Abstract
Glaucophytes are primary symbiotic algae with unique plastids called cyanelles, whose structure is most similar to ancestral cyanobacteria among plastids in photosynthetic organisms. Here we compare the regulation of photosynthesis in glaucophyte with that in cyanobacteria in the aim of elucidating the changes caused by the symbiosis in the interaction between photosynthetic electron transfer and other metabolic pathways. Chlorophyll fluorescence measurements of the glaucophyte Cyanophora paradoxa NIES-547 indicated that plastoquinone (PQ) pool in photosynthetic electron transfer was reduced in the dark by chlororespiration. The levels of nonphotochemical quenching of chlorophyll fluorescence was high in the dark but decreased under low light, and increased again under high light. This type of concave light dependence was quite similar to that observed in cyanobacteria. Moreover, the addition of ionophore hardly affected nonphotochemical quenching, suggesting state transition as a main component of the regulatory system in C. paradoxa. These results suggest that cyanelles of C. paradoxa retain many of the characteristics observed in their ancestral cyanobacteria. From the viewpoint of metabolic interactions, C. paradoxa is the primary symbiotic algae most similar to cyanobacteria than other lineages of photosynthetic organisms.
Approximately 2.5 billion years ago, cyanobacteria have evolved to use water molecule as electron donor for photosynthesis1. Change of photosynthetic machineries of cyanobacteria to those of anoxygenic photosynthetic bacteria is quite drastic: photosynthetic pigments are converted from bacteriochlorophylls to chlorophylls, two photosystems are connected in series to form linear electron flow, and water oxidizing complex is devised to split water molecules2. Ability of cyanobacteria to use water as electron donor for photosynthesis allows them to thrive on the entire surface of the earth. Evolution of photosynthesis after the emergence of the cyanobacteria looks less drastic, at least from a viewpoint of photosynthetic reaction centre. Structure of reaction centre complexes and the mechanisms of charge separation are almost identical between prokaryotic cyanobacteria and eukaryotic algae/land plants. This commonality of photosynthesis between two totally different domains of organisms can be explained by endosymbiosis theory3. All the plastids in eukaryotic cells originated from a single endosymbiosis event involving a eukaryote and a cyanobacterium4 in about one billion years ago5. After the event, three primary photosynthetic eukaryotes (green algae, red algae and glaucophytes) diverge from a common ancestor of eukaryotic photosynthetic organism.
Although photosynthetic reaction centre is well conserved among different algal groups as well as in cyanobacteria, their photosynthetic pigments and peripheral antenna systems are quite diverse6, which, in turn, result in the diversity of regulatory mechanisms for light harvesting systems7. Several different regulatory mechanisms are employed in cyanobacteria, which use phycobilisome (PBS) for their peripheral antenna. One mechanism is state transition, a distribution system of light energy from PBS to reaction centres, which is regulated by the redox state of plastoquinone (PQ) pool8. Another mechanism is energy dissipation system within PBS using orange carotenoid protein (OCP), which is activated by strong blue light9. Energy dissipation within PBS is also reported to be induced through the decoupling of PBS upon excessive irradiance or short heat stress10. Although approximately 80% of PBS-containing cyanobacteria use OCP9, eukaryotic algae, including red algae and glaucophytes that use PBS for peripheral antenna as cyanobacteria, lost OCP genes: instead, many eukaryotic algae acquire various energy dependent quenching mechanisms7. For example, the green alga Chlamydomonas reinhardtii uses light-harvesting complex stress-related protein 3 (LHCSR3) for energy dissipation system under high light condition11, while land plants use xanthophyll cycle12 and PsbS protein13 for the same purpose. Diatom, a secondary symbiotic alga in red lineage, uses diadinoxanthin cycle in place of xanthophyll cycle14.
The symbiosis producing eukaryotic algae must have triggered different kind of changes, i.e. interactions between chloroplasts derived from cyanobacteria and cytosol of host cells. Interactions between photosynthesis and other metabolisms, e.g. respiratory, nitrogen and carbon metabolism, are quite direct in photosynthetic prokaryotes such as cyanobacteria. Cyanobacteria do not have organelles and all the metabolic pathways can directly interact with one another within a cell. Particularly, photosynthetic electron transport and respiratory electron transport share several electron transfer components such as PQ, cytochrome b6/f complex and cytochrome c15,16. Respiratory NAD(P)H dehydrogenase (NDH)-1 complexes directly transfer electrons to PQ pool in photosynthetic electron transfer chain17,18. Thus, PQ pool is reduced in the dark by respiration in many cyanobacterial species8,18,19,20.
On the other hand, photosynthetic and respiratory electron transport chains are separated into organelles in eukaryotic cells; photosynthesis in chloroplasts and respiration in mitochondria. Nevertheless, the interaction between photosynthesis and respiration still exists in the form of chlororespiration21. In chloroplasts of land plants, plastidial NDH-1 complexes can transfer electrons from stromal NADPH to PQ pool22,23,24, while plastid terminal oxidases (PTOX) pull out electrons from PQ pool and transfer them to molecular oxygen25,26. Eukaryotic algae also possess PTOX27, but plastidial ndh genes have not been widely reported in plastid genome of eukaryotic algae28. In Chlamydomonas, however, a dehydrogenase component has been identified recently and was attributed to a type II NADPH dehydrogenase (NDA2)29.
The existence of chlororespiration in plastid may affect the redox state of PQ pool, just as in the case of the effect of respiration in cyanobacteria. In case of eukaryotic algae, however, the effect of chlororespiration on the redox state of PQ pool in the dark seems to be controversial. In case of land plants, the PQ pool is oxidized in the dark30,31. PQ reduction is known to induce the regulations of distribution system of light energy (state transition) but such regulatory change is not observed in the dark32. In eukaryotic algae, the redox state of PQ pool in the dark seems to be oxidized in some species33,34, but highly reduced in a few species35,36. For some cases, different research groups report different results for the same species37,38. As for glaucophytes, virtually no information is available.
Glaucophytes have plastids that are most structurally similar to the ancestral cyanobacteria among the three lineages of primary symbiotic algae. The plastids of glaucophytes, usually called as cyanelles, are thought as “living fossils“39 because the cyanelles keep several features of cyanobacteria such as PBS for peripheral antenna40, lack of membrane-intrinsic light-harvesting chlorophyll protein complexes (LHCs)41, peptidoglycan wall42 and carboxysomes, organelle-like polyhedral bodies involved in CO2 fixation39,43. These features, except for the presence of PBS, cannot be observed in the other two primary photosynthetic eukaryotes. On the other hand, likewise other algal plastid genome, genome size of cyanelle is small and approximately 1/10 compared to that of cyanobacteria44,45. In spite of these structural similarities between cyanobacteria and glaucophytes, recent comparative genomics and phylogenetic studies have not conclusively resolved the branching position of the glaucophytes, and the early branching history of the three primary photosynthetic lineages is still uncertain46,47. There is a report for photosynthetic activity of glaucophytes48 but there is no report on the regulatory aspects of photosynthesis: existence of chlororespiration in glaucophytes is not clear and its effect on the redox state of photosynthetic electron transport is totally unknown. For the understanding of the diversity of photosynthetic regulation and metabolic interaction among primary symbiotic algae, lack of information of these aspects in glaucophytes should be made up for.
Here we investigate the effect of chlororespiration on photosynthesis in the glaucophyte Cyanophora paradoxa NIES-547 through the measurements of chlorophyll fluorescence. The results clearly indicate that the effect of chlororespiration on photosynthesis in C. paradoxa is surprisingly similar to the interaction between respiration and photosynthesis in cyanobacteria. The chlorophyll fluorescence measurements also reveal that the main regulatory mechanism of the light harvesting systems is state transition even under photoautotrophic high light condition, just in the case of cyanobacteria. These results suggest that cyanelles of C. paradoxa retain many of the characteristics observed in their ancestral cyanobacteria. From the viewpoint of metabolic interactions, C. paradoxa is the primary symbiotic algae most similar to cyanobacteria.
Results
PQ pool is reduced in the dark in Cyanophora paradoxa
Effect of chlororespiration on photosynthesis should be reflected in the redox poise of PQ pool in the dark. Reduction of PQ pool induces state transition, resulting in more energy allocation to PSI, the extent of which could be estimated by chlorophyll fluorescence measurement of the cells at liquid nitrogen temperature. Upon PBS excitation at 625 nm of glaucophyte C. paradoxa cells, both PSI fluorescence (at 725 nm) and PSII fluorescence (at 685/695 nm) were observed reflecting the energy transfer from PBS to both photosystems (Fig. 1A). Illumination of the cells in the presence of DCMU fully oxidized the PQ pool and brought the cells to State 1, resulting in the high PSII fluorescence/PSI fluorescence ratio (F695/F725) of 0.904 ± 0.025 that reflects preferential energy allocation to PSII from PBS (Fig. 1A, red solid line). Dark acclimation of cells in the presence of KCN reduces the PQ pool through respiration in cyanobacteria18 or through chlororespiration in green algae21,49. This was also the case in glaucophyte: the cells of C. paradoxa were locked in State 2 with low F695/F725 ratio of 0.697 ± 0.013 (Fig. 1A, black dotted line). The simple dark acclimation of the cells in the absence of KCN resulted in the F695/F725 ratio of 0.750 ± 0.014 (Fig. 1A, black solid line). If we assume a liner relationship between the redox of PQ pool and F695/F725 ratio, approximately 70% of PQ pool would be reduced in the dark in C. paradoxa. C. paradoxa would have also the ability of spill-over type state transition, since much smaller but similar differences were also observed for the chlorophyll excitation at 435 nm (Fig. 1B).
Figure 1.
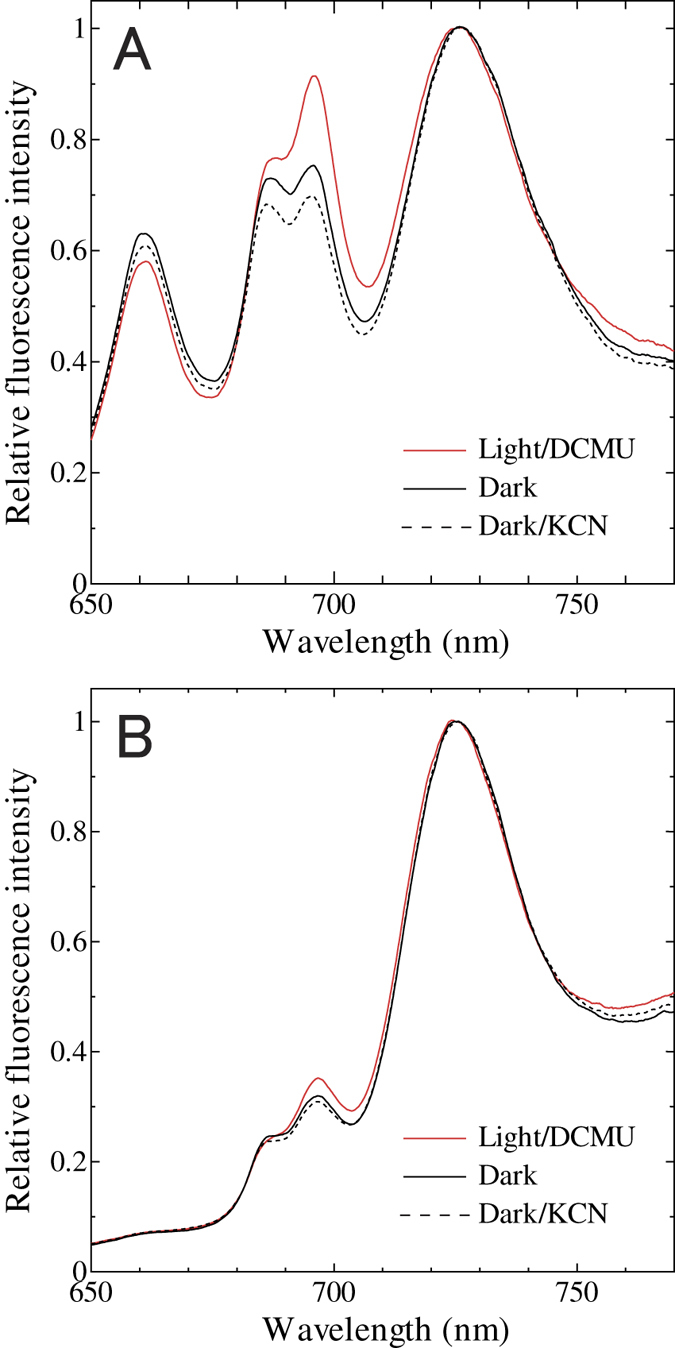
Chlorophyll fluorescence emission spectra determined at 77 K with phycocyanin excitation at 625 nm (A) or chlorophyll excitation at 435 nm (B). Black solid line, dark-adapted cells without any addition; black dotted line, dark-adapted cells in the presence of 1 mM KCN; red solid line, illuminated cells in the presence of 10 μM DCMU. Averages of spectra with three independent cultures are presented.
In land plants, Fv/Fm, calculated as (Fm-Fo)/Fm, is widely used for the parameter representing the efficiency of PSII function, since this parameter could be simply determined from the room temperature fluorescence level of dark-acclimated samples (Fo) and that upon the saturating pulse (Fm). However, if state transition is induced in the dark acclimated cells of C. paradoxa, fluorescence level upon the application of saturating light pulse in the dark (Fm’dark) should be already quenched and lower than the level of true Fm determined under illumination in the presence of DCMU. This is the case and the level of (Fv’/Fm’)dark, i.e. “apparent Fv/Fm” level determined for the dark acclimated cells, was smaller than the true Fv/Fm level (Table 1). This quenching of the chlorophyll fluorescence in dark acclimated C. paradoxa cells was not relieved by the addition of nigericin, an ionophore that would collapse proton gradient (Table 1). Thus, the fluorescence quenching in the dark could not be ascribed to energy dependent quenching triggered by the proton gradient across the thylakoid membrane but to decrease of cross-section of PSII through state transition triggered by the reduction of the PQ pool. In other words, the PQ pool is reduced by chlororespiration, and PBS is functionally disconnected from PSII (i.e. being in State 2) in the dark acclimated cells of C. paradoxa.
Table 1. Photosynthetic parameters of C. paradoxa.
| Fv’/Fm’dark | Fv/Fm | |
|---|---|---|
| control | 0.457 (±0.021) | 0.580 (±0.010) |
| Nigericin (10 μM) | 0.480 (±0.013) | 0.595 (±0.008) |
(Fv’/Fm’)dark was calculated as (Fm’dark–Fo)/Fm’dark. Values represent the average ± standard deviation with three independent cultures.
PQ pool would be oxidized upon illumination of the C. paradoxa cells by blue light, since PSI is preferentially excited by chlorophyll-absorbing light, presumably due to very high PSI/PSII ratio in C. paradoxa (Fig. 1B). Under weak blue light at 44.6 μmol m−2 s−1, the level of Fm’ (Fig. 2A, the leftmost open arrowhead) was much higher than that in the dark acclimated cells (Fm’dark) (the leftmost filled arrowhead) and became closer to the Fm level determined in the presence of DCMU. Apparently, the chlorophyll quenching was reversed upon the oxidation of the PQ pool by weak blue light. On the other hand, the subsequent higher blue light illumination (72.8, 145 and 288 μmol m−2 s−1) did not cause any further changes in the levels of Fm’ (Fig. 2A, the remaining open arrowheads). The result suggests that energy dependent quenching is not induced even under strong blue light illumination in C. paradoxa. Under blue light, the levels of the nonphotochemical quenching (NPQ) parameter, calculated as Fm/Fm’−150, decreased to one third of that in the dark (Fig. 2B). After turning off of the blue light, Fm’ was quenched to the initial Fm’dark level again in subsequent five minutes (Fig. 2A, the remaining filled arrowheads and Fig. 2B), suggesting the reversible transition to State 2 in the dark, presumably due to the electron flow to PQ pool through chlororespiration.
Figure 2.
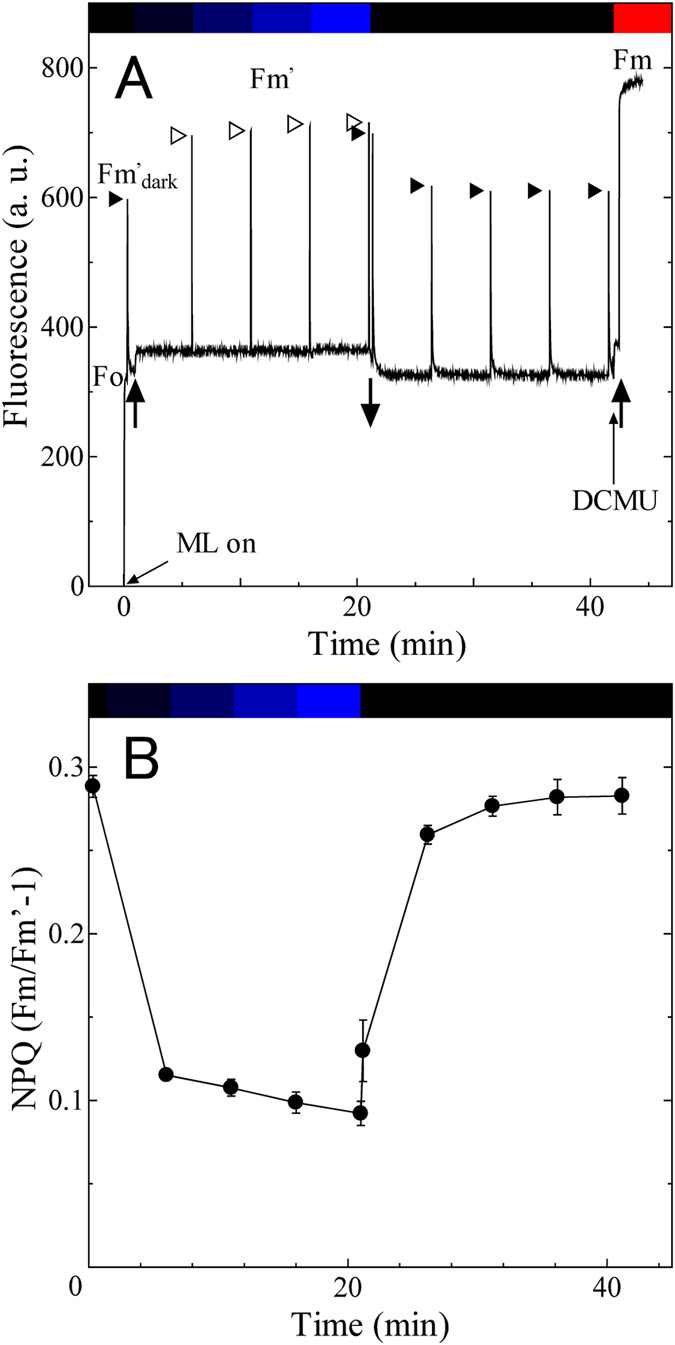
Quenching analysis of the chlorophyll fluorescence kinetics of C. paradoxa (A), and the change in NPQ calculated from the chlorophyll fluorescence kinetics (B). Actinic light was turned on at the time point indicated by solid upward arrows and off at the time point indicated by a downward arrow. DCMU was added at the time point indicated by a thin upward arrow. The bar on the top of the figure indicates illumination condition; dark (black), blue light (blue) or red light at 562 μmol m−2 s−1 (red). The change in the deepness of the blue colour represents different photon flux densities (44.6, 72.8, 145 and 288 μmol m−2 s−1) each applied for 5 min in step-wise manner. Averages of NPQ in three independent cultures are presented and vertical bars indicate standard deviation in panel B. See material and method for details.
State transition is still the main photoregulatory mechanism even under high light for 4–5 minutes
In the case of cyanobacteria, not only blue light that preferentially excites PSI but also weak white or red light that excites both PSI and PSII are known to oxidize PQ pool. Since illumination by higher light reduces the PQ pool, NPQ values are high in the dark as well as under high light but low under growth light condition, resulting in the concave dependence on actinic light intensity8. This concave dependence of NPQ is observed in a wide range of cyanobacteria20, including a model cyanobacterium Synechocystis sp. PCC6803 (Fig. 3, open circles). We found that glaucophyte shows similar light dependence of NPQ (Fig. 3). When red actinic light was used, the levels of NPQ of glaucophyte cells were high in the dark and under high light, with minimum NPQ values around the actinic light at 31.5 μmol m−2 s−1 (Fig. 3, black filled circles). Under red actinic light, the main component of NPQ in cyanobacteria is shown to be state transition8,20. This seems to be also true for glaucophytes, since similar concave change was observed for the actinic light dependence of state transition estimated by relative chlorophyll fluorescence of PSI to that of PSII (F725/F695) determined at 77 K upon phycocyanin excitation (Fig. 3, red filled circles).
Figure 3. Red actinic light (peak at 660 nm) dependence of NPQ in C. paradoxa (black filled circles) and Synechocystis sp. PCC 6803 (open circles) at room temperature and white light dependence of state transition (red filled circles, corresponding to right vertical axis) estimated by the ratio of PSI fluorescence (725 nm) to PSII fluorescence (695 nm) determined at 77 K.
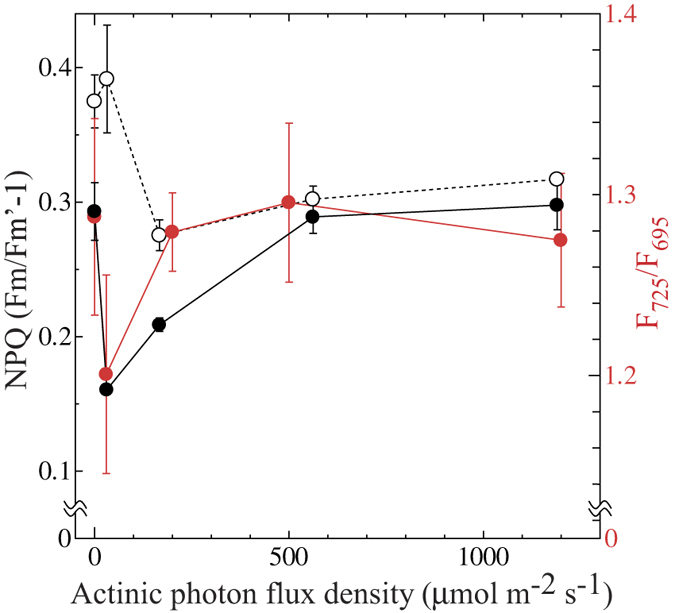
Averages of at least three independent cultures are presented respectively and vertical bars indicate standard deviation.
Apparently the main cause of the chlorophyll quenching was not energy dependent quenching but state transition. This assumption was further tested by the addition of an ionophore, nigericin, under high red light condition. After the addition of nigericin (10 or 50 μM) under strong red actinic light (562 μmol m−2 s−1), the level of Fm’ was only slightly affected even though the level of Fs gradually increased (Fig. 4B,C). The calculated NPQ in the presence of 10 μM nigericin (0.338 ± 0.030) was not significantly different from that before the addition of nigericin (0.406 ± 0.033) or that in the presence of mock control (ethanol) (0.361 ± 0.034). Thus, the low level of Fm’ under strong light for several minutes could be fully ascribed to state transition, not to energy-dependent quenching, similarly to the case of Fm’ in the dark presented in Table 1.
Figure 4. The effect of ionophore (nigericin) on chlorophyll fluorescence kinetics.
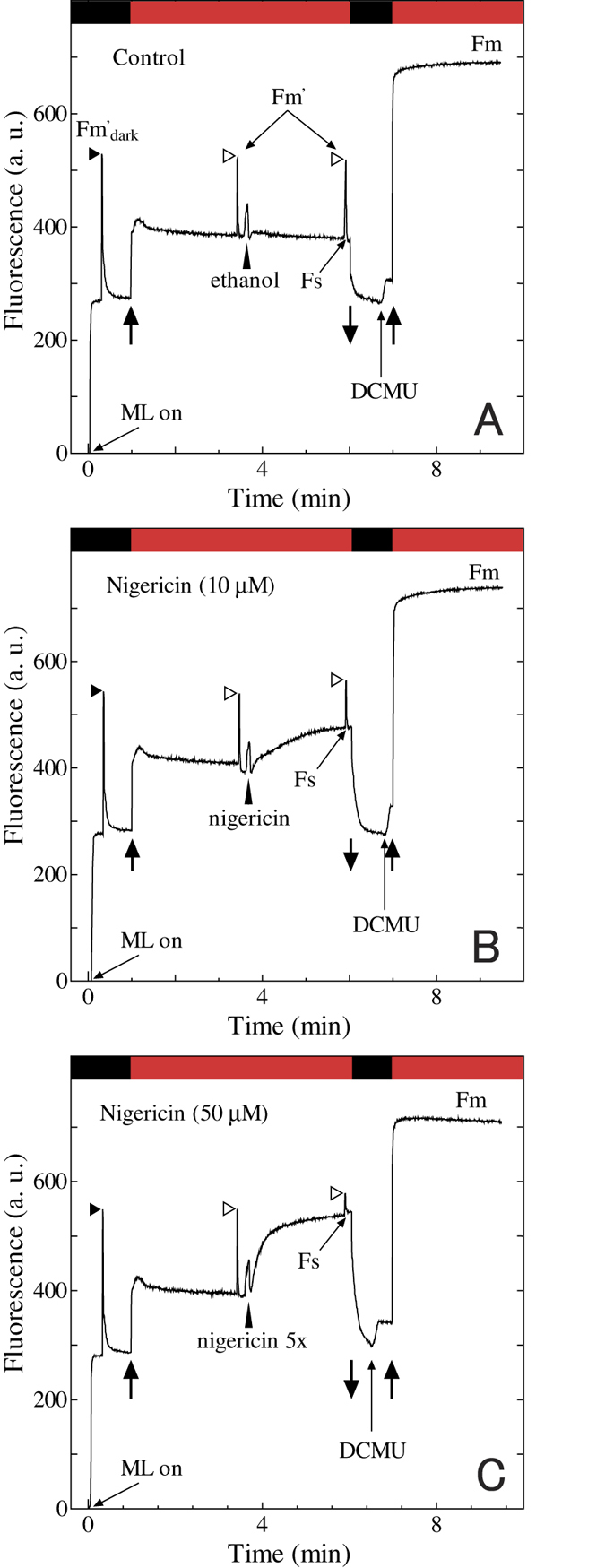
After 2.5 min from turning on of red actinic light (solid upward arrow), saturating light was applied to obtain first Fm’. Soon after, ethanol for mock control (A) or nigericin (final concentration at 10 μM in the panel B experiment or at 50 μM in the panel C experiment) was added. After the level of fluorescence settled down to the steady state (Fs), saturating light was applied to obtain second Fm’. Then, red actinic light temporary turned off (solid downward arrow), and DCMU was added (thin upward arrow). Finally, red actinic light was turned on again and bring the cells to State 1 for the determination of Fm.
It must be noted, however, that the illumination of longer duration (180 min) with higher photon flux density (2000 μmol m−2 s−1) induced the reduction of chlorophyll fluorescence at 695 nm from PSII (F695) in compensation for the increase of 660 nm fluorescence from allophycocyanin and 685 nm fluorescence (Fig. 5, red line), suggesting the decoupling of PBS. Such increase was not observed in the cells treated with lower photon flux densities (1200 μmol m−2 s−1 and 500 μmol m−2 s−1) (Fig. 5, blue lines and black broken line, respectively). On the other hand, relative decease of F695 was observed upon illumination at 1200 μmol m−2 s−1 for 180 min but not at 500 μmol m−2 s−1, possibly reflecting some quenching mechanism working at 1200 μmol m−2 s−1 in PSII reaction centre.
Figure 5. Effects of high light on 77 K chlorophyll fluorescence emission spectra with phycocyanin excitation at 625 nm.
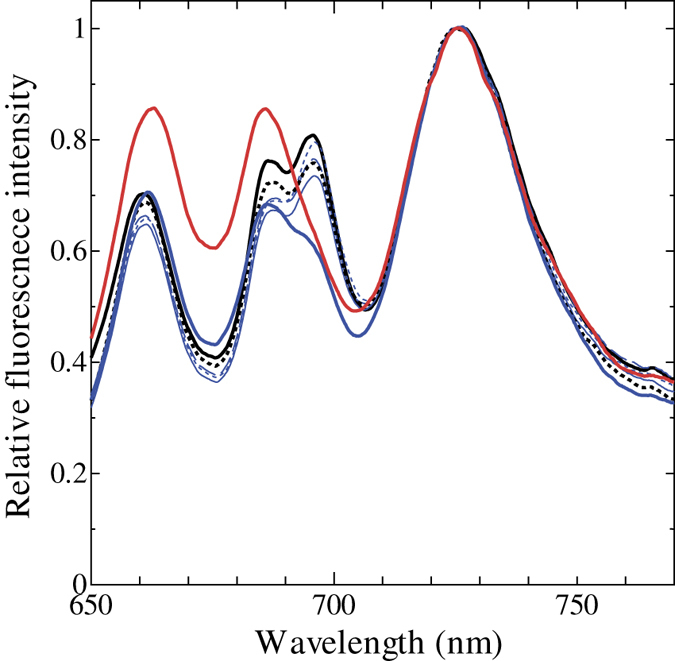
Black solid line, dark-adapted cells; black broken line, cells treated with illumination at 500 μmol m−2 s−1 for 180 min, blue lines, cells treated with 1200 μmol m−2 s−1 for 4 min (dotted line), 30 min (dashed line), 60 min (thin line) and 180 min (bold line); red line, cells treated with 2000 μmol m−2 s−1 for 180 min. Averages of spectra with three independent cultures are presented.
Red-shifted phycobilisome absorption in C. paradoxa
Although the concave dependence of NPQ on the actinic light is similar between cyanobacteria and C. paradoxa, the level of actinic light that gives the minimum NPQ level seems to be different: While NPQ in cyanobacteria is minimum at the excitation around growth light level8,20 (see Fig. 3, open circles for Synechocystis sp. PCC 6803), NPQ in C. paradoxa gave minimum at 31.5 μmol m−2 s−1 that was approximately 1/6–1/7 of the growth light (Fig. 3). This difference could be partly ascribed to the difference in the absorbance of the photosynthetic pigments, since absorption spectrum of the intact cells of C. paradoxa at room temperature (Fig. 6) showed red shifted absorption peak of PBS at 636 nm, which is more close to the wavelength of the actinic light from red LED employed in this study (650 nm) compared with the absorption peak of cyanobacterial PBS (625 nm). The absorption spectra of C. paradoxa cells reported in the past also appears to indicate the presence of red shifted PBS51, although basic pigment composition (C-phycocyanin and allophycocyanin) of PBS is similar between cyanobacteria and C. paradoxa52.
Figure 6. Absorption spectrum of the intact cells in the growth medium at room temperature.
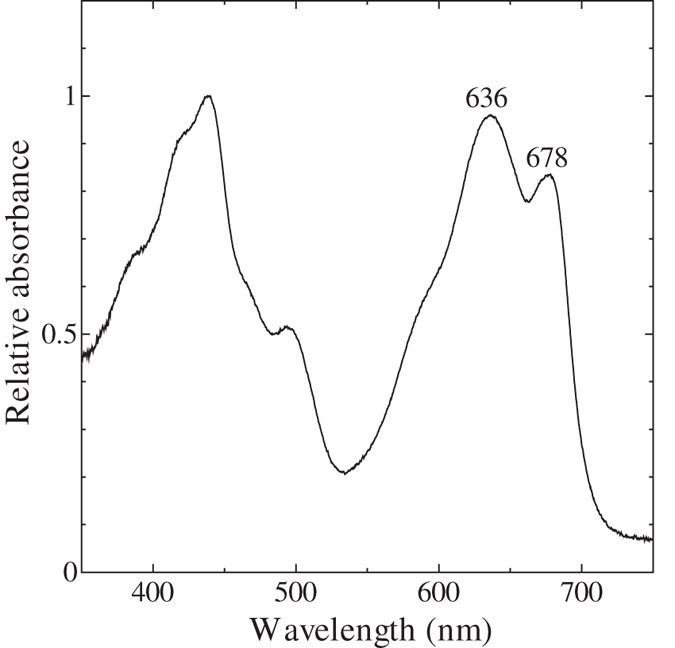
Average of spectrum with three independent cultures is presented.
Discussion
PQ pool in prokaryotic cyanobacteria is known to be reduced in the dark due to the interaction between photosynthesis and respiration8, although there are some exceptions for certain cyanobacterial species that adapt to low light environments20. Here we show that the PQ pool is reduced in the dark in the eukaryotic glaucophyte, Cyanophora paradoxa. The level of NPQ in the dark was almost same as that under high actinic light condition, suggesting the highly reduced PQ pool in the dark (Fig. 3, black filled circles). The reduction of PQ pool in the dark was also supported by the chlorophyll fluorescence spectra determined at 77 K (Fig. 1A,B). Chlororespiratory electron flow to PQ pool must have substantial rate, since turning off of the blue actinic light that preferentially excites PSI triggered reduction of PQ pool in five minutes (Fig. 2A).
PQ pool of many eukaryotic photosynthetic organisms is known to be poised in a moderately oxidized state30,33,34 even though they do chlororespiration. On the other hand, PQ pool is reported to be highly reduced in the dark in a few eukaryotic algae such as chrysophyte Ochromonas danica35 and euglenophyte Euglena gracilis36. It is rather difficult to judge whether the highly reduced PQ pool is dependent on species because the two studies reporting high reduction of PQ pool employed photoheterotrophic growth condition. In Chlamydomonas reinhardtii, PQ pool is oxidized in the dark34 but becomes reduced upon addition of organic carbon source such as acetate53. Furthermore, the poise of PQ pool of Chlamydomonas in the dark can be affected by many other experimental conditions; inhibition of mitochondrial respiration21,49, nitrogen starvation54 and hyperosmotic condition55. Apparently, the redox poise of PQ pool in eukaryotic algae is dependent on environmental condition. In the case of C. paradoxa, however, PQ pool was already reduced under photoautotrophic condition without any stress (Fig. 1A,B) as in cyanobacteria. In terms of redox poise of PQ pool, C. paradoxa seems to be more similar to cyanobacteria than to other eukaryotic algae.
According to the model of chlororespiration56, the mechanism of PQ reduction is as follows: First, NAD(P)H is provided by metabolic reactions in stromal side of chloroplast. This NAD(P)H reduces PQ by some plastidial NDH complexes. In addition, like in cyanobacteria57, PQ might be also reduced by succinate through thylakoid succinate dehydrogenase (SDH). As for the source of NAD(P)H in glaucophyte, glycolytic pathway is a candidate. The cyanelle of glaucophyte have almost complete set of glycolytic enzymes except for hexokinase and phosphofructokinase according to a proteomic analysis58. Thus, at least, the second half of glycolytic pathway (3-phosphoglyceraldehyde to pyruvate) can produce NAD(P)H in cyanelle. In addition, the cyanelle have an isoenzyme of glucose 6-phosphate dehydrogenase (G6PDH) involved in the oxidative pentose-phosphate pathway (OPPP), and this isoenzyme is reversibly inhibited by dithiothreitol (DTT)59. Through this enzyme NAD(P)H can be produced by catabolic action in stromal side of cyanelle in the dark.
The import of organic substances to chloroplasts from mitochondria through cytosol is reported to be important for chlororespiration60,61. On the other hand, isolated cyanelles do not exhibit malate/oxaloacetate exchange activity62 so that cyanelles may not have malate valve to discharge stromal NADPH to cytosol/mitochondria as reducing equivalents. As a result, the stromal side of cyanelles could be more reduced than the plastids of green and red algae, and electrons may be more easily transferred to PQ pool. As for electron donor to PQ pool, cyanelle (plastid) genome do not have plastidial NDH complexes gene28, similar to most of eukaryotic algae but different from cyanobacteria and land plants. When we looked at C. paradoxa complete genome46, genes with moderate similarities with cyanobacterial (Synechocystis sp. PCC6803) ndh genes, i.e. ndhI (sll0520), ndhK (slr1280, sll8031) and ndhM (sll1623), and those with cyanobacterial sdh gene (slr1233) could be found. However, the localization of the product of these genes was not known. We also looked for the homologs of Chlamydomonas NDA2 gene in C. paradoxa genome but found only sequences matched to the part of the NDA2 gene.
More reduced PQ pool in C. paradoxa than that in other algae could be also brought about by the lower activity of PTOX. PTOX activity must be important as a determinant of redox poise of PQ pool in Chlamydomonas, since the knockout mutant of Chlamydomonas defective in PTOX2, a major oxidase involved in chlororespiration in this organism, shows the phenotype of reduced PQ pool in the dark34. It is suggested that PTOX has complex evolutionary history with several independent duplication events27. C. paradoxa genome contains only a sequence that shows low similarity to PTOX of red algae, green algae and cyanobacteria. On the other hand, genome of another glaucophyte, Glaucocystis nostochinearum, contains a gene with much higher similarity with red and green algal PTOX sequence. It would be worth to compare the redox state of PQ pool of Glaucocystis nostochinearum with other algal species in the dark, in order to see whether the PTOX activity is the universally determinant of the redox poise of PQ pool in the dark among eukaryotic algae.
The reduced PQ pool in the dark in C. paradoxa (Figs 1, 2 and 3) could be interpreted as a protective mechanism from photoinhibition of PSI. As the mechanism of PSI photoinhibition, it is proposed that active oxygen species, which are produced through the reduction of oxygen molecule in the acceptor side of PSI, directly destroys iron-sulphur cluster of PSI63. In other words, the combination of inefficient electron transport in the downstream of PSI and the presence of excitation pressure to PSI from non-downregulating PSII is the cause of PSI photoinhibition, which would be fatal problem for obligate photoautotrophs such as C. paradoxa. In order to avoid the photoinhibition of PSI, photosynthetic organisms have several layers of protection mechanisms such as cyclic electron flow and down regulation of PSII64. For example, PGR5-dependent cyclic electron transport is essential for protection of PSI under fluctuating light in Arabidopsis thaliana65. Cyclic electron transport is especially important in the transition period from dark to light, since Calvin-Benson cycle is inactivated in the dark, leading to the inefficient electron transport in the downstream of PSI66. Furthermore, the double mutant strain of Chlamydomonas, Crpgrl1npq4 deficient in both cyclic electron flow and energy dependent quenching mediated by LHCSR3 is reported to be more susceptible to PSI photoinhibition than the single cyclic electron flow deficient mutant Crpgr167. The down regulation of PSII activity seems to be essential for the protection of PSI from photoinhibition. C. paradoxa, which does not develop energy dependent quenching (Fig. 4), may have reduced PQ pool in the dark in order to decrease the reducing pressure to PSI upon onset of light illumination by downregulating PSII as well as by inducing state transition in advance. Although physiological function of chlororespiration is still under discussion27,60, it may serve for the photoprotective mechanism under fluctuating light through the reduction of PQ pool in the dark in C. paradoxa, and this may be also true for the PQ reduction in cyanobacteria through respiration.
During the course of evolution from cyanobacteria to green algae and finally to land plants, relative importance of state transition in light acclimation seems to decrease while that of energy dependent quenching seems to increase. In the case of green algae, about 80% of light harvesting capacity is controlled by state transition68, while only 20–25% of light harvesting capacity relies on state transition in land plants69. Here we show that the main mechanism of the light acclimation in C. paradoxa is state transition as discussed above. It would be reasonable to assume that the contribution of regulated energy dependent quenching to NPQ in C. paradoxa is negligible for the following three reasons. First, the NPQ level in high light condition was comparable to the level in the dark (Fig. 3), suggesting that no additional quenching mechanism is induced under high light condition at least for several minutes. Secondly, increase in the photon flux density of blue light illumination did not cause any further changes in the levels of Fm’ (Fig. 2A). It must be also noted that C. paradoxa does not have OCP genes7. Thirdly, the Fm’ level under high red light condition was largely unaffected by the addition of the ionophore, nigericin. Instead, the significant increase of Fs level was observed when ionophore was added (Fig. 4B,C). The cause of the increase of Fs level would be ascribed to the reduction of electron transfer components that is induced by the suppressed carbon assimilation due to ATP shortage that is, in turn, induced by the collapse of ΔpH by the ionophore. It must be noted that the extent of the energy dependent quenching is regulated by several factors: the photon flux density of growth light in green algae70,71 and growth phase in chromophyte alga O. danica35 as well as in cryptophyte alga Guillardia theta72. Even though we grew the cells of C. paradoxa under relatively high light to the exponential phase for the experiments, we cannot deny the possibility that this alga shows energy dependent quenching for some specific growth environment.
Allophycocyanin fluorescence at 660 nm relative to PSI fluorescence determined at 77 K increased (Fig. 5) presumably due to decoupling of PBS. Although decoupling of PBS is observed in cyanobacteria10 as well as in red algae73, our results indicate that the condition for the decoupling of PBS in Glaucophyte is rather nonphysiological for this alga, i.e. 2000 μmol m−2 s−1 for 180 min. On the other hand, the decrease of chlorophyll fluorescence at 695 nm was observed under less severe condition, i.e. 1200 μmol m−2 s−1 for 180 min, which is still rather harsh condition for this alga. Although this quenching of fluorescence may be comparable to the reaction centre-based quenching observed in many red algal species33,74,75,76, kinetics of induction is totally different between red algae and C. paradoxa: reaction centre-based quenching in red algae is induced very fast (saturating multi-turnover light pulse is enough to induce this quenching) while high light for several minutes is not enough to induce fluorescence quenching at 695 nm in C. paradoxa. Considering that C. paradoxa cannot grow under continuous light at 1200 or 2000 μmol m−2 s−1, decoupling of PBS and/or reaction centre-based quenching in C. paradoxa would be a kind of damage brought about by extreme high light rather than regulatory energy dissipation system.
Although red algae share many physiological characteristics with glaucophytes and cyanobacteria, red algae are different from glaucophytes and cyanobacteria in the metabolism of ascorbic acid77, which is known to be important for xanthophyll cycle78. C. paradoxa seems to be the only photosynthetic eukaryote that lacks any isoform of ascorbate peroxidase, and cellular concentration of ascorbate in C. paradoxa is reported to be either very low or null77. Thus, ascorbate-dependent xanthophyll cycle would be absent in C. paradoxa, which is in accordance with the lack of energy dependent quenching demonstrated here. Interestingly, cyanobacteria also do not appear to use ascorbate for photoprotection79,80. The strategy of photoprotection in glaucophyte would be quite different from that of red algae and might be similar to cyanobacteria.
Material and Method
Strain and growth conditions
Cyanophora paradoxa strain NIES-547 was obtained from the Microbial Culture Collection of the National Institute for Environmental Studies (Tsukuba, Japan). Liquid cultures of the C. paradoxa cells were grown at 26 °C in C medium81. Cell cultures were bubbled with filtered air under continuous illumination at 200 μmol m−2 s−1 from white fluorescence tubes. The typical doubling time of C. paradoxa in logarithmic phase (OD750 = 0.04–1.4) was 22.8 h (±0.20). Cells were sampled in the logarithmic phase at OD750 = 0.4–0.8.
Culture conditions for Synechocystis sp. PCC 6803 were similar to those for C. paradoxa, except for temperature (30 °C) and medium (BG-11)82. The typical doubling time of Synechocystis sp. PCC 6803 in logarithmic phase (OD750 = 0.03–0.76) was 6.23 h (±0.013). Cells were sampled in the logarithmic phase at OD750 = 0.18–0.31.
Chlorophyll fluorescence emission spectra
Chlorophyll fluorescence emission spectra were determined at 77 K with a fluorescence spectrometer (FP-8500, JASCO, Japan) with a low temperature attachment (PU-830, JASCO, Japan)18. Cell suspensions were adjusted to a concentration of 2 μg chlorophyll ml−1 in growth medium. Chlorophyll concentration was determined by extraction with 100% methanol83. Prior to the measurements, the cells were dark-adapted for 15 min with or without KCN (1 mM) (Fig. 1A,B), illuminated by while light (30, 200 and 500 μmol m−2 s−1) for 4 min (Fig. 3), or illuminated by white light at 500 μmol m−2 s−1 for 180 min, at 1200 μmol m−2 s−1 for 4, 30, 60 and 180 min or at 2000 μmol m−2 s−1 for 180 min (Fig. 5) from a light source (PICL-NRX, NIPPON P-I). The effect of 10 μM DCMU was also tested for the measurements of cells illuminated at 500 μmol m−2 s−1 (Fig. 1A,B). The samples were excited by 625 nm light for phycocyanin excitation and 435 nm for chlorophyll excitation with excitation slit width at 10 nm. The fluorescence spectra were recorded with fluorescence slit width at 2.5 nm and resolution of 0.2 nm. The spectra were corrected for the sensitivity of photomultiplier and spectrum of light source using a secondary standard light source (ESC-842, JASCO, Japan). Chlorophyll fluorescence emission spectra were normalized at their respective maxima at around 725 nm.
Chlorophyll fluorescence measurements by pulse-amplitude modulation
Chlorophyll fluorescence was measured by pulse-amplitude modulation with a fluorometer (WATER-PAM, Waltz, Germany). Cell suspension of C. paradoxa was adjusted to a concentration of 1 μg chlorophyll ml−1 in growth medium. The cell suspension was continuously stirred during the experimental procedure including the time for dark-acclimation to avoid oxygen deficiency. Cells in 2 ml liquid culture were dark-acclimated for 15 min and minimum fluorescence level (Fo) was determined with measuring light (peak at 650 nm). A pulse of saturating light (0.8 s) was given to dark-acclimated cells to determine Fm’dark. Subsequently, one of the following 3 types of the experiments was conducted. (1) Cells were illuminated by 562 μmol m−2 s−1 red actinic light (peak at 660 nm) for 2.5 min in the presence of 10 μM DCMU and saturating light was given to monitor the level of maximum fluorescence (Fm) (Table 1, Fig. 3), which is necessary to calculate NPQ that represents redox poise of PQ pool. Nigericin (final concentration at 10 μM) was added just before the dark-acclimation if necessary (Table 1). (2) Cells were illuminated by blue light (peak at 460 nm) at four different photon flux densities (44.6, 72.8, 145 and 288 μmol m−2 s−1), each applied for 5 min in step-wise manner, to monitor fluorescence under each steady state condition to investigate the effect of blue actinic light. At the end of each blue light illumination, the saturating light was applied to monitor the level of Fm’ under respective light conditions. Following the turning off of the blue light, the pulses of the saturating light were applied to monitor the recovery kinetics of Fm’ at 10 sec, 5 min, 10 min, 15 min and 20 min in the dark. Finally, Fm was obtained by the addition of 10 μM DCMU under red actinic light (Fig. 2A). (3) Red actinic light at respective photon flux densities (31.5, 167, 562 or 1190 μmol m−2 s−1) was applied to the cells for 5 min to monitor fluorescence under steady state condition to investigate the relationship between the photon flux densities of red actinic light and the levels of NPQ. At the end of red actinic light illumination, the saturating light was applied to monitor maximum fluorescence of the light acclimated cells (Fm’). Then, red actinic light was turned off and cells were relieved in the dark for 5 min from the effect of actinic light and the saturating light was given again. Finally, Fm was obtained by the addition of 10 μM DCMU under the red actinic light (Fig. 3). Experimental conditions for Synechocystis sp. PCC 6803 were identical to those for C. paradoxa (Fig. 3). To test the effect of ionophore, the cells were illuminated by strong red light (562 μmol m−2s−1), and we first obtained the level of Fm’ by a pulse of saturating light 2.5 min after the onset of illumination. Then, 2 μl of ethanol as a mock control (Fig. 4A) or nigericin (2 μl or 10 μl; final concentration at 10 μM or 50 μM, respectively) (Fig. 4B,C) was added, and 2.5 min later, a pulse of saturating light was applied again to obtain second Fm’ level. Fluorescence parameters were calculated as the following: Fv/Fm’dark = (Fm’dark − Fo)/Fm’dark, NPQ = Fm/Fm’ − 150.
Absorbance spectrum
Absorbance spectrum was determined with a spectrophotometer (V-650, JASCO, Japan) equipped with integrating sphere (ISV-722, JASCO, Japan)18 at room temperature. Absorbance of cell suspensions was determined in a cuvette with light path length of 5 mm. Absorbance spectrum was normalized at its maximum.
Additional Information
How to cite this article: Misumi, M. and Sonoike, K. Characterization of the influence of chlororespiration on the regulation of photosynthesis in the glaucophyte Cyanophora paradoxa. Sci. Rep. 7, 46100; doi: 10.1038/srep46100 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This work was supported by Grant-in-Aid for Scientific Research on Innovative Areas (No. 16H06552 and No. 16H06553 to K.S.) and Grant-in-Aid for Scientific Research (B) (No. 16H04809 to K.S.). We thank Dr. Takako Ogawa for the critical reading of the manuscript.
Footnotes
The authors declare no competing financial interests.
Author Contributions M.M. and K.S. designed the experiments. M.M. cultured the strain, performed the experiments, analysed the data and drafted the manuscript. M.M. and K.S. interpreted the data. K.S. revised the manuscript and contributed extensively to its finalization.
References
- Kopp R. E., Kirschvink J. L., Hilburn I. A. & Nash C. Z. The Paleoproterozoic snowball Earth: a climate disaster triggered by the evolution of oxygenic photosynthesis. Proc. Natl. Acad. Sci. USA 102, 11131–11136 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship R. E. & Hartman H. The origin and evolution of oxygenic photosynthesis. Trends Biochem. Sci. 23, 94–97 (1998). [DOI] [PubMed] [Google Scholar]
- Margulis L. Origin of eukaryotic cells (Yale University Press, New Haven, 1970). [Google Scholar]
- Rodríguez-Ezpeleta N. et al. Monophyly of primary photosynthetic eukaryotes: green plants, red algae, and glaucophytes. Curr. Biol. 15, 1325–1330 (2005). [DOI] [PubMed] [Google Scholar]
- Douzery E. J. P., Snell E. A., Bapteste E., Delsuc F. & Philippe H. The timing of eukaryotic evolution: does a relaxed molecular clock reconcile proteins and fossils? Proc. Natl. Acad. Sci. USA 101, 15386–15391 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durnford D. G. et al. A phylogenetic assessment of the eukaryotic light-harvesting antenna proteins, with implications for plastid evolution. J. Mol. Evol. 48, 59–68 (1999). [DOI] [PubMed] [Google Scholar]
- Niyogi K. K. & Truong T. B. Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Curr. Opin. Plant Biol. 16, 307–314 (2013). [DOI] [PubMed] [Google Scholar]
- Campbell D. & Öquist G. Predicting light acclimation in cyanobacteria from nonphotochemical quenching of photosystem II fluorescence which reflects state transition in these organisms. Plant Physiol. 111, 1293–1298 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirilovsky D. & Kerfeld C. A. The orange carotenoid protein in photoprotection of photosystem II in cyanobacteria. Biochim. Biophys. Acta-Bioenergetics 1817, 158–166 (2012). [DOI] [PubMed] [Google Scholar]
- Stoitchkova K. et al. Heat-and light-induced reorganizations in the phycobilisome antenna of Synechocystis sp. PCC 6803. Thermo-optic effect. Biochim. Biophys. Acta-Bioenergetics 1767, 750–756 (2007). [DOI] [PubMed] [Google Scholar]
- Peers G. et al. An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature 462, 518–521 (2009). [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B. & Adams W. W. III. The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1, 21–26 (1996). [Google Scholar]
- Li X. P. et al. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403, 391–395 (2000). [DOI] [PubMed] [Google Scholar]
- Olaizola M., La Roche J., Kolber Z. & Falkowski P. G. Non-photochemical fluorescence quenching and the diadinoxanthin cycle in a marine diatom. Photosyn. Res. 41, 357–370 (1994). [DOI] [PubMed] [Google Scholar]
- Aoki M. & Katoh S. Oxidation and reduction of plastoquinone by photosynthetic and respiratory electron transport in a cyanobacterium Synechococcus sp. Biochim. Biophys. Acta-Bioenergetics 682, 307–314 (1982). [Google Scholar]
- Peschek G. A. & Schmetterer G. Evidence for plastoquinol-cytochrome f/b563 reductase as a common electron donor to P700 and cytochrome oxidase in cyanobacteria. Biochem. Biophys. Res. Commun. 108, 1188–1195 (1982). [DOI] [PubMed] [Google Scholar]
- Mi H., Endo T., Schreiber U., Ogawa T. & Asada K. Electron donation from cyclic and respiratory flows to the photosynthetic intersystem chain is mediated by pyridine nucleotide dehydrogenase in the cyanobacterium Synechocystis PCC 6803. Plant Cell Physiol. 33, 1233–1237 (1992). [Google Scholar]
- Ogawa T., Harada T., Ozaki H. & Sonoike K. Disruption of the ndhF1 gene affects Chl fluorescence through state transition in the cyanobacterium Synechocystis sp. PCC 6803, resulting in apparent high efficiency of photosynthesis. Plant Cell Physiol. 54, 1164–1171 (2013). [DOI] [PubMed] [Google Scholar]
- Mullineaux C. W. & Allen J. F. State 1-State 2 transitions in the cyanobacterium Synechococcus 6301 are controlled by the redox state of electron carriers between photosystems I and II. Photosyn. Res. 23, 297–311 (1990). [DOI] [PubMed] [Google Scholar]
- Misumi M., Katoh H., Tomo T. & Sonoike K. Relationship between photochemical quenching and non-photochemical quenching in six species of cyanobacteria reveals species difference in redox state and species commonality in energy dissipation. Plant Cell Physiol. 57, 1510–1517 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennoun P. Evidence for a respiratory chain in the chloroplast. Proc. Natl. Acad. Sci. USA 79, 4352–4356 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K. et al. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 5, 2043–2049 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama K. et al. Chloroplast gene organization deduced from complete sequence of liverwort Marchantia polymorpha chloroplast DNA. Nature 322, 572–574 (1986). [Google Scholar]
- Burrows P. A., Sazanov L. A., Svab Z., Maliga P. & Nixon P. J. Identification of a functional respiratory complex in chloroplasts through analysis of tobacco mutants containing disrupted plastid ndh genes. EMBO J. 17, 868–876 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carol P. et al. Mutations in the Arabidopsis gene IMMUTANS cause a variegated phenotype by inactivating a chloroplast terminal oxidase associated with phytoene desaturation. Plant Cell 11, 57–68 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Wright D. A., Wetzel C., Voytas D. F. & Rodermel S. The IMMUTANS variegation locus of Arabidopsis defines a mitochondrial alternative oxidase homolog that functions during early chloroplast biogenesis. Plant Cell. 11, 43–55 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrocki W. J., Tourasse N. J., Taly A., Rappaport F. & Wollman F. A. The plastid terminal oxidase: its elusive function points to multiple contributions to plastid physiology. Annu. Rev. Plant Biol. 66, 49–74 (2015). [DOI] [PubMed] [Google Scholar]
- Martín M. & Sabater B. Plastid ndh genes in plant evolution. Plant Physiol. Biochem. 48, 636–645 (2010). [DOI] [PubMed] [Google Scholar]
- Jans F. et al. A type II NAD (P) H dehydrogenase mediates light-independent plastoquinone reduction in the chloroplast of Chlamydomonas. Proc. Natl. Acad. Sci. USA 105, 20546–20551 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruk J. & Karpinski S. An HPLC-based method of estimation of the total redox state of plastoquinone in chloroplasts, the size of the photochemically active plastoquinone-pool and its redox state in thylakoids of Arabidopsis. Biochim. Biophys. Acta-Bioenergetics 1757, 1669–1675 (2006). [DOI] [PubMed] [Google Scholar]
- Trouillard M. et al. Kinetic properties and physiological role of the plastoquinone terminal oxidase (PTOX) in a vascular plant. Biochim. Biophys. Acta-Bioenergetics 1817, 2140–2148 (2012). [DOI] [PubMed] [Google Scholar]
- Bellafiore S., Barneche F., Peltier G. & Rochaix J. D. State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 433, 892–895 (2005). [DOI] [PubMed] [Google Scholar]
- Delphin E., Duval J. C., Etienne A. L. & Kirilovsky D. State transitions or ΔpH-dependent quenching of photosystem II fluorescence in red algae. Biochemistry 35, 9435–9445 (1996). [DOI] [PubMed] [Google Scholar]
- Houille-Vernes L., Rappaport F., Wollman F. A., Alric J. & Johnson X. Plastid terminal oxidase 2 (PTOX2) is the major oxidase involved in chlororespiration in Chlamydomonas. Proc. Natl. Acad. Sci. USA 108, 20820–20825 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs P. B. & Biggins J. Regulation of the distribution of excitation energy in Ochromonas danica, an organism containing a chlorophyll-A/C/carotenoid light harvesting antenna. Photosyn. Res. 21, 81–91 (1989). [DOI] [PubMed] [Google Scholar]
- Doege M., Ohmann E. & Tschiersch H. Chlorophyll fluorescence quenching in the alga Euglena gracilis. Photosyn. Res. 63, 159–170 (2000). [DOI] [PubMed] [Google Scholar]
- Ting C. S. & Owens T. G. Photochemical and nonphotochemical fluorescence quenching processes in the diatom Phaeodactylum tricornutum. Plant Physiol. 101, 1323–1330 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber U. Chlorophyll fluorescence: new instruments for special applications. Photosynthesis: mechanisms and effects (ed. Garab G.) 5, 4253–4258 (Kluwer Academic Publishers, Dordrecht, 1998). [Google Scholar]
- Fathinejad S. et al. A carboxysomal carbon-concentrating mechanism in the cyanelles of the ‘coelacanth’ of the algal world, Cyanophora paradoxa? Physiol. Plant. 133, 27–32 (2008). [DOI] [PubMed] [Google Scholar]
- Giddings T. H., Wasmann C. & Staehelin L. A. Structure of the thylakoids and envelope membranes of the cyanelles of Cyanophora paradoxa. Plant Physiol. 71, 409–419 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H., Shibata M., Yasutomi K., Kashino Y. & Satoh K. Identification of photosystem I components from a glaucocystophyte, Cyanophora paradoxa: The PsaD protein has an N-terminal stretch homologous to higher plants. Photosyn. Res. 65, 207–217 (2000). [DOI] [PubMed] [Google Scholar]
- Schenk H. E. A. Notizen: Nachweis einer lysozymempfindlichen Stützmembran der Endocyanellen von Cyanophora Paradoxa Korschikoff. Z. Naturforsch. 25B, 656–657 (1970). [Google Scholar]
- Burey S. C. et al. Acclimation to low [CO2] by an inorganic carbon-concentrating mechanism in Cyanophora paradoxa. Plant Cell Environ. 30, 1422–1435 (2007). [DOI] [PubMed] [Google Scholar]
- Herdman M. & Stanier R. Y. The cyanelle: chloroplast or endosymbiotic prokaryote? FEMS Microbiol. Lett. 1, 7–11 (1977). [Google Scholar]
- Stirewalt V. L., Michalowski C. B., Löffelhardt W., Bohnert H. J. & Bryant D. A. Nucleotide sequence of the cyanelle genome from Cyanophora paradoxa. Plant Mol. Biol. Rep. 13, 327–332 (1995). [Google Scholar]
- Price D. C. et al. Cyanophora paradoxa genome elucidates origin of photosynthesis in algae and plants. Science 335, 843–847 (2012). [DOI] [PubMed] [Google Scholar]
- Mackiewicz P. & Gagat P. Monophyly of Archaeplastida supergroup and relationships among its lineages in the light of phylogenetic and phylogenomic studies. Are we close to a consensus? Acta. Soc. Bot. Pol. 83, 399–407 (2014). [Google Scholar]
- Floener L. & Bothe H. Metabolic activities in Cyanophora paradoxa and its cyanelles: II. Photosynthesis and respiration. Planta 156, 78–83 (1982). [DOI] [PubMed] [Google Scholar]
- Gans P. & Wollman F. A. The effect of cyanide on state transitions in Chlamydomonas reinhardtii. Biochim. Biophys. Acta-Bioenergetics 1228, 51–57 (1995). [Google Scholar]
- Bilger W. & Björkman O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosyn. Res. 25, 173–185 (1990). [DOI] [PubMed] [Google Scholar]
- Schenk H. E. A., Bayer M. G. & Maier T. Nitrate assimilation and regulation of biosynthesis and disintegration of phycobiliproteids by Cyanophora paradoxa. Indications for a nitrogen store function of the phycobiliproteids. Endocytobiosis Cell Res. 4, 167–176 (1987). [Google Scholar]
- Chapman D. J. The pigments of the symbiotic algae (cyanomes) of Cyanophora paradoxa and Glaucocystis nostochinearum and two Rhodophyceae, Porphyridium aerugineum and Asterocytis ramosa. Arch. Mikrobiol. 55, 17–25 (1966). [Google Scholar]
- Endo T. & Asada K. Dark induction of the non-photochemical quenching of chlorophyll fluorescence by acetate in Chlamydomonas reinhardtii. Plant Cell Physiol. 37, 551–555 (1996). [Google Scholar]
- Peltier G. & Schmidt G. W. Chlororespiration: an adaptation to nitrogen deficiency in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 88, 4791–4795 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T., Schreiber U. & Asada K. Suppression of quantum yield of photosystem II by hyperosmotic stress in Chlamydomonas reinhardtii. Plant Cell Physiol. 36, 1253–1258 (1995). [Google Scholar]
- Nixon P. J. & Rich P. R. Chlororespiratory pathways and their physiological significance. In The Structure and Function of Plastids (eds. Wise R. R., Hoober J. K.) 237–251 (Springer Dordrecht, 2007).
- Cooley J. W. & Vermaas W. F. J. Succinate dehydrogenase and other respiratory pathways in thylakoid membranes of Synechocystis sp. strain PCC 6803: capacity comparisons and physiological function. J. Bacteriol. 183, 4251–4258 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchinelli F. et al. Proteomic analysis of the Cyanophora paradoxa muroplast provides clues on early events in plastid endosymbiosis. Planta 237, 637–651 (2013). [DOI] [PubMed] [Google Scholar]
- Fester T. & Schenk H. E. A. Glucose-6-phosphate dehydrogenase isoenzymes from Cyanophora paradoxa: Examination of their metabolic integration within the meta-endocytobiotic system. In Eukaryotism and Symbiosis (eds. Schenk H. E. A., Herrmann R. G., Jeon K. W., Müller N. E., Schwemmler W.) 243–251 (Springer Berlin Heidelberg, 1997).
- Peltier G. & Cournac L. Chlororespiration. Annu. Rev. Plant Biol. 53, 523–550 (2002). [DOI] [PubMed] [Google Scholar]
- Scheibe R. Malate valves to balance cellular energy supply. Physiol. Plant. 120, 21–26 (2004). [DOI] [PubMed] [Google Scholar]
- Schlichting R., Zimmer W. & Bothe H. Exchange of metabolites in Cyanophora paradoxa and its cyanelles. Bot. Acta 103, 392–398 (1990). [Google Scholar]
- Sonoike K. Photoinhibition of photosystem I. Physiol. Plant. 142, 56–64 (2011). [DOI] [PubMed] [Google Scholar]
- Chaux F., Peltier G. & Johnson X. A security network in PSI photoprotection: regulation of photosynthetic control, NPQ and O2 photoreduction by cyclic electron flow. Front. Plant Sci. 6, 875; 10.3389/fpls.2015.00875 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suorsa M. et al. Proton gradient regulation5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell 24, 2934–2948 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A., Miyake C. & Yokota A. Physiological functions of the water-water cycle (Mehler reaction) and the cyclic electron flow around PSI in rice leaves. Plant Cell physiol. 43, 1017–1026 (2002). [DOI] [PubMed] [Google Scholar]
- Kukuczka B. et al. Proton gradient regulation5-like1-mediated cyclic electron flow is crucial for acclimation to anoxia and complementary to nonphotochemical quenching in stress adaptation. Plant physiol. 165, 1604–1617 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delosme R., Olive J. & Wollman F. A. Changes in light energy distribution upon state transitions: an in vivo photoacoustic study of the wild type and photosynthesis mutants from Chlamydomonas reinhardtii. Biochim. Biophys. Acta-Bioenergetics 1273, 150–158 (1996). [Google Scholar]
- Allen J. F. Protein phosphorylation in regulation of photosynthesis. Biochim. Biophys. Acta-Bioenergetics 1098, 275–335 (1992). [DOI] [PubMed] [Google Scholar]
- Bonente G., Pippa S., Castellano S., Bassi R. & Ballottari M. Acclimation of Chlamydomonas reinhardtii to different growth irradiances. J. Biol. Chem. 287, 5833–5847 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaas T. et al. Non-photochemical quenching and xanthophyll cycle activities in six green algal species suggest mechanistic differences in the process of excess energy dissipation. J. Plant Physiol. 172, 92–103 (2015). [DOI] [PubMed] [Google Scholar]
- Cheregi O., Kotabová E., Prášil O., Schröder W. P., Kaňa R. & Funk C. Presence of state transitions in the cryptophyte alga Guillardia theta. J. Exp. Bot. 66, 6461–6470 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. N. et al. Light-induced energetic decoupling as a mechanism for phycobilisome-related energy dissipation in red algae: a single molecule study. PLoS One 3, e3134, 10.1371/journal.pone.0003134 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delphin E., Duval J. C., Etienne A. L. & Kirilovsky D. ΔpH-dependent photosystem II fluorescence quenching induced by saturating, multiturnover pulses in red algae. Plant Physiol. 118, 103–113 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert H., Andersson M. & Snoeijs P. Relationship between photosynthesis and non-photochemical quenching of chlorophyll fluorescence in two red algae with different carotenoid compositions. Mar. Biol. 149, 1003–1013 (2006). [Google Scholar]
- Krupnik T. et al. A reaction center-dependent photoprotection mechanism in a highly robust photosystem II from an extremophilic red alga, Cyanidioschyzon merolae. J. Biol. Chem. 288, 23529–23542 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler G., Ishikawa T., Pornsaksit V. & Smirnoff N. Evolution of alternative biosynthetic pathways for vitamin C following plastid acquisition in photosynthetic eukaryotes. eLife 4, e06369, 10.7554/eLife.06369 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth S. Z., Schansker G. & Garab G. The physiological roles and metabolism of ascorbate in chloroplasts. Physiol. Plant. 148, 161–175 (2013). [DOI] [PubMed] [Google Scholar]
- Latifi A., Ruiz M. & Zhang C. C. Oxidative stress in cyanobacteria. FEMS Microbiol. Rev. 33, 258–278 (2009). [DOI] [PubMed] [Google Scholar]
- Bernroitner M., Zamocky M., Furtmüller P. G., Peschek G. A. & Obinger C. Occurrence, phylogeny, structure, and function of catalases and peroxidases in cyanobacteria. J. Exp. Bot. 60, 423–440 (2009). [DOI] [PubMed] [Google Scholar]
- Ichimura T. Sexual cell division and conjugation-papilla formation in sexual reproduction of Closterium strigosum. In International Symposium on Seaweed Research 7th, Sapporo (1971).
- Allen M. M. Simple conditions for growth of unicellular blue‐green algae on plates. J. Phycol. 4, 1–4 (1968). [DOI] [PubMed] [Google Scholar]
- Grimme L. H. & Boardman N. K. Photochemical activities of a particle fraction P1 obtained from the green alga Chlorella fusca. Biochem. Biophys. Res. Commun. 49, 1617–1623 (1972). [DOI] [PubMed] [Google Scholar]


