (Grenoble, France). Voxel size is 6.5 µm for CCC 202.1 and 30.45 µm for CCC 162.21. Reconstructed volumes were reduced using binning of 2×2 pixels for CCC 202.1 and CCC 162.21 (13 µm and 60.90 µm, respectively). Both specimens were imaged with a high-quality pink beam using the ID19 W150 wiggler at a gap of 50 mm filtered by 2 mm of aluminium, 0.25 mm of copper, 0.2 mm of gold for CCC 202.1 and 0.25 of tungsten for CCC 162.21. The scintillator was a 250 mm-thick LuAG : Ce (lutetium–aluminium–garnet) crystal. The detector was a FreLoN 2 K charge-coupled device (CCD) camera mounted on a lens system. For a sufficient propagation phase-contrast effect, a distance of 3 m between the sample and the detector was used.
Images were reconstructed and exported into 16-bit TIFF stacks using the phoenix datos|x 2.0 reconstruction. Segmentation and three-dimensional rendering were made at the Palaeontology Imaging Unit of the MNHN Département Histoire de la Terre/UMR 7207 CR2P CNRS/MNHN/UPMC and at the Laboratório de Ictiologia Tempo e Espaço of the Universidade do Estado do Rio de Janeiro with the software MIMICS Innovation Suite 16.0 and 18.0 (Materialise).
Histological thin sections of the oesophagus and the vestigial lung (=oesophageal diverticulum) of L. chalumnae were prepared by Millot, Anthony and Robineau (1978) using azocarmine, haematoxylin/eosin and cajal colorations. These histological thin sections were prepared from specimen CCC 5 (MNHN C5, adult male of 127 cm TL, caught in Comoro Islands in 1954). This material is part of the historical material of extant coelacanths housed in the Collection of Comparative Anatomy of the MNHN, France, for which there is unfortunately no further detailed protocol archived.
Abbreviations: SAIAB, South African Institute for Aquatic Biodiversity, Grahamstown (South Africa); MNHN, Muséum national d'Histoire naturelle, Paris (France); ZSM, Zoologische Staatssammlung, München (Germany); CCC, Coelacanth Conservation Council.
3. Results
Here, we describe the general morphology through partial dissections (figure 1), three-dimensional reconstructions (figure 1), histology (figures 2–4) and virtual thin-sections of long-propagation phase-contrast synchrotron X-ray microtomography (figure 4) of the lung of various developmental stages of the extant coelacanth L. chalumnae.
Figure 1.
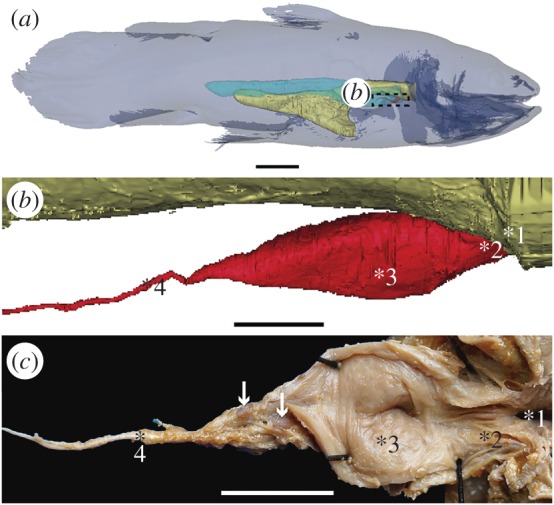
The vestigial lung of the extant coelacanth Latimeria chalumnae. (a) Three-dimensional reconstruction of the adult specimen CCC 22 (130 cm TL) in right lateral view. (b) Details of the three-dimensional reconstruction of the lung of the adult specimen CCC 28, corresponding to the boxed area in (a). (c) Partial dissection of the lung of the adult specimen CCC 3, exhibiting its lumen in the ventral view. Yellow, oesophagus and stomach; red, vestigial lung; blue, fatty organ. White arrows point to two hard but flexible plates. 1, 2, 3, 4 indicate the four successive areas of the vestigial lung. Scale bars, 10 cm (a); 1 cm (b); 0.5 cm (c).
Figure 2.
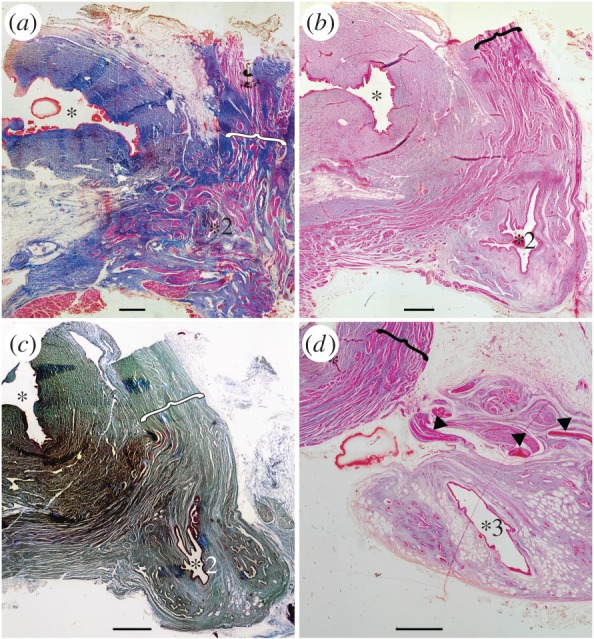
Histological thin sections of the anterior part of the vestigial lung, from adult specimen CCC 5. Oesophagus at the top left (asterisks indicate the lumen) and lung in the bottom right (numbered asterisks localize the lumen relative to the lung in figure 1b,c). (a) Vestigial lung at the level of its origin (asterisk 2 of figure 1) with disorganized muscle bundles (brackets) surrounding this organ. (b,c) Anterior portion (two successive sections) of the lung still in close proximity with the oesophagus, showing invaginations in the lung walls (asterisk 2 of figure 1). Muscle bundles distributed in an organized network (brackets). (d) Vestigial lung completely dissociated from the oesophagus (asterisk 3 of figure 1), presenting the clearly reduced invaginations of the lung walls. Arrowheads indicating the hard, but flexible, plates. Scales bar 0.1 cm (a–d). (a,b,d, azocarmin; c, cajal colorations).
Figure 4.
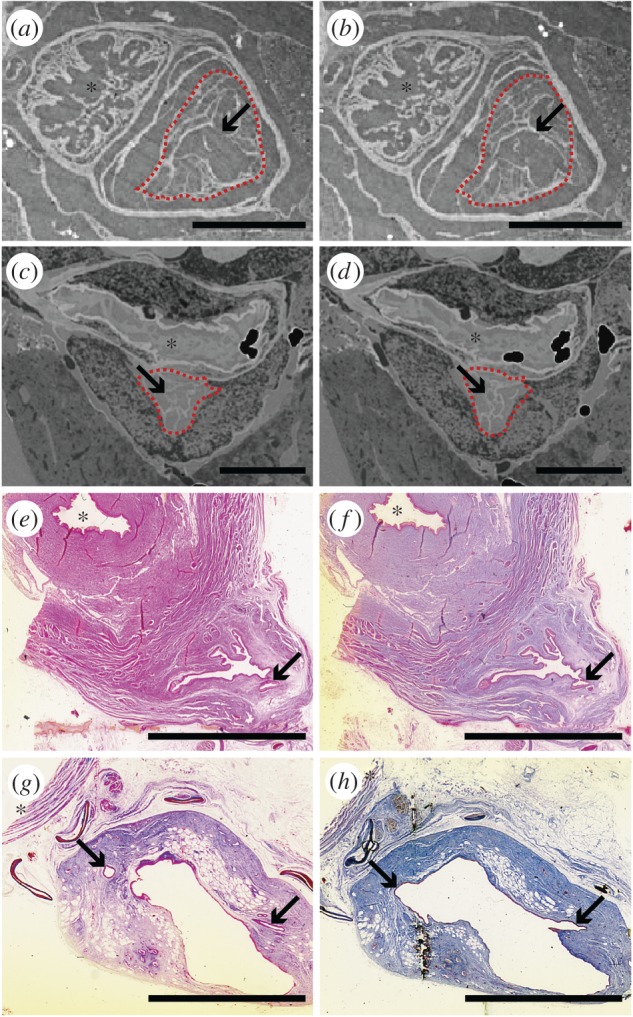
The compartmentalization of the extant coelacanth lung at different ontogenetic stages. (a,b) Sections of synchrotron X-ray microtomography of the early embryo CCC 202.1. (c,d) Sections of long-propagation phase-contrast synchrotron X-ray microtomography of the late embryo without yolk sac CCC 162.21. (e–h) Histological thin sections of the adult specimen CCC 5. Red dashed line, vestigial lung. Arrows indicating compartmentalized structures suggesting alveolation. Asterisks correspond to the oesophageal lumen. (e–g, azocarmin; h, cajal colorations). Scale bars, 1 mm (a,b); 5 mm (c–h).
3.1. General morphology
The pulmonary complex of L. chalumnae is reduced to an oesophageal diverticulum originating in the ventromedial region of the anterior portion of the oesophagus. It is entirely included in the anteriormost part of a long tubular organ filled with fat (here called fatty organ) (figure 1a), also with a ventral origin in relation to the anterior part of the oesophagus [17,18]. Some major morphological features characterize the oesophageal diverticulum of L. chalumnae as a vestigial lung, such as its ventral position to the oesophagus and the presence of a non-obliterated opening between the oesophagus and the lung (figure 1b,c, asterisk 1) [18].
The vestigial lung of L. chalumnae is unpaired and present, in adult specimens, a unique morphology with a clear division in a short anterior chamber and a long and thin posterior residual cord (figure 1b,c, asterisks 3 and 4), highlighted by the presence of a septum observed from partial dissections and segmentation of high-resolution computerized axial tomography scans. Owing to the rarity of coelacanth specimens, particularly of their first ontogenetic stages, deposited in collections worldwide, we have not dissected embryo and juvenile individuals, and could not verify the presence of this septum in these ontogenetic stages.
The lung of adult individuals does not present alveolar septa, but only some invaginations in the anterior part of the anterior chamber (figures 1c, 2a−d, 3c,e and 4e−h), observed from dissections and histological thin sections described below. Virtual sections of high-quality tomography facilities of the earlier known embryo of L. chalumnae CCC 202.1 (4 cm TL), in which the lung exhibits some features still compatible with a potentially functional lung [18], and of the late embryo without yolk sac CCC 162.21 (35.6 cm TL) have evidenced the presence of compartmentalized structures throughout the length of the lung (figure 4a−d), suggesting the presence of alveolation in these first ontogenetic stages.
Figure 3.
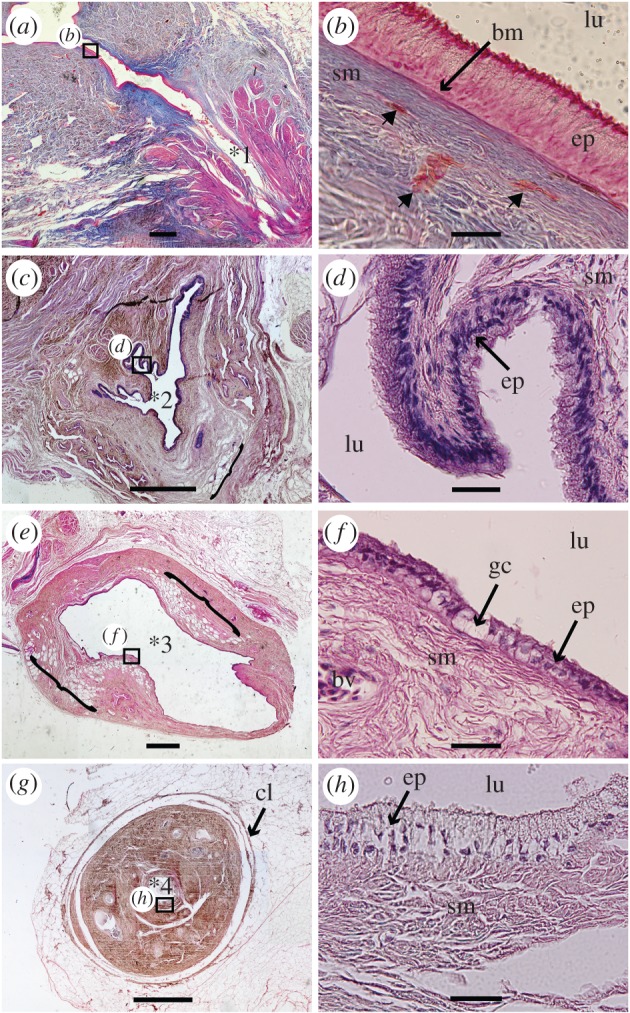
Histological thin sections of the vestigial lung of the adult specimen CCC 5. (a) The section shows the small and non-obliterated pneumatic duct. (b) Close-up of the oesophageal epithelium from the boxed area in (a), composed of a pseudostratified layer of epithelial ciliated cells. The epithelium (ep) lies on the submucosa constituted of intermingled muscular fibres, and blood vessels (arrowheads). (c) Section with the pleated epithelium of the lung, still linked to the oesophagus (figure 2b,c). (d) Close-up of the respiratory epithelium of the vestigial lung, from the boxed area in (c), showing the ciliated cells lying on the submucosa. (e) Section at the level of the middle portion of the vestigial lung fully dissociated from the oesophagus (figure 2d), still showing some minute invaginations and several areas with fat cells (brackets). (f) Close-up of the epithelium of the middle portion of the lung from the boxed area in (e) showing some rare ciliated cells and goblet cells. (g) Residual cord (asterisk 4 of figure 1). (h) Close-up of the epithelium of the residual cord from the boxed area in (g). Scale bars, 0.1 cm (a,c,e,g); 0.04 mm (b,d,f,h). Asterisks correspond to the same area indicated in figures 1 and 2. bm, basal membrane; bv, blood vessels; cl, conjunctive layers; ep, epithelium; gc, goblet cells; lu, lumen; sm, submucosa. (a,b, azocarmin; c–h, haematoxylin-eosin colorations).
The vestigial lung has multiple conjunctive layers covering its whole length, a feature also observed from sections of high-resolution computerized axial tomography scans (from adult specimens CCC 3 and CCC 28). Inside the pulmonary sheath, there are dense layers of fat, more compact and thinner than those present in the fatty organ. The fatty organ presents also multiple sheaths covering its multiple fatty lobes. Glottal ridges have not been observed.
Based on thin sections, ‘pulmonary arteries’ have been previously described [23] for the pulmonary complex of L. chalumnae, but these structures have been recently identified [18] as small but dense plates that surround the vestigial lung of Latimeria (arrows and arrowheads in figures 1c and 2d, respectively). These small plates could be homologous to the calcified plates of Palaeozoic and Mesozoic coelacanth lungs [18]. During the development of this work, we have not identified the presence of true pulmonary arteries in L. chalumnae, probably due to the poor conservation of the pre-dissected material.
3.2. Histology
Regarding the histological and anatomical antero-posterior organization of the vestigial lung from adult specimens, that is from the region close to the oesophageal wall to the residual cord, we can define four areas (figures 1–3): the zone of the small pneumatic duct and the area immediately after it, still on the oesophageal wall (figure 3a, asterisk 1); the diverticulum area located just outside of the oesophagus (figure 2a–c; figure 3c, asterisk 2), but still linked to it (figures 2a–c and 3c); the anterior chamber where the vestigial lung is completely dissociated from the oesophagus (figures 2d and 3e, asterisk 3); and the residual cord (figure 3g, asterisk 4).
The vestigial lung at the level of its origin exhibits a small and pleated lumen, sharing the same features of the oesophagus mucosa and submucosa (figure 2a). The muscularis mucosa presents transversal and longitudinal muscle bundles in a diverse organization, depending on the degree of development. In this section, there are disorganized muscle bundles surrounding the lung (brackets in figure 2a). All sections of the oesophagus present a mucosa composed of a pseudostratified epithelium with tall columnar ciliated cells intercalated by goblet cells and a submucosa with collagenous and elastic fibres (figure 3a,b). The presence of a small, unobstructed and muscular duct connecting the oesophagus with the vestigial lung is highlighted by histological thin sections and dissections (figures 1c and 3a). The muscles that adjoin the glottis (figure 3a) may have had the function of control of the glottis opening during air swallow and expiration, also described and suggested for the ABOs of Lepisosteus oculatus and for the lung of Protopterus dolloi [3,26]. This function has disappeared in the extant coelacanth L. chalumnae that inhabits moderately deep waters.
The second area is represented by the anterior portion of the lung still in close proximity with the oesophagus. It reveals the presence of a pseudostratified and pleated epithelium, composed of tall columnar ciliated cells intercalated by goblet cells (figures 2b,c, 3c,d and 4e,f). The submucosa of the lung presents collagenous and elastic fibres, numerous blood vessels and a small number of vacuolar sectors for fat reserve (brackets in figure 3c). The muscularis mucosa presents muscle bundles distributed in an organized network, beginning the differentiation between the oesophagus and the lung (brackets in figure 2b,c).
In the third area, the vestigial lung is completely dissociated from the oesophagus (figures 2d and 3e). The lumen of the anterior chamber of the vestigial lung is almost not pleated (figure 4g,h) and the mucosa presents a reduced number of tall columnar, and sometimes cuboidal, ciliated cells in the mucosa intercalating with goblet cells (figures 2d and 3e). The submucosa of this portion is composed of collagenous and elastic fibres, numerous blood vessels and a greater amount of vacuolar sectors for fat reserve, indicating the beginning of the vestigial feature (figure 3e,f). The diameter of the diverticulum lumen in this area increases over the previous histological thin section.
The most posterior region corresponds to the residual cord [23] and presents a fibrous submucosa with some blood vessels, a mucosa with goblet cells and with a reduced number of ciliated cells, as well as an extremely reduced lumen (figure 3g,h).
4. Discussion
Among the extant fauna, many aquatic vertebrate taxa—such as aquatic tetrapods, sarcopterygian fishes[18–21,26–30], polypterids [14,16,31,32], holosteans [2,3,33–35] and some teleosts [36,37]—are able to make gas exchange employing many different ABOs [1,3].
Although morphologically distinguishable, lungs and physostomous gas bladders of aquatic vertebrates display some structures with analogous function that allow, or increase, the gas exchange, such as: anteriorly non-obliterated duct; respiratory epithelium (composed of ciliated cells, goblet cells and pneumocytes); compartmentalization (including the presence of alveoli for some taxa, such as the marine tetrapods and lungfishes Neoceratodus, Protopterus and Lepidosiren) [1,19,38]; and rich vascularization.
The extant coelacanth lung is unpaired, well vascularized and originated from the ventral portion of the oesophagus by the presence of a non-obliterated opening [18]. Although this lung exhibits some respiratory features by the presence of ciliated cells intercalated by goblet cells in the adult specimens here analysed, it lacks compartmentalization and pneumocytes.
The histological arrangement of the pleated anteriormost portion of the anterior chamber of the lung of adult specimens is similar to the histology of the oesophagus, by the presence of a pleated epithelium composed of ciliated cells intercalated by goblet cells (figures 1c, 2b,c and 3c,d), and, therefore, reveals it as a structure derived from the oesophagus with an arrested differentiation into an ABO. The presence of invaginations in the anterior chamber of the vestigial lung of Latimeria in this adult stage is the only characteristic that can be interpreted as a vestige of alveolization (figure 4). By contrast, embryos CCC 202.1 and CCC 162.21 present compartmentalized structures throughout the length of the lung (figure 4a–d). This structural organization may suggest the presence of alveolation in these ontogenetic stages and confirms the marked reduction of the lung throughout different developmental stages.
Histological features of the vestigial lung of the adult L. chalumnae are structurally similar to the conducting portion (e.g. central duct) of the respiratory systems of some other functional ABOs of actinopterygians and sarcopterygians, for instance in the holostean Lepisosteus and the lungfish Protopterus [3,26,31], and stores functional features for conduction of the air. Nonetheless, extant air-breathing actinopterygian and sarcopterygian fishes that have functional ABOs present, in general, the respiratory portion constituted by compartments and pneumocytes [3,26].
The vestigial stage of the lung of L. chalumnae has been recently interpreted by the negative allometric growth of this organ when compared with the growth of the fatty organ and the TL [18]. The vestigial feature of this organ is here supported also by anatomical and histological features observed in adult specimens, such as the absence of compartments and pneumocytes. Although functional single-chambered lungs have been reported for polypterids [1,31], amphibians and the majority of adult lepidosaurs, the simplification of this organ seems to have appeared secondarily, and all the remaining amniotes retain multichambered lungs, which increases the functional surface and hence offers advantages for efficient respiration in terrestrial environments [39]. Some fossil coelacanth genera (e.g. Axelrodichthys from the Cretaceous) show a well-developed calcified lung with one or two constrictions [17], suggesting a multichambered lung.
The negative allometric growth added to the histological and anatomical features of the lung, with an arrested differentiation for a functional respiratory complex, offers new insights into the vestigial stage of this organ in the extant coelacanth L. chalumnae, which inhabits moderately deep waters, and whose main oxygen supply is provided through gills [40].
5. Conclusion
The extant coelacanth lung presents a vestigial feature at the adult stage, morphologically represented by a reduced size, a short anterior chamber and a proportionally long residual cord. Our detailed structural study and the previously described allometric growth of this lung support the arrested differentiation of this organ to a functional respiratory complex. This vestigial lung is structurally similar to the conducting portion of other ABOs, but lacks true compartments and pneumocytes, the main components of the respiratory portion in osteichthyans.
Supplementary Material
Acknowledgements
The synchrotron experiments were performed on the ID19 beamline at the European Synchrotron Radiation Facility (ESRF), Grenoble, France. We are grateful to P. Tafforeau at ESRF for providing assistance in using beamline ID19, and to Hugo Dutel, who collected the synchrotron microtomography data. We warmly thank R. Bills and A. Paterson (SAIAB, Grahamstown, South Africa) and D. Neumann (München, Germany) for the loans of the valuable specimens CCC 202.1 (SAIAB 76199) and CCC 162-21 (ZSM 28409), respectively. We thank the UMS 2700 ‘Outils et méthodes de la systématique intégrative’, CNRS-MNHN, Paris, and the analytic platform AST-RX, ‘Plateau technique d'Accès Scientifique à la Tomographie à Rayons X’, MNHN, Paris, for the tomography acquisitions of the extant coelacanth specimens. We also thank the staff of the Collection of ‘Pièces Anatomiques en Fluides’ of the MNHN, Paris, for their help. We are grateful to the referees and to the editors K. Padian (Subject Editor) and A. Power (Editorial Coordinator).
Ethics
All examined material is housed in the following public collections: Collection of Comparative Anatomy of the Muséum national d'Histoire naturelle, Paris (MNHN, France); South African Institute for Aquatic Biodiversity, Grahamstown (SAIAB, South Africa); and Zoologische Staatssammlung, München (ZSM, Germany). Specimens were caught previously to this project, between 1953 and 2005, in Tanzania, Mozambique and Comoro Islands. No special permission was required to publish results from the specimens deposited in these scientific collections.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
C.C., G.C. and P.M.B. designed the research, which was conducted by C.C. C.C. processed microtomography data and prepared the figures. M.H., C.C. and F.J.M. dissected the material. C.C. and F.J.M. studied the histological material. G.C. collected CT microtomography data. All authors contributed to the interpretation of the results and the drafting of the manuscript, and gave their final approval for publication.
Competing interests
The authors have no competing interests.
Funding
C.C.'s research has been partially supported by CNPq (142252/2011-5) and Capes fellowships (proc. no. BEX 0346/13-6 and PNPD/Capes). P.M.B. was supported by grants from CNPq (304082/2013-9). C.C. and P.M.B. thank also the support of FAPERJ (E-26/111.818/2013). This research has been partially supported by Muséum national d'Histoire naturelle (UMR 7207 CR2P, BOREA CP026 and UMR 7179).
References
- 1.Graham JB. 1997. Air breathing fishes: evolution, diversity and adaptation. San Diego, CA: Academic Press. [Google Scholar]
- 2.Farmer CG, Jackson DC. 1998. Air-Breathing during activity in the fishes Amia calva and Lepisosteus oculatus. J. Exp. Biol. 201, 943–948. [DOI] [PubMed] [Google Scholar]
- 3.Icardo JM, Colvee E, Lauriano ER, Capillo G, Guerrera MC, Zaccone G. 2015. The structure of the gas bladder of the spotted gar, Lepisosteus oculatus. J. Morphol. 276, 90–101. (doi:10.1002/jmor.20323) [DOI] [PubMed] [Google Scholar]
- 4.Gardiner B. 1980. Tetrapod ancestry: a reappraisal. In The terrestrial environment (ed. Panchen AL.), pp. 177–185. London, UK: Academic Press. [Google Scholar]
- 5.Panchen AL. 1980. The terrestrial environment and the origin of land vertebrates. London, UK: Academic Press. [Google Scholar]
- 6.Little C. 1983. The colonisation of land: origins and adaptations of terrestrial animals. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 7.Little C. 1990. The terrestrial invasion. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 8.Liem KF. 1988. Form and function of lungs: the evolution of air breathing mechanisms. Am. Zool. 28, 739–759. (doi:10.1093/icn/28.2.739) [Google Scholar]
- 9.Gordon MS, Olson EC. 1994. The invasions of the land. New York, NY: Columbia University Press. [Google Scholar]
- 10.Long JA. 1995. The rise of fishes: 500 million years of evolution. Baltimore, MD: The Johns Hopkins University Press. [Google Scholar]
- 11.Roux E. 2002. Origine et évolution de l'appareil respiratoire aérien des Vertébrés. Rev. Mal. Respir. 19, 601–615. [PubMed] [Google Scholar]
- 12.Perry SF, Wilson RJA, Straus C, Harris MB, Remmers JE. 2001. Which came first, the lung or the breath? Comp. Biochem. and Physiol. 129, 37–47. (doi:10.1016/S1095-6433(01)00304-X) [DOI] [PubMed] [Google Scholar]
- 13.Perry SF, Sander M. 2004. Reconstructing the evolution of the respiratory apparatus in tetrapods. Resp. Physiol. Neurobi. 144, 125–139. (doi:10.1016/j.resp.2004.06.018) [DOI] [PubMed] [Google Scholar]
- 14.Smet W. 1966. Le développement des sacs aériens des Polyptères. Acta Zool. 47, 151–183. (doi:10.1111/j.1463-6395.1966.tb00744.x) [Google Scholar]
- 15.Lechleuthner A, Schumacher U, Negele RD, Welsch U. 1989. Lungs of Polypterus and Erpetoichthys. J. Morphol. 201, 161–178. (doi:10.1002/jmor.1052010206) [DOI] [PubMed] [Google Scholar]
- 16.Graham JB, Wegner NC, Miller LA, Jew CJ, Lai NC, Berquist RM, Frank LR, Long JA. 2014. Spiracular air-breathing in polypterid fishes and its implications for aerial respiration in stem tetrapods. Nat. Commun. 5, 3022 (doi:10.1038/ncomms4022) [DOI] [PubMed] [Google Scholar]
- 17.Brito PM, Meunier FJ, Clément G, Geffard-Kuriyama D. 2010. The histological structure of the calcified lung of the fossil coelacanth Axelrodichthys araripensis (Actinistia: Mawsoniidae). Palaeontology 53, 1281–1290. (doi:10.1111/j.1475-4983.2010.01015.x) [Google Scholar]
- 18.Cupello C, Brito PM, Herbin M, Meunier FJ, Janvier P, Dutel H, Clément G. 2015. Allometric growth in the extant coelacanth lung during ontogenetic development. Nat. Commun. 6, 8222 (doi:10.1038/ncomms9222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grigg GC. 1965. Studies of the Queensland lungfish, Neoceratodus forsteri (Krefft). I. Anatomy, histology, and functioning of the lung. Aust. J. Zool. 13, 243–253. (doi:10.1071/ZO9650243) [Google Scholar]
- 20.Grigg GC. 1965. Studies of the Queensland lungfish, Neoceratodus forsteri (Krefft). III. Aerial respiration in relation to habits. Aust. J. Zool. 13, 413–421. (doi:10.1071/ZO9650413) [Google Scholar]
- 21.Perry SF, Euverman R, Wang T, Loong AM, Chew SF, Ip YK, Gilmour KM. 2008. Control of breathing in African lungfish (Protopterus dolloi): A comparison of aquatic and cocooned (terrestrialized) animals. Resp. Physiol. Neurobi. 160, 8–17. (doi:10.1016/j.resp.2007.06.015) [DOI] [PubMed] [Google Scholar]
- 22.Smith JLB. 1939. A living fish of Mesozoic type. Nature 143, 455–456. (doi:10.1038/143455a0) [Google Scholar]
- 23.Millot J, Anthony T, Robineau D. 1978. Anatomie de Latimeria chalumnae III. Paris, France: CNRS. [Google Scholar]
- 24.Bruton MN, Coutouvidis SE. 1991. An inventory of all known specimens of the coelacanth Latimeria chalumnae, with comments on trends in the catches. Environ. Biol. of Fish. 32, 371–390. (doi:10.1007/BF00007467) [Google Scholar]
- 25.Nulens R, Scott L, Herbin M. 2011. An updated inventory of all known specimens of the coelacanth Latimeria spp. S. Afr. Inst. Aquatic Biodiver. 3, 1–52. [Google Scholar]
- 26.Poll M. 1962. Étude sur la structure adulte et la formation des sacs pulmonaires des Protoptères. Ann. Mus R. Afr. Cent 108, 129–172. [Google Scholar]
- 27.Smith HW. 1931. Observations on the African lung-fish, Protopterus aethiopicus, and on evolution from water to land environment. Ecology 12, 164–181. (doi:10.2307/1932938) [Google Scholar]
- 28.Johansen K, Lenfant C, Grigg GC. 1967. Respiratory control in the lungfish, Neoceratodus forsteri. Comp. Biochem. Physiol. 20, 835–854. (doi:10.1016/0010-406X(67)90057-6) [Google Scholar]
- 29.Johansen K, Lenfant C. 1967. Respiratory function in the South American lungfish, Lepidosiren paradoxa (Fitz). J. Exp. Biol. 46, 205–218. [DOI] [PubMed] [Google Scholar]
- 30.Kind PK, Grigg GC, Booth DT. 2002. Physiological responses to prolonged aquatic hypoxia in the Queensland lungfish Neoceratodus forsteri. Resp. Physiol. Neurobi. 132, 179–190. (doi:10.1016/S1569-9048(02)00113-1) [DOI] [PubMed] [Google Scholar]
- 31.Poll M, Dewattines C. 1967. Etude systématique des appareils respiratoire et circulatoire des Polypteridae. Ann. Mus R. Afr. Cent 158, 1–63. [Google Scholar]
- 32.Brainerd EL, Liem KF, Samper CT. 1989. Air ventilation by recoil aspiration in polypterid fishes. Science 246, 1593–1595. (doi:10.1126/science.246.4937.1593) [DOI] [PubMed] [Google Scholar]
- 33.Johansen J, Hanson D, Lenfant C. 1970. Respiration in a primitive air breather, Amia calva. Resp. Physiol. 9, 162–174. (doi:10.1016/0034-5687(70)90068-X) [DOI] [PubMed] [Google Scholar]
- 34.Randall DJ, Cameron JN, Daxboeck C, Smatresk N. 1981. Aspects of bimodal gas exchange in the bowfin, Amia calva L. (Actinopterygii: Amiiformes). Resp. Physiol. 43, 339–348. (doi:10.1016/0034-5687(81)90114-6) [DOI] [PubMed] [Google Scholar]
- 35.Zaccone D, Dabrowski K, Lauriano ER, de Pasquale A, Macri D, Satora L, Lanteri G. 2012. The simultaneous presence of neuroepithelial cells and neuroepithelial bodies in the respiratory gas bladder of the longnose gar, Lepisosteus osseus, and the spotted gar, L. oculatus. Acta Histochem. 114, 370–378. (doi:10.1016/j.acthis.2011.07.006) [DOI] [PubMed] [Google Scholar]
- 36.Marlier G. 1938. Considérations sur les organes accessoires servant à la respiration aérienne chez les Téléostéens. Ann. Soc. R. Zool. Bel. 69, 163–185. [Google Scholar]
- 37.Greenwood PH, Liem KF. 1984. Aspiratory respiration in Arapaima gigas (teleostei, osteoglossomorpha): a reappraisal. J. Zool. 203, 411–425. (doi:10.1111/j.1469-7998.1984.tb02341.x) [Google Scholar]
- 38.Maina JN. 1987. The morphology of the lung of the African lungfish, Protopterus aethiopicus: a scanning electron-microscopic study. Cell Tissue Res. 250, 191–196. (doi:10.1007/BF00214671) [DOI] [PubMed] [Google Scholar]
- 39.Lambertz M, Grommes K, Kohlsdorf T, Perry SF. 2015. Lungs of the first amniotes: why simple if they can be complex? Biol. Lett. 11, 20140848 (doi:10.1098/rsbl.2014.0848) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Millot J. 1954. Le troisième Coelacanthe (Le Naturaliste Malgache. Premier Supplément). Paris, France.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.


