Abstract
Aim
Status epilepticus (SE) results in the generation of reactive oxygen species (ROS), which contribute to seizure-induced brain injury. It is well known that oxidative stress plays a pivotal role in status epilepticus (SE). Thymoquinone (TQ) is a bioactive monomer extracted from black cumin (Nigella sativa) seed oil that has anti-inflammatory, anti-cancer, and antioxidant activity in various diseases. This study evaluated the protective effects of TQ on brain injury in a lithium-pilocarpine rat model of SE and investigated the underlying mechanism related to antioxidative pathway.
Methods
Electroencephalogram and Racine scale were used to value seizure severity. Passive-avoidance test was used to determine learning and memory function. Moreover, anti-oxidative activity of TQ was observed using Western blot and super oxide dismutase (SOD) activity assay.
Results
Latency to SE increased in the TQ-pretreated group compared with rats in the model group, while the total power was significantly lower. Seizure severity measured on the Racine scale was significantly lower in the TQ group compared with the model group. Results of behavioral experiments suggest that TQ may also have a protective effect on learning and memory function. Investigation of the protective mechanism of TQ showed that TQ-pretreatment significantly increased the expression of Nrf2, HO-1 proteins and SOD in the hippocampus.
Conclusion
These findings showed that TQ attenuated brain injury induced by SE via an anti-oxidative pathway.
Keywords: brain injury, Nrf2, oxidative stress, status epilepticus, thymoquinone, status epilepticus
Introduction
Status epilepticus (SE) is a common, lifethreatening neurologic disorder characterized by acute, prolonged epileptic seizures. The incidence rate of SE is about 0.05 to 0.1% in the general population and 1.1 to 14% in the epileptic population. SE has a poor prognosis because seizures that cannot be terminated quickly result in irreversible brain injury. It is known that multiple factors are involved in the pathogenesis of brain injury induced by SE, one of which is oxidative stress [1–2]. The generation of excess free radicals and increase in lipid peroxidation that occur during SE damage brain tissue, therefore, antioxidants might be effective for treatment of SE [3-5]. Thymoquinone (TQ), a bioactive monomer derived from black seed (Nigella sativa) oil, has significant antioxidant effects [6-8]. It has been shown to suppress oxidative stress and attenuate seizure activity and lower hippocampal neuronal loss in an epilepsy model and in children with refractory seizures [9–11]. We speculate that TQ alleviates the brain injury of SE by modulating oxidative stress. It is thought that the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway plays a key defensive role against oxidative stress in epilepsy [12–14]. This study evaluated the protective effects of TQ on brain injury in a status epilepticus rat model, and the underlying mechanism related to antioxidative pathway using biochemistry techniques and behavioral and electrophysiological tests.
Materials and methods
Reagents
Pilocarpine, Lithium chloride, and TQ were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Animals and study groups
Male Sprague-Dawley (SD) rats weighing 200–230 g were purchased from the Animal Center of the Chinese Academy of Sciences (Shanghai, China). All animal experiments were carried out following the Guidelines for Animal Experiments of the Chinese Academy of Medical Sciences and were approved by the ethics committee for animal care of Jinshan Hospital. Rats were housed in a standard animal room with three animals in each cage and 12 h of light per day. The temperature was maintained at 20±2°C, the relative humidity at 60%. Animals were randomly divided into three groups: normal control, status epilepticus model (model) and status epilepticus + thymoquinone (TQ)(n=11). Rats in the TQ group were administered TQ 10 mg/kg twice by intraperitoneal injection 24 hours and 1 hour prior to injection of pilocarpine. Rats in the model group were administered equivalent volumes of normal saline.
Establishment of a status epilepticus rat model
Rats were intraperitoneally injected with 1% lithium chloride (3 mg/kg) followed by 1% pilocarpine (30 mg/kg) 20 hours later. Preliminary observation confirmed that seizures would appear 15 min after pilocarpine injection. Seizure behaviors after kindling were classified using the Racine scale as Stage I, mouth and facial movements; Stage II, head nodding; Stage III, forelimb clonus; Stage IV, rearing; and Stage V, rearing and falling [15]. Rats with consecutive Stage IV seizures for 60 min was injected diazepam to terminate the seizures. Passive avoidance task were carried out after the model was established for 48 hours.
Electroencephalograms and seizure behavior
Rats were immobilized on the stereotaxic instrument after intraperitoneal anesthesia with 10% chloral hydrate. To record EEGs, stainless steel screw electrodes were placed epidurally into the bone. The recording electrode was placed 2 mm lateral and 2 mm posterior to the bregma. The reference electrode was 2 mm lateral and −2 mm posterior to the bregma, and the ground electrode was −10 mm lateral and 0 mm posterior to the bregma. Two stainless steel screws were fixed on the bone with dental acrylic. After waiting 3 days for the animals to recover, EEG signals were recorded monopolarly using an animal Bluetooth digital electroencephalograph (BT-8, Nuocheng, Shanghai, China) in freely moving rats. The latency to first seizure and total power (frequency and amplitude) were recorded. Behavioral changes were recorded by video cameras, and seizures induced by lithium chloride-pilocarpine were scored according to the Racine scale.
Passive-avoidance test
Passive-avoidance tests were performed in two identical light and dark square boxes. On day 24, the rats were initially placed in the light chamber; after 10 seconds, the door between the compartments was opened. When rats entered the dark compartment, the door was closed and an electrical foot shock (0.1 mA/10 g body weight) was delivered through stainless steel rods for 2 sec (one training trial). Twentyfour hours after the training trial, the rats were again placed in the light compartment and the step-through latency to enter the dark compartment was measured [17].
Western blot assay
Total protein was extracted from hippocampal and cortical tissue with 1× RIPA lysis buffer (Beyotime Institute of Biotechnology, China) supplemented with 1 mM PMSF. Protein samples were run on SDS-PAGE gels and transferred to PVDF membranes. The membrane was incubated with anti-Nrf2 rabbit polyclonal antibody (1:500; Santa Cruz Biotechnology, USA), anti-HO-1 mouse monoclonal antibody (1:500; Abcam, USA), and anti-alpha tublin primary antibody (1:5000; Proteintech group, USA) at 4°C overnight. Membranes were subsequently incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit (1:5000; Proteintech group) or goat anti-mouse (1:5000, Proteintech group) secondary antibody at room temperature for 2 h. Signals were detected by electrochemiluminescence (ECL-Plus; Merck Millipore, Darmstadt, Germany) and quantified using a Bio-Rad 2000 gel imaging system with QUANTITY ONE software (Bio-Rad Laboratories, Hercules, CA, USA).
Assay of super oxide dismutase (SOD) activity
The inhibition rate of SOD was assayed with SOD detection kit (DOJINDO, Shanghai, China) following the manufacturer’s instructions and OD value was read by Bio-Rad 680 microplate reader (Bio-Rad Laboratories).
Statistical analysis
Data were expressed as means±standard deviation (SD). Mean values were compared using the paired-sample t-test or one-way analysis of variance (ANOVA). Behavioral scores were tested for significance using Kendall’s rank correlation analysis. All statistical analyses were performed using SPSS 11.5 software (SSPS, Inc., Chicago, IL, USA). Differences were considered significant when p < 0.05.
Results
Rat model of SE
In the model group, two rats died during establishment of the model. The remaining 11 rats were successfully modeled. No rats died in the TQ group.
Effect of TQ on EEG
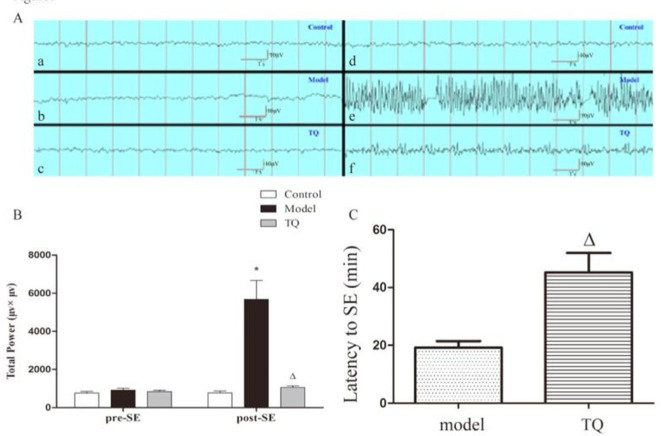
As shown in Figure 1, the latency to SE in the TQ group after pilocarpine administration was longer than that in the model group (p<0.05). In addition, the total power was significantly lower in the TQ group than in the model group (p<0.05).
Figure 1.
Total power was significantly lower in the TQ group after pilocarpine administration than in the model group (A, B). Pre-SE EEG of control group (a), model group (b), and TQ group (c) rats; post-SE EEG of control group (d), model group (e), and TQ group (f) rats. The latency to SE in the TQ group was longer than that in model group. *model group vs. control group, p<0.05, ΔTQ group vs. model group, p<0.05.
Effect of TQ on seizure behavior
Seizure behavior was measured by Racine scale grade. As shown in Figure 2, Grade IV, and V seizures were observed in the model group. In the TQ group, nearly all seizures were Grades II and III, only one seizure was Grade IV. Compared with the model group, the Racine grades of the TQ group were significantly lower (p<0.05). The data suggest that the overall seizure behavior was milder in the TQ group.
Figure 2.
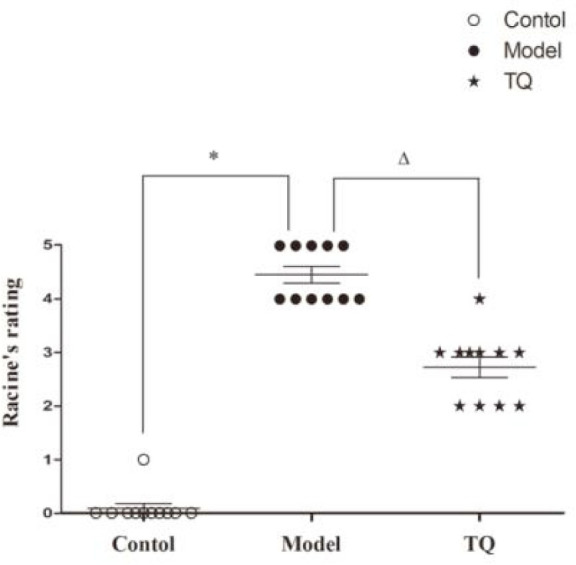
Effect of TQ on the epileptic behavior of status epileptic rats. *model group vs. control group, p<0.05, ΔTQ group vs. model group, p<0.05.
Effect of TQ on cognitive function
The step-through test was used to estimate the effect of TQ on cognitive function. As shown in Figure 3, post-SE latent period in model group rats was significantly shorter than that observed pre-SE, thus indicating that SE impaired learning and memory. Compared with the rats in model group, the post-SE escape latency in TQ group rats was significantly longer (p <0.05). These results indicated the improvement of spatial learning and memory in TQ group.
Figure 3.
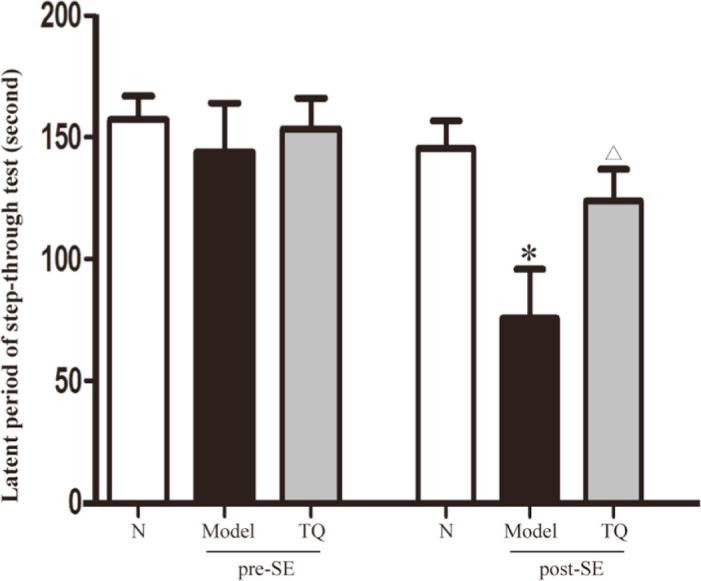
Effect of TQ on the epileptic behavior of SE rats. Latent period of the step-through test. *model group vs. control group, p<0.05 Δ TQ group vs. model group, p<0.05.
Antioxidative effect of TQ
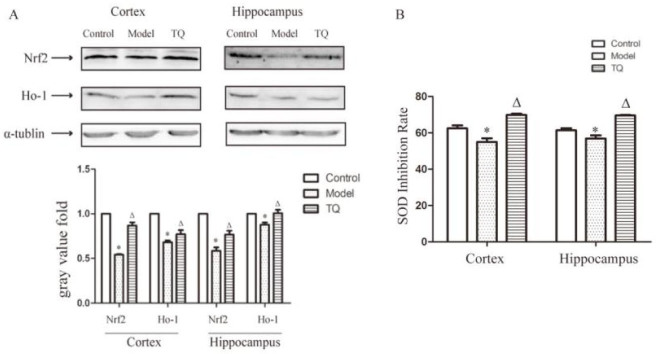
We assayed Nrf2, HO-1, and SOD levels in the hippocampus and cortex to determine whether TQ inhibited the oxidative stress induced by SE. Western blots showed that the levels of Nrf2 and HO-1 protein increased significantly in the TQ group compared with the model group (p < 0.05) (Figure 4A,B). SOD inhibition rate was higher in the TQ group than that in the model group (p < 0.05) (Figure 4C).
Figure 4.
TQ increased Nrf2, HO-1 and SOD inhibition rate in the cortex and hippocampus of SE rats. (A) Protein extracted from hippocampal and cortical tissues was assayed by western blotting. Nrf2, HO-1 expression in SE rat brains was significantly lower than in control group rats. TQ significantly downregulated the expression of Nrf2 and HO-1. (B) The inhibition rate of SOD. *Model group vs. control group; ΔTQ group vs. model group, p < 0.05.
Discussion
The International League Against Epilepsy (ILAE) defines SE as a condition resulting either from the failure of the mechanisms responsible for seizure termination or from the initiation of mechanisms that lead to abnormally prolonged seizures. It is obvious that SE can lead to long-term consequences, including neuronal death and neuronal injury [18]. The brain injury induced by SE is unavoidable. It has been reported that a single SE seizure can reduce cognitive function of patients by 8 to 26% [19]. Clinical studies have demonstrated that SE patients present neurological deficits associated with seizures that correspond to imaging changes, such as high T2WI signals, and brain atrophy [20]. The existence of pathological changes of hippocampus, limbic system, cortex and cerebellar tissue have been confirmed by studies in both animal models and SE patients [21,22]. SE can induce increased oxygen consumption, apnea, and vasomotor dysfunction, which lead to cerebral hypoxia. Subsequent to brain injury, other pathological events such as excitatory neurotoxicity, inflammation, and changes in the microenvironment of glial cells and neurons cells contribute to the brain injury caused by SE [23–25].
Recent evidence shows that oxidative stress plays a key role in SE [26,27]. Under normal circumstances, brain tissue continuously produces ROS, such as NO, hydrogen peroxide, and superoxide. At the same time, many antioxidant enzymes are released in brain tissue, including HO-1, GSH and SOD. To protect brain tissue from damage by ROS, a delicate balance is maintained. When ROS increase rapidly, the balance is disturbed, and oxidative damage occured [28]. In animal models, SE can lead to increased oxidative stress in the brain [26]. Oxygen free radicals are produced by the abnormal metabolism in the brain as a consequence of SE, and further aggravate brain injury. It is well known that mitochondrial damage is a key step in the pathophysiological increase of excitatory neurotransmitter synthesis and promotion of neuronal apoptosis in the brain [27]. A number of studies have indicated that inhibiting oxidative stress has a protective effect on the brain injury induced by SE [29,30].
TQ is a bioactive monomer isolated from the oil of black cumin (Nigella sativa) seed, and is traditionally used for treating hypertension, asthma, dysentery and eczema [31]. TQ has multiple bioactive properties, including anticancer, anti-inflammatory and antioxidant effects [6–8]. Recently, it was shown that TQ is potent mitochondria-targeted antioxidants effective in treating a large number of agerelated pathologies [32, 33]. Previous studies in a temporal lobe epilepsy model epilepsy have shown that TQ reduced seizures and protected hippocampal neurons against apoptosis [9,10]. Other studies have evaluated TQ for treatment of refractory epilepsy in children [11]. We thus speculated that TQ has a protective effect on SE. We found that TQ had an anticonvulsant effect as measured by better representation of EEG, and a decrease in seizure severity. In addition, results of the behavioral experiments suggest that TQ may also have a protective effect on learning and memory function. In our study, we used passive-avoidance testing to evaluate the learning and memory function in a state of fear.
The protective mechanism of TQ was evaluated in rats with pilocarpine-induced SE. We also assayed the expression of antioxidants (Nrf2⊠HO-1 and SOD) in brain tissue. The expression level of proteins is difference between cortex tissue and hippocampus tissue, so we detected the expression in both cortex tissue and hippocampus tissue. The results showed that Nrf2 and HO-1 proteins and SOD were highly expressed in TQ group rats, and indicate that TQ may reduce the brain injuries caused by SE by Nrf2-mediated activation of antioxidant defense. Nrf2 is a key transcription factor involved in antioxidant response, and can thus protect cells from toxic substances and pathogens [34]. Nrf2 belongs to a subset of basic leucine zipper genes. Under normal conditions, Nrf2 is tethered in the cytoplasm by the Keap1 protein. Activated Nrf2 translocates into the nucleus, where it binds to a small Maf protein and activates the transcription of target genes, such as heme oxygenase-1 (HO-1), NADPH: quinine oxidoreductase 1 and SOD. HO-1 is a rate-limiting enzyme for degrading heme into biliverdin, carbon monoxide (CO), and free iron [35]. As antioxidant enzymes, HO-1 and SOD can protect against oxidative stress. The crucial role of Nrf2 and its downstream products in the antioxidant defense has been demonstrated in various diseases [13,36].
Overall, our results indicate that TQ has a protective effect on brain injury induced by pilocarpine. The mechanism may be mediated by modulation of an antioxidative pathway. The study provides experimental evidence to support further evaluation of of TQ as a potential therapeutic drug for treatment of SE.
Acknowledgments
This investigation was supported by grants from the Traditional Chinese Medicine Research Fund of Shanghai Municipal Commission of Health and Family Planning (2014JP007A) to YC.
Footnotes
Competing interests The authors have no competing interests to declare.
References
- [1].Waldbaum S., Liang L.P., Patel M.. Persistent impairment of mitochondrial and tissue redox status during lithium-pilocarpine-induced epileptogenesis. J. Neurochem. 2010;115:1172–1182. doi: 10.1111/j.1471-4159.2010.07013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Liu J., Wang A., Li L., Huang Y., Xue P., Hao A.. Oxidative stress mediates hippocampal neuron death in rats after lithium-pilocarpine-induced status epilepticus. Seizure. 2010;19:165–172. doi: 10.1016/j.seizure.2010.01.010. [DOI] [PubMed] [Google Scholar]
- [3].Ahmad M., Abu-Taweel G.M., Aboshaiqah A.E., Ajarem J.S. and oxidative stress in rat lithium-pilocarpine model of status epilepticus. Oxid Med Cell Longev; 2014. The effects of quinacrine, proglumide, and pentoxifylline on seizure activity, cognitive deficit; p. 630509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gao F., Gao Y., Liu Y.F., Wang L., Li Y.J.. Berberine exerts an anticonvulsant effect and ameliorates memory impairment and oxidative stress in a pilocarpine-induced epilepsy model in the rat. Neuropsychiatr Dis Treat. 2014;13:2139–2145. doi: 10.2147/NDT.S73210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Smilin Bell Aseervatham G., Sivasudha T., Suganya M., Rameshkumar A., Jeyadevi R.. Trichosanthes tricuspidata modulates oxidative toxicity in brain hippocampus against pilocarpine induced status epilepticus in mice. Neurochem Res. 2013;38:1715–1725. doi: 10.1007/s11064-013-1075-3. [DOI] [PubMed] [Google Scholar]
- [6].Darakhshan S., Bidmeshki Pour A., Hosseinzadeh Colagar A., Sisakhtnezhad S.. Thymoquinone and its therapeutic potentials. Pharmacol Res. 2015;95–96:138–158. doi: 10.1016/j.phrs.2015.03.011. [DOI] [PubMed] [Google Scholar]
- [7].Galaly S.R., Ahmed O.M., Mahmoud A.M.. Thymoquinone and curcumin prevent gentamicin-induced liver injury by attenuating oxidative stress, inflammation and apoptosis. J. Physiol Pharmacol. 2014;65:823–832. [PubMed] [Google Scholar]
- [8].Woo C.C., Hsu A., Kumar A.P., Sethi G., Tan K.H.. Thymoquinone inhibits tumor growth and induces apoptosis in a breast cancer xenograft mouse model: the role of p38 MAPK and ROS. PLoS One. 2013;8:e75356. doi: 10.1371/journal.pone.0075356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ullah I., Badshah H., Naseer M.I., Lee H.Y., Kim M.O.. Thymoquinone and vitamin C attenuates pentylenetetrazole-induced seizures via activation of GABAB1 receptor in adult rats cortex and hippocampus. Neuromolecular Med. 2015;17:35–46. doi: 10.1007/s12017-014-8337-3. [DOI] [PubMed] [Google Scholar]
- [10].Dariani S., Baluchnejadmojarad T., Roghani M.. Thymoquinone attenuates astrogliosis, neurodegeneration, mossy fiber sprouting, and oxidative stress in a model of temporal lobe epilepsy. J. Mol Neurosci. 2013;51:679–686. doi: 10.1007/s12031-013-0043-3. [DOI] [PubMed] [Google Scholar]
- [11].Akhondian J., Kianifar H., Raoofziaee M., Moayedpour A., Toosi M.B., Khajedaluee M.. The effect of thymoquinone on intractable pediatric seizures (pilot study) Epilepsy Res. 2011;93:39–43. doi: 10.1016/j.eplepsyres.2010.10.010. [DOI] [PubMed] [Google Scholar]
- [12].Wang W., Wu Y., Zhang G., Fang H., Wang H., Zang H.. et al. Activation of Nrf2-ARE signal pathway protects the brain from damage induced by epileptic seizure. Brain Res. 2014;1544:54–61. doi: 10.1016/j.brainres.2013.12.004. [DOI] [PubMed] [Google Scholar]
- [13].Mazzuferi M., Kumar G., Van Eyll J., Danis B., Foerch P., Kaminski R.M.. Nrf2 defense pathway: Experimental evidence for its protective role in epilepsy. Ann Neurol. 2013;74:560–568. doi: 10.1002/ana.23940. [DOI] [PubMed] [Google Scholar]
- [14].Wang W., Wang W.P., Zhang G.L., Wu Y.F., Xie T., Kan M.C.. et al. Activation of Nrf2-ARE signal pathway in hippocampus of amygdala kindling rats. Neurosci Lett. 2013;543:58–63. doi: 10.1016/j.neulet.2013.03.038. [DOI] [PubMed] [Google Scholar]
- [15].Racine R.J.. Modification of seizure activity by electrical stimulation. II. Motor seizure. J. Electroenceph Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- [16].Chen L., Yang X., Zhou X., Wang C., Gong X., Chen B.. et al. Hyperactivity and impaired attention in Gamma aminobutyric acid transporter subtype 1 gene knockout mice. Acta Neuropsychiatr. 2015;27:368–374. doi: 10.1017/neu.2015.37. [DOI] [PubMed] [Google Scholar]
- [17].Kuo C.T., Lin Y.W., Tang N.Y., Cheng C.Y., Hsieh C.L.. Electric stimulation of the ears ameliorated learning and memory impairment in rats with cerebral ischemia-reperfusion injury. Sci Rep. 2016;6:20381. doi: 10.1038/srep20381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Trinka E., Cock H., Hesdorffer D., Rossetti A.O., Scheffer I.E., Shinnar S.. et al. A definition and classification of status epilepticus-Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia. 2015;56:1515–1523. doi: 10.1111/epi.13121. [DOI] [PubMed] [Google Scholar]
- [19].Helmstaedter C.. Cognitive outcome of status epilepticus in adults. Epilepsia. 2007;8:85–90. doi: 10.1111/j.1528-1167.2007.01360.x. [DOI] [PubMed] [Google Scholar]
- [20].Siepmann T., Barlinn K., Penzlin A.I., Illigens B.M., Kitzler H., Bodechtel U.. Subsequent Bilateral Hippocampal Diffusion Restriction and Atrophy in Repeated Status Epilepticus. Neurodiagn J. 2015;55:243–50. doi: 10.1080/21646821.2015.1071143. [DOI] [PubMed] [Google Scholar]
- [21].Kumar G., Mittal S., Moudgil S.S., Kupsky W.J., Shah A.K.. Histopathological evidence that hippocampal atrophy following status epilepticus is a result of neuronal necrosis. J. Neurol Sci. 2013;334:186–191. doi: 10.1016/j.jns.2013.08.016. [DOI] [PubMed] [Google Scholar]
- [22].Seinfeld S., Goodkin H.P., Shinnar S.. Status Epilepticus. Cold Spring Harb Perspect Med. 2016;6:a022830. doi: 10.1101/cshperspect.a022830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Johnson E.A., Guignet M.A., Dao T.L., Hamilton T.A., Kan R.K.. Interleukin-18 expression increases in response to neurovascular damage following soman-induced status epilepticus in rats. J Inflamm (Lond) 2015;12:43. doi: 10.1186/s12950-015-0089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Reddy D.S., Kuruba R.. Experimental models of status epilepticus and neuronal injury for evaluation of therapeutic interventions. Int J Mol Sci. 2013;14:18284–18318. doi: 10.3390/ijms140918284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang C., Xie N., Wang Y., Li Y., Ge X., Wang M.. Role of the Mitochondrial Calcium Uniporter in Rat Hippocampal Neuronal Death After Pilocarpine-Induced Status Epilepticus. Neurochem Res. 2015;40:1739–1746. doi: 10.1007/s11064-015-1657-3. [DOI] [PubMed] [Google Scholar]
- [26].Williams S., Hamil N., Abramov A.Y., Walker M.C., Kovac S.. Status epilepticus results in persistent overproduction of reactive oxygen species, inhibition of which is neuroprotective. Neuroscience. 2015;303:160–165. doi: 10.1016/j.neuroscience.2015.07.005. [DOI] [PubMed] [Google Scholar]
- [27].Liu J., Wang A., Li L., Huang Y., Xue P., Hao A.. Oxidative stress mediates hippocampal neuron death in rats after lithium-pilocarpine-induced status epilepticus. Seizure. 2010;19:165–172. doi: 10.1016/j.seizure.2010.01.010. [DOI] [PubMed] [Google Scholar]
- [28].Celi P., Gabai G.. Oxidant/Antioxidant Balance in Animal Nutrition and Health: The Role of Protein Oxidation. Front Vet Sci. 2015;2:48. doi: 10.3389/fvets.2015.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Si P.P., Zhen J.L., Cai Y.L., Wang W.J., Wang W.P.. Salidroside protects against kainic acid-induced status epilepticus via suppressing oxidative stress. Neurosci Lett. 2016;618:19–24. doi: 10.1016/j.neulet.2016.02.056. [DOI] [PubMed] [Google Scholar]
- [30].Hsieh P.F., Hou C.W., Yao P.W., Wu S.P., Peng Y.F., Shen M.L.. et al. Sesamin ameliorates oxidative stress and mortality in kainic acid-induced status epilepticus by inhibition of MAPK and COX-2 activation. J. Neuroinflammation. 2011;8:57. doi: 10.1186/1742-2094-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Banerjee S., Padhye S., Azmi A., Wang Z., Philip P.A., Kucuk O.. et al. Review on molecular and therapeutic potential of thymoquinone in cancer. Nutr Cancer. 2010;62:938–946. doi: 10.1080/01635581.2010.509832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Severina I.I., Severin F.F., Korshunova G.A., Sumbatyan N.V., Ilyasova T.M., Simonyan R.A.. et al. In search of novel highly active mitochondria-targeted antioxidants: thymoquinone and its cationic derivatives. FEBS Lett. 2013;587:2018–2024. doi: 10.1016/j.febslet.2013.04.043. [DOI] [PubMed] [Google Scholar]
- [33].Silachev D.N., Plotnikov E.Y., Zorova L.D., Pevzner I.B., Sumbatyan N.V., Korshunova G.A.. et al. Neuroprotective Effects of Mitochondria-Targeted Plastoquinone and Thymoquinone in a Rat Model of Brain Ischemia/Reperfusion Injury. Molecules. 2015;20:14487–14503. doi: 10.3390/molecules200814487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Espinosa-Diez C., Miguel V., Mennerich D., Kietzmann T., Sánchez-Pérez P., Cadenas S.. et al. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015;6:183–197. doi: 10.1016/j.redox.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Na H.K., Surh Y.J.. Oncogenic potential of Nrf2 and its principal target protein heme oxygenase-1. Free Radic Biol Med. 2014;67:353–365. doi: 10.1016/j.freeradbiomed.2013.10.819. [DOI] [PubMed] [Google Scholar]
- [36].Chapple S.J., Siow R.C., Mann G.E.. Crosstalk between Nrf2 and the proteasome: therapeutic potential of Nrf2 inducers in vascular disease and aging. Int J Biochem Cell Biol. 2012;44:1315–1320. doi: 10.1016/j.biocel.2012.04.021. [DOI] [PubMed] [Google Scholar]