Abstract
In Drosophila melanogaster the most widely used technique to drive gene expression is the binary UAS/Gal4 system. We show here that a set of nervous system specific enhancers (elav, D42/Toll-6, OK6/RapGAP1) display ectopic activity in epithelial tissues during development, which is seldom considered in experimental studies. This ectopic activity is variable, unstable and influenced by the primary sequence of the enhancer and the insertion site in the chromosome. In addition, the ectopic activity is independent of the protein expressed, Gal4, as it is reproduced also with the expression of Gal80. Another enhancer, LN2 from the sex lethal (Sxl) gene, shows sex-dependent features in its ectopic expression. Feminization of LN2 expressing males does not alter the male specific pattern indicating that the sexual dimorphism of LN2 expression is an intrinsic feature of this enhancer. Other X chromosome enhancers corresponding to genes not related to sex determination do not show sexual dimorphism in their ectopic expressions. Although variable and unstable, the ectopic activation of enhancer-Gal4 lines seems to be regulated in terms of tissue and intensity. To characterize the full domain of expression of enhancer-Gal4 constructs is relevant for the design of transgenic animal models and biotechnology tools, as well as for the correct interpretation of developmental and behavioural studies in which Gal4 lines are used.
Keywords: gene expression, neuroscience, Drosophila, development, Gal4
1. Introduction
The yeast transcription factor Gal4 in combination with artificial gene constructs placed under the control of UAS regulatory sequences became a powerful experimental tool when converted into transgenes in other species [1–3]. In Drosophila, this binary UAS/Gal4 system has been extensively used for over two decades [1]. The specificity of the expression domains of Gal4 lines has allowed cellular resolution in most of these studies, representing a major advance over the formerly used genetic mosaics obtained by somatic recombination [4,5]. Beyond the cellular studies, mostly addressing developmental questions, the UAS/Gal4 system has been used also for organismal studies in the fields of neurobiology and behaviour [6–9]. In all cases, however, the space and time specificity of the Gal4 line was the cornerstone of the experiment rationale. Thus, Gal4 lines were described on the bases of their canonical expression domains using a UAS reporter referred to one or several developmental stages. These domains were interpreted as instructions dictated by enhancers located nearby the site of insertion of the Gal4 construct. These lines are generally known as enhancer trap Gal4 lines. In addition, characterized enhancers of a given gene were used to create Gal4 lines with the desired expression domain [10,11]. These lines are referred to as synthetic promoter Gal4 lines.
Enhancers are short (up to 400-bp) DNA sequences that can activate transcription at target promoters located in their vicinity [12–14]. The first transcriptional enhancer was characterized more than 30 years ago, when a viral DNA sequence was shown to activate transcription of the rabbit haemoglobin beta1 gene, independently from its orientation and position relative to the promoter [15,16]. Eukaryotic chromatin can loop to permit enhancer--promoter interactions in still poorly understood three-dimensional structures [14,17]. Recent genomic studies using various versions of 3C and 4C chromatin immunoprecipitation assays revealed the widespread phenomenon of gene regulation by enhancers while other studies identified specific signatures (histone modifications and associated proteins) of enhancers that greatly facilitate analysis of the databases (reviewed in [18]).
To determine if the expression domains of Gal4 lines are constant along time, and as specific as reported, we analysed several Drosophila lines considered to be nervous system specific. Most of them showed transient expression in other tissues. This ectopic activation of enhancers, however, did not necessarily imply the expression of the corresponding genes. Pressure to find utilitarian uses of the limited knowledge on the normal mechanisms of gene expression has led to the production of enhancer-based genetic tools intended to drive expression of engineered genes. However, the range of spurious effects of these applications is rarely analysed in depth, and this justifies further efforts to study how enhancers, both native and synthetic, attain their expression domains.
On the other hand, the temporal analysis of the expression domain of native and synthetic enhancers may help to understand the dynamic process of gene expression along development. Contrary to the widely accepted view that gene expression is a deterministic event, meaning that the final outcome is determined by the initial conditions, our results seem to favour the proposal that, during development, enhancers undergo changing molecular conditions that trigger their variable and ectopic expression. The variability of these conditions is progressively reduced leading to two mutually exclusive events, the extinction of the enhancer's aberrant expression or the consolidation of its canonical expression domain which finally allows transcription of the corresponding native gene and, thus, cell fate determination.
2. Results
We selected a collection of Drosophila enhancer-Gal4 lines (electronic supplementary material, table S1) described in the literature to be active specifically in the nervous system, to determine if their expression pattern was indeed restricted to the nervous system all along development. For this study we analysed three different scenarios: (i) the activation of enhancers in the original position [E in electronic supplementary material, table S1, elavc155, D42, OK6, NP2426 (LN2), c105, 796 and Repo], (ii) enhancers with their corresponding promoter built into transgenic reporters [P in electronic supplementary material, table S1, elav (II), elav (III) and phantom]; and (iii) enhancers with a synthetic promoter (P-DSCP in electronic supplementary material, table S1, GMR10B11 and GMR 78G09) (electronic supplementary material, figure S1).
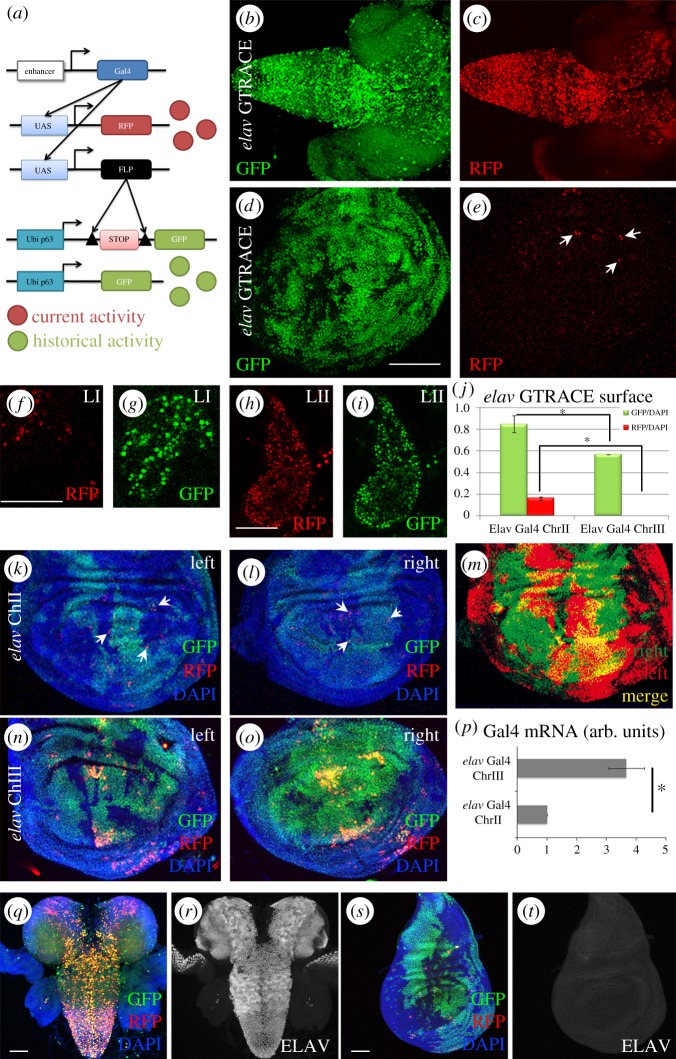
The G-TRACE technique has been instrumental in this study [19]. Briefly summarized, it consists of three constructs that contain: UAS-RFP fluorescent protein, UAS-Flipase and Act-FRT-STOP-FRT-GFP, respectively. The system reports the temporal activation of the enhancer-Gal4 under study (figure 1a, modified from [19]). The enhancer-Gal4 activity induces the expression of the UAS-Flipase (Flp) and UAS-RFP (red) constructs. The Flp enzyme recognizes FRT sites and removes the STOP cassette, allowing the expression of Act>GFP (green) in these cells and their progeny. Thus, we can determine the current expression of an enhancer-Gal4 at the moment of dissection (red, RFP) and its historical expression during development (Act>GFP (green)). The inventors of this technique showed already that some enhancer-Gal4 lines exhibit divergence of activity at different stages of development within the same tissue [19].
Figure 1.
Activation of the neural elav enhancer in wing disc cells. (a) Schematic description of the G-TRACE technique. An enhancer controls the expression of Gal4 (blue) which results in a red reporter signal (RFP). This red signal will be maintained as long as the enhancer is active, thus, it reflects the current expression domain. On the other hand, the first time in development when the enhancer becomes active the flipase encoding construct is also activated (black box). The flipase, through the excision of a STOP cassette (black triangles), allows the expression of a GFP-encoding construct (green box). This reporter is now controlled by a ubiquitous p63 promoter, thus becoming independent from the original enhancer. This GFP reporter signal represents the historical expression domain. (b–e) G-TRACE data from elav enhancer in larval brains (b,c) and wing discs (d,e). Note that the enhancer is not CNS specific. (f–i) In vivo images of early developmental stages activation of elav enhancer in first instar (f,g) and second instar (h,i) larvae. (j–o) Quantification (j) of G-TRACE pattern of elav enhancer comparing two different elav-Gal4 insertions, chromosome II (k–m) and chromosome III (n,o). Note that the ectopic elav enhancer expression in the wing disc is not consistent between left and right sides of the same animal. This is evidence of the variable nature of the ectopic expression. (p) Gal4 mRNA quantitative RT-PCRs from chromosome II and chromosome III elav-Gal4 lines. (q,r) G-TRACE data for elav enhancer (q) and anti-ELAV staining (r) in larval brain. (s,t) G-TRACE data for elav enhancer (s) and anti-ELAV staining (t) in larval wing imaginal disc. Note that the ELAV protein is CNS specific. Scale bar, 50 µm. Statistics: t-test *p < 0,05. n = 5 wing discs/sample. Arrows in (e) indicate active enhancer cells (RFP).
2.1. The nervous system elav enhancer is active in epithelial cells
The Drosophila gene elav, orthologue of human ELAVL gene family, is expressed throughout the brain [20–24]. We confirmed the reported elav-Gal4 expression in brain neurons (figure 1b,c). The current (red) and historical (green) records of elav enhancer activity show a high correspondence between both signals. These data indicate that cells fated to be neurons in the brain are determined during development, and that this fate is maintained during CNS development.
To determine the specificity of this elav enhancer, we analysed also epithelial tissues. The data showed unexpected widespread GFP positive cells (figure 1d) and randomly distributed RFP positive cells in wing imaginal discs (figure 1e) compared to the negative control (sibling flies without the Gal4 construct) (electronic supplementary material, figure S2a,b). In addition, we analysed the elav-Gal4 enhancer using the Act>STOP>Gal4; UAS-GFP line (see Materials and methods). The data show that elav enhancer is active in wing imaginal disc cells (electronic supplementary material, figure S2c,d) consistent with the previous result. To further validate the initial observation, we used another Gal4 line inserted adjacent to the endogenous elav-Gal4 enhancer (elavc155) and the G-TRACE reporter line inserted in chromosome III. In this case, GFP and RFP positive cells were also identified in the wing disc in a pattern similar to the one shown by the previous elav construct. Moreover, a detailed analysis of elav enhancer activation also showed activity in halter and leg imaginal discs and tracheal cells (electronic supplementary material, figure S2e–l). Together, these results indicate that the elav enhancer, both in foreign or native locations, is active in epithelial cells during development (GFP cells) and maintains its activity by the third larval instar, albeit to a lesser extent [RFP cells are fewer than GFP cells (electronic supplementary material, figure S2f–l)]. It seems that some wing disc cells switched on this enhancer sometime early in development and still maintained it on by third larval instar (GFP + RFP, yellow cells), while others have switched it off (GFP, green only cells) and others have switched it on lately (RFP, red only cells). Most likely, the RFP signal (red only), occurred several hours before wing discs fixation/dissection and has not had the time to express the FRT/FLP-GFP reporter, a sequential process that takes longer than the RFP reporting (figure 1a).
To determine at what stage during development this ectopic enhancer activation occurs, we analysed first and second instar larvae (24 and 48 h after egg laying, AEL) (figure 1f–i). G-TRACE reporters showed that the elav enhancer is active as early as first (figure 1f,g) and second (figure 1h,i) instar larvae. Red and green cells are found in the wing disc suggesting that the enhancer activity is triggered at the initial stages of development in this cell system.
To further analyse the temporal activation of elav enhancer in this ectopic domain, we used wg-Gal4 (wingless) in combination with the G-TRACE system. In second instar larvae, wg expression is confined to the wing pouch of the wing imaginal disc (electronic supplementary material, figure S2m). However, later during development, this domain is restricted to concentric rings in third instar larval discs (electronic supplementary material, figure S2n) [25,26]. These two different expression patterns allowed us to define the temporal activation of the elav enhancer. We combined wg-Gal4/G-TRACE with a repressor of Gal4 activity, Gal80, under the regulation of elav enhancer, aiming to determine the activation of elav enhancer by the effect of elav-Gal80 suppressor on wg-Gal4/G-TRACE pattern. Our results confirm that the wg enhancer is active early during development in wing pouch cells (green in electronic supplementary material, figure S2o,p) and, at the third instar larvae stage, it is restricted to its canonical wg domain (green in electronic supplementary material, figure S2n and yellow in electronic supplementary material, figure S2o,p). Also, elav-Gal80 eliminates the GFP signal corresponding to early wg-Gal4 activity, suggesting that the elav enhancer is active during the early stages of development. However, elav-Gal80 does not suppress RFP signal at third instar stage (electronic supplementary material, figure S2q,r), confirming that the elav enhancer is active during early stages of development in the wing disc.
Next, to discard an effect due to the chromosomal site of insertion, we compared G-TRACE patterns of the same elav-Gal4 construct but inserted in two different sites: chromosome II and chromosome III (electronic supplementary material, table S1). All experiments were carried out in parallel and the results indicate that both elav-Gal4 enhancer insertions have activity during development (GFP cells in figure 1j–o). However, the insertion in chromosome II is expressed in 60% of the wing cells while the insertion in chromosome III is expressed in 80% of them (figure 1j). Moreover, chromosome III insertion has RFP-reported activity in 20% of wing cells (figure 1j,n,o) but activity for the chromosome II insertion is restricted to less than 1% cells (figure 1j,k,l). In summary, the data indicate that even though the elav enhancer shows ectopic expression in epithelial cells always, the genomic insertion site affects the extent of this ectopic domain.
We noticed that the expression pattern of GFP throughout different discs is not reproducible in different larvae. This variability could result from heterogeneous environmental conditions among individuals or cell systems. To further evaluate this feature, we compared left and right wing discs from the same individual. Although the number of GFP positive cells is roughly comparable between the two wing discs of the same animal, the pattern differs (figure 1k–o). This result supports the notion that the enhancer activation during development is not deterministic. We cannot ascertain whether the GFP positive cells activated the elav enhancer on a cell autonomous manner, or if they constitute a lineage from one or very few cells that activated the enhancer early in development. However, the fact that the expression pattern is highly variable within the same animal suggests that this is actually a non-clonal event.
Concerning the canonical domain of expression in the CNS, both insertions of the elav enhancer show the same pattern in brain cells. However, we addressed possible differences in the intensity of this expression by qRT-PCR. The data show that the expression of the chromosome III insertion is five times higher than that of the chromosome II (figure 1p). Together, it seems that the insertion site determines the extent of the ectopic domain as well as the intensity of its expression.
Finally, to determine if the ectopic wing disc expression of the elav enhancer induces the expression of the ELAV protein, we stained larval tissues with a specific anti-ELAV antibody. Third instar larval brains show that, as expected, elav enhancer is active in neurons (figure 1q) and brain cells express ELAV protein (figure 1r). However, while wing discs show elav enhancer activity (figure 1s), we did not detect ELAV protein (figure 1t). These results indicate that the ectopic activation of elav enhancer does not lead to detectable protein expression.
2.2. Toll-6 and OK6 neuronal enhancers are transiently active in epithelial cells in early development
To assess if the phenomenon observed with the elav enhancer is general to other enhancers, we selected additional cases also described as CNS specific. Line D42-Gal4 corresponds to a Toll-6 gene enhancer [27]. As described, neurons from the third instar larval brain had activated D42 during early development (historical GFP, figure 2a, compared to current RFP, figure 2b). Similar to elav, D42 is also activated in wing disc cells during early development but not in third instar larvae (no RFP cells) (figure 2c,d). In addition, D42-Gal4 domain in the wing disc is also different between left and right discs within the same animal (figure 2c,d). The historical GFP positive cells in the vicinity of the anterior--posterior (A/P) border of the wing disc appear to align with the A/P compartment border. To determine if this is the case, we stained for β-galactosidase in dpp-LacZ expressing discs that mark this boundary (figure 2e,f). The GFP cells distribution does not correspond with the dpp enhancer signal.
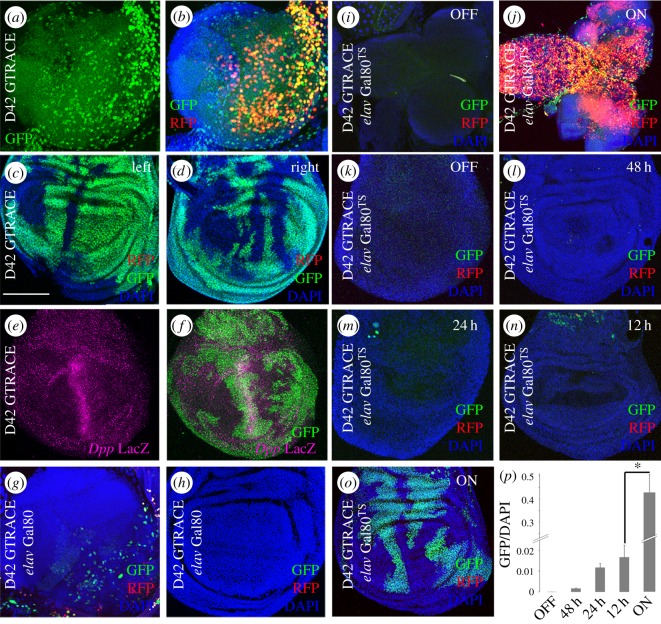
Figure 2.
Toll-6 enhancer in brain and wing cells during development. (a–d) G-TRACE signal from third instar larval brain (a,b) and wing disc (c,d) of the D42-Gal4 line inserted in Toll-6. (e,f) Historical expression of D42 enhancer (GFP) versus LacZ-reported Dpp expression (Dpp-LacZ, magenta). (g,h) elav-Gal80 suppresses D42-Gal4 activity in the brain (g) and wing disc (h). (i–o) Temporal expression experiments in D42-Gal4>G-TRACE>tub-Gal80TS brain (i,j) and wing discs (k–o) maintained at 17°C ‘OFF’ (Gal4 silenced), 48 h, 24 h or 12 h OFF or maintained at 29°C ‘ON’ (Gal4 active). (p) Quantification of GFP positive cells per time point. Student's t-test *p < 0.05. n = 5 wing discs/time point. Bar in (c) = 50 µm.
OK6-Gal4 is another widely used Gal4 line expressed in motor neurons [27] and the Gal4 insertion is located 34 base pairs upstream of the transcription start site of the RapGAP1 gene (see electronic supplementary material, table S1). We investigated the expression pattern of this driver (electronic supplementary material, figure S3). As reported, OK6 is active in the brain (electronic supplementary material, figure S3a–c), but there are GFP positive cells in the wing discs also (electronic supplementary material, figure S3d–f), revealing earlier activity of this enhancer. Comparing the wing disc expression of the three enhancers, elav, D42 and OK6, it is important to realize that the extent of their ectopic expressions is different among them. Thus, ectopic enhancer activity in wing cells is a variable and general phenomenon characteristic of each enhancer and its genomic site.
To discard that the expressions identified by G-TRACE could result from a leaky Gal4 expression, we repeated some experiments incorporating the Gal4 repressor, Gal80. We combined D42>G-TRACE with elav-Gal80 and analysed the GFP and RFP reporters. Signals in the brain are silenced by elav-Gal80 (figure 2g compared to figure 2a,b). This is a validation of the Gal80 effect on the canonical D42 expression domain in the brain. Next, to validate the inhibitory activity of elav-Gal80 during the enhancer ectopic activation, we analysed the wing discs. The ectopic wing expression (figure 2c,d) is also fully repressed by elav-Gal80 (figure 2h). This result confirms that the phenomenon of ectopic expression is not dependent on the protein expressed, Gal4 or Gal80, but it is a property of the enhancer and its environment.
Next, to determine the temporal activation of the D42 enhancer, we used a thermo-sensitive form of the repressor, Gal80TS, (electronic supplementary material, figure S4) and analysed the resulting G-TRACE pattern. As controls, we maintained the larvae at 17°C (negative control, figure 2i) or at 29°C (positive control, figure 2j). The negative control shows no G-TRACE signal and the positive control yields the full enhancer expression in the brain. We set five time points (OFF, 12hOFF, 24hOFF, 48hOFF and ON) corresponding to the hours that the system is kept at the restrictive temperature and, hence, silenced (electronic supplementary material, figure S4). The data show that, if the system is silenced during 48 h, 24 h or 12 h of larval development, the ectopic expression of D42 does not occur (figure 2k–p). Thus, D42 activation occurs during the first 12 hours of larval development, mainly.
2.3. The ectopic activation of enhancers does not lead to expression of their native gene
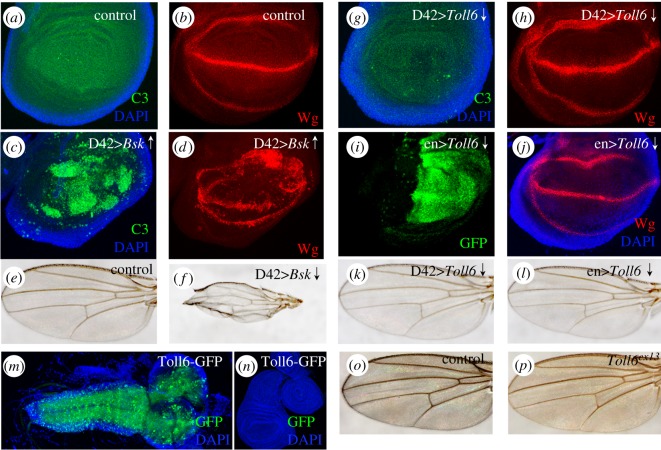
To determine if the ectopic enhancer expression implies a transient expression of the corresponding native gene, we induced apoptosis by driving a core component of the JNK pathway, Basket (Bsk), [28] under D42-Gal4 control. Wing imaginal discs were stained with anti-caspase 3 (C3) to detect apoptosis, and with anti-Wingless (WG) to monitor wing disc development. Epithelial activation of D42-Gal4/Toll-6 leads to Bsk-dependent apoptosis, as revealed by C3 staining, and to abnormalities in the Wg immune pattern which resulted in defective adult wings (figure 3a–f). Further, to resolve if Toll-6 gene expression is necessary for the development of the wing disc, we expressed a Toll-6 specific RNAi under the control of D42-Gal4. We did not detect any apoptosis or morphological defect and the Wg expression pattern was normal (figure 3g,h). To further demonstrate if Toll-6 is necessary for wing development, we used an independent Gal4 (engrailed, en) to drive Toll-6 RNAi expression in the posterior compartment of the wing disc (GFP in figure 3i–l). The expression of this RNAi with this independent driver did not affect the wing in any noticeable feature. Next, we used a Toll-6-GFP protein trap (Mi{MIC}Toll-6MI02127) to detect endogenous Toll-6 protein expression. We observed the canonical Toll-6-GFP signal in the brain (figure 3m). However, no signal was detected in imaginal disc (figure 3n). These results are in line with reported data showing no detectable Toll-6 mRNA in wing imaginal discs [29]. To further determine that Toll-6 expression is dispensable for wing development, we compared wild-type and Toll-6ex13 mutant wings (figure 3o,p). Mutant wings do not display morphological defects indicating that Toll-6 expression is not necessary for wing development. Thus, even though the Toll-6 enhancer D42 is active in the wing disc during development, Toll-6 is not switched on in the wing, at least to the point of manifesting a visible phenotype in disc size, shape or Wg pattern.
Figure 3.
D42 enhancer is expressed in wing cells but its gene Toll-6 is not. (a–d) Caspase 3 (C3) (green) and Wingless expression (red) in wild-type (a,b) and D42-Gal4>Bsk (c,d). Note the extensive apoptosis (c) and distorted Wg pattern (d) caused by Bsk. (e,f) Resulting adult wings with morphological abnormalities due to the Bsk-elicited apoptosis. (g–j) Caspase 3 (C3) (green) and Wingless pattern (red) in D42>Toll-6 RNAi (g,h) wing disc. (i,j) Wingless pattern (red) in en>Toll-6 RNAi (GFP) wing disc. (k,l) Resulting adult wings. Note the lack of morphological effects after inactivating Toll-6 in the posterior wing. (m,n) Toll-6-GFP protein trap (Mi{MIC}Toll-6MI02127) shows signal in larval brain (m) but not in imaginal discs (n). (o,p) Control and Toll-6ex16 mutant adult wings. Cell nuclei are marked in blue (DAPI).
2.4. Sequence-dependent enhancer activation
We aimed to determine the contribution of the enhancer's sequence to its unstable early activation. To that end, we took advantage of the enhancer-Gal4 insertions directed to the exact same and insulated chromosomal site with no detectable Gal4 basal activity [30]. Under these conditions, promoters are subject to the same chromatin structural determinants except for the enhancer nucleotide sequence. We compared two same-site/different-sequence insertions: GMR10B11 and GMR78G09 (see electronic supplementary material, table S1). Both insertions are in the 3 L chromosome arm (68A4 polytene band) and show specific activity in the nervous system. G-TRACE experiments confirmed that both enhancer domains are restricted to the nervous system during development. Nevertheless, although GMR10B11 is active in larval brain cells, its historical (GFP) and current (RFP) expressions are not identical (figure 4a–c). This demonstrates that GMR10B11 expression is transient in certain larval brain cells. GMR78G09 is also expressed in the brain although in fewer cells (about 28 cells, RFP, in third instar larvae). In this case, a group of about 14 cells activated this enhancer in the ventral ganglion during development but not at third instar larvae (figure 4d–f, arrowheads). These results indicate that the nucleotide sequence of the enhancer is determinant to establish the time and number of cells that will display transient expression during development. In the cases of GMR10B11 and GMR78G09 the non-canonical expression domain is transient but not ectopic since it occurs within the same tissue.
Figure 4.
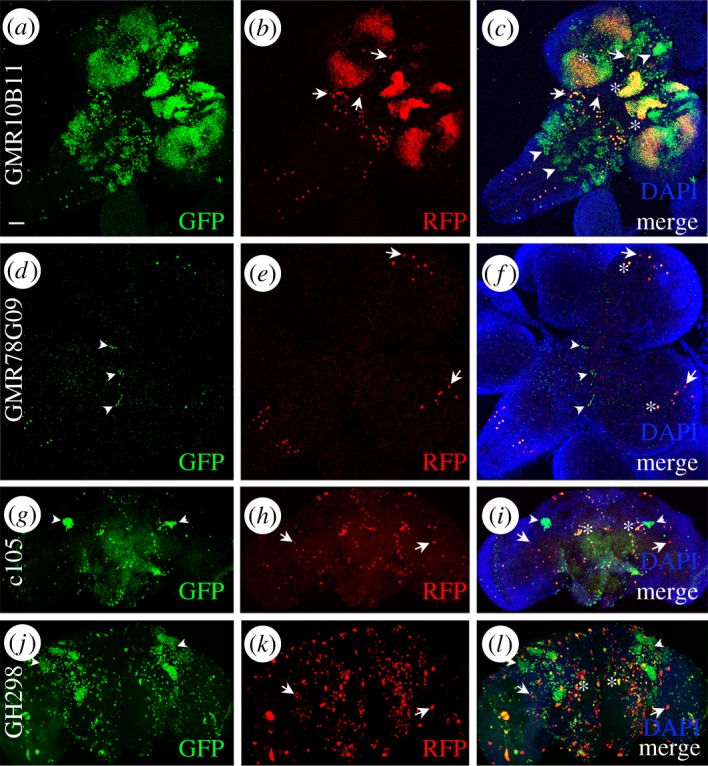
Transient expression of nervous system specific enhancers. GMR10B11-Gal4 (a–c) and GMR78G09-Gal4 (d–f) expression revealed by G-TRACE in larval CNS. c105-Gal4 (g–i) and GH298-Gal4 (j–l) expression in the adult brain. Note the differences between the historical (GFP) and current (RFP) expression domains in the four enhancers. Since all cells correspond to the CNS, this difference must be considered ‘transient’, rather than ‘ectopic’. Arrows indicate cells with active enhancer (RFP), arrowheads indicate historical enhancer activity (GFP) and asterisks indicate cells with coincident historical and current expressions (yellow). Bar in (a) = 50 µm.
2.5. Do all nervous system enhancers have epithelial ectopic activity?
Among the nervous system Gal4 enhancers tested here (electronic supplementary material, table S1) we observed that some enhancers were restricted to the nervous system and showed no activity in wing imaginal disc cells. Enhancers such as GMR78G09 were restricted to a few cells in the larval brain (figure 4d–f), showing a reduced number of cells with historical expression, which is different from the expression at the moment of dissection (third instar larvae). To verify if some enhancers are less susceptible to ectopic activity than others, we searched for Gal4 constructs whose activity in the mature adult brain is restricted to the brain. c105-Gal4 is active in a small number of cells during pupariation and adulthood [8] (see figure 4g–i and electronic supplementary material, table S1) and GH298-Gal4 is active in local olfactory interneurons from larva and adult brain [31,32] (see figure 4j–l and electronic supplementary material, table S1). Our G-TRACE data show that there are some GFP positive cells dispersed in the adult brain and two groups of cells that had transient activity of the enhancer c105 (figure 4g–i, arrowheads). Also, GH298 was active in clusters of cells along the brain (GFP) (figure 4j–l, arrowheads). We could not detect ectopic expression in other tissues for these two enhancers.
Further, we searched for domains that are active in a large number of brain cells but with a cellular identity different from neurons. The gene reverse polarity (repo) is active in glial cells and their precursors during development and throughout adulthood. repo-Gal4 G-TRACE experiments show specific activity in the brain (electronic supplementary material, figure S5) with a full correspondence between historical and actual enhancer activity. Moreover, no ectopic expression could be detected in epithelial wing disc cells. That is, the enhancer repo represents a case with neither ectopic nor transient expression. Thus, within the limits of the subset analysed here, all neuronal enhancers display a historical domain which is larger than that of the final stages of development. This extended domain may be ectopic, in most cases, and/or transient. The repo enhancer is an exception to this trend. Is this exception marking a difference between neuron and non-neuron cells?
2.6. A neurosecretory specific enhancer also shows transient and ectopic activity
To further analyse neural cells, other than neurons, we studied a case from the phantom (phm) gene (electronic supplementary material, table S1). This gene is thought to be specifically expressed in neurosecretory cells of the prothoracic gland (PG). However, we recently showed that the phm-Gal4 driver is expressed in margin cells of the wing disc [33]. The G-TRACE experiments confirm that cells in third instar larvae activate the phm enhancer in the PG and in the wing disc (figure 5a–d). The wing disc also showed historical GFP positive cells at earlier stages of development (figure 5c,d). Thus, this enhancer of phantom, has ectopic activity in epithelial cells early in development.
Figure 5.
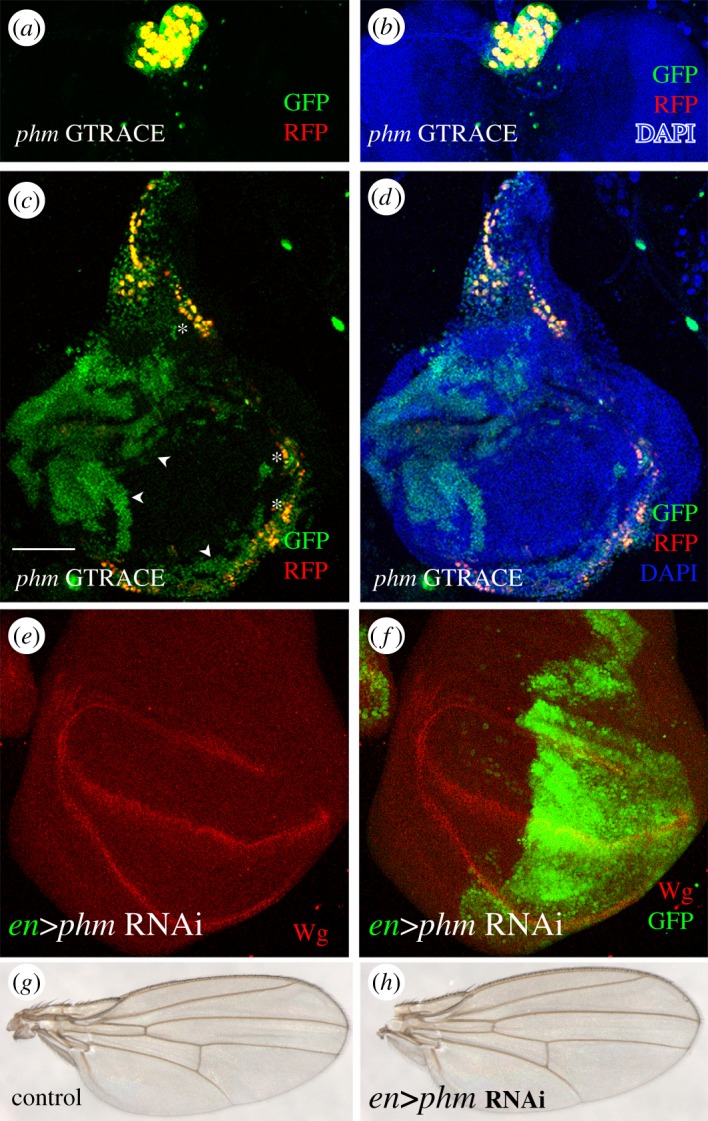
The neurosecretory cells specific phantom enhancer. (a,b) Neurosecretory cells of the larval prothoracic gland expressing the phantom-Gal4 enhancer. The expression at the third instar larvae (RFP) is coincident with the historical trace during development (GFP) resulting in all cells yellow. (c,d) By contrast, the ectopic expression in the wing disc shows a historical trace (GFP) that is larger than the current expression at third instar larvae (RFP). Note cells with GFP only signal (arrow heads) and others with coincident GFP + RFP signal (asterisks). (e,f) Akin to Toll-6 (see figure 3), knocking down phantom by RNAi in the posterior wing compartment (en-Gal4>phm-RNAi) does not alter disc development as judged by the normal expression of Wingless (red). (g,h) Adult wings of genotypes from (e) and (f). Note the lack of morphological defects. Bar in (c) = 50 µm.
We have shown above that Toll-6 expression is not necessary for wing development (figure 3). To investigate this issue in the case of phm enhancer, we expressed a phantom-RNAi under the control of an independent early enhancer (engrailed-Gal4) to knock down phantom in the wing disc. Wing disc morphology, size or the expression of the morphogenetic protein Wingless were not affected by the ectopic expression of the phantom enhancer (figure 5e,f). In addition, phm knockdown (en-Gal4>phmRNAi) does not affect adult wing formation (figure 5g,h). These data suggest that, similar to Toll-6, even though phantom enhancer is active in the wing disc, phantom gene expression is not required for wing cell development.
2.7. Sexual dimorphism in the transient enhancer expression
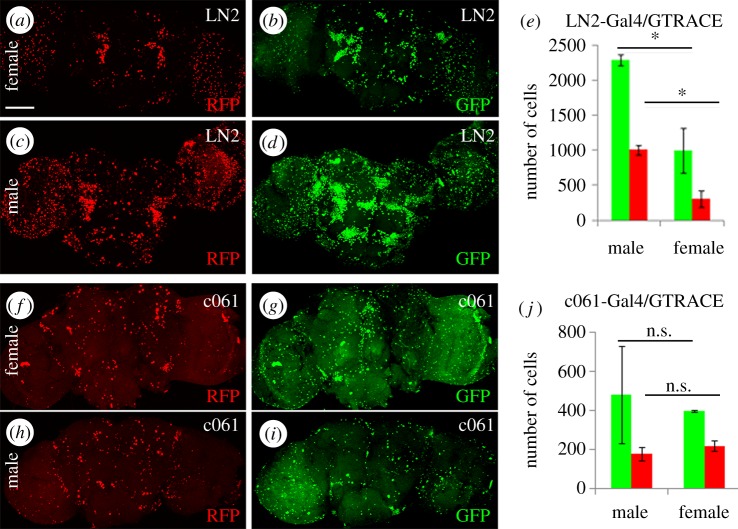
In our study, we included the LN2-Gal4 line that is inserted in the 5′region of the gene sex-lethal (Sxl) aiming to explore the behaviour of an enhancer in the context of sex. LN2-Gal4 is expressed in a set of olfactory local and projection neurons that arise from the lateral neuroblasts [34]. We carried out G-TRACE experiments to verify that LN2-Gal4 expression is restricted to the adult brain and we did not find early expression during embryonic or larval stages. In the adult brain, we found expression concentrated in two groups of neurons symmetrically distributed; presumably olfactory interneurons as it was previously described (figure 6a, arrows). Some additional neurons also activate LN2-Gal4, particularly in the optic lobes (figure 6a). However, when the historical expression of LN2-Gal4 was analysed, the number of neurons with earlier expression was noticeably more extensive than the current expression domain (figure 6b,d,e). Since LN2 was not expressed during embryonic or larval stages, these supernumerary neurons must have activated the enhancer either during metamorphosis or during the initial hours of adulthood. Thus, similar to GMR78G09 and GMR10B11 above, LN2 shows transient, rather than ectopic, expression.
Figure 6.
Sex differences in enhancer expression domains. G-TRACE analysis of 4-day-old adult brains expressing LN2-Gal4 (a–e) or c061-Gal4 (f–j). Female (a,b) and male (c,d) adult brains express the LN2 enhancer during development (GFP) and in its current pattern (RFP). (e) Quantification of LN2 active cells. (f–i) c061-Gal4 expression pattern. Female (f,g) and male (h,i) adult brains show activity during development (GFP) and in adulthood (RFP) but, contrary to the case of LN2, there is no evidence of sex differences in the extent of the expression domain. (j) Quantification of c061 data. Statistics: Student's t-test *p < 0.05. Number of brains/sample = 5. Bar in (a) = 50 µm.
Further analysis revealed that males and females show differences in the activity of this enhancer. Males display more LN2 expressing cells per brain than females (figure 6c,d,e) and the transcription of Gal4 in LN2-Gal4 is stronger in males than in females as shown by PCR assays (electronic supplementary material, figure S6h). Since the Sxl gene is located in the sexually dimorphic X chromosome, we analysed if this effect could result from chromosomal dosage compensation. To that end, we tested another neural Gal4 line inserted in the X chromosome, c061. This line is expressed in the fan-shaped body, dorsal protocerebrum, mushroom body and dopaminergic neurons [35–37]. The data from adult brains showed a significant difference between the historical and current expression domains, the former being more extensive than the latter in line with all other enhancers of this study (figure 6f–j). However, no sex differences were detected in the expression domains of c061 (figure 6j) or in the transcription levels of Gal4 (electronic supplementary material, figure S6). To further validate these results, two additional X-chromosome neural enhancers were tested, 796-Gal4 and elavc155-Gal4. These lines are P-element insertions in the endogenous ccb [38] and elav [39] genes, respectively (electronic supplementary material, table S1). 796-Gal4 flies were crossed by UAS-RFP to mark cell membranes and UAS-synaptobrevin-GFP, to visualize synaptic zones. No sex dimorphism was observed in the volumes of either of these cellular domains (electronic supplementary material, figure S6a–e). In addition, we compared elavc155-Gal4/G-TRACE male and female ectopic expression in wing imaginal discs (electronic supplementary material, figure S2h). No sex differences were detected either in the historical (green) or in the current (red) ectopic activation pattern of this enhancer (electronic supplementary material, figure S2f,g).
To clarify if the sexual dimorphism observed in LN2-Gal4 was determined by the sex of the cell or by the peculiar nature of this enhancer, we feminized these cells by co-expressing a construct from the transformer gene, UAS-traF [40]. In this genotype, the feminized males still show the high number of LN2-Gal4 expressing neurons as regular males do (electronic supplementary material, figure S6i,j). Thus, the differential expression of LN2 is an intrinsic feature of this enhancer rather than an indirect consequence of the sex-determining function of Sxl.
3. Discussion
This study has revealed that enhancers may be activated in ectopic domains early in development. This non-canonical expression is always transient although it can be sustained until the end of the last larval stage. However, we have not found evidence that the transient expression of an enhancer leads to the transient expression of the corresponding gene. The type and extent of the ectopic expression depend on the enhancer sequence, as well as on the genomic localization site.
In spite of the unstable expression pattern at early stages of development, all enhancers analysed in this study eventually consolidate into a canonical expression domain that corresponds to the nervous system. Within the enhancer set analysed here, ectopic domains include preferentially the imaginal discs (see electronic supplementary material, figure S2f–i). Whether this feature is related to the common ectodermal origin of neural and epithelial tissues is an issue worth analysing in the future. Other tissues were routinely screened during the dissection but found mostly negative. Within the nervous system, some enhancers show ectopic expression meaning that the historical trace of activity spans more neural cells than the final expression domain. We refer to these cases as transient domains to differentiate them from the bona fide ectopic expression meaning a different tissue. Transient and ectopic, however, may be considered two variants of the same phenomenon that differs from the canonical expression.
As enhancers modulate transcriptional activity of promoters, it is important to emphasize that the phenomenon of transient and/or ectopic enhancer activation is reproduced with different promoters. In particular, the study of elav was performed with three different transgenic lines. The P-element inserted in elav locus (elavc155) accounts for native enhancers and promoter. By contrast, elav-Gal4 transgenic lines inserted in chromosome II and III contain the native sequence of elav enhancer plus a minimal promoter from the P-element. Despite these differences, in the three cases ectopic activation occurred.
Enhancer activity is determined by chromatin structures and, ultimately, by the binding of protein complexes to DNA. The Sex lethal enhancer LN2 exhibits sexual dimorphism in its ectopic expression domain. LN2 is expressed in more cells in males than in females, both in the ectopic and in the canonical expression domains. All other enhancers analysed do not seem to show sexual dimorphism. Sex lethal is known to be regulated by splicing mechanisms that determine transcript isoform expression according to sex [41,42]. Thus, the sex-dependent regulation of LN2 identified here suggests that a different mechanism must operate on the activation of this enhancer with respect to that of other enhancers. This alternative mechanism is independent of the sex identity of the cell and it is regulated by intrinsic characteristics of the enhancer because the feminization of male cells did not alter the sexual dimorphism in its expression. It is plausible that this feature might reflect sex differences in chromatin structure at the LN2 locus or in the repertoire of binding transcription factors involved in sex determination. The speculations proposed here for the LN2 enhancer of Sxl would be akin to the genomic specializations described for general sex determination in plants and animals including Drosophila [43–45]. In any case, it seems that the mechanisms that underlie unstable enhancer expression are diverse but not unspecific.
The ectopic expression identified through the G-TRACE system is not artefactual because it is effective to trigger the subsequent expression of the coupled gene, illustrated here with basket. Thus, we can conclude that the observations provided by the G-TRACE technique reflect the normal course of events during development. That accepted, the question arises of what type of filter the genome uses to extinguish the unstable expression to finally consolidate a canonical expression domain. Since ectopic domains may consist of single isolated cells, to relatively large cohorts of adjacent cells, extinction by signalling from neighbouring cells seems unlikely. For an autonomous cell, perhaps chromatin locus specific mechanism seems more likely. The case of sex-dependent LN2 enhancer may indicate that the activation and extinction mechanisms are site specific. We hypothesize that the process of enhancer activation follows a sequential history in which the early steps are largely variable and refinement into the canonical domain is progressively built. The molecular microenvironment at the enhancer site, rather than enhancer sequence, affects the early steps of this process. However, the final expression domain is determined by the enhancer sequence. The two examples of the elav enhancer analysed here support this conclusion. It is worth noting that the initial steps of this process, although variable, cannot be considered random throughout the organism. The ectopic wing expression domains of elav, for example, although variable among and within larvae, always affect the wing disc and not the fat body, for instance. Thus, it is likely that the first step in the enhancer activation process may be constrained already to some extent. This study leaves open, however, the issue of the mechanism to identify and extinguish the ectopic expression of enhancers. The pillar of evolutionary change is variability and subsequent selection. Thus, we can envision that the unstable activation of enhancers, although regulated to some extent, could be modified by any number of factors (e.g. hybridization, heterochromatin rearrangements, viral infections, environmental clues, etc.) in a way that would escape the filter mechanism for expression extinction and, thus, eventually result in a change of the canonical expression domain of a gen.
The initial instability in enhancer expression may be relevant to properly evaluate the use of genetic engineering in biotechnology and the interpretation of a plethora of developmental biology studies where Gal4 lines are used. It seems appropriate to carry out extensive analyses on the historical expression of enhancers before launching studies based on the utilitarian use of gen constructs in the, so-called, selective expression domains.
4. Material and methods
4.1. Drosophila genetics
All fly stocks were maintained at 25°C (unless otherwise specified) on a 12/12 h light/dark cycle at constant humidity in standard medium. The following stocks were used: elav-Gal4c155 (BL#458), elav-Gal4 (BL#8765), elav-Gal4 (BL#8760), D42-Gal4 (BL#8816), repo-Gal4 (BL#7415), GH298-Gal4 (BL#37294), c105 (BL#30822), c061-Gal4 (BL#30845), GMR10B11-Gal4 (BL#48247), GMR78G09-Gal4 (BL#40015), G-TRACE (BL#28280), G-TRACE (BL#28281), UAS-bsk (BL#9310), Tubulin-Gal80TS (BL#7019), tub-GAL80TS (BL#7019) Toll-6ex13 (BL#64072), Toll-6-GFP Mi{MIC}Toll-6MI02127 (BL# 34467), P{Act5C-Gal4} (BL#3954) and (BL#42713), UAS-Tra.F (BL#4590) are from Bloomington Drosophila Stock Center. Line LN2-Gal4 (NP2426-Gal4, Kyoto#104198) is from Kyoto Stock center, phantom-Gal4 is a gift from M.B. O'Connor, elav-Gal80 is a gift from G. Morata, 796-Gal4 [38] was generated in our group and OK6-Gal4 [27] is a gift from C. O'Kane.
4.2. Immunostaining
Third instar larvae and adult brains were dissected and fixed with 4% formaldehyde in phosphate-buffered saline for 20 minutes, washed three times with 0.1% triton, and mounted in Vectashield mounting medium with DAPI, or incubated with primary antibodies anti-active-caspase 3 (1/100, Cell Signaling), anti β-gal (1/50, DSHB), anti-elav (1/50, DSHB) or anti-Wingless (1/20, DSHB), and secondary antibodies Alexa 568 or 647 (Life Technologies). Preparations were imaged by confocal microscopy with Leica SP5 microscope. Fluorescence quantification was performed with Imaris software. Images were processed with ImageJ.
4.3. Statistical analysis
Statistical significance was calculated using a Student's two-tailed t-test, with significant differences between compared groups noted by *p < 0.05.
4.4. Live imaging
First and second instar larval wing imaginal discs were visualized and imaged following a previously published protocol [46].
4.5. Quantitative RT-PCR
Total RNA was isolated from 15 fly heads per genotype (Trizol, Invitrogen); cDNAs were synthesized with M-MLV RT (Invitrogen). Gal4 Taqman probe (Sc04172924_s1) and RNA-pol II (housekeeping gene) Taqman probe (Dm02134593) were used (Applied Biosystems). qPCR analysis was done using 7500 Real Time PCR System (Applied Biosystems) with cycling conditions of 95°C for 10 min and 40 cycles of 95°C for 15 s and 55°C for 1 min. qPCR results were analysed with 7500 v.2.0.6 software (Applied Biosystems).
4.6. Statement on data and reagent availability
All the strains and reagents are available in the repositories indicated in Materials and methods section. Electronic supplementary material, table S1 contains all the Gal4 lines tested.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We appreciate fly strains and reagents from Drs M.B. O'Connor, M. Calleja, G. Morata, and from the Kyoto and Bloomington Stock Centers. Critical comments from Drs F. Martín and C. Estella are most appreciated.
Authors' contributions
S.C.-T. designed and performed the experiments, analysed data and wrote the manuscript. M.A. performed the experiments and analysed data. A.F. designed experiments and wrote the manuscript.
Competing interests
The authors declare no conflict of interest and consent to participate in the study.
Funding
S.C.-T. holds a contract from the Ramón y Cajal program RYC-2012-11410. Research has been funded by grant BFU2012-38191 (A.F.) and BFU2015-65685P (S.C.-T. & A.F.) from the Spanish Ministry of Economy.
References
- 1.Brand AH, Perrimon N. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415. [DOI] [PubMed] [Google Scholar]
- 2.Ornitz DM, Moreadith RW, Leder P. 1991. Binary system for regulating transgene expression in mice: targeting int-2 gene expression with yeast GAL4/UAS control elements. Proc. Natl Acad. Sci. USA 88, 698–702. (doi:10.1073/pnas.88.3.698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheer N, Campos-Ortega JA. 1999. Use of the Gal4-UAS technique for targeted gene expression in the zebrafish. Mech. Dev. 80, 153–158. (doi:10.1016/S0925-4773(98)00209-3) [DOI] [PubMed] [Google Scholar]
- 4.Ronen A. 1964. Interchromosomal effects on somatic recombination in Drosophila melanogaster. Genetics 50, 649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stern C. 1969. Somatic recombination within the white locus of Drosophila melanogaster. Genetics 62, 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casas-Tinto S, Zhang Y, Sanchez-Garcia J, Gomez-Velazquez M, Rincon-Limas DE, Fernandez-Funez P. 2011. The ER stress factor XBP1s prevents amyloid-beta neurotoxicity. Hum. Mol. Genet. 20, 2144–2160. (doi:10.1093/hmg/ddr100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez-Funez P, Casas-Tinto S, Zhang Y, Gomez-Velazquez M, Morales-Garza MA, Cepeda-Nieto AC, Castilla J, Soto C, Rincon-Limas DE. 2009. In vivo generation of neurotoxic prion protein: role for hsp70 in accumulation of misfolded isoforms. PLoS Genet. 5, e1000507 (doi:10.1371/journal.pgen.1000507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin-Pena A, Acebes A, Rodriguez JR, Chevalier V, Casas-Tinto S, Triphan T, Strauss R, Ferrus A. 2014. Cell types and coincident synapses in the ellipsoid body of Drosophila. Eur. J. Neurosci. 39, 1586–1601. (doi:10.1111/ejn.12537) [DOI] [PubMed] [Google Scholar]
- 9.Yamanaka N, Romero NM, Martin FA, Rewitz KF, Sun M, O'Connor MB, Leopold P. 2013. Neuroendocrine control of Drosophila larval light preference. Science 341, 1113–1116. (doi:10.1126/science.1241210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo L, Liao YJ, Jan LY, Jan YN. 1994. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 8, 1787–1802. (doi:10.1101/gad.8.15.1787) [DOI] [PubMed] [Google Scholar]
- 11.Ono H, et al. 2006. Spook and Spookier code for stage-specific components of the ecdysone biosynthetic pathway in Diptera. Dev. Biol. 298, 555–570. (doi:10.1016/j.ydbio.2006.07.023) [DOI] [PubMed] [Google Scholar]
- 12.Bondarenko VA, Liu YV, Jiang YI, Studitsky VM. 2003. Communication over a large distance: enhancers and insulators. Biochem. Cell Biol. 81, 241–251. (doi:10.1139/o03-051) [DOI] [PubMed] [Google Scholar]
- 13.Casas-Tinto S, Marr MT II, Andreu P, Puig O. 2007. Characterization of the Drosophila insulin receptor promoter. Biochim. Biophys. Acta 1769, 236–243. (doi:10.1016/j.bbaexp.2007.03.003) [DOI] [PubMed] [Google Scholar]
- 14.Kulaeva OI, Nizovtseva EV, Polikanov YS, Ulianov SV, Studitsky VM. 2012. Distant activation of transcription: mechanisms of enhancer action. Mol. Cell. Biol. 32, 4892–4897. (doi:10.1128/MCB.01127-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banerji J, Rusconi S, Schaffner W. 1981. Expression of a beta-globin gene is enhanced by remote SV40 DNA sequences. Cell 27, 299–308. (doi:10.1016/0092-8674(81)90413-X) [DOI] [PubMed] [Google Scholar]
- 16.Shlyueva D, Stampfel G, Stark A. 2014. Transcriptional enhancers: from properties to genome-wide predictions. Nat. Rev. Genet. 15, 272–286. (doi:10.1038/nrg3682) [DOI] [PubMed] [Google Scholar]
- 17.Krivega I, Dean A. 2012. Enhancer and promoter interactions: long distance calls. Curr. Opin. Genet. Dev. 22, 79–85. (doi:10.1016/j.gde.2011.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maston GA, Landt SG, Snyder M, Green MR. 2012. Characterization of enhancer function from genome-wide analyses. Annu. Rev. Genomics Hum. Genet. 13, 29–57. (doi:10.1146/annurev-genom-090711-163723) [DOI] [PubMed] [Google Scholar]
- 19.Evans CJ, et al. 2009. G-TRACE: rapid Gal4-based cell lineage analysis in Drosophila. Nat. Methods 6, 603–605. (doi:10.1038/nmeth.1356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han J, Knops JF, Longshore JW, King PH. 1996. Localization of human elav-like neuronal protein 1 (Hel-N1) on chromosome 9p21 by chromosome microdissection polymerase chain reaction and fluorescence in situ hybridization. Genomics 36, 189–191. (doi10.1006/geno.1996.0444) [DOI] [PubMed] [Google Scholar]
- 21.Muresu R, Baldini A, Gress T, Posner JB, Furneaux HM, Siniscalco M. 1994. Mapping of the gene coding for a paraneoplastic encephalomyelitis antigen (Hud) to human-chromosome site-1p34. Cytogenet. Cell Genet. 65, 177–178. (doi:10.1159/000133626) [DOI] [PubMed] [Google Scholar]
- 22.Robinow S, White K. 1988. The locus elav of Drosophila melanogaster is expressed in neurons at all developmental stages. Dev. Biol. 126, 294–303. (doi:10.1016/0012-1606(88)90139-X) [DOI] [PubMed] [Google Scholar]
- 23.Robinow S, White K. 1991. Characterization and spatial distribution of the ELAV protein during Drosophila melanogaster development. J. Neurobiol. 22, 443–461. (doi:10.1002/neu.480220503) [DOI] [PubMed] [Google Scholar]
- 24.Van Tine BA, Knops JF, Butler A, Deloukas P, Shaw GM, King PH. 1998. Localization of HuC (ELAVL3) to chromosome 19p13.2 by fluorescence in situ hybridization utilizing a novel tyramide labeling technique. Genomics 53, 296–299. (doi:10.1006/geno.1998.5468) [DOI] [PubMed] [Google Scholar]
- 25.Alexandre C, Baena-Lopez A, Vincent JP. 2014. Patterning and growth control by membrane-tethered Wingless. Nature 505, 180–185. (doi:10.1038/nature12879) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin FA, Morata G. 2006. Compartments and the control of growth in the Drosophila wing imaginal disc. Development 133, 4421–4426. (doi:10.1242/dev.02618) [DOI] [PubMed] [Google Scholar]
- 27.Sanyal S. 2009. Genomic mapping and expression patterns of C380, OK6 and D42 enhancer trap lines in the larval nervous system of Drosophila. Gene Expr. Patterns 9, 371–380. (doi:10.1016/j.gep.2009.01.002) [DOI] [PubMed] [Google Scholar]
- 28.Moreno E, Yan M, Basler K. 2002. Evolution of TNF signaling mechanisms: JNK-dependent apoptosis triggered by Eiger, the Drosophila homolog of the TNF superfamily. Curr. Biol. 12, 1263–1268. (doi:10.1016/S0960-9822(02)00954-5) [DOI] [PubMed] [Google Scholar]
- 29.Yagi Y, Nishida Y, Ip YT. 2010. Functional analysis of Toll-related genes in Drosophila. Dev. Growth Differ. 52, 771–783. (doi:10.1111/j.1440-169X.2010.01213.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfeiffer BD, et al. 2008. Tools for neuroanatomy and neurogenetics in Drosophila. Proc. Natl Acad. Sci. USA 105, 9715–9720. (doi:10.1073/pnas.0803697105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Acebes A, Devaud JM, Arnes M, Ferrus A. 2012. Central adaptation to odorants depends on PI3 K levels in local interneurons of the antennal lobe. J. Neurosci. 32, 417–422. (doi:10.1523/JNEUROSCI.2921-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stocker RF, Heimbeck G, Gendre N, de Belle JS. 1997. Neuroblast ablation in Drosophila P[GAL4] lines reveals origins of olfactory interneurons. J. Neurobiol. 32, 443–456. (doi:10.1002/(SICI)1097-4695(199705)32:5<443::AID-NEU1>3.0.CO;2-5) [DOI] [PubMed] [Google Scholar]
- 33.Mansilla A, Martin FA, Martin D, Ferrus A. 2015. Ligand-independent requirements of steroid receptors EcR and USP for cell survival. Cell Death Differ. 23, 405–416. (doi:10.1038/cdd.2015.108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Das A, Sen S, Lichtneckert R, Okada R, Ito K, Rodrigues V, Reichert H. 2008. Drosophila olfactory local interneurons and projection neurons derive from a common neuroblast lineage specified by the empty spiracles gene. Neural Dev. 3, 33 (doi:10.1186/1749-8104-3-33) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keleman K, Vrontou E, Kruttner S, Yu JY, Kurtovic-Kozaric A, Dickson BJ. 2012. Dopamine neurons modulate pheromone responses in Drosophila courtship learning. Nature 489, 145–149. (doi:10.1038/nature11345) [DOI] [PubMed] [Google Scholar]
- 36.Krashes MJ, DasGupta S, Vreede A, White B, Armstrong JD, Waddell S. 2009. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell 139, 416–427. (doi:10.1016/j.cell.2009.08.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Q, Liu S, Kodama L, Driscoll MR, Wu MN. 2012. Two dopaminergic neurons signal to the dorsal fan-shaped body to promote wakefulness in Drosophila. Curr. Biol. 22, 2114–2123. (doi:10.1016/j.cub.2012.09.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin-Pena A, Acebes A, Rodriguez JR, Sorribes A, de Polavieja GG, Fernandez-Funez P, Ferrus A. 2006. Age-independent synaptogenesis by phosphoinositide 3 kinase. J. Neurosci. 26, 10 199–10 208. (doi:10.1523/JNEUROSCI.1223-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin DM, Goodman CS. 1994. Ectopic and increased expression of Fasciclin II alters motoneuron growth cone guidance. Neuron 13, 507–523. (doi:10.1016/0896-6273(94)90022-1) [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Hoxha V, Lama C, Dinh BH, Vo CN, Dauwalder B. 2011. The hector G-protein coupled receptor is required in a subset of fruitless neurons for male courtship behavior. PLoS ONE 6, e28269 (doi:10.1371/journal.pone.0028269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bashaw GJ, Baker BS. 1997. The regulation of the Drosophila msl-2 gene reveals a function for Sex-lethal in translational control. Cell 89, 789–798. (doi:10.1016/S0092-8674(00)80262-7) [DOI] [PubMed] [Google Scholar]
- 42.Cline TW, Dorsett M, Sun S, Harrison MM, Dines J, Sefton L, Megna L. 2010. Evolution of the Drosophila feminizing switch gene Sex-lethal. Genetics 186, 1321–1336. (doi:10.1534/genetics.110.121202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baroux C, Autran D. 2015. Chromatin dynamics during cellular differentiation in the female reproductive lineage of flowering plants. Plant J. 83, 160–176. (doi:10.1111/tpj.12890) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyer RE, Algazeery A, Capri M, Brazier H, Ferry C, Ait-Ahmed O. 2014. Drosophila Yemanuclein associates with the cohesin and synaptonemal complexes. J. Cell Sci. 127, 4658–4666. (doi:10.1242/jcs.152520) [DOI] [PubMed] [Google Scholar]
- 45.Murphy MW, et al. 2015. An ancient protein-DNA interaction underlying metazoan sex determination. Nat. Struct. Mol. Biol. 22, 442–451. (doi:10.1038/nsmb.3032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nienhaus U, Aegerter-Wilmsen T, Aegerter CM. 2012. In-vivo imaging of the Drosophila wing imaginal disc over time: novel insights on growth and boundary formation. PLoS ONE 7, e47594 (doi:10.1371/journal.pone.0047594) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.