Abstract
Various conditions, including bacterial infection, can promote osteonecrosis. For example, following invasive dental therapy with anti-bone resorptive agents, some patients develop osteonecrosis in the jaw; however, pathological mechanisms underlying these outcomes remain unknown. Here, we show that administration of anti-resorptive agents such as the bisphosphonate alendronate accelerates osteonecrosis promoted by infectious osteomyelitis. Potent suppression of bone turnover by these types of agents is considered critical for osteonecrosis development; however, using mouse models we found that acceleration of bone turnover by teriparatide injection did not prevent osteonecrosis but rather converted osteoclast progenitors to macrophages expressing inflammatory cytokines, which were required for osteonecrosis development. In fact, we demonstrate that TNFα-, IL-1α/β- or IL-6-deficient mice as well as wild-type mice administered a TNFα-inhibitor were significantly resistant to development of osteonecrosis accompanying infectious myelitis, even under bisphosphonate treatment. Our data provide new insight into mechanisms underlying osteonecrosis and suggest new ways to prevent it.
Osteonecrosis is characterized by osteocyte death, as marked by the appearance of empty lacunae in cortical bone1,2, and develops in response to trauma, radiation, bacterial infection or ischemia3,4,5,6,7,8,9. Most osteonecrosis is intractable and often causes bone fragility fractures or bone deformities10,11,12. Osteonecrosis of the jaw (ONJ) was initially reported in patients who had undergone invasive dental treatment such as tooth extraction or implantation and had been administered long term the bisphosphonate alendronate, which potently inhibits bone resorption and suppresses bone turnover13,14. Such osteonecrosis was initially termed bisphosphonate-related ONJ (BRONJ)15. ONJ was subsequently seen in patients treated with desnosumab, an antibody targeting receptor activator of nuclear factor kappa B ligand (RANKL), a different anti-resorptive agent. As a result, ONJ seen in patients treated with anti-resorptive agents in general is now called ARONJ (anti-resorptive agent-related ONJ)16. To date, ARONJ is reported in patients with either osteoporosis or metastatic bone tumors who were administered strong anti-bone resorbing agents, including but not limited to bisphosphonates and denosumab16. ARONJ is intractable and causes severe deficits in quality of life17; thus preventing osteonecrosis is critical for maintenance of activity of daily living in ARONJ patients.
Given these outcomes and that metastatic bone cancer patients administered particularly high doses of anti-resorbing agents frequently exhibit ARONJ, investigators have concluded that either long-term usage or high doses of these drugs promotes a condition known as severely suppressed bone turnover (SSBT), which associated with osteonecrosis development18,19. Interestingly, treatment with teriparatide, a parathyroid hormone (PTH) peptide that activates bone turnover, is reportedly effective in treating ARONJ patients20,21.
In addition to SSBT, local infection is thought to promote ARONJ8, since jaws are near the oral cavity where >1011 bacteria/cm3 exist and may be released following invasive dental treatment. ARONJ is also seen in individuals with poor oral hygiene or diabetes myelitis or rheumatoid arthritis (RA) patients, who are often at high risk for infection8,12. At present, a relationship between the use of anti-resorbing agents and infection is not known.
To date, several ONJ animal models have been reported22,23,24,25,26. For example, immune-deficient beige nu/nu Xid (III) mice exhibit ARONJ-like phenotypes following tooth extraction and treatment with the bisphosphonate zoledronate and the steroid dexamethasone27. ONJ is also reportedly seen in bisphosphonate-treated rats23, dogs28 and other species29. In contrast, ONJ is not seen in C57/BL6 wild-type mice undergoing tooth extraction and zoledronate treatment, although mice exhibit delayed wound healing30. The activity of regulatory T cells (Tregs)27 or γδ T cells31 reportedly promotes ARONJ development, although it is unclear how these activities promote osteonecrosis development.
Here, we hypothesized that ARONJ develops from a combination of infectious myelitis and treatment of anti-resorptive agents. Using mice, we show that alendronate treatment exacerbates osteonecrosis development in femur in cases of infectious osteomyelitis. Accelerating bone turnover by teriparatide administration did not prevent osteonecrosis development in this model. Following alendronate treatment of mice with infectious osteomyelitis, osteocytes underwent apoptosis. Moreover, in the presence of alendronate, osteoclast progenitor cells were converted to TNFα-, IL-6- and IL-1β-expressing cells in vitro, and blocking TNFα, either by gene targeting or treatment with the TNFα-inhibitor etanercept, significantly inhibited osteonecrosis development, even in alendronate-treated mice with infectious osteomyelitis mice. IL-6- or IL-1α/β-deficient mice were also significantly resistant to osteonecrosis development in this model. Our data provide insight into osteonecrosis pathogenesis and suggest therapeutic targets potentially useful to antagonize ONJ, even in the presence of anti-resorptive therapy.
Results
Anti-bone resorbing agents accelerate osteonecrosis development in the presence of infectious osteomyelitis
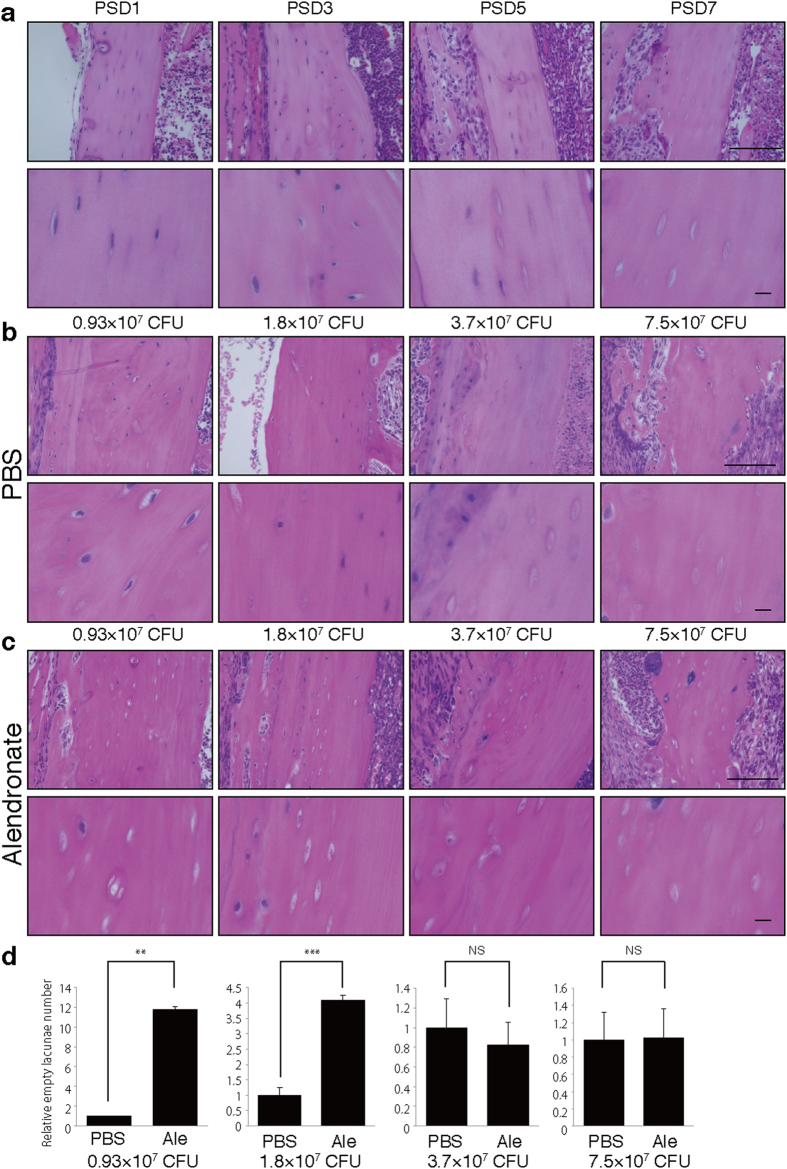
To assess effects of anti-resorptive bisphosphonates on osteonecrosis, we created a murine model of osteonecrosis by first pre-treating wild-type mice with alendronate (Ale) by injection and then surgically infecting the left femurs with Streptococcus aureus (SA) (Fig. 1). Left femurs were sham-operated and served as non-infected controls. Osteonecrosis, as marked by the appearance of empty lacunae in cortical bone, was not seen in the sham-operated femur of alendronate-treated mice (Alendronate/PBS) (Fig. S1) but appeared in mice treated with a combination of SA and alendronate in a time- and SA titer-dependent manner (Fig. S1a–d). This observation suggests that administration of alendronate alone is not sufficient for osteonecrosis development. Higher SA titers (>3.7 × 107 CFU) promoted osteonecrosis development in the absence of alendronate (PBS group) (Fig. 1b and d). However, osteonecrosis developed even at lower SA titers (<1.8 × 107 CFU) in alendronate-treated mice (Fig. 1c and d), suggesting that alendronate accelerates osteonecrosis development.
Figure 1. Alendronate treatment accelerates osteonecrosis development.
Wild-type mice were administered alendronate (Ale) or vehicle (PBS) for two weeks. Then, infectious osteomyelitis was established by direct surgical application of 7.5 × 107 (a) or indicated (b–d) colony-forming units (CFU) of Streptococcus aureus (SA) to femurs. After indicated (a) or seven (b–d) days post surgery (post surgery day, PSD), sections of cortical bone were stained with HE, observed microscopically (a-c), and the number of empty versus whole lacunae was scored. Data represents relative proportion of empty versus whole lacunae in cortical bone ± SD (n = 4). Scale bars = 100 μm (upper) and 10 μm (lower panels). **P < 0.01; ***P < 0.001; NS, not significant. Representative data of at least two independent experiments are shown.
Similar to outcomes seen in alendronate-treated mice, osteonecrosis developed in mice administered anti-RANKL antibody, a different anti-resorptive agent (Fig. S2), suggesting that in the presence of osteomyelitis, treatment with any anti-bone resorbing agent promotes osteonecrosis development.
Overall, osteonecrosis was induced in osteocytes in ratio indicated in Fig. S1 in 100% of mice treated with a combination of SA and anti-bone resorptive agents.
Osteocytes in alendronate-treated mice with infectious osteomyelitis undergo apoptosis
Treatment of patients with teriparatide, a bioactive form of PTH, reportedly antagonizes SSBT20,21. Thus, we administered teriparatide to alendronate-treated osteomyelitis model mice by subcutaneous injection at the time of SA infection and then twice weekly (Fig. S3). Osteonecrosis development was, however, not blocked by teriparatide treatment (Ale + PTH) but rather developed at levels comparable to those seen in mice not treated with teriparatide (Ale) (Fig. S3), suggesting that SSBT in this context does not promote osteonecrosis development. Moreover, bone turnover as evaluated by osteoid production was inhibited by alendronate treatment but reversed by PTH (Fig. S4a). In contrast, proliferation of bone lining osteoblastic cells, as analyzed by staining for proliferating cell nuclear antigen (PCNA), was inhibited by alendronate and not fully rescued by PTH treatment (Fig. S4b). Teriparatide administration did not promote up-regulation of bone-turnover markers in sera in patients treated with alendronate32. However, administration of alendronate alone did not promote osteonecrosis development, strongly suggesting that SSBT is not the primary cause of osteonecrosis.
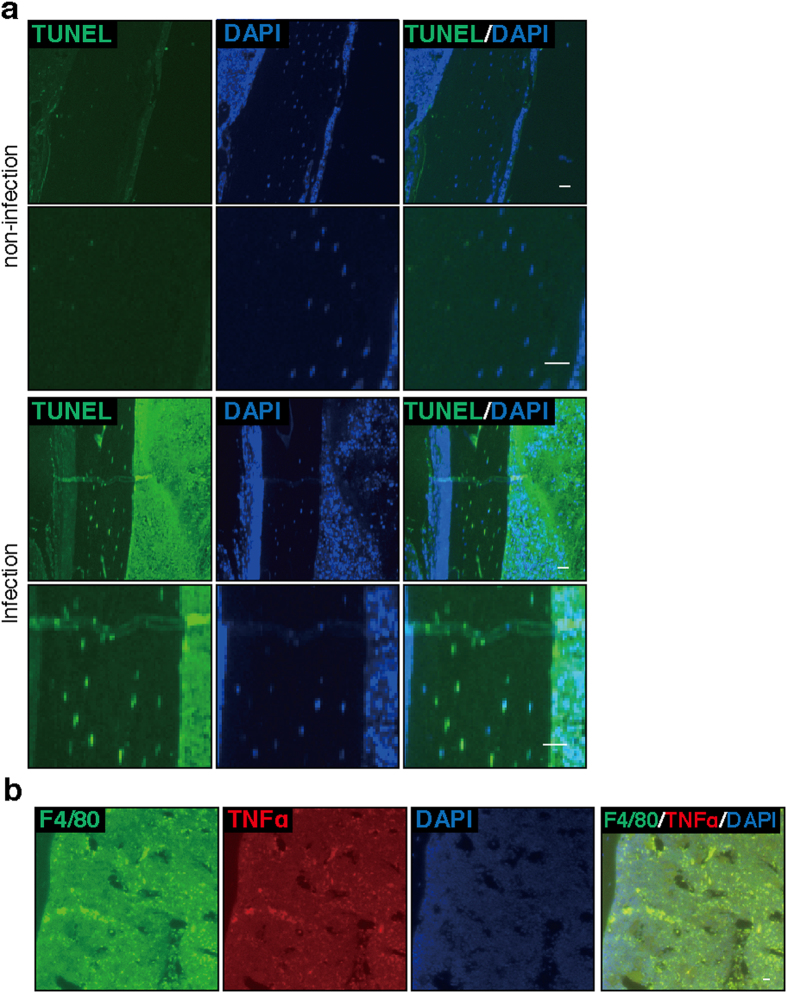
Nonetheless, we found that osteonecrosis developed on the SA-infected but not the control, uninfected side of mice injected with alendronate (Fig. S5), suggesting that disease emerges from local rather than systemic factors in alendronate-administered, SA-infected mice. Furthermore, we observed apoptosis, as detected by a TUNEL assay, in osteocytes of infected and alendronate-treated mice (Fig. 2a). TNFα is a strong inducer of apoptosis33. In accordance, immunofluorescence analysis revealed TNFα expression in F4/80-positive macrophages in osteomyelitis mice treated with alendronate (Fig. 2b), suggesting an association of alendronate treatment with TNFα expression. Thus, we focused on TNFα rather than SSBT as a potential underlying cause of osteonecrosis seen in the presence of anti-resorptive agents.
Figure 2. Alendronate treatment promotes osteocyte apoptosis and stimulates TNFα expression in macrophages.
Wild-type mice were administered alendronate for two weeks. Then, osteomyelitis (infection) was established in left femurs, as in Fig. 1. Right femurs were sham-operated and served as controls (non-infection). Seven days later, bone sections were prepared and labeled with Biotin-dUTP using terminal deoxynucleotidyl trans (TdT), followed by Avidin-DTAF as TUNEL staining (TUNEL) to identify apoptotic cells (a). Sections were doubly-stained with Alexa488-conjugated rat anti-mouse F4/80 and goat anti-mouse TNFα, followed by Alexa488-conjugated donkey anti-rat Ig’ and Alexa568-conjugated donkey anti-goat Ig’. (b). Nuclei were stained with DAPI. Sections were observed under a fluorescence microscope. Bar = 10 μm. Representative data of at least two independent experiments are shown.
Infection in the presence of alendronate treatment converts osteoclast progenitors to TNFα-expressing cells
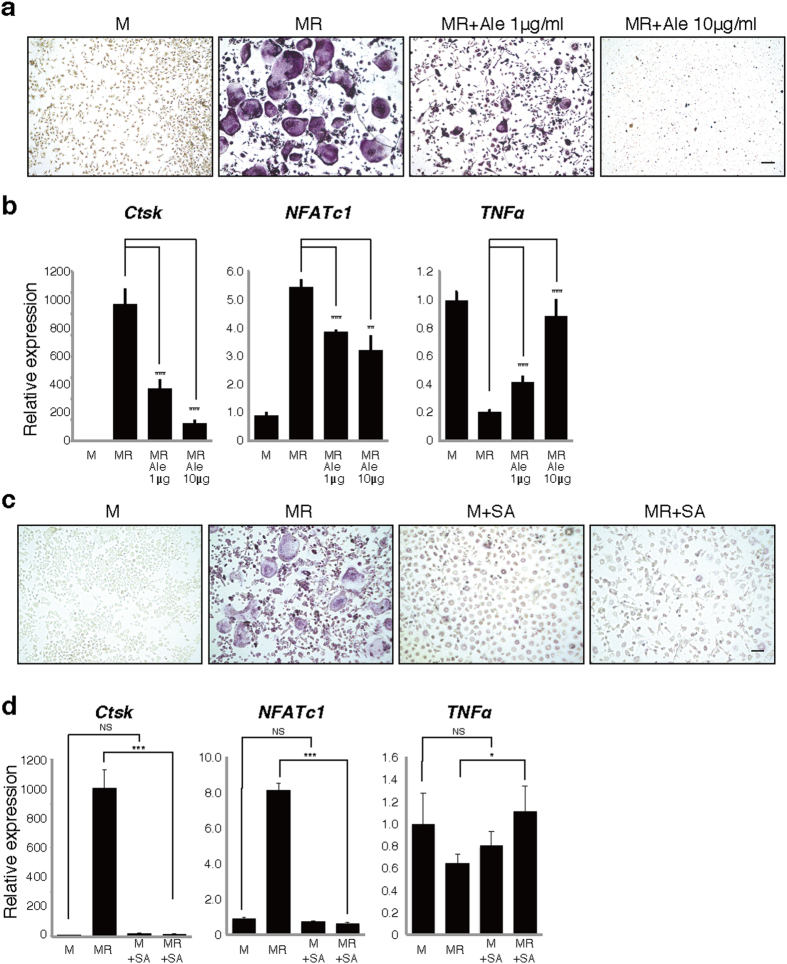
To determine potential effects of anti-resorptive agents on osteoclast progenitors, we isolated M-CSF-dependent bone marrow macrophages (BMMs) as osteoclast progenitors and then cultured with M-CSF (M) and RANKL (R) in the presence or absence of various alendronate concentrations (Fig. 3). Interestingly, TNFα expression in BMMs was significantly downregulated by RANKL along with osteoclast differentiation, as indicated by induction of Cathepsin K (Ctsk) and nuclear factor of activated T cells 1 (NFAtc1), but was significantly stimulated by alendronate in a dose-dependent manner inversely correlated with inhibition of osteoclastogenesis (Fig. 3a and b).
Figure 3. Alendronate treatment or Streptococcus aureus infection increases TNFα expression and inhibits osteoclastogenesis.
Osteoclast progenitors were isolated from wild-type mice and cultured in the presence or absence of M-CSF (M) and RANKL (R) with or without 1 or 10 μg/ml alendronate (Ale) (a,b) or Streptococcus aureus lysate (SA) (c,d). Osteoclast formation was evaluated by TRAP staining (a,c), or realtime PCR to analyze expression of Cathepsin K (Ctsk) and NFATc1 (b,d). TNFα expression was also analyzed by realtime PCR(b,d). Scale bar = 100 μm. Data represent mean Ctsk, NFATc1 or TNFα expression relative to β-actin ± SD (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant. Representative data of at least two independent experiments are shown.
Bacterial infection also strongly induces TNFα expression34. Indeed, TNFα expression was significantly upregulated by treatment of cultured BMMs with SA lysate even in the presence of RANKL (Fig. 3c and d).
Similar to SA, we also observed development of osteonecrosis following of development of infectious osteomyelitis by infection of femurs of alendronate-treated mice with Porphyromonas gingivaris (PG), a periodontal bacteria (Fig. S6a and b). In vitro, osteoclastogenesis was significantly inhibited while TNFα expression was significantly upregulated by PG treatment in osteoclast progenitor cells (Fig. S6c and d), as seen in SA-treated cells. These results suggest combined infection plus treatment with anti-resorptive agents promotes TNFα expression in osteoclast progenitors, and anti-resorptive agents convert the osteoclast progenitor cells into TNFα-expressing macrophages even in the presence of RANKL.
TNFα is required for osteonecrosis in osteomyelitis mice administered alendronate
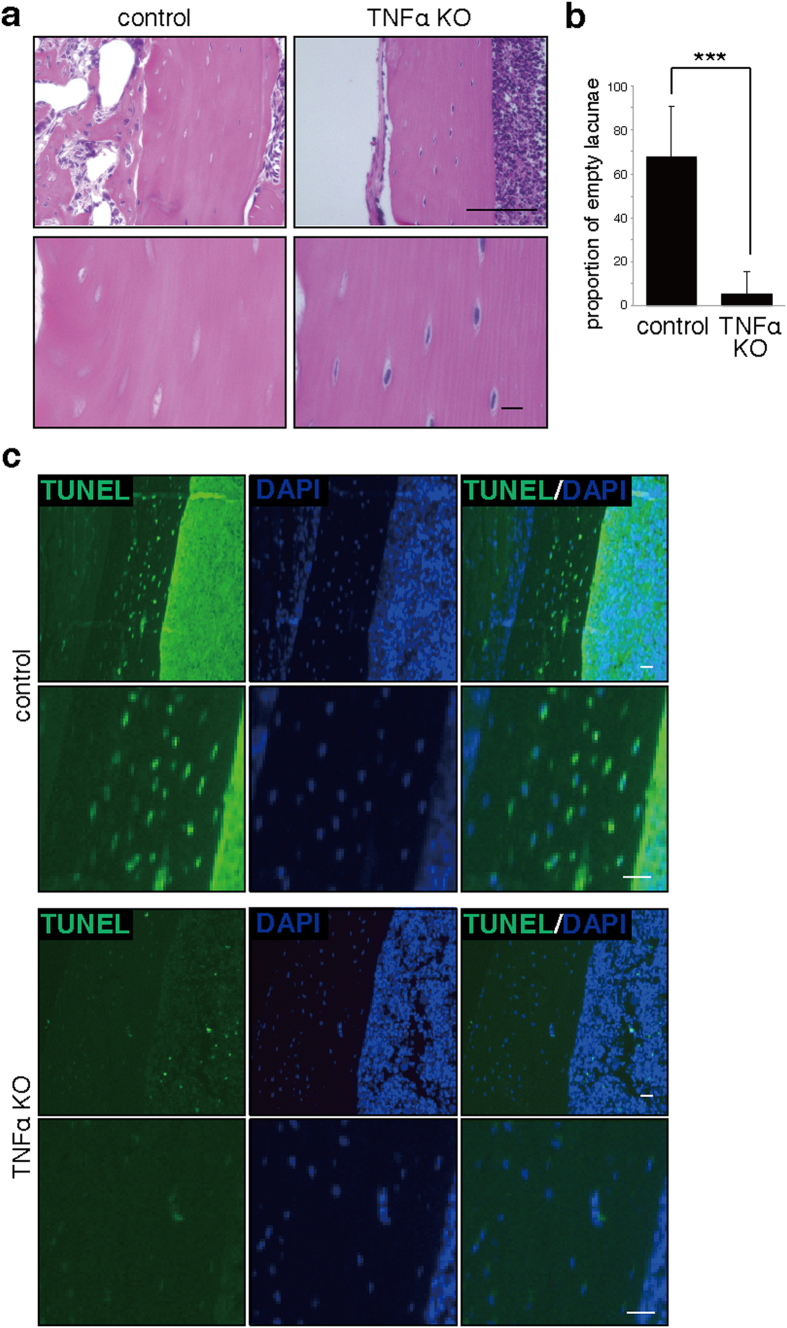
To determine whether TNFα is required for osteonecrosis development in our model system, we induced infectious osteomyelitis using a comparable experimental protocol in TNFα-deficient (TNFα KO) mice administered alendronate (Fig. 4). We observed that osteonecrosis promoted by infection was significantly blocked in TNFα-deficient mice (Fig. 4a and b). Likewise, the number of either apoptotic TUNEL-positive or necrotic ssDNA-positive osteocytes decreased significantly in TNFα-deficient mice (Fig. 4c and Fig. S7), suggesting that TNFα is required for apoptosis induction.
Figure 4. TNFα is required for osteocyte apoptosis accompanying osteonecrosis.
Wild-type (control) or TNFα-deficient (TNFα KO) mice were administered alendronate for two weeks. Infectious osteomyelitis was then established by Streptococcus aureus infection of left femurs of control (wild-type) or TNFα KO mice. One week later, cortical bone sections were prepared and stained with HE (a) or TUNEL (c), and the proportion of empty to whole lacunae in cortical bone was determined (b). DAPI served as a nuclear stain (c). Data shows the mean percentage (%) of empty versus whole lacunae ± SD (n = 4, ***P < 0.001). Scale bars = 100 (upper in (a) or 10 μm (lower panels in (a), and all panels in (c). Representative data of at least two independent experiments are shown.
In experiments with wild-type mice, we also observed significant upregulation of the pro-inflammatory cytokines IL-1β, IL-6, IL-17a and IL-17f in infectious osteomyelitis tissue relative to control femurs in SA-infected, alendronate-treated (SA+, Ale+) versus infected but non-treated (SA+, Ale−) controls (Fig. S8). These results suggest that inflammatory cytokine expression by infection was further stimulated by alendronate treatment. Interestingly, osteonecrosis induced by infectious osteomyelitis plus alendronate administration was significantly blocked in either IL-1α/β (IL-1 KO)- or IL-6-deficient (IL-6 KO) mice, and was weakly attenuated in IL-17A/F-deficient (IL-17 KO) mice (Fig. S9).
Administration of TNFα antagonists or vitamin D analogues antagonizes osteonecrosis in infected mice treated with anti-resorptive agents
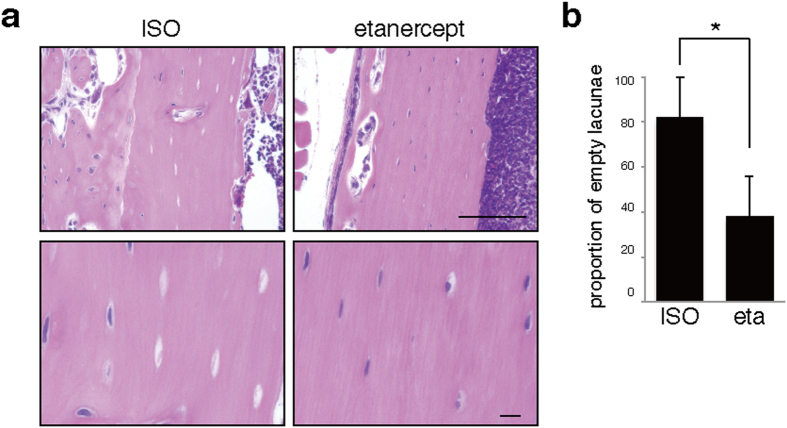
To develop potential treatments against osteonecrosis brought on by infectious osteomyelitis and treatment with anti-resorptive agents, we administered etanercept, a TNFα-inhibitor, subcutaneously one week before surgery and subsequently twice a week to alendronate-treated model mice infected in the left femur with SA (Fig. 5). Etanercept treatment significantly blocked osteonecrosis development in the infected femur (Fig. 5).
Figure 5. Blocking TNFα significantly antagonizes osteonecrosis development.
Wild-type mice were administered alendronate for two weeks. Then, infectious osteomyelitis was established by Streptococcus aureus infection of left femurs. The TNFα inhibitor etanercept (eta) or ISO type control (ISO) was subcutaneously injected one week before surgery and then subsequently twice a week. Seven days after surgery, cortical bone sections of left femurs were prepared and stained with HE (a), and the proportion of empty versus whole lacunae was calculated (b). Scale bars = 100 (upper) or 10 μm (lower panels). Data shows the mean percentage (%) of empty versus whole lacunae ± SD (n = 4, *P < 0.05). Representative data of at least two independent experiments are shown.
Active vitamin D, 1,25(OH)2D3 (VD3), or its analogue ED71, is often administered with anti-resorptive agents to osteoporosis patients35,36. We observed that administration of either VD3 or ED71 two weeks before surgery and subsequently twice weekly significantly blocked osteonecrosis in alendronate-treated mice with infectious osteomyelitis (Fig. S10). These studies suggest overall that either blocking TNFα or treatment with vitamin D analogues can prevent osteonecrosis development due to infection in mice treated with anti-resorptive agents.
Finally, we asked ectopic bone formation, which frequently occurs in ARONJ patients, resulting in facial deformities, a serious concern for patients17. We detected ectopic bone formation beneath the periosteum in mice treated systemically with alendronate on the side exhibiting infectious osteomyelitis but not on the control side (Fig. S11a). We observed similar outcomes following anti-RANKL treatment in the presence of SA infection or in alendronate-treated mice infected with PG (Fig. S11b and c). Our findings in mice are likely consistent with clinical observations, as ectopic bone formation in ARONJ patients is limited to the jaw, even in the presence of anti-resorptive treatment37,38,39. Interestingly, in our model of infectious osteomyelitis in the presence of alendronate, ectopic bone formation under the periosteum was significantly blocked by targeting of TNFα- or IL-1α/β or by treatment with a TNFα inhibitor (Fig. S11d and e). By contrast, ectopic bone formation was not blocked by treatment with PTH or vitamin D analogues (Fig. S11f and g).
Discussion
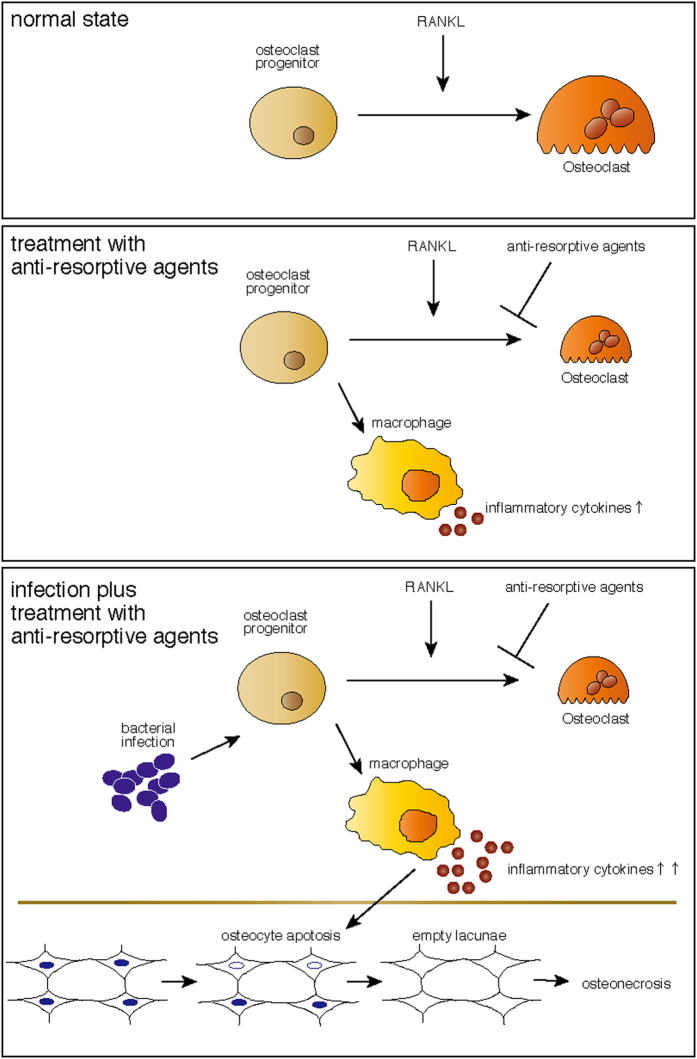
Treatment with anti-resorptive agents may promote ARONJ, and there is debate over whether these types of drugs should be withdrawn to prevent ARONJ prior to invasive oral treatment, despite fracture risk. In this study we show that alendronate treatment significantly exacerbates infectious osteomyelitis-induced osteonecrosis development. We found that osteonecrosis development was not blocked by teriparatide, which promotes bone turnover, suggesting that low bone turnover does not underlie osteonecrosis. Instead, we show that osteonecrosis development in infectious osteomyelitis mice administered alendronate was significantly blocked by gene targeting of either TNFα-, IL-6 or IL-1α/β, or by treatment with a TNFα inhibitor. Moreover we observed that osteoclast progenitors were converted to inflammatory cytokine-expressing cells following alendronate treatment, even in the presence of RANKL. Thus, pro-inflammatory cytokines may represent therapeutic targets to prevent osteonecrosis induced by infectious osteomyelitis in patients treated with anti-resorptive therapy (Fig. 6).
Figure 6. A schematic model for osteonecrosis development by osteomyelitis in the presence of administration of anti-resorptive agents.
(upper) In the normal state, osteoclast progenitors differentiate into osteoclasts by RANKL exposure. (middle) Treatment with anti-resorptive agents converts osteoclast progenitors to inflammatory cytokine-expressing macrophages. (lower) Bacterial infection further promotes inflammatory cytokine expression, which in turn promotes osteocyte apoptosis, leading to osteonecrosis.
ARONJ development in patients is limited to jaws, even in SSBT conditions promoted by treatment with anti-resorptive agents. Indeed, in our model, alendronate treatment alone did not promote osteonecrosis development, and osteonecrosis occurred in infectious osteomyelitis but not on the non-infectious control side in mice treated with alendronate, suggesting that local inflammation underlies osteonecrosis. Indeed, systemic inflammation is seen in RA patients, who are frequently treated with anti-resorptive agents to antagonize secondary osteoporosis. However, not all RA patients develop ARONJ, suggesting that strong local inflammation induced by infection plus potential conversion of osteoclast progenitors to inflammatory macrophages is a primary contributor to osteonecrosis.
We also found that although either VD3 or ED71 could prevent infectious myelitis from developing into osteonecrosis in the presence of alendronate treatment (Fig. S10), neither agent inhibited inflammatory cytokine expression by either macrophages or osteoclasts (Fig. S12). These results suggest that vitamin D or vitamin D analogues prevent osteonecrosis development via cells other than macrophages or osteoclasts or by mechanisms different from those that regulate inflammation. Further studies are needed to determine how VD3 and ED71 block osteonecrosis development.
To date, several ARONJ models have been established in mice. Osteonecrosis was reportedly seen following administration of the bisphosphonate zoledronate, following tooth extraction in wild-type mice27,40,41,42,43,44. These methods are considered more straightforward techniques to investigate ARONJ development compared with our models and thus represent a limitation of our study. Meanwhile, over 5–15 weeks of observation, osteonecrosis development in these models occurred in 0–40% of osteocytes27,40,41,42,43,44 in approximately 10% of wild-type mice26; however, our model promotes osteonecrosis development in three weeks in 100% of wild-type mice, and 60–80% of lacunae observed in the model were empty. This enabled us to analyze effects of inflammatory cytokines on osteonecrosis development. Infectious myelitis of the jaw is considered a cause of ARONJ development in humans who have undergone invasive dental treatment and are being treated with bisphosphonates8,45. Thus models combining infectious myelitis with bisphosphonate administration are useful to understand mechanisms underlying ARONJ development. Further studies are needed to fully understand pathological mechanisms underlying ARONJ development.
Our study suggests that it is critical to control inflammation in cases of invasive dental treatment in patients administered anti-resorptive agents to prevent ARONJ and ectopic bone formation. Anti-TNFα drugs or reagents that block IL-6 or IL-1 receptor agonists now clinically available may serve as useful therapies in this context.
Methods
Mice
Wild-type mice were purchased from Sankyo Labo Service (Tokyo, Japan). TNFα-deficient mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). IL-1α−/− IL-1β−/−, IL-17-, and IL-6-deficient mice were prepared as described1,2,3. Animals were maintained under specific pathogen-free conditions in animal facilities certified by the Keio University Institutional Animal Care and Use Committee, and animal protocols were approved by that committee. Animals were housed up to 5 mice per cage and kept on a 12 h light/dark cycle. Water and food was available ad libitum. All animal studies were performed in accordance with the Guidelines of the Keio University animal care committee.
Immunofluorescence
Surgical sections of bone tissue were stained using a MEBSTAIN Apoptosis TUNEL Kit Direct (MEDICAL & BIOLOGICAL LABORATORIES CO., LTD., Nagoya, Japan), or stained with anti-single standard DNA (ssDNA) Rabbit IgG (#18731 1:50; IBL, Gunma, Japan) followed by Alexa488-conjugated goat anti-rabbit Ig’ (#A-11034 1:200; Invitrogen, Carlsbad, CA). Sections were also stained with Alexa Fluor 488-conjugated rat anti-mouse F4/80 (#B116640 1:100 BioLegend, San Diego, CA, USA) and goat anti-mouse TNFα (#K2911 1:100 Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), followed by Alexa488-conjugated donkey anti-rat Ig’ (#A-21208 1:200; Invitrogen, Carlsbad, CA) and Alexa568-conjugated donkey anti-goat Ig’ (#A-11057 1:200; Invitrogen, Carlsbad, CA). Sections were also stained with purified mouse anti-PCNA (#610664 1:100; BD Transduction Laboratories, Franklin Lakes, New Jersey) followed by Alexa488-conjugated goat anti-mouse Ig’ (#A-11029 1:200; Invitrogen, Carlsbad, CA). DAPI (#D1306 1:750; Wako Pure Chemicals Industries, Osaka, Japan) was used as a nuclear stain.
In vitro osteoclast formation
Bone marrow cells isolated from wild-type mouse femurs and tibias were cultured 72 h in MEM (Sigma-Aldrich Co.) containing 10% (vol/vol) heat-inactivated FBS (JRH Biosciences) and GlutaMax (Invitrogen Corp.) supplemented with M-CSF (50 ng/mL, Kyowa Hakko Kirin Co.). Subsequently, adherent cells were collected and cultured in 96-well plates (1 × 105 cells per well) under indicated conditions containing M-CSF (50 ng/mL) and recombinant soluble RANKL (25 ng/mL, PeproTech Ltd.) with or without a SA or PG lysate. Lysates were prepared using RIPA buffer (1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 150 mM NaCl, 5 mM EDTA, 1 mM dithiothreitol, 10 mM Tris-HCl, pH7.5) supplemented with a protease inhibitor cocktail (Sigma-Aldrich Co.) and MG-132 (EMD Millipore Corporation). Medium was changed every 2 days. Osteoclastogenesis was evaluated by TRAP staining, as described4,5.
Quantitative PCR
Total RNAs were isolated from bone marrow cultures using TRIzol reagent (Invitrogen Corp.), and cDNA synthesis was performed using oligo(dT) primers and reverse transcriptase (Wako Pure Chemicals Industries). Quantitative PCR was performed using SYBR Premix ExTaq II reagent and a DICE Thermal cycler (Takara Bio Inc.), according to the manufacturer’s instructions. β-actin (Actb) expression served as an internal control. Primers for realtime PCR were as follows.
β-actin-forward: 5′-TGAGAGGGAAATCGTGCGTGAC-3′
β-actin-reverse: 5′-AAGAAGGAAGGCTGGAAAAGAG-3′
Ctsk-forward: 5′-ACGGAGGCATTGACTCTGAAGATG-3′
Ctsk-reverse: 5′-GGAAGCACCAACGAGAGGAGAAAT-3′
NFATc1-forward: 5′-CAAGTCTCACCACAGGGCTCACTA-3′
NFATc1-reverse: 5′-GCGTGAGAGGTTCATTCTCCAAGT-3′
TNFα-forward: 5′- AAGCCTGTAGCCCACGTCGT-3′
TNFα-reverse: 5′-GGCACCACTAGTTGGTTGTCTTTG -3′
IL-1β-forward: 5′-AAGTTGACGGACCCCAAAAGAT-3′
IL-1β-reverse: 5′-AGCTCTTGTTGATGTGCTGCTG-3′
IL-6-forward: 5′-GTCCTTAGCCACTCCTTCTG-3′
IL-6-reverse: 5′-CAAAGCCAGAGTCCTTCAGAG-3′
IL-17a-forward: 5′-TGTGAAGGTCAACCTCAAAGTCT-3′
IL-17a-reverse: 5′-CCCAGATCACAGAGGGATATCTA-3′
IL-17f-forward: 5′-TACTTCCTGAGGGAAGAAGCAG-3′
IL-17f-reverse: 5′-GCAAGTCCCAACATCAACAGTA-3′
Statistical analysis
Results are expressed as means ± s.d. Statistical significance of differences between groups was evaluated using Student’s t-test (*P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant, throughout the paper).
Additional Information
How to cite this article: Morita, M. et al. Elevation of pro-inflammatory cytokine levels following anti-resorptive drug treatment is required for osteonecrosis development in infectious osteomyelitis. Sci. Rep. 7, 46322; doi: 10.1038/srep46322 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
T. Miyamoto was supported by a grant-in-aid for Scientific Research in Japan and a grant from the Japan Agency for Medical Research and Development. Y. Sato and K. Miyamoto were supported by a grant-in-aid for Scientific Research in Japan. This study was supported in part by a grant-in-aid for Scientific Research and a grant from the Translational Research Network Program.
Footnotes
The authors declare no competing financial interests.
Author Contributions M.M. (Morita) performed cell culture and animal experiments. R.I., M.M. (Matsumoto), M.N., H.K., T.N. and T.M. designed the study. Y.S. and T.K. prepared animals for experiments. R.W., T.O., S.N., Y.K., K.M. and T.M. analyzed data. K.I. (Ishihara) prepared PG. Y.I. established IL-17A/F-deficient mice. K.I. (Ishii) performed and supervised animal experiments. T.M. wrote the manuscript with input from all authors. All authors discussed the results and commented on the manuscript.
References
- Frost H. M. In vivo osteocyte death. J Bone Joint Surg Am 42-A, 138–143 (1960). [PubMed] [Google Scholar]
- Fondi C. & Franchi A. Definition of bone necrosis by the pathologist. Clin Cases Miner Bone Metab 4, 21–26 (2007). [PMC free article] [PubMed] [Google Scholar]
- Hermodsson I. On the problem of trauma and aseptic osteonecrosis. Acta radiol 28, 257–268 (1947). [DOI] [PubMed] [Google Scholar]
- Phemister D. B. Treatment of the necrotic head of the femur in adults. J Bone Joint Surg Am 31A, 55–66 (1949). [PubMed] [Google Scholar]
- Almquist E. E. Kienbock’s disease. Clin Orthop Relat Res 68–78 (1986). [PubMed] [Google Scholar]
- Marx R. E. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg 41, 283–288 (1983). [DOI] [PubMed] [Google Scholar]
- Milner P. F. et al. Sickle cell disease as a cause of osteonecrosis of the femoral head. N Engl J Med 325, 1476–1481, doi: 10.1056/NEJM199111213252104 (1991). [DOI] [PubMed] [Google Scholar]
- Hinson A. M., Smith C. W., Siegel E. R. & Stack B. C. Jr. Is bisphosphonate-related osteonecrosis of the jaw an infection? A histological and microbiological ten-year summary. Int J Dent 2014, 452737, doi: 10.1155/2014/452737 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almazrooa S. A. & Woo S. B. Bisphosphonate and nonbisphosphonate-associated osteonecrosis of the jaw: a review. J Am Dent Assoc 140, 864–875 (2009). [DOI] [PubMed] [Google Scholar]
- Mont M. A., Jones L. C. & Hungerford D. S. Nontraumatic osteonecrosis of the femoral head: ten years later. J Bone Joint Surg Am 88, 1117–1132, doi: 10.2106/JBJS.E.01041 (2006). [DOI] [PubMed] [Google Scholar]
- Schnetzke M. et al. Quality of Reduction Influences Outcome After Locked-Plate Fixation of Proximal Humeral Type-C Fractures. J Bone Joint Surg Am 98, 1777–1785, doi: 10.2106/JBJS.16.00112 (2016). [DOI] [PubMed] [Google Scholar]
- Khan A. A. et al. Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Miner Res 30, 3–23, doi: 10.1002/jbmr.2405 (2015). [DOI] [PubMed] [Google Scholar]
- Holzinger D. et al. Effect of dental implants on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg 72, 1937 e1931-1938, doi: 10.1016/j.joms.2014.04.037 (2014). [DOI] [PubMed] [Google Scholar]
- Matsuo A. et al. Evaluation of dental implants as a risk factor for the development of bisphosphonate-related osteonecrosis of the jaw in breast cancer patients. Odontology 104, 363–371, doi: 10.1007/s10266-015-0207-4 (2016). [DOI] [PubMed] [Google Scholar]
- Ruggiero S. L. Guidelines for the diagnosis of bisphosphonate-related osteonecrosis of the jaw (BRONJ). Clin Cases Miner Bone Metab 4, 37–42 (2007). [PMC free article] [PubMed] [Google Scholar]
- Hellstein J. W. et al. Managing the care of patients receiving antiresorptive therapy for prevention and treatment of osteoporosis: executive summary of recommendations from the American Dental Association Council on Scientific Affairs. J Am Dent Assoc 142, 1243–1251 (2011). [DOI] [PubMed] [Google Scholar]
- Miksad R. A. et al. Quality of life implications of bisphosphonate-associated osteonecrosis of the jaw. Oncologist 16, 121–132, doi: 10.1634/theoncologist.2010-0183 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero S. L., Mehrotra B., Rosenberg T. J. & Engroff S. L. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg 62, 527–534 (2004). [DOI] [PubMed] [Google Scholar]
- Odvina C. V. et al. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab 90, 1294–1301, doi: 10.1210/jc.2004-0952 (2005). [DOI] [PubMed] [Google Scholar]
- Song K. E. et al. A probable case of oral bisphosphonate-associated osteonecrosis of the jaw and recovery with parathyroid hormone treatment. Curr Ther Res Clin Exp 69, 356–362, doi: 10.1016/j.curtheres.2008.08.003 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A. & Seeman E. Teriparatide therapy for alendronate-associated osteonecrosis of the jaw. N Engl J Med 363, 2473–2474, doi: 10.1056/NEJMc1002684 (2010). [DOI] [PubMed] [Google Scholar]
- Allen M. R. Medication-Related Osteonecrosis of the Jaw: Basic and Translational Science Updates. Oral Maxillofac Surg Clin North Am 27, 497–508, doi: 10.1016/j.coms.2015.06.002 (2015). [DOI] [PubMed] [Google Scholar]
- Aghaloo T. L. et al. RANKL inhibitors induce osteonecrosis of the jaw in mice with periapical disease. J Bone Miner Res 29, 843–854, doi: 10.1002/jbmr.2097 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Molon R. S. et al. OPG-Fc but Not Zoledronic Acid Discontinuation Reverses Osteonecrosis of the Jaws (ONJ) in Mice. J Bone Miner Res 30, 1627–1640, doi: 10.1002/jbmr.2490 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi M. et al. Introducing a protocol to create bisphosphonate-related osteonecrosis of the jaw in rat animal model. J Craniomaxillofac Surg 44, 271–278, doi: 10.1016/j.jcms.2015.12.010 (2016). [DOI] [PubMed] [Google Scholar]
- Zhang Q. et al. Bisphosphonate Induces Osteonecrosis of the Jaw in Diabetic Mice via NLRP3/Caspase-1-Dependent IL-1beta Mechanism. J Bone Miner Res 30, 2300–2312, doi: 10.1002/jbmr.2577 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuiri T. et al. Cell-based immunotherapy with mesenchymal stem cells cures bisphosphonate-related osteonecrosis of the jaw-like disease in mice. J Bone Miner Res 25, 1668–1679, doi: 10.1002/jbmr.37 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr D. B. & Allen M. R. Mandibular necrosis in beagle dogs treated with bisphosphonates. Orthod Craniofac Res 12, 221–228, doi: 10.1111/j.1601-6343.2009.01456.x (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D., Hamlet S., Petcu E. & Ivanovski S. Animal models for bisphosphonate-related osteonecrosis of the jaws–an appraisal. Oral Dis 19, 747–754, doi: 10.1111/odi.12067 (2013). [DOI] [PubMed] [Google Scholar]
- Kobayashi Y. et al. Zoledronic acid delays wound healing of the tooth extraction socket, inhibits oral epithelial cell migration, and promotes proliferation and adhesion to hydroxyapatite of oral bacteria, without causing osteonecrosis of the jaw, in mice. J Bone Miner Metab 28, 165–175, doi: 10.1007/s00774-009-0128-9 (2010). [DOI] [PubMed] [Google Scholar]
- Movila A. et al. Possible pathogenic engagement of soluble Semaphorin 4D produced by gammadeltaT cells in medication-related osteonecrosis of the jaw (MRONJ). Biochem Biophys Res Commun 480, 42–47, doi: 10.1016/j.bbrc.2016.10.012 (2016). [DOI] [PubMed] [Google Scholar]
- Finkelstein J. S. et al. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med 349, 1216–1226, doi: 10.1056/NEJMoa035725 (2003). [DOI] [PubMed] [Google Scholar]
- Laster S. M., Wood J. G. & Gooding L. R. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol 141, 2629–2634 (1988). [PubMed] [Google Scholar]
- Blanchard D. K., Djeu J. Y., Klein T. W., Friedman H. & Stewart W. E. 2nd. Induction of tumor necrosis factor by Legionella pneumophila. Infect Immun 55, 433–437 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubodera N., Tsuji N., Uchiyama Y. & Endo K. A new active vitamin D analog, ED-71, causes increase in bone mass with preferential effects on bone in osteoporotic patients. J Cell Biochem 88, 286–289, doi: 10.1002/jcb.10346 (2003). [DOI] [PubMed] [Google Scholar]
- Matsumoto T. et al. A new active vitamin D3 analog, eldecalcitol, prevents the risk of osteoporotic fractures–a randomized, active comparator, double-blind study. Bone 49, 605–612, doi: 10.1016/j.bone.2011.07.011 (2011). [DOI] [PubMed] [Google Scholar]
- Phal P. M., Myall R. W., Assael L. A. & Weissman J. L. Imaging findings of bisphosphonate-associated osteonecrosis of the jaws. AJNR Am J Neuroradiol 28, 1139–1145, doi: 10.3174/ajnr.A0518 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi S. D., Scoletta M., Cassione F. B., Migliaretti G. & Mozzati M. Computerized tomographic findings in bisphosphonate-associated osteonecrosis of the jaw in patients with cancer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 104, 249–258, doi: 10.1016/j.tripleo.2007.01.040 (2007). [DOI] [PubMed] [Google Scholar]
- Bedogni A. et al. Bisphosphonate-associated jawbone osteonecrosis: a correlation between imaging techniques and histopathology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 105, 358–364, doi: 10.1016/j.tripleo.2007.08.040 (2008). [DOI] [PubMed] [Google Scholar]
- Bi Y. et al. Bisphosphonates cause osteonecrosis of the jaw-like disease in mice. Am J Pathol 177, 280–290, doi: 10.2353/ajpath.2010.090592 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B. et al. Periapical disease and bisphosphonates induce osteonecrosis of the jaws in mice. J Bone Miner Res 28, 1631–1640, doi: 10.1002/jbmr.1894 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. et al. Osteonecrosis of the Jaw developed in mice: disease variants Regulated by gammadelta T Cells In Oral Mucosal Barrier Immunity. J Biol Chem 290, 17349–17366, doi: 10.1074/jbc.M115.652305 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soundia A. et al. Osteonecrosis of the jaws (ONJ) in mice after extraction of teeth with periradicular disease. Bone 90, 133–141, doi: 10.1016/j.bone.2016.06.011 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y. et al. Plasticity of Myeloid Cells during Oral Barrier Wound Healing and the Development of Bisphosphonate-related Osteonecrosis of the Jaw. J Biol Chem 291, 20602–20616, doi: 10.1074/jbc.M116.735795 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedghizadeh P. P. et al. Microbial biofilms in osteomyelitis of the jaw and osteonecrosis of the jaw secondary to bisphosphonate therapy. J Am Dent Assoc 140, 1259–1265 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.