Abstract
Purpose
Cooperative studies support complete metastasectomy in osteosarcoma (OS). Pre-operative CT is used to identify and quantify metastases and can facilitate minimally invasive techniques. Here we assess the accuracy of pre-operative CT compared to findings at thoracotomy and its change over time.
Methods
We reviewed OS thoracotomies performed at our institution from 1996-2015. The number of metastases identified on pre-operative chest CT was compared to the number of metastases seen on pathology (both metastases with viable cells and non-viable, osteoid-only metastases).
Results
Eighty-eight patients underwent 161 thoracotomies with a median of 14 days (range, 1-85) between CT and surgery, a median of 2 CT-identified lesions (range, 0-15), and a median of 4 resected lesions (range, 1-25). In 56 (34.8%) cases, more metastases were found surgically than were seen on CT, and among these, 34 (21.1%) had a greater number of viable metastases. There was poor overall correlation between CT and pathology findings (Kendall Tau-b = 0.506), regardless of CT slice thickness, decade of thoracotomy, or total number of CT-identified lesions.
Conclusions
CT accuracy in pre-operatively quantifying OS pulmonary metastases has not improved in recent decades. Consequently, we recommend an open technique with direct lung palpation for complete identification and resection of OS pulmonary metastases.
Keywords: Osteosarcoma, pulmonary metastasis, thoracotomy, pediatric surgery, metastasectomy
Introduction
In recent cooperative group trials, overall survival in osteosarcoma (OS) has been approximately 75%[1]. Unfortunately, survival is drastically lower in patients with relapsed OS [2, 3], 75% of whom relapse with metastases in the lungs [4, 5]. Over the last four decades, significant progress has been made in the treatment of pulmonary metastases in OS, bringing the overall survival from 0% in 1970 [6] to 17-34% in the most recent published series [7-11]. Prognosis in patients with pulmonary metastases has repeatedly been shown to depend on the extent of treatment-induced necrosis in the lesions, disease-free interval before relapse, the total number of metastases, and unilateral versus bilateral involvement. The single most important prognostic factor, however, is the ability to achieve complete resection of the metastases, with little or no benefit derived from systemic therapy [2, 7-20].
Concomitant with the ongoing advances in treatment of pulmonary OS metastases, rapid progress has been made in minimally invasive surgery. Given its benefits with respect to post-operative pain and length of stay, its application to childhood cancer has expanded [21]. Use of minimally invasive techniques in OS metastasectomy, however, has been limited by difficulty visualizing and/or locating the metastases without palpation. Over the last two decades, multiple techniques have been developed to circumvent this problem in metastatic childhood cancers, including OS. Intraoperative thoracoscopic ultrasound, pre-operative tattooing of lesions, and pre-operative wire localization have all been shown to successfully localize pulmonary lesions identified on pre-operative CT and allow resection in the majority of patients [22-25]. Unfortunately, minimally invasive resection of pulmonary metastases is still inherently limited to the lesions identified on pre-operative CT scan, despite all of the aforementioned technologies.
We previously published a series showing that pre-operative CT scanning underestimated the total number of histologically proven OS metastases in 35% of the thoracotomies performed [26]. In this study we have significantly expanded our series in order to further analyze the overall accuracy of pre-operative CT and the change in that accuracy over the last two decades, given the significant advances in CT imaging. This retrospective study uses data from thoracotomies performed by the Pediatric Surgical Service at Memorial Sloan Kettering Cancer Center over the last 20 years.
1. Patients and Methods
1.1 Inclusion Criteria
With institutional review board approval, we retrospectively identified and analyzed records of all thoracotomies performed for metastatic OS by the Pediatric Surgical Service at Memorial Sloan Kettering Cancer Center from May 1996 to 2015 (n = 176). As shown in Figure 1, patients who underwent palliative thoracotomies and those with hilar lymph node, mediastinal, parietal pleura, or chest wall involvement were excluded (n = 8). Thoracotomies with miliary disease (>25 lesions) on pre-operative CT or at operative resection were excluded because of the difficulties posed by confluence of lesions (n = 4). Patients lacking complete pre-operative CT imaging of the lungs were also excluded (n = 3). A total of 161 thoracotomies in 88 patients were included in the analysis.
Figure 1.
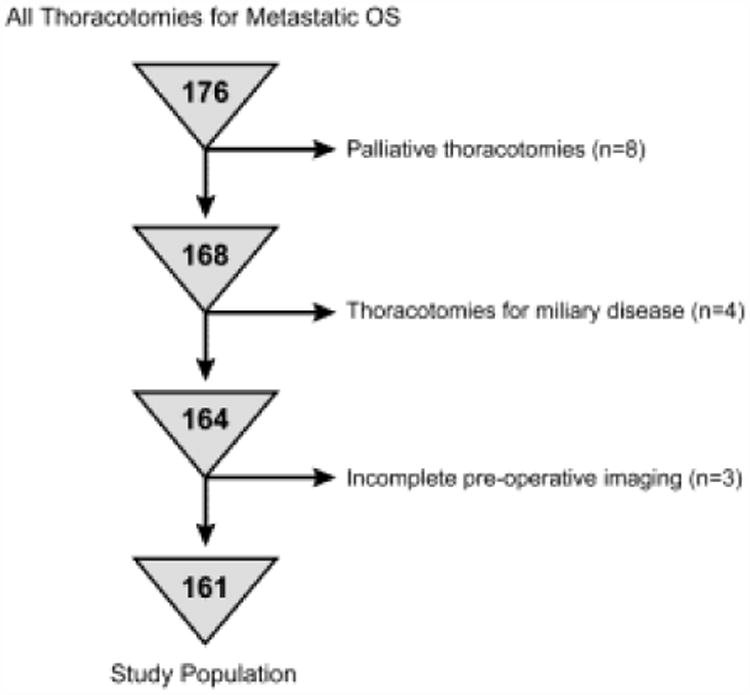
Flow chart showing reasons for patient exclusion.
1.2 Operative Procedures
At our institution, we perform staged bilateral thoracotomies when metastatic OS is suspected on pre-operative CT-imaging. Our practice is to proceed with a contralateral thoracotomy if the patient is found to have OS metastases on the initial side of the lesion excision, whether or not there is evidence of contralateral disease on CT [27]. We normally perform the contralateral thoracotomy within 2-4 weeks or after WBC and platelet recovery from intervening chemotherapy, if necessary. In this series, vertical transaxillary thoracotomy (n = 12) and posterolateral muscle-sparing thoracotomy (n = 149) were used. After mobilization, a systematic whole-lung palpation technique was employed by each operating surgeon to identify and systematically remove all palpable lesions, regardless of size or intraoperative pathology. The lung was palpated 2-3 times by all participating surgeons after the last palpable lesion was removed to confirm complete resection of metastases.
1.3 Radiology Review
CT scans used in this study had a maximum slice thickness of 5 mm. If 1.25-mm thickness reconstructions were available, they were used for enumeration of lesions and their use was recorded for comparison. If maximum intensity projection sequences were available, they were used in conjunction with standard projection, and a consensus count was obtained prior to our data analyses. Scans were reviewed by either two pediatric radiologists or one pediatric radiologist and one pediatric surgeon. When developing the consensus counts, reviewers were blinded to the initial CT-scan reading, all comparison scans, and the outcome of each thoracotomy. Lack of specific anatomic labeling of resected specimens prevented lesion-for-lesion comparison of CT attributes to pathology, so any CT identifying an equal or greater number of lesions than the number of metastases resected at thoracotomy was considered a predictive pre-operative CT. Overestimation, while increasing the number of resections, was assumed to have no effect on oncologic outcome.
1.4 Pathologic Analysis
Pathologic evaluation of all resected lung specimens was performed by standard light microscopy. If a wedge contained multiple individual lesions, each was counted in the pathologic total. Confluent lesions and lesions that required excision of additional margin to achieve complete resection were counted as a single lesion. The degree of necrosis was evaluated in each metastatic lesion and a percent viability was estimated [28]. Metastases were considered viable if any obviously viable OS cells were identified. Bony osteoid metastases devoid of viable cells were counted as non-viable metastases. Non-metastatic lesions resected were also characterized and tallied.
1.5 Statistical Analysis
Non-normal distributions of CT lesions, resected lesions, and pathologically confirmed metastases are described using the median, range, and quartiles of each distribution. Age, number of CT lesions, size of the lesions on pathology, and intervals between CT and thoracotomy were compared across subgroups using the Wilcoxon signed rank test. Kendall Tau-b correlation coefficients were calculated to describe the correlation between the number of CT-identified lesions and the total number of metastases on pathology. All statistical analyses were performed using R software (version 3.1.1 2014; R Project for Statistical Computing, Vienna, Austria; www.r-project.org).
2. Results
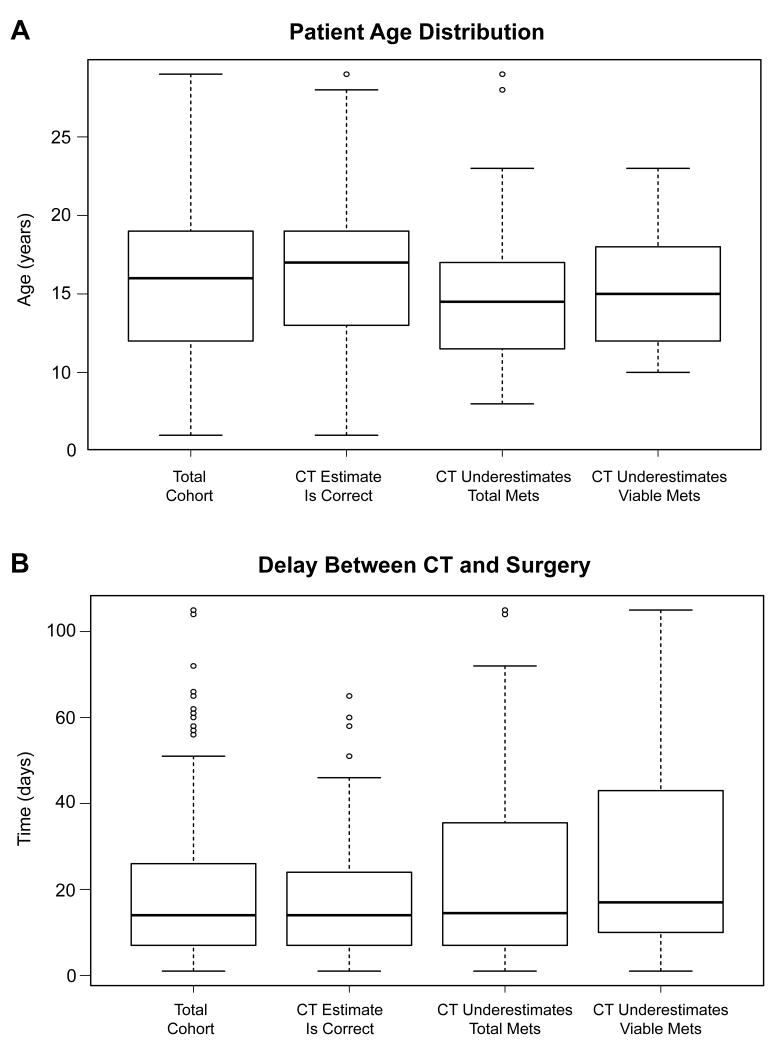
As described in Table 1, 49 patients (56%) were male and 39 (44%) were female. The median number of thoracotomies per patient was 2 (range 1-5). Median age at thoracotomy for the whole cohort was 16 years (range 6-29).The age of patients with predictive CTs (median 17 y, range 6-29) and patients with CTs underestimating viable metastases (median 15 y, range 10-23) were similarly distributed (p=0.38). However, patients with CTs underestimating total metastases were younger (median 14.5 y, range 8-29) than patients with predictive CTs (p=0.005). These differing variables are presented in whisker-and-box plots in Figure 2. Sex and race were similarly distributed within each group.
Table 1. Comparisons of patient and pre-operative CT characteristics for each patient group.
| Total Cohort | CT predicted # of mets | CT underestimated # of total mets | CT underestimated # of viable mets | |
|---|---|---|---|---|
| N | 161 | 105 | 56 | 34 |
| Age | 16, 6-29 | 17, 6-29 | 14.5, 8-29* | 15, 10-23 |
| Male | 92 | 61 | 31 | 21 |
| CT Lesion # | 2, 0-15 | 2, 0-13 | 2, 0-15 | 3, 0-15 |
| Lesion Size (mm) | 4, <1-70 | 7, <1-70 | 3, <1-55* | 4, <1-48* |
| Time to Tx | 14, 1-85 | 14, 1-66 | 15.5, 1-85 | 19, 1-85* |
significantly different from predictive CT group, p<0.05
Figure 2.
Whisker-and-box plots showing the distribution of age of patients (A) and the interval between CT and surgery (B) in the entire cohort and the study groups divided by the accuracy of the pre-operative CT scan.
There were 498 lesions identified on pre-operative CTs (median 2 per CT, range 0-15), and for 20% of these CTs, thin-cut reconstructions were available for comparison. The number of lesions on CT did not differ significantly between predictive CT scans (median 2, range 0-13) and CTs underestimating total metastases (median 2, range 0-15) or those underestimating viable metastases (median 3, range 0-15). A median of 14 days (range 1-85) passed between the CT and thoracotomy. This interval was similar for predictive CTs (median 14 days, range 1-66) and CTs underestimating total metastases (median 15.5 days, range 1-85), but the interval from CT to surgery was longer for CTs underestimating viable metastases (median 18 days, range 1-85) than for predictive CTs (p=0.03). The distribution of this variable is also described further in Figure 2.
In 161 thoracotomies, 836 lesions were resected, of which 559 (67%) were osteosarcoma metastases. The median number of resected lesions per thoracotomy was 4 (range 1-25), and the median lesion size was 4 mm (range <1mm - 70mm). On pathology, 424 (51%) of the metastases were viable and 120 (14%) were osteoid only. The most common pathologic diagnoses for all resections, including the remaining 277 non-metastatic lesions, are listed in Table 2. The total number of lesions resected was similar, regardless of whether the pre-operative CTs were predictive (median 2 lesions, range 0-13), whether they underestimated total metastases (median 2, range 0-15), or whether they underestimated viable metastases (median 3, range 0-15). The size of the lesions resected was significantly larger in thoracotomies with predictive CTs (median 0.7mm, range 0.1-7.0) than for thoracotomies with CTs underestimating total metastases (median 0.3mm, range 0.1-5.5, p<0.01) and cases in which CTs underestimated viable metastases (median 0.4mm, range 0.1-4.8, p<0.01).
Table 2. Common histopathology for the 836 resected lesions.
| Pathology of resected lesion | Total number (% of resections) |
|---|---|
| Osteosarcoma | 559 (66.9%) |
| Viable | 424 (50.7%) |
| Non-viable | 135 (14.4%) |
| Fibrotic lung | 95 (11.4%) |
| Normal lung tissue | 83 (9.9%) |
| Lymph node | 55 (6.6%) |
| Congestion/Hemorrhage | 20 (2.5%) |
| Benign calcification | 12 (1.4%) |
| Granuloma | 12 (1.4%) |
Table 3 shows the frequency of pre-operative CT underestimation of total and viable OS metastases found at thoracotomy and the Kendall Tau-b correlation coefficient (graphically depicted in Figure 3) between those same findings, with subgroup analysis by decade, number of lesions identified on CT, and slice thickness. In the full cohort, 56 (34.8%) of 161 pre-operative CTs underestimated the number of total metastases, and 34 (21.1%) underestimated the number of viable metastases, giving sensitivities of 62.9% for identification of total metastases and 72.6% for identification of viable metastases. The pre-operative CT correctly predicted the total number of OS metastases in 57 thoracotomies (35.4%) and overestimated the total in 48 thoracotomies (29.8%). The Kendall Tau-b correlation coefficient between the number of CT-identified lesions and the total number of OS metastases for the full cohort was 0.506. The subgroup analysis showed consistent underestimation of the OS metastases by the pre-operative CT scan and persistently poor correlation, regardless of the decade of the thoracotomy, the slice thickness, or the number of pre-operative CT-identified lesions.
Table 3.
Comparisons of the numbers of CT-identified lesions for each thoracotomy and the pathologic findings, including Kendall Tau-b correlation coefficients where possible, with subanalyses by time period (top), number of CT lesions (middle), and slice thickness (bottom).
| CT Predicted # of mets | CT Underestimated | Total Operations | Kendall Tau Coeff. | |||
|---|---|---|---|---|---|---|
| Viable Mets | Total Mets | |||||
|
| ||||||
| Date Of Surgery | Entire 20 years | 105 | 34 (21.1%) | 56 (34.8%) | 161 | 0.506 |
| 1996-2005 | 47 | 17 (23.6%) | 25 (34.7%) | 72 | 0.470 | |
| 2006-2015 | 58 | 17 (19.1%) | 31 (34.8%) | 89 | 0.522 | |
|
| ||||||
| Lesions On CT | 0 mets on CT | 5 | 3 (27.3%) | 6 (54.5%) | 11 | NA |
| 1 met on CT | 33 | 8 (16.7%) | 15 (31.3%) | 48 | NA | |
| >1 mets on CT | 67 | 23 (22.5%) | 35 (34.3%) | 102 | 0.360 | |
|
| ||||||
| CT Slice Size | 1.25 mm | 18 | 9 (28.1%) | 14 (43.8%) | 32 | 0.446 |
| 5 mm | 87 | 25 (19.4%) | 42 (32.6%) | 129 | 0.524 | |
Figure 3.
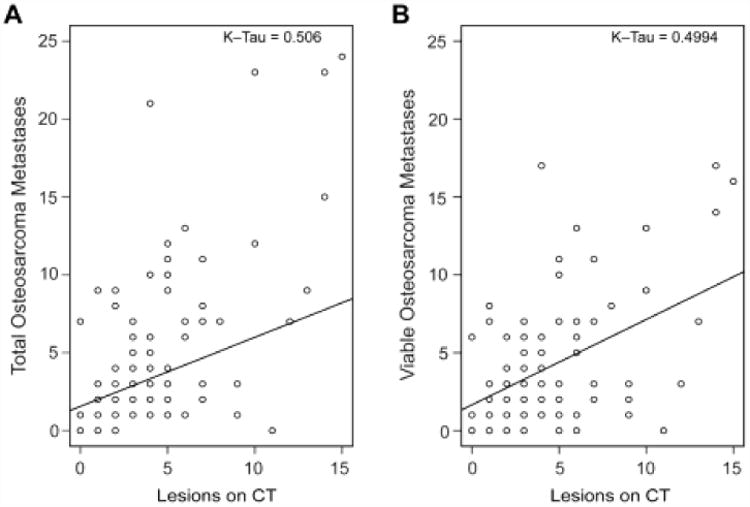
Scatter plots showing correlation between number of lesions identified on CT and the total number of metastases found at thoracotomy (A) and number of viable metastases found at thoracotomy for the entire cohort.
Eleven of the 161 thoracotomies were performed with the preoperative prediction of no lesions on because those patients had a previous thoracotomy on the side contralateral to pathology-proven OS metastases. Nevertheless, 6 (54.5%) of those 11 patients had viable or non-viable metastases at thoracotomy, and 3 (27.3%) of the 11 had viable OS metastases on the side that had a negative pre-operative CT. Forty-eight pre-operative CTs predicted 1 lesion at thoracotomy, but underestimated the total number of metastases in 15 (31.3%) and the number of viable metastases in 8 (16.7%).
Discussion
Shortly after its invention, CT became the preferred method for diagnosing pulmonary metastases because its sensitivity was superior to standard tomography [29]. However, despite its higher level of sensitivity, multiple studies have shown that CT is not adequate for enumerating OS lung metastases [26, 30, 31]. Given the abundance of trials showing that complete metastasectomy is the single most important determinant of survival after pulmonary relapse [2, 7-20], we hypothesized that this lack of sensitivity would lead to unresected metastases if the surgeon limited his/her resections to lesions seen on CT.
When applying minimally invasive techniques to OS metastasectomy, the pre-operative CT is the only guide available to the surgeon and radiologist for determining which lesions should be resected. New techniques for the pre-operative or intra-operative marking of lung lesions have been successful in allowing minimally invasive resection of lung lesions in several pediatric cancers [22-25], but those resections are still limited by the sensitivity of the CT. That limited sensitivity could lead to incomplete resection of metastatic disease in patients with OS pulmonary metastases.
In this expanded series, we again see that pre-operative CT fails to identify approximately one-third of total OS metastases and one-fifth of viable OS metastases ultimately found during thoracotomy. Despite improved technology and thinner CT slice thickness, we found no improvement in the sensitivity of pre-operative CT or the correlation between the number of lesions on CT and the number of metastases resected through direct lung palpation over the last two decades. Poor correlation and sensitivity were also found when grouping by the number of lesions on CT. This finding is similar to that from the International Registry of Lung Metastases, which showed that CT underestimated metastases in 25% of 2988 patients [32], but contradictory to more recent trials evaluating minimally invasive techniques in highly selected patients [33]. There are statistically significant differences in age and time to surgery between some groups; however, these differences do not appear clinically significant when absolute values and distributions are reviewed (Figure 2). The significantly smaller size of the lesions in patients whose pre-operative CTs underestimated the number of metastases is expected, and partially explain why the CTs were inaccurate.
The retrospective nature of this study limits the direct evaluation of specific shortcomings of CT and the analysis of which lesions are more or less likely to be undetected before surgery, but we believe it clearly shows that pre-operative CT still fails to identify a significant number of OS pulmonary metastases. This study, unfortunately, cannot address the most controversial question in metastatic OS‒ specifically, whether these missed metastases at operation affect disease-free or overall survival. While numerous published analyses have raised concerns about the sensitivity of CT in metastatic OS [26, 29-31], and have emphasized the importance of complete resection in long-term outcome in OS [2, 7-17, 19, 20], there have been no studies analyzing the effect of surgical approach on overall survival. Recently, some studies have supported the possibility of minimally invasive techniques in highly selected populations [33] and questioned the need for contralateral thoracotomy with a negative contralateral CT [34]; however, they are limited by small numbers and highly selected populations. The Children's Oncology Group is currently developing the design of a prospective, multi-institutional trial with the goal of answering some of these difficult questions.
Conclusion
We found that pre-operative CT routinely fails to identify all metastases in one-third of patients and viable metastases in one-fifth of patients, and that pre-operative CT findings correlate poorly with operative findings at thoracotomy. These rates of missed metastases and poor correlations have not changed during the last two decades, and are not affected by the total number of lesions on CT or CT slice thickness. No studies to date have adequately evaluated the effect of surgical technique on oncologic outcome. Ultimately, future studies will be needed to answer that question, but based on the available data, we recommend open surgery with direct palpation of the lung for the surgical management of pulmonary metastases in OS.
Acknowledgments
Funding: Research at Memorial Sloan Kettering Cancer Center is supported in part by a Cancer Center Support Grant from the National Institutes of Health/National Cancer Institute (#P30 CA008748).
Footnotes
Contribution(s) of Authors: Study conception and design (TEH, MPL), acquisition of data (TEH, WJH, BAF, VP, PAM, AJC, APP, MPL), analysis and interpretation of data (TEH, APP, MPL), drafting of manuscript (TEH, MPL), critical revision of manuscript (TEH, WJH, BAF, PAM, AJC, APP, MPL).
Level of Evidence: Level IV, retrospective study with no comparison group
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival--a report from the Children's Oncology Group. J Clin Oncol. 2008;26:633–638. doi: 10.1200/JCO.2008.14.0095. [DOI] [PubMed] [Google Scholar]
- 2.Saeter G, Hoie J, Stenwig AE, et al. Systemic relapse of patients with osteogenic sarcoma. Prognostic factors for long term survival. Cancer. 1995;75:1084–1093. doi: 10.1002/1097-0142(19950301)75:5<1084::aid-cncr2820750506>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 3.Meyer WH, Schell MJ, Kumar AP, et al. Thoracotomy for pulmonary metastatic osteosarcoma. An analysis of prognostic indicators of survival. Cancer. 1987;59:374–379. doi: 10.1002/1097-0142(19870115)59:2<374::aid-cncr2820590235>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Chi SN, Conklin LS, Qin J, et al. The patterns of relapse in osteosarcoma: the Memorial Sloan-Kettering experience. Pediatr Blood Cancer. 2004;42:46–51. doi: 10.1002/pbc.10420. [DOI] [PubMed] [Google Scholar]
- 5.Duffaud F, Digue L, Mercier C, et al. Recurrences following primary osteosarcoma in adolescents and adults previously treated with chemotherapy. Eur J Cancer. 2003;39:2050–2057. doi: 10.1016/s0959-8049(03)00435-0. [DOI] [PubMed] [Google Scholar]
- 6.Marcove RC, Mike V, Hajek JV, et al. Osteogenic sarcoma under the age of twenty-one. A review of one hundred and forty-five operative cases. J Bone Joint Surg Am. 1970;52:411–423. [PubMed] [Google Scholar]
- 7.Slade AD, Warneke CL, Hughes DP, et al. Effect of concurrent metastatic disease on survival in children and adolescents undergoing lung resection for metastatic osteosarcoma. J Pediatr Surg. 2015;50:157–160. doi: 10.1016/j.jpedsurg.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 8.Leary SE, Wozniak AW, Billups CA, et al. Survival of pediatric patients after relapsed osteosarcoma: the St. Jude Children's Research Hospital experience. Cancer. 2013;119:2645–2653. doi: 10.1002/cncr.28111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou AJ, Kleinerman ES, Krailo MD, et al. Addition of muramyl tripeptide to chemotherapy for patients with newly diagnosed metastatic osteosarcoma: a report from the Children's Oncology Group. Cancer. 2009;115:5339–5348. doi: 10.1002/cncr.24566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gelderblom H, Jinks RC, Sydes M, et al. Survival after recurrent osteosarcoma: data from 3 European Osteosarcoma Intergroup (EOI) randomized controlled trials. Eur J Cancer. 2011;47:895–902. doi: 10.1016/j.ejca.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 11.Buddingh EP, Anninga JK, Versteegh MI, et al. Prognostic factors in pulmonary metastasized high-grade osteosarcoma. Pediatr Blood Cancer. 2010;54:216–221. doi: 10.1002/pbc.22293. [DOI] [PubMed] [Google Scholar]
- 12.Harting MT, Blakely ML, Jaffe N, et al. Long-term survival after aggressive resection of pulmonary metastases among children and adolescents with osteosarcoma. J Pediatr Surg. 2006;41:194–199. doi: 10.1016/j.jpedsurg.2005.10.089. [DOI] [PubMed] [Google Scholar]
- 13.Kager L, Zoubek A, Potschger U, et al. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2003;21:2011–2018. doi: 10.1200/JCO.2003.08.132. [DOI] [PubMed] [Google Scholar]
- 14.Horan TA, Santiago FF, Araujo LM. The benefit of pulmonary metastectomy for bone and soft tissue sarcomas. Int Surg. 2000;85:185–189. [PubMed] [Google Scholar]
- 15.Beattie EJ, Harvey JC, Marcove R, et al. Results of multiple pulmonary resections for metastatic osteogenic sarcoma after two decades. J Surg Oncol. 1991;46:154–155. doi: 10.1002/jso.2930460305. [DOI] [PubMed] [Google Scholar]
- 16.Goorin AM, Delorey MJ, Lack EE, et al. Prognostic significance of complete surgical resection of pulmonary metastases in patients with osteogenic sarcoma: analysis of 32 patients. J Clin Oncol. 1984;2:425–431. doi: 10.1200/JCO.1984.2.5.425. [DOI] [PubMed] [Google Scholar]
- 17.Putnam JB, Jr, Roth JA, Wesley MN, et al. Survival following aggressive resection of pulmonary metastases from osteogenic sarcoma: analysis of prognostic factors. Ann Thorac Surg. 1983;36:516–523. doi: 10.1016/s0003-4975(10)60679-0. [DOI] [PubMed] [Google Scholar]
- 18.Schaller RT, Jr, Haas J, Schaller J, et al. Improved survival in children with osteosarcoma following resection of pulmonary metastases. J Pediatr Surg. 1982;17:546–550. doi: 10.1016/s0022-3468(82)80106-1. [DOI] [PubMed] [Google Scholar]
- 19.Han MT, Telander RL, Pairolero PC, et al. Aggressive thoracotomy for pulmonary metastatic osteogenic sarcoma in children and young adolescents. J Pediatr Surg. 1981;16:928–933. doi: 10.1016/s0022-3468(81)80848-2. [DOI] [PubMed] [Google Scholar]
- 20.Hankins FD, DeSanto DA. Treatment of osteogenic sarcoma that had metastasized to the lungs--25-year survival of a patient. Western J Med. 1980;132:245–248. [PMC free article] [PubMed] [Google Scholar]
- 21.Smith TJ, Rothenberg SS, Brooks M, et al. Thoracoscopic surgery in childhood cancer. J Pediatr Hematol Oncol. 2002;24:429–435. doi: 10.1097/00043426-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Waldhausen JH, Shaw DW, Hall DG, et al. Needle localization for thoracoscopic resection of small pulmonary nodules in children. J Pediatr Surg. 1997;32:1624–1625. doi: 10.1016/s0022-3468(97)90468-1. [DOI] [PubMed] [Google Scholar]
- 23.Partrick DA, Bensard DD, Teitelbaum DH, et al. Successful thoracoscopic lung biopsy in children utilizing preoperative CT-guided localization. J Pediatr Surg. 2002;37:970–973. doi: 10.1053/jpsu.2002.33820. [DOI] [PubMed] [Google Scholar]
- 24.Gow KW, Saad DF, Koontz C, et al. Minimally invasive thoracoscopic ultrasound for localization of pulmonary nodules in children. J Pediatr Surg. 2008;43:2315–2322. doi: 10.1016/j.jpedsurg.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 25.Parida L, Fernandez-Pineda I, Uffman J, et al. Thoracoscopic resection of computed tomographylocalized lung nodules in children. J Pediatr Surg. 2013;48:750–756. doi: 10.1016/j.jpedsurg.2012.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kayton ML, Huvos AG, Casher J, et al. Computed tomographic scan of the chest underestimates the number of metastatic lesions in osteosarcoma. J Pediatr Surg. 2006;41:200–206. doi: 10.1016/j.jpedsurg.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 27.Su WT, Chewning J, Abramson S, et al. Surgical management and outcome of osteosarcoma patients with unilateral pulmonary metastases. J Pediatr Surg. 2004;39:418–423. doi: 10.1016/j.jpedsurg.2003.11.030. discussion 418-423. [DOI] [PubMed] [Google Scholar]
- 28.Huvos AG, Rosen G, Marcove RC. Primary osteogenic sarcoma: pathologic aspects in 20 patients after treatment with chemotherapy en bloc resection, and prosthetic bone replacement. Arch Pathol Lab Med. 1977;101:14–18. [PubMed] [Google Scholar]
- 29.Chang AE, Schaner EG, Conkle DM, et al. Evaluation of computed tomography in the detection of pulmonary metastases: a prospective study. Cancer. 1979;43:913–916. doi: 10.1002/1097-0142(197903)43:3<913::aid-cncr2820430319>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 30.McCarville MB, Kaste SC, Cain AM, et al. Prognostic factors and imaging patterns of recurrent pulmonary nodules after thoracotomy in children with osteosarcoma. Cancer. 2001;91:1170–1176. doi: 10.1002/1097-0142(20010315)91:6<1170::aid-cncr1114>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 31.Parsons AM, Detterbeck FC, Parker LA. Accuracy of helical CT in the detection of pulmonary metastases: is intraoperative palpation still necessary? Ann Thorac Surg. 2004;78:1910–1916. doi: 10.1016/j.athoracsur.2004.05.065. discussion 1916-1918. [DOI] [PubMed] [Google Scholar]
- 32.Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thoracic Cardiovasc Surg. 1997;113:37–49. doi: 10.1016/s0022-5223(97)70397-0. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez-Pineda I, Daw NC, McCarville B, et al. Patients with osteosarcoma with a single pulmonary nodule on computed tomography: a single-institution experience. J Pediatr Surg. 2012;47:1250–1254. doi: 10.1016/j.jpedsurg.2012.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karplus G, McCarville MB, Smeltzer MP, et al. Should contralateral exploratory thoracotomy be advocated for children with osteosarcoma and early unilateral pulmonary metastases? J Pediatr Surg. 2009;44:665–671. doi: 10.1016/j.jpedsurg.2008.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]