Abstract
Purpose
In this longitudinal study we explored the relationships between plasma n-3 and n-6 polyunsaturated fatty acids (PUFAs) and Δ5 and Δ6 desaturase activities (D5D and D6D, respectively) and fasting lipids in youth with type 1 diabetes (T1D).
Methods
Incident cases of T1D in youth <20 years of age who were seen for a baseline study visit (N=914) and a 1-year follow-up visit (N=416) were included. Fasting blood samples were obtained at each visit and plasma phospholipid n-6 PUFAs were measured, which included linoleic acid (LA), dihomo-γ-linolenic acid (DGLA) and arachidonic acid (AA); n-3 PUFAs included α-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). Estimated D5D and D6D were calculated as FA product-to-precursor ratios, where D5D= AA/DGLA and D6D = DGLA/LA. To examine the longitudinal relationships between long chain PUFAs, desaturase activities and fasting plasma lipids in youth with T1D mixed effects models were used for each individual PUFAs, D5D and D6D, adjusted for demographics, clinic site, diabetes duration, insulin regimen, insulin dose/kg, HbA1c, insulin sensitivity score, and body mass index with random effects to account for the repeated measurements.
Findings
Favorable lipid associations were found between LA and low-density lipoprotein (LDL) cholesterol (β= −0.58, P<0.05); AA, plasma triglycerides (TG) (β= −0.04, P<0.05) and TG/ high-density lipoprotein (HDL)-C ratio (β= −0.04, P<0.05); and D5D, plasma TG (β= −0.2, P<0.05) and TG/HDL-cholesterol ratio (β= −0.23, P<0.05). Findings were mixed for the n-3 PUFAs and DGLA: ALA was positively associated with plasma TG (β= 0.33, P<0.05) and HDL cholesterol (β= 9.86, P<0.05); EPA was positively associated with total cholesterol (β= 8.17, P<0.05), LDL cholesterol (β=5.74, P<0.01) and HDL cholesterol (β= 2.27, P<0.01); and DGLA was positively associated with TG/HDL-cholesterol ratio (β= 0.05, P<0.05)
Conclusion
Findings suggest that the most abundant PUFA, LA as well as its metabolic bi-product AA, may be important targets for CVD lipid risk factor reduction in youth with T1D.
Keywords: polyunsaturated fatty acids, desaturase, lipids, cardiovascular disease, type 1 diabetes
1. Introduction
Dietary intake of n-3 and n-6 polyunsaturated fatty acids (PUFAs) may have beneficial effects on cardiovascular health, although some controversy exists in this regard. For example, observational and intervention studies have shown that long chain n-3 PUFAs tend to reduce serum triglycerides (TG), but may increase low density lipoprotein (LDL) cholesterol (C) (1, 2). Further, cross-sectional analysis and controlled trials show diets rich in n-6 PUFAs lower LDL-C, but may also lower high density lipoprotein (HDL)-C (3, 4). Increasing PUFA dietary intake has been associated with positive health outcomes in healthy individuals without diabetes (1, 2, 4). Current recommendations for individuals at high risk for cardiovascular disease (CVD), including those with diabetes, support increasing food sources of n-3 PUFAs including α-linolenic acid (ALA, 18:3n-3), eicosapentanoic acid (EPA, 20:5n-3), and docosahexanoic acid (DHA, 22:6 n-3) to favorably alter serum lipids and for the prevention of heart disease (5). However few studies exist showing health benefits in individuals with diabetes, particularly those with type 1 diabetes (T1D).
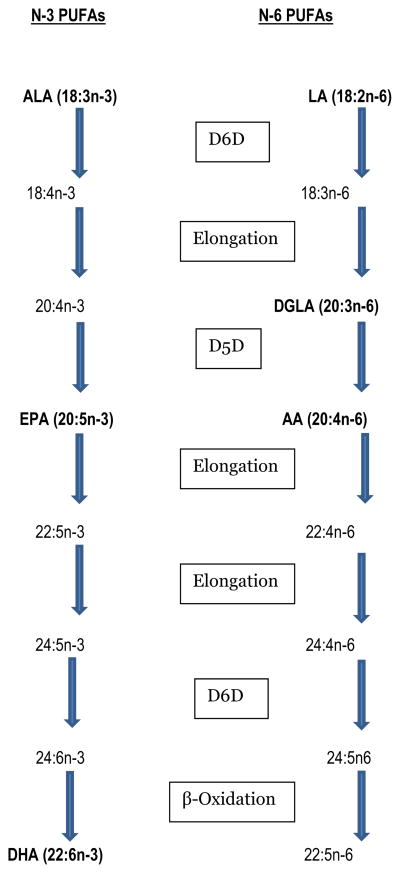
PUFAs modify plasma lipids through several intracellular mechanisms including modulation of cellular signaling pathways and transcription factors that control fatty acid synthesis, beta oxidation, and lipid transport and clearance (3, 6). Total plasma PUFAs can alter lipid metabolism, but individual circulating PUFAs exert differential effects such that the relative proportion of one PUFA compared to another, even within the same class (n-3 or n-6), may impact health and well-being (3, 4). As evidence, several clinical studies have shown that the n-6 PUFA di-homo- γ - linolenic acid (DGLA, 20:3n-6) but not the less saturated n-6 PUFA arachidonic acid (AA, 20:4n-6) was related to dyslipidemia and a high degree of insulin resistance in patients with type 2 diabetes (T2D) (3, 4). While dietary intake of n-3 and n-6 PUFA is an important determinate of the relative amount of these fatty acids in vivo, long chain PUFAs can be further metabolized endogenously (see Figure 1). Therefore, activities of enzymes that regulate fatty acid desaturation processes also play a critical role in the availability of long chain n-3 and n-6 PUFAs. Product-to-precursor ratios of certain PUFAs can be used to estimate enzyme activity of desaturases involved in PUFA metabolism (7), and applying these ratios may be of particular relevance to diabetes risk management, as insulin induces the genes encoding desaturase enzymes (7, 8). For example, acute hypoinsulinemia that often accompanies T1D has been shown to contribute to altered desaturase levels and lower endogenous formation of PUFAs (9). Whether these metabolic perturbations resulting in dysregulation of PUFA metabolism also have adverse effects on plasma lipids of individuals with T1D has not been investigated.
Figure 1.
The n-3 and n-6 polyunsaturated fatty acid (PUFA) metabolic pathways where LA = Linoleic Acid; DGLA = Dihomo-γ-linolenic acid; AA = Arachidonic acid; ALA =α-linolenic acid; EPA = Eicosapentaenoic acid; DHA = Docosahexaenoic acid; D5D = delta 5 desaturation; D6D = delta 6 desaturation; the highlighted PUFAs were those measured in plasma phospholipids in the present study.
Presently, longitudinal studies are lacking on relationships between long chain PUFAs, desaturase activities and fasting plasma lipids in youth with T1D. In the current study, we examined these associations in a large, multi-ethnic sample of youth with T1D. In these analyses, consideration was given to relevant confounders such as hemoglobin A1c (HbA1c), insulin treatment regimen, and body mass index (BMI) to provide a more precise estimate of the relationships between n-3 and n-6 PUFAs and each lipid parameter.
2. Methods
2.1. Participants
The SEARCH for Diabetes in Youth study is an ongoing multi-center, observational study of the epidemiology of childhood diabetes (10). Additional diet data were collected by the SEARCH Nutrition Ancillary Study (SNAS), funded in 2008 with data collection completed in 2011, designed to test hypotheses related to nutritional determinants of CVD risk in youth with T1D. Both SEARCH and SNAS are reviewed and approved annually by local institutional review boards that have jurisdiction over the local study populations. Parents of participants under age 18 years at the time of data collection provided written informed consent and age-eligible participants provided assent; all participants aged 18 years or older provided written informed consent.
The present study was a longitudinal analysis of data from the 2002–2005 incident cohort in the main SEARCH study. Data were available on 977 children 1–20 years of age with T1D who participated in the SEARCH baseline exam. Included participants had plasma PUFAs and fasting plasma lipid data at the initial visit. Participants excluded from analysis included: T1D duration <3 months at baseline (N=58); fasting lipids not available at baseline (N=1); hypertriglyceridemia at baseline (TG>400 mg/dl, N=1); and outlying plasma PUFAs values (> 3 IQR above the 75th percentile or 3 IQR <25th percentile, N=3). This yielded an N=914 at baseline; 419 of these had 12-month follow-up fatty acid and fasting lipid data available. Three follow-up samples were excluded due to hypertriglyceridemia yielding a final follow-up sample of N=416.
2.2. Data Collection
Participants were invited to attend an in-person baseline exam where demographic, anthropometric, laboratory and clinical data were collected. Those who completed the baseline exam where subsequently invited to participate in a follow-up visit 1-year later consisting of the same elements as the baseline exam. Blood samples were obtained under conditions of metabolic stability, defined as no episode of diabetic ketoacidosis during the previous month. Specimens were processed at the site and shipped within 24 hours to the Northwest Lipid Metabolism and Diabetes Research Laboratories in Seattle, Washington. All measures were conducted by trained, certified staff in accordance with standardized study protocols (available at www.searchfordiabetes.org). A more detailed description of the measures considered in the present study is described in the following sections.
2.3. Outcomes
Plasma lipid profiles
Measurement of plasma cholesterol (C), TG, and HDL- C were performed enzymatically on a Hitachi 917 autoanalyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN). LDL- C was calculated by the Friedewald equation (11). The ratio of TG to HDL-C was calculated as a predictor of small, dense LDL particle size (12).
2.4. Exposures
PUFAs: total lipids were extracted by the Bligh Dyer method and phospholipids (PLs) were separated from all other lipids by one dimensional thin layer chromatography. The PL extract was then saponified and trans-methylated using the method of Tacconi and Wurtman (13). Gas chromatography was performed on samples dissolved in undecane using conditions modified from Lemaitre et al (14). Data were analyzed with ChemStation Firmware A.01.09 (Agilent Technologies Inc., Palo Alto CA). The coefficients of variation in the quality control pooled samples were as follows: 2% for linoleic acid (LA, 18:2n-6), 5.7% for DGLA, 2.7% for AA, 9.6% for ALA, 9.9% for EPA and 11.5% for DHA. Details of the methods are published elsewhere (15). Individual plasma PLs are expressed as percentage (by weight) of total fatty acids detected. Desaturase activities: delta 5 desaturase (D5D) and delta 6 desaturase (D6D) activities were estimated from product-to-precursor ratios of individual fatty acids in plasma PL. In this study, D5D activity = AA /DGLA and D6D = DGLA /LA. Estimation of desaturase activity using this approach is well accepted in the literature (16).
2.5. Covariates
Demographics: were self-reported and included birth date, gender, race/ethnicity, highest level of parent education in the household, and diabetes related factors (duration of disease, insulin regimen type, units of insulin/kg, clinical site). BMI: weight and height were measured twice in a standardized manner and averaged. BMI was calculated as weight in kg divided by height in m2, and age and gender specific BMI z-scores were derived based on the Centers for Disease Control and Prevention national standards (17). HbA1c: was measured on whole blood samples with an automated ion-exchange high-performance liquid chromatography instrument (Tosoh Bioscience, Montgomeryville, PA). Insulin sensitivity score (ISS): a surrogate measure of insulin resistance was estimated using the following equation: loge ISS = 4.64725 – 0.02032 (waist circumference, cm)–0.09779 (HbA1c, %)–0.00235 (TG, mg/dl). A detailed description of the development and validation of this equation has been published elsewhere (18).
2.6. Statistical analysis
Statistical analyses were performed using SAS for Windows (version 9.2; SAS Institute, Cary NC). There were 914 individuals in the dataset and N=32 were missing ALA, N=8 were missing EPA and N=1 was missing DGLA. Missing fatty acid percentages may result from an undetected peak, which is associated with a small value. Ignoring these unobserved values from the dataset can cause bias. The literature suggests that when observations are undetectable (Environmental Protection Agency guideline suggests <15%) (19), it is appropriate to impute the non-detects with a small number; half the detection limit is a common choice. The detection limit for these data is not well-defined, as the units are “percent of total” and may vary across individuals. To circumvent this, we replaced the non-detectable values for ALA and DGLA with half the lowest observed value. As previously described, DGLA was used in the estimation of D5D and D6D, and the use of the imputed value yielded an outlier in both cases. Consequently, the missing desaturase value was not imputed. The final sample size was N=914 for each fatty acid and N=913 for each desaturase. At follow-up, there were 416 observations available for each fatty acid (i.e., neither the participant with the outlying D5D or the missing DGLA had a follow-up measure).
Demographic characteristics were described with means and standard deviations (SD) for continuous variables and frequencies for categorical variables. Log transformations were used for triglycerides, the proportions of EPA and DHA in plasma PL, and estimated D5D and D6D activities to improve normality as residuals. A mixed effects model was fit for the relationship between fatty acid or desaturase exposure and concurrent outcome. The mixed effects model allows for correlation between observations within a participant. Two models were fit: unadjusted and adjusted for demographics (age, gender, race, parent education, clinic site), diabetes-related factors (disease duration, insulin regimen type, insulin dose per kilogram, HbA1c and insulin sensitivity score), and BMI z-score. A third model was run on a subset of participants with available food frequency data (for children >10 years; N=491 at baseline and N=207 at 1-year). For the third model, the adjusted repeated measures model was re-fit for each PUFA and plasma lipid outcome to include saturated fat intake. All models included random effects to account for the repeated measurements. Statistical significance of each nutritional exposure/lipid relationship was established with p<0.05.
3. Results
Demographics and clinical characteristics of the population studied are shown in Table 1. Our sample consisted of 914 youth with T1D who were predominately Caucasians with a mean age of 11 years and who were, on average, 10.9 months post-diagnosis. Forty-five percent were managed with basal bolus regimens including insulin pump therapy or a combination of long + short/rapid acting insulin with injections 3 or more times per day. Average HbA1c values were 7.8% with 11.2% (N=100) having HbA1c in excess of 9.5%. Table 2 shows the relative proportion of individual PUFAs in plasma PL, D5D and D6D activities estimated by fatty acid product-to-precursor ratios, and lipid profiles of the sample.
Table 1.
Baseline demographics and clinical characteristics in youth with Type 1 Diabetes
| Characteristic/Measure | Total N | Mean ± SD or N (%) |
|---|---|---|
| Age (years) | 914 | 11.0 (4.0) |
| Race | 914 | |
| African American | 90 (9.9) | |
| Caucasian | 721 (78.9) | |
| Other | 103 (11.3) | |
| Gender | 914 | |
| Males | 478 (52.3) | |
| Diabetes Duration (months) | 914 | 10.9 (6.2) |
| HbA1c (%) | 912 | 7.8 (1.6) |
| Insulin dose (Units/kg) | 881 | 0.67 (0.36) |
| Insulin sensitivity | 886 | 2.3 (0.36) |
| Insulin regimen type | 906 | |
| Pump users | 96 (10.6) | |
| Long + short/rapid, 3 or more x/ day | 308 (33.9) | |
| Other regimens a | 502 (55.4) | |
| BMI z-score (kg/m2) | 892 | 0.5 (1.0) |
Other regimens include long acting + any other combination of insulins with injections 2 or more times per day (8%); any combination of insulins excluding long acting with injections 3 or more times per day (15%); and any insulin or insulin combinations excluding long acting with injections 1 or 2 times per day (32%).
Table 2.
Baseline lipid profiles and nutritional exposures in youth with Type 1 Diabetes
| Characteristic/Measure | Total N | Mean ± SD or N (%) |
|---|---|---|
| Nutritional Exposures | ||
| Plasma Phospholipid Fatty Acids (weight %) | ||
| Linoleic Acid (18:2n-6) | 914 | 25.5 (2.7) |
| Di-homo-gamma-linoleic acid (20:3n-6) | 914 | 3.1 (0.7) |
| Arachidonic Acid (20:4n-6) | 914 | 13.1 (1.9) |
| Alpha-linolenic Acid (18:3n-3) | 914 | 0.2 (0.1) |
| Eicosapentanoic Acid (20:5n-3) | 914 | 0.4 (0.3) |
| Docosahexaenoic acid (22:6n-3) | 914 | 2.4 (0.8) |
| Estimated Desaturase Activities | ||
| Delta-5 desaturase (20:4n-6/20:3n-6) | 913 | 4.5 (1.3) |
| Delta-6 desaturase (20:3n-6/18:2n-6) | 913 | 0.1 (0.03) |
| Lipid Profile (mg/dl) | 914 | |
| Triglycerides | 63.1 (34.7) | |
| Total cholesterol | 162.1 (31.1) | |
| LDL-cholesterol | 95.2 (25.5) | |
| HDL-cholesterol | 54.3 (12.5) | |
| Triglyceride/HDL-cholesterol ratio | 1.3 (0.9) | |
Results of mixed effects models to examine associations between PUFAs, desaturases and lipid outcomes are shown in Table 3 and Table 4. In unadjusted models (Table 3), favorable associations were found for the following n-6 PUFAs, desaturases and lipids: LA was positively related with HDL-C (p<0.01); AA was negatively associated with TG (p<0.05), total C (p<0.01) and TG/HDL-C ratio (p<0.05); DGLA and D6D were negatively associated with total C (p<0.01 for both); and D5D was negatively related with TG (p<0.05) and TG/HDL-C ratio (p<0.05) and positively associated with HDL-C (p<0.01). Of the n-6 PUFAs, only DGLA showed an adverse lipid association with HDL-C (p<0.05) and TG/HDL-C (p<0.05). For the n-3 PUFAs, adverse lipid associations were found for ALA and EPA. Specifically, ALA was positively associated with TG (p<0.05), total C (p<0.01) and TG/HDL-C ratio (p<0.05) and EPA was positively associated with total C (p<0.01) and LDL–C (p<0.01).
Table 3.
Repeated measures association of plasma PL (weight %) with TG, total C, LDL-C, HDL-C, and TG/HDL-C ratio in youth with T1D, unadjusted
| Plasma PL n-6 PUFA | Plasma PL n-3 PUFA | Estimated Desaturase Activity | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| LA | DGLA | AA | ALA | EPA | DHA | D5D | D6D | |
| β (SE) for outcomes | ||||||||
| Log-TG | 0.001 (0.005) | 0.01 (0.02) | −0.06 (0.01)* | 0.85 (0.18)* | 0.018 (0.026) | −0.06 (0.04) | −0.25 (0.05)* | −0.01 (0.04) |
| Total C | 0.11 (0.3) | −3.42 (1.21)** | −1.63 (0.44)** | 34.97 (12.17)** | 5.2 (1.77)** | −0.43 (2.61) | 2.25 (3.09) | −7.82 (2.91)** |
| HDL-C | 0.33 (0.12)** | −2.18 (0.5)* | 0.01 (0.18) | 8.25 (5.02) | 0.99 (0.73) | −1.13 (1.08) | 4.64 (1.27)** | −4.9 (1.19)* |
| LDL-C | −0.29 (0.25) | −1.34 (1.01) | −0.66 (0.36) | 10.84 (10.1) | 3.94 (1.47)** | 2.09 (2.16) | 1.36 (2.56) | −2.27 (2.41) |
| TG/HDL-C | −0.005 (0.006) | 0.05 (0.02)* | −0.06 (0.01)* | 0.68 (0.23)** | −0.001 (0.033) | −0.05 (0.05) | −0.33 (0.06)* | 0.08 (0.05) |
Abbreviations: LA = linoleic Acid (18:2n-6);DGLA = Di-homo-gamma-linoleic acid (20:3n-6); AA = Arachidonic Acid (20:4n-6); ALA - Alpha-linolenic Acid (18:3n-3); EPA = Eicosapentanoic Acid (20:5n-3); DHA = Docosahexaenoic acid (22:6n-3); D5D =Delta-5 desaturase calculated as AA/DGLA; D6D = Delta-6 desaturase calculated as DGLA/LA; HDL = high density lipoprotein; LDL = low density lipoprotein; C = cholesterol; TG = triglyceride; PL = phospholipid; T1D = type 1 diabetes; PUFA = polyunsaturated fatty acid; SE = standard error;
p<0.05;
p<0.01
Table 4.
Repeated measures association of plasma PL (weight %) with TG, Total C, LDL-C, HDL-C, and TG/HDL ratio in youth with T1D, adjusted for potential confounders a
| Plasma PL n-6 PUFA | Plasma PL n-3 PUFA | Estimated Desaturase Activity | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| LA | DGLA | AA | ALA | EPA | DHA | D5D | D6D | |
| β (SE) for outcomes | ||||||||
| Log-TG | −0.003 (0.004) | 0.03 (0.01) | −0.04 (0.01)* | 0.33 (0.14)* | 0.007 (0.02) | −0.04 (0.03) | −0.2 (0.04)* | 0.06 (0.04) |
| Total C | −0.36 (0.31) | −0.12 (1.31) | −0.68 (0.46) | 19.23 (12.13) | 8.17 (1.73)* | 1.68 (2.63) | −2.6 (3.23) | 1.61 (3.2) |
| HDL-C | 0.21 (0.13) | −0.98 (0.52) | 0.04 (0.18) | 9.86 (4.85)* | 2.27 (0.7)** | −2 (1.05) | 1.72 (1.29) | −2.36 (1.28) |
| LDL-C | −0.58 (0.27)* | 0.49 (1.12) | −0.11 (0.39) | 1.34 (10.37) | 5.74 (1.48)** | 4.58 (2.25)* | −1.31 (2.75) | 3.52 (2.73) |
| TG/HDL-C | −0.007 (0.005) | 0.05 (0.02)* | −0.04 (0.01)* | 0.14 (0.18) | −0.03 (0.03) | −0.01 (0.04) | −0.23 (0.05)* | 0.1 (0.05)* |
Abbreviations: LA = linoleic Acid (18:2n-6);DGLA = Di-homo-gamma-linoleic acid (20:3n-6); AA = Arachidonic Acid (20:4n-6); ALA - Alpha-linolenic Acid (18:3n-3); EPA = Eicosapentanoic Acid (20:5n-3); DHA = Docosahexaenoic acid (22:6n-3); D5D =Delta-5 desaturase calculated as AA/DGLA; D6D = Delta-6 desaturase calculated as DGLA/LA; HDL = high density lipoprotein; LDL = low density lipoprotein; C = cholesterol; TG = triglyceride; PL = phospholipid; T1D = type 1 diabetes; PUFA = polyunsaturated fatty acid; SE = standard error;
Repeated measures mixed models adjusted for demographics (age, parental education, race, sex, clinic), diabetes-related factors (duration, insulin regimen, dose per kg, HbA1c, and insulin sensitivity score) and BMI z-scores;
p<0.05
p<0.01
In adjusted models (Table 4), the overall finding of favorable lipid associations with LA, AA and D5D remained; mixed effects for the n-3 PUFAs, DGLA and D6D were identified. Specifically, LA was negatively associated with LDL-C (p<0.05); AA was negatively associated with TG (p<0.05) and TG/HDL-C ratio (p<0.05); and D5D was inversely associated with TG (p<0.05) and TG/HDL-C ratio (p<0.05). For the n-3 PUFAs, ALA was positively associated with TG (p<0.05) and HDL-C (p<0.05); EPA was positively associated with total C (p<0.05), LDL–C (p<0.01), and HDL-C (p<0.05); and DHA was positively associated with LDL-C (p<0.05). For DGLA and D6D, positive associations with TG/HDL-C ratio (p<0.05) were identified.
Given the unexpected adverse relationships between the n-3 PUFAs and plasma lipids and to determine whether saturated fat intake could be a potential mediator for these observed associations, adjusted models were re-fit to include dietary saturated fat intake (grams/day) in the subset of the sample with food frequency data available. The adjustment for saturated fat did not change any of the findings of significance as reported in Table 4 (data not shown). For all models except AA and total C, the beta coefficients changed less than 0.5% and the p-values for these relationships remained unchanged to 3 decimal places. For AA and total C, the beta coefficient changed 13%, however the effect was not significant.
4. Discussion
There is ongoing debate regarding the cardiovascular effects of n-3 and n-6 PUFAs and key enzymes involved in their metabolism. The debate stems from controversial evidence regarding whether too much of these fatty acids and the relative dietary proportion of each may have beneficial or adverse effects on the cardiovascular system (1–4). This debate is clinically relevant to youth with T1D who are prone to CVD and its complications (20). A major finding from the present analyses was that the n-6 PUFAs LA and AA were favorably associated with plasma lipids after adjustment for important demographic and clinical characteristics, whereas findings were mixed relative to DGLA and the n-3 PUFAs. Desaturase activities were related to plasma lipids, but not in a similar manner, suggesting different functional roles for D5D and D6D relative to lipid metabolism. These results suggest that desaturase activities and plasma PL PUFA profile of youth with T1D may be important targets for CVD lipid risk factor reduction in this at-risk population.
In this study, a higher proportion of the n-6 PUFAs LA and AA were related to lower LDL-C and TG concentrations, respectively, suggesting a CVD protective effect. AA was also inversely related to TG/HDL-C ratio, a predictor of atherogenic small, dense LDL-C (12). In contrast, the n-6 PUFA DGLA, which is the precursor to AA (see Figure 1), was directly related to the TG/HDL-C ratio. This finding suggests that regulation of D5D activity, which catalyzes the desaturation of DGLA to AA, may be an important determinant of the TG/HDL-C ratio and LDL particle size in T1D. In the literature, some (21, 22) but not all studies (4, 23) have shown n-6 PUFAs are related to beneficial effects on plasma lipids. Recent data from the HEPFAT trial (24) showed that high intake of n-6 PUFAs were related to a decrease in cholesterol biosynthesis and low hepatic fat content and exportation, which may explain our findings of favorable n-6 PUFA relationships with plasma lipids. From our results, the calculated LDL-cholesterol lowering effect from LA was small (1.6 mg/dl change in LDL-cholesterol per unit change in LA). Whether this effect is clinically meaningful remains to be determined. Dietary LA is the most abundant of the n-6 PUFA (25), derived from vegetable oils. In randomized controlled trials, safflower and corn oil, which are richest in LA, were shown to have the most potent cholesterol lowering effects of the vegetable oils (22). Preformed AA is limited and derived from animal products such as meats, poultry and eggs. LA can be endogenously converted to AA after successive desaturation and elongation reactions, but this conversion is limited in humans (Figure 1) (26). Therefore, determining alternative methods to increase plasma PL AA may be important for CV risk reduction, although some caution is needed as AA can also be endogenously converted to bioactive eicosanoids, which produce chemical mediators that have diverse inflammatory and immune modulating effects (4, 27).
The unfavorable positive association between the n-3 PUFA ALA and blood TG in our study was unexpected, but very little research has been done with ALA specifically (28). The literature supports a TG lowering effect of the longer chain n-3 PUFAs EPA and DHA (1, 2); however the TG lowering effects usually occur with supplemental doses of EPA and DHA in the range of 2–4 g/day (29, 30) and ALA is not typically evaluated. No prior information exists between ALA and plasma lipids in youth with T1D, although several researchers have shown higher levels of plasma PL ALA and lower EPA and DHA in T1D adults compared to healthy controls suggesting altered synthesis of LC n-3 PUFAs from the precursor ALA (31, 32). Therefore, imbalances in EPA/DHA to ALA ratios in this sample due to disease related factors may provide some explanation for the unexpected findings. Alternatively, ALA may have its own specific physiological effects relative to lipid metabolism that are independent of being a precursor for EPA (33, 34). Others have shown that in contrast to EPA and DHA, fasting VLDL concentrations were not reduced with ALA feeding (34). Decreased hepatic synthesis of VLDL is the primary mechanism whereby EPA and DHA exert TG lowering effects (6). A related increase in the activity of lecithin-cholesterol acyl-transfer protein by ALA, as seen by others (35), could explain the favorable positive association between ALA and HDL-cholesterol in the present study. This enzyme promotes the esterification and internalization of cholesterol into HDL, thereby increasing HDL-C levels.
Our findings that PL EPA and DHA were unfavorably related to LDL-C and that PL EPA was favorably related to HDL-C and unrelated to TG is in line with several recent reviews of the controversial effects of EPA and DHA on plasma lipids in healthy individuals (36, 37). LC n-3 PUFAs are thought to modify plasma lipids in a number of ways, including decreasing hepatic VLDL TG synthesis through modulation of transcription factors (e.g., SREBP-1c and PPARα) involved in lipogenic and beta-oxidation pathways (38). N-3 PUFAs also reportedly increase lipoprotein lipase activity, promoting increased clearance of TG and conversion of VLDL to LDL (39). This is thought to be the likely mechanism for the LDL-raising effect of LC n-3 PUFAs (37). Additionally, cholesterol ester transfer protein has been shown to be increased by EPA and DHA leading to enhanced lipid exchange between LDL and HDL particles (39). In most studies, alterations in the function of these enzymes and transcription factors are achieved when supplemental doses of EPA and DHA are provided (3). Although supplementation does not explain the findings of the present investigation, it is possible that those with relatively high EPA in the sample also had a higher proportion of saturated fat in the diet. A positive relationship between saturated fat, LDL-C and HDL-C is well established (40). To determine whether the relationship between EPA and LDL- and HDL-C observed in the present study could be mediated by dietary saturated fat intake, we re-fit our adjusted repeated measures models for EPA and plasma lipids to include saturated fat intake from a subset of participants with available food frequency data (n=497). Findings from this sub-analysis do not support saturated fat as a likely explanation of our results.
Similar to previous studies in healthy adolescents (20), our results show a favorable association between estimated D5D and plasma lipids, whereas adverse associations were found for D6D. D5D and D6D activities may have divergent effects on the FA composition of cell membranes, which affect many aspects of glucose control including insulin receptor binding, glucose transport activity, and intracellular signaling (9). The activities of D5D and D6D appear to be of high relevance for the development of T2D, as higher D5D activity has been associated with decreased disease risk, while D6D activity has been directly linked to increased risk (41, 42). To our knowledge, this is the first study to examine estimated desaturase activities and CVD lipid risk factors in T1D. Given the favorable association between D5D and plasma lipids in our sample, further studies are required to better understand how the desaturase enzymes relate to cardiovascular health in T1D.
The strengths of the study include the large, diverse sample of adolescents with T1D studied. Additionally, confounding was assessed in our statistical models by including variables known to influence fatty acid metabolism-CVD lipid risk factor associations in youth with diabetes; however the possibility for residual confounding remains. The repeated measures analysis used in this investigation provides increased power over existing cross-sectional analyses in examining associations between fatty acid exposures and plasma lipid risk factors. Intra-individual variation as well as inter-individual variation was taken into account in mixed models, thus providing increased validity for the associations identified. It should be noted, however, that the repeated measures analysis is limited in deducing causality. Another limitation was the fact that desaturase activities were not directly measured but rather estimated using product-to-precursor ratios. Although desaturase activity can be measured directly in animals by measurement of the rate of conversion of radiolabeled precursor fatty acids to their respective products (7), for ethical and practical reasons it cannot be measured in humans. Finally, imputation was used to replace undetectable plasma PL PUFAs to avoid bias in the results; best practices were used in this regard.
5. Conclusions
The present study provides novel findings regarding relationships between n-3 and n-6 PUFAs, desaturases, and plasma lipids/lipoproteins in T1D. Our findings of potential beneficial effects of LA, AA and D5D and mixed effects of the n-3 PUFAs, DGLA and D6D on plasma lipids suggest interplay between the types of dietary fat consumed, endogenous fatty acid synthesis, and diabetes that is important in the development and management of the CVD risk profile. The practical interpretation of these data is that vegetable oils rich in the most abundant n-6 PUFAs, such as LA rich-corn oil and safflower oil, may provide some benefit relative to lipid profile in T1D. Future studies are needed to clarify whether the observed associations are related to long-term health outcomes in youth with T1D.
Acknowledgments
The SEARCH for Diabetes in Youth Study is indebted to the many youth and their families, and their health care providers, whose participation made this study possible.
Grant Support: SEARCH for Diabetes in Youth is funded by the Centers for Disease Control and Prevention (PA numbers 00097, DP-05-069, and DP-10-001) and supported by the National Institute of Diabetes and Digestive and Kidney Diseases.
Site Contract Numbers: Kaiser Permanente Southern California (U48/CCU919219, U01 DP000246, and U18DP002714), University of Colorado Denver (U48/CCU819241-3, U01 DP000247, and U18DP000247-06A1), Children’s Hospital Medical Center (Cincinnati) (U48/CCU519239, U01 DP000248, and 1U18DP002709), University of North Carolina at Chapel Hill (U48/CCU419249, U01 DP000254, and U18DP002708), University of Washington School of Medicine (U58/CCU019235-4, U01 DP000244, and U18DP002710-01), and Wake Forest University School of Medicine (U48/CCU919219, U01 DP000250, and 200-2010-35171).
The authors wish to acknowledge the involvement of General Clinical Research Centers (GCRC) at the South Carolina Clinical & Translational Research (SCTR) Institute, at the Medical University of South Carolina (NIH/NCRR Grant number UL1RR029882); Seattle Children’s Hospital (NIH CTSA Grant UL1 TR00423 of the University of Washington); University of Colorado Pediatric Clinical and Translational Research Center (CTRC) (Grant Number UL1 TR000154) and the Barbara Davis Center at the University of Colorado at Denver (DERC NIH P30 DK57516); and the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 8 UL1 TR000077; and the Children with Medical Handicaps program managed by the Ohio Department of Health.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases.
List of Abbreviations
- Apo B
apolipoprotein B
- AA (C20:4n-6)
arachidonic acid
- ALA (C18:3n-3)
alpha linolenic acid
- BMI
body mass index
- C
Cholesterol
- CVD
cardiovascular disease
- D5D
Δ5 desaturase
- D6D
Δ6 desaturase
- DGLA (C20:3n-6)
dihomo-γ-linolenic acid
- DHA (C22:6n-3)
docosahexaenoic acid
- EPA (C20:5n-3)
eicosapentaenoic acid
- HDL
high density lipoprotein
- LA (C18:2n-6)
linoleic acid
- LC
long chain
- LDL
low density lipoprotein
- PL
phospholipid
- PUFA
polyunsaturated fatty acid
- SNAS
SEARCH Nutrition Ancillary Study
- T1D
Type 1 diabetes
- T2D
Type 2 diabetes
- TG
Triglyceride
- VLDL
Very Low Density Lipoprotein
Footnotes
Conflict of Interests: There are no conflicts of interests to report.
Author’s Contributions: SC contributed to conception and design, interpretation of data, drafted the manuscript, and agreed to be accountable for all aspects of the work; JC contributed to the analysis and interpretation of data; IK, AP, AS, JT, TS contributed to the interpretation of data and in revising the manuscript critically for intellectual content; LD contributed to acquisition and interpretation of data and in revising the manuscript critically for intellectual content; EM-D contributed to conception and design, interpretation of data, revising the manuscript critically for intellectual content, and gave final approval for the work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sarah C. Couch, 3202 Eden Avenue, French Building East, Room 364, University of Cincinnati Medical Center, Cincinnati, OH 45267-0394.
Jamie Crandell, Carrington Hall #7460, School of Nursing and Department of Biostatistics, UNC Chapel Hill, NC 27599.
Irena King, MSC 10 5550, University of New Mexico, Albuquerque, NM 87131.
Abigail Peairs, 3202 Eden Avenue, French Building East, Room 364, University of Cincinnati Medical Center, Cincinnati, OH 45267-0394.
Amy S Shah, 3333 Burnett Avenue, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH 4522.
Lawrence M Dolan, 3333 Burnett Avenue, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH 45229.
Janet Tooze, 1 Medical Center Blvd, Wake Forest School of Medicine, Winston-Salem, NC 27157.
Tessa Crume, 13001 E. 17th Place, University of Colorado, Aurora, CO 80045.
Elizabeth Mayer-Davis, 1700 Martin Luther King Drive, Departments of Nutrition and Medicine, UNC, Chapel Hill, NC 27599.
References
- 1.Riediger ND, Othman RA, Suh M, Moghadasian MH. A systematic review of the roles of n-3 fatty acids in health and disease. J Am Diet Assoc. 2009;109:668–679. doi: 10.1016/j.jada.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 2.Cottin SC, Sanders TA, Hall WL. The differential effects of EPA and DHA on cardiovascular risk factors. Proc Nutr Soc. 2011;70:215–231. doi: 10.1017/S0029665111000061. [DOI] [PubMed] [Google Scholar]
- 3.Baum SJ, Kris-Etherton PM, Willett WC, Lichtenstein AH, Rudel LL, Maki KC, Whelan J, Ramsden CE, Block RC. Fatty acids in cardiovascular health and disease: A comprehensive update. J Clin Lipid. 2012;6:216–234. doi: 10.1016/j.jacl.2012.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czernichow S, Thomas D, Bruckert N-6 fatty acids and cardiovascular health: a review of the evidence for dietary intake recommendations. Br J Nutr. 2010;104:788–796. doi: 10.1017/S0007114510002096. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association. Standards of medical care in diabetes–2014. V. Part E. Medical Nutrition Therapy. Diabetes Care. 2014;37(Suppl 1):S28–S30. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 6.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 7.Kroger J, Schulze MB. Recent insights into the relation of 5 desaturase and 6 desaturase activity to the development of type 2 diabetes. Curr Opin Lipidol. 2012;23:4–10. doi: 10.1097/MOL.0b013e32834d2dc5. [DOI] [PubMed] [Google Scholar]
- 8.Vessby B, Gustafsson IB, Tengblad S, Boberg M, Andersson A. Desaturation and elongation of fatty acids and insulin action. Ann NY Acad Sci. 2002;967:183–195. doi: 10.1111/j.1749-6632.2002.tb04275.x. [DOI] [PubMed] [Google Scholar]
- 9.Szabo E, Marosvolgyi T, Kozari A, Erhardt E, Soltesz G, Decsi T. Long chain polyunsaturated fatty acids in a diabetic teenager during and after nine repeated episodes of diabetic ketoacidosis. Pediatr Diabetes. 2009;10(3):209–12. doi: 10.1111/j.1399-5448.2008.00487.x. [DOI] [PubMed] [Google Scholar]
- 10.SEARCH Study Group. SEARCH for Diabetes in Youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials. 2004;25:458–471. doi: 10.1016/j.cct.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultra-centrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 12.Tsimihodimos V, Gazi I, Kostara C, Tselepis AD, Elisaf M. Plasma lipoproteins and triacylglycerol are predictors of small, sense LDL particles. Lipid. 2007;42:403–409. doi: 10.1007/s11745-007-3050-8. [DOI] [PubMed] [Google Scholar]
- 13.Tacconi M, Wurtman RJ. Rat brain phosphatype 1 diabetesyl-N-N-dimethylethanolamine is rich in polyunsaturated fatty acids. Am J Clin Nutr. 2006;83:227–236. [Google Scholar]
- 14.Lemaitre RN, King IB, Patterson RE, Psaty BM, Kestin M, Heckbert SR. Assessment of trans-fatty acid intake with a food frequency questionnaire and validation with adipose tissue levels of trans-fatty acids. Am J Epidemiol. 1998;148:1085–1093. doi: 10.1093/oxfordjournals.aje.a009586. [DOI] [PubMed] [Google Scholar]
- 15.King IB, Lemaitre RN, Kestin M. Effects of a low-fat diet on fatty acid composition in red cells, plasma phospholipids and cholesterol esters: investigation of a biomarker of total fat intake. Am J Clin Nutr. 2006;83:227–236. doi: 10.1093/ajcn/83.2.227. [DOI] [PubMed] [Google Scholar]
- 16.Warensjo E, Sundstrom J, Vessby B, Cedarholm T, Riserus U. Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: A population-based prospective study. Am J Clin Nutr. 2008;88:203–209. doi: 10.1093/ajcn/88.1.203. [DOI] [PubMed] [Google Scholar]
- 17.Kuczmarski RJ, Ogden Cl, Guo SS, Grummer-Strawn LM, Fiegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CI. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat. 2002;11:10190. [PubMed] [Google Scholar]
- 18.Dabelea D, D’Agostino RB, Jr, Mason CC, West N, Hamman RF, Mayer-Davis EJ. Development, validation and use of an insulin sensitivity score in youths with diabetes: the SEARCH for Diabetes in Youth study. Diabetologia. 2011;54:78–86. doi: 10.1007/s00125-010-1911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.US Environmental Protection Agency. US EPA. Office of Pesticide Programs; 2000. Assigning values to non-detectable/non-quantifiable pesticide residues in human health food exposure assessment. [Google Scholar]
- 20.Steffen LM, Vessby B, Jacobs DR, Steinberger J, Moran A, Hong C-P, Sinaiko AR. Serum phospholipid and cholesterol ester fatty acids and estimated desaturase activities are related to overweight and cardiovascular risk in adolescents. Int J Obes (Lond) 2008;32:1297–1304. doi: 10.1038/ijo.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- 22.Kris-Etherton P, Fleming J, Harris WS. The debate about n-6 polyunsaturated fatty acid recommendations for cardiovascular health. J Am Diet Assoc. 2010;110:201–205. doi: 10.1016/j.jada.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Ramsden CE, Hibbeln JR, Majchrzak SF, Davis JM. N-6 fatty acid-specific and mixed polyunsaturated dietary interventions have different effects on CHD risk: a meta-analysis of randomized controlled trials. Br J Nutr. 2010;104:1586–1600. doi: 10.1017/S0007114510004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bjermo H, Iggman D, Kullberg J, Dahlman I, Johansson L, Persson L, Berglund J, Pulkki K, Basu S, Uusitupa M. Effects of n–6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: a randomized controlled trial. Am J Clin Nutr. 2012;95:1003–1012. doi: 10.3945/ajcn.111.030114. [DOI] [PubMed] [Google Scholar]
- 25.Patterson E, Wall R, Fitzgerald E, Ross RP, Stanton C. Health implications of high dietary omega-6 polyunsaturated fatty acids. J Nutr Metab. 2012;2012:1–16. doi: 10.1155/2012/539426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emken EA, Adlof RO, Gulley RM. Dietary linoleic acid influences destaturation and acylation of deuterium-labeled linoleic and alpha linolenic acids in young adult males. Biochim Biophys Acta. 1994;1213:277–288. doi: 10.1016/0005-2760(94)00054-9. [DOI] [PubMed] [Google Scholar]
- 27.Calder PC. Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie. 2009;91:791–795. doi: 10.1016/j.biochi.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Pan A, Chen M, Chowdhury R, Wu J, Sun Q, Campos H, Mozaffarian D, Hu F. Alpha-linolenic acid and risk of cardiovascular disease: a systematic review and meta-analysis. Am J Clin Nutr. 2012;96:1262–1273. doi: 10.3945/ajcn.112.044040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arterburn IM, Hall EB, Oken H. Distribution, inter-conversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83:1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 30.Desci T, Kennedy K. Sex-specific differences in essential fatty acid metabolism. Am J Clin Nutr. 2011;94:1914S–9S. doi: 10.3945/ajcn.110.000893. [DOI] [PubMed] [Google Scholar]
- 31.Decsi T, Szabo E, Burus I, Marosvolgyi T, Kozari A, Erhardt E, Soltesz G. Low contribution of n-3 polyunsaturated fatty acids in plasma and erythrocyte membrane lipids in diabetic young adults. Prostaglandins, Leukotrienes EFA. 2007;76:159–164. doi: 10.1016/j.plefa.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Tilvis RS, Miettinen TA. Fatty acid compositions of serum lipids, erythrocytes and platelets in insulin-dependent diabetic women. J Clin Endocrinol Metab. 1985;61:741–745. doi: 10.1210/jcem-61-4-741. [DOI] [PubMed] [Google Scholar]
- 33.Harris WS. Cardiovascular risk and alpha-linoleic acid. Circulation. 2008;118:323–324. doi: 10.1161/CIRCULATIONAHA.108.791467. [DOI] [PubMed] [Google Scholar]
- 34.Egert S, Kannenberg F, Somoza V, Erbersdobler HF, Wahrburg U. Dietary alpha-linolenic acid, EPA and DHA have differential effects on LDL fatty acid composition but similar effects on serum lipid profiles in normolipidemic humans. J Nutr. 2009;139:861–868. doi: 10.3945/jn.108.103861. [DOI] [PubMed] [Google Scholar]
- 35.Vaysse-Boue C, Dabadie H, Peuchant E, Le Ruyet P, Mendy F, Gin F, Combe N. Moderate dietary intake of myristic and alpha-linolenic acid increases lecithin-cholesterol acyltransferase activity in humans. Lipids. 2007;42:717–722. doi: 10.1007/s11745-007-3074-0. [DOI] [PubMed] [Google Scholar]
- 36.Superko HR, Superko SM, Nasir K, Agatston A, Garrett BC. Omega-3 fatty acid blood levels: clinical significance and controversy. Circulation. 2013;128:2154–2161. doi: 10.1161/CIRCULATIONAHA.113.002731. [DOI] [PubMed] [Google Scholar]
- 37.Wei M, Jacobson TA. Effects of eicosapentaenoic acid versus docosahexanoic acid on serum lipids: A systematic review and meta-analysis. Curr Atheroscler Rep. 2011;13:474–483. doi: 10.1007/s11883-011-0210-3. [DOI] [PubMed] [Google Scholar]
- 38.Jump DB. Fatty acid regulation of hepatic lipid metabolism. Curr Opinion Clin Nutr Metab. 2011;14:115–120. doi: 10.1097/MCO.0b013e328342991c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krauss RM. Lipoprotein sub-fractions and cardiovascular disease risk. Curr Opin Lipidol. 2010;21:30–40. doi: 10.1097/MOL.0b013e32833b7756. [DOI] [PubMed] [Google Scholar]
- 40.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fat, carbohydrate, and cardiovascular disease. Am J Clin Nutr. 2010;91:502–509. doi: 10.3945/ajcn.2008.26285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hodge AM, English DR, O’Dea K, Sinclair AJ, Makrides M, Gibson RA, Giles GG. Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: interpreting the role of linoleic acid. Am J Clin Nutr. 2007;86:189–197. doi: 10.1093/ajcn/86.1.189. [DOI] [PubMed] [Google Scholar]
- 42.Krachler B, Norberg M, Erikson JW, Hallmans G, Johansson I, Vessby B, Weinehall L, Lindahl B. Fatty acid profile of the erythrocyte membrane preceding development of type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis. 2008;18:503–510. doi: 10.1016/j.numecd.2007.04.005. [DOI] [PubMed] [Google Scholar]