Abstract
Fusarium graminearum is the major causal agent of fusarium head blight in wheat, a serious disease worldwide. Linoleic acid isomerase (LAI) catalyses the transformation of linoleic acid (LA) to conjugated linoleic acid (CLA), which is beneficial for human health. We characterised a cis-12 LAI gene of F. graminearum (FGSG_02668; FgLAI12), which was downregulated by salicylic acid (SA), a plant defence hormone. Disruption of FgLAI12 in F. graminearum resulted in decreased accumulation of cis-9,trans-11 CLA, enhanced sensitivity to SA, and increased accumulation of LA and SA in wheat spikes during infection. In addition, mycelial growth, accumulation of deoxynivalenol, and pathogenicity in wheat spikes were reduced. Re-introduction of a functional FgLAI12 gene into ΔFgLAI12 recovered the wild-type phenotype. Fluorescent microscopic analysis showed that FgLAI12 protein was usually expressed in the septa zone of conidia and the vacuole of hyphae, but was expressed in the cell membrane of hyphae in response to exogenous LA, which may be an element of LA metabolism during infection by F. graminearum. The cis-12 LAI enzyme encoded by FgLAI12 is critical for fungal response to SA, mycelial growth and virulence in wheat. The gene FgLAI12 is potentially valuable for biotechnological synthesis of cis-9,trans-11 CLA.
Fusarium graminearum (teleomorph Gibberella zeae [Schwein.] Petch) is an ascomycete fungus that causes fusarium head blight (FHB) in wheat. FHB can lead to yield loss and accumulation of trichothecene mycotoxins (predominantly deoxynivalenol [DON]) in wheat seeds, which threatens human and animal health1,2,3. Control of FHB by using natural resistance and traditional breeding in wheat is difficult, and thus FHB remains a serious disease worldwide4,5.
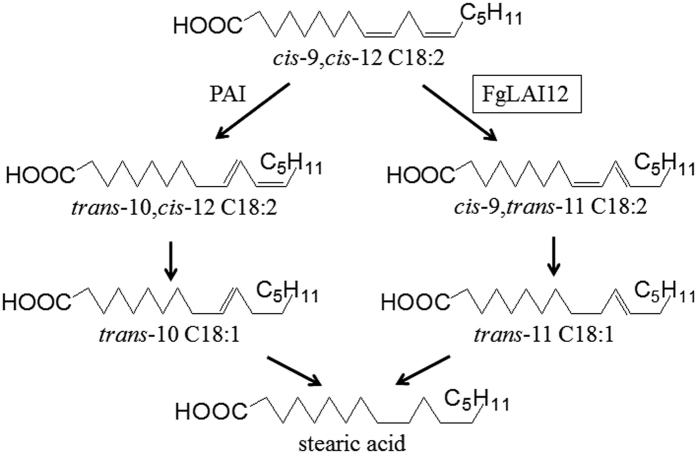
Linoleic acid (LA; cis-9,cis-12 C18:2) plays an important role in wheat resistance to F. graminearum infection. Accumulation of LA is greater in FHB-resistant wheat lines than in susceptible lines6, suggesting that LA may contribute to FHB resistance. Enhanced accumulation of LA and other free fatty acids can reinforce the cuticle, which acts as a barrier to pathogen entry7. LA is metabolised by linoleate diol synthase (LDS)8,9 and linoleic acid isomerase (LAI) in fungi (Fig. 1). LDS has been well studied in fungi, and is involved in fungal development, reproduction, synthesis of trichothecenes and cytochrome metabolism8,9,12,13. However, limited knowledge on LAI in fungi is available.
Figure 1. Schematic illustration of LA isomers.
FgLAI12 is marked by a black box. PAI, Propionibacterium acnes cytosolic LA isomerase10,11.
Activity of LAI was first analysed in the bacterium Butyrivibrio fibrisolvens14 and subsequently in the red alga Ptilota filicina15. To date, the enzyme has been detected mostly frequently in bacteria16,17. LAI catalyses the transformation of LA to conjugated linoleic acid (CLA) in microbes. Two categories of LAI (i.e., cis-9 and cis-12) have been identified: cis-9 LAI catalyses conversion of LA to trans-10,cis-12 CLA (trans-10,cis-12 C18:2); and cis-12 LAI transforms LA to cis-9,trans-11 CLA (cis-9,trans-11 C18:2). Trans-10,cis-12 CLA and cis-9,trans-11 CLA are then transformed into trans-10 C18:1 and trans-11 C18:1, respectively, by unknown enzymes (Fig. 1). In fungi, the gene encoding cis-9 LAI (PAI) was identified in Propionibacterium acnes10,11,18. Activity of cis-12 LAI was measured in Clostridium sporogenes; however, no cis-12 LAI gene has yet been reported19.
In this study, we characterised a cis-12 LAI gene of F. graminearum (FGSG_02668; FgLAI12; cis-12 LAI of F. graminearum), which was downregulated by salicylic acid (SA), a plant defence hormone20. The objectives were to investigate the function of FgLAI12 in LA metabolism and to clarify the gene’s effects on fungal response to SA, fungal development, synthesis of DON and pathogenicity in wheat.
Results
Deletion and complementation of FgLAI12
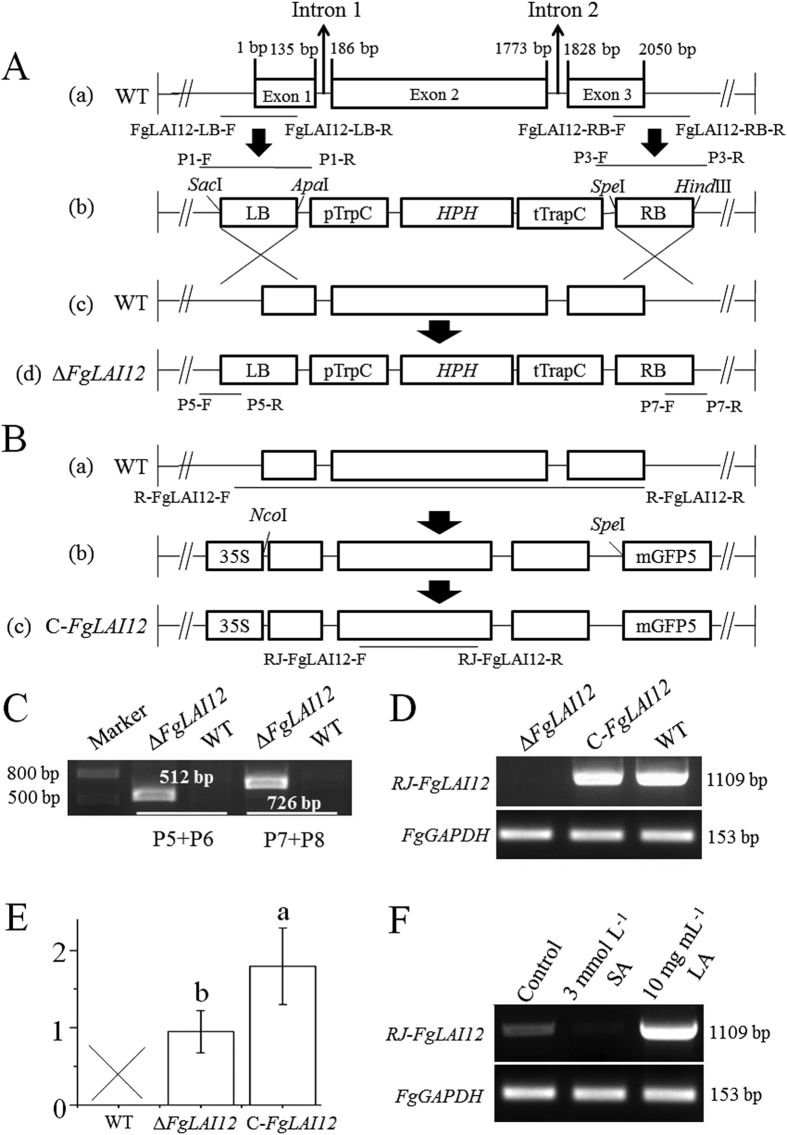
The FgLAI12 gene contains three exons and two introns (Fig. 2A). To determine the function of FgLAI12 in F. graminearum, knockout mutants (ΔFgLAI12) and complementation mutants (C-FgLAI12) were created (Fig. 2A,B). The mutants were verified by PCR (Fig. 2C,D) and sequencing (data not shown).
Figure 2. Deletion and complementation of FgLAI12 in F. graminearum.
(A) The left border (LB) and right border (RB) were amplified from the wild type (a). ΔFgLAI12 mutant (d) was created by recombination between the knockout vector (b) and FgLAI12 (c). (B) The nucleotide sequence of FgLAI12 was cloned (a), and ligated into the complementation vector (b). The T-DNA region of the complementation vector was recombined into ΔFgLAI12 to create C-FgLAI12 (c). SacI, ApaI, SpeI, HindIII, NcoI and SpeI indicate the restriction enzymes used. (C) PCR verification of ΔFgLAI12 using the primer pairs P5 forward + P5 reverse and P7 forward + P7 reverse. (D) Verification of expression of FgLAI12 using the primer pair RJ-FgLAI12 forward + RJ-FgLAI12 reverse. FgGAPDH were used as a control. (E) Copy numbers of HPH determined by qPCR. Different letters (a and b) above each column indicate a significant difference (P < 0.05; n = 3). (F) Effect of SA and LA on expression of FgLAI12 in hyphae, as determined using the primer pair RJ-FgLAI12 (Table 1). Hyphae were collected on the 4th day after adding SA and LA. FgGAPDH was used as a reference. “F”, forward; “R”, reverse.
The copy number of integrated T-DNA inserts was determined by targeting hygromycin B phosphotransferase genes (HPH). As expected, HPH was not detected in the wild type (WT). One and two normalised copies of HPH were detected in the ΔFgLAI12 and C-FgLAI12 mutants, respectively, confirming the single insertion of the T-DNA construct for gene knockout and complementation (Fig. 2E).
Expression of FgLAI12 under treatment with 3 mmol L−1 SA or 10 mg mL−1 LA was determined by reverse transcription-PCR (RT-PCR). As expected, FgLAI12 expression was downregulated by SA and upregulated by LA (Fig. 2F).
FgLAI12 catalyses conversion of LA to cis-9,trans-11 CLA
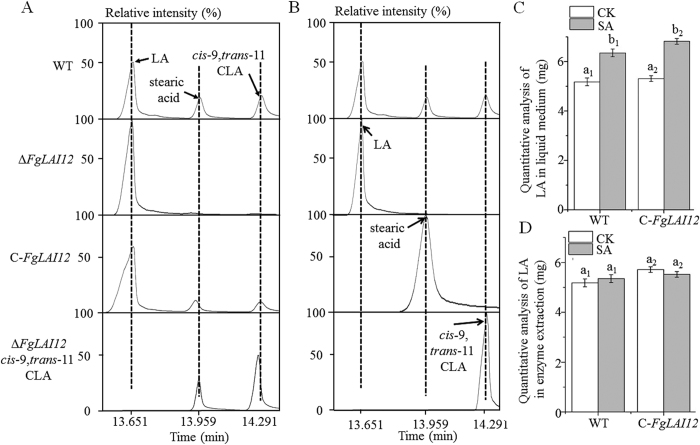
Three peaks were detected in gas chromatography–mass spectrometry (GC-MS) analysis of compounds derived from LA (Fig. 3A), namely LA (the first peak), stearic acid (the second peak) and cis-9,trans-11 CLA (the third peak), by matching with GC-MS spectrometry gallery. The peaks for LA, stearic acid and cis-9,trans-11 CLA in GC-MS were confirmed by using of corresponding standard chemicals (Fig. 3B). In the presence of exogenous LA, enzyme extracts from mycelia of WT and C-FgLAI12 produced stearic acid and cis-9,trans-11 CLA, compared with no cis-9,trans-11 CLA and a small quantity of stearic acid for the extract from ΔFgLAI12. After addition of cis-9,trans-11 CLA, the enzyme extract from ΔFgLAI12 produced stearic acid as for the WT. FgLAI12 contains no known conserved domain of transcription factors, further suggesting that FgLAI12 is directly responsible for conversion of LA to cis-9,trans-11 CLA. These results showed that FgLAI12 encoded a LAI enzyme that catalysed the transformation of LA to cis-9,trans-11 CLA (Fig. 1), and that FgLAI12 played a major role in the isomerism of LA in F. graminearum under the experimental conditions.
Figure 3. FgLAI12 encodes a cis-12 LA isomerase.
(A) GC-MS analysis of chemicals derived from LA. (B) The peaks for LA, stearic acid and cis-9,trans-11 CLA when using standard chemicals. (C) Quantitative analysis of LA added to liquid medium containing growing mycelia. (D) Quantitative analysis of LA added to enzyme extract from mycelia. The same amount of mycelia was used. Different letters above each column indicate a significant difference (P < 0.05; n = 3). “a1, b1” and “a2, b2” are used to show the significance within WT and C-FgLAI12 treatments, respectively, since there are more than one treatments in the same chart.
SA inhibited the expression of FgLAI1220 (Fig. 2F). To further confirm the function of FgLAI12 and clarify the relationship between SA and FgLAI12, 3 mL mSNA-2 liquid medium was supplemented with 10 mg SA and cultures of WT, ΔFgLAI12 and C-FgLAI12 were incubated for 24 h before collection of mycelia. Compared with cultures that lacked SA, the enzyme extracts from WT and C-FgLAI12 metabolised a smaller quantity of LA in the presence of SA (Fig. 3C). As expected, following addition of 10 mg SA to the enzyme extract, no significant difference in LA concentration was observed in the presence and absence of SA (Fig. 3D). These results indicated that FgLAI12 was a target for manipulation of LA metabolism by SA in F. graminearum.
Influence of FgLAI12 on mycelial growth and conidial morphology
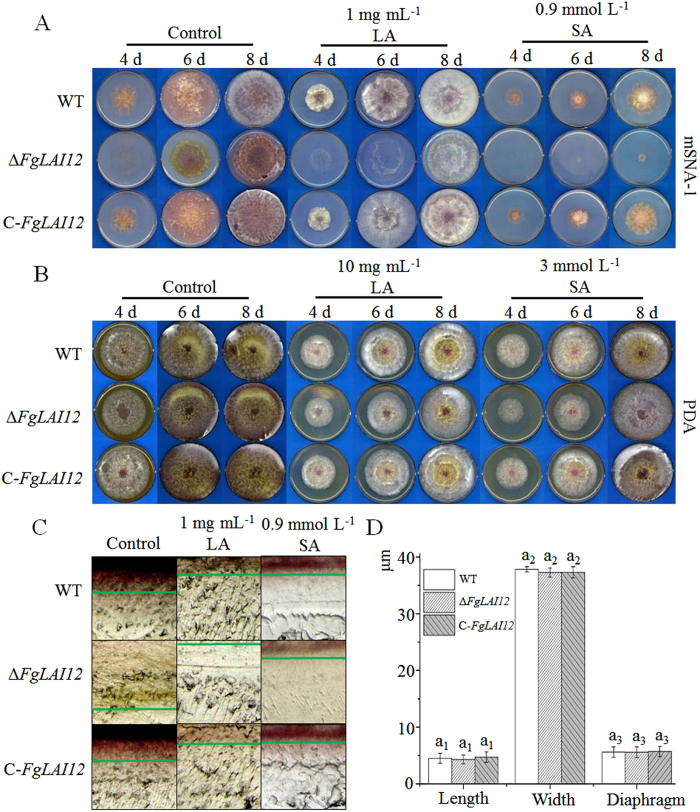
To observe the changes in the growth phenotype caused by disruption of FgLAI12 in F. graminearum, the WT, ΔFgLAI12 and C-FgLAI12 strains were inoculated on modified Synthetischer Nährstoffarmer Agar-1 (mSNA-1) (Fig. 4A) and potato dextrose agar (PDA) plates (Fig. 4B). On mSNA-1 plates, ΔFgLAI12 formed much less aerial mycelium compared with WT and C-FgLAI12. On the 6th day after inoculation, aerial mycelium of ΔFgLAI12 was clearly observed (control in Fig. 4A). Similarly, ΔFgLAI12 mycelia grew more slowly than those of WT and C-FgLAI12 on PDA plates, as indicated on the 4th day after inoculation (control in Fig. 4B). Compared with the control, the spread of mycelia was inhibited by LA (LA treatment in Fig. 4A,B). Consistent with the role of FgLAI12 in LA metabolism, the spread and density of ΔFgLAI12 mycelia were more strongly inhibited by LA. Mycelial growth of ΔFgLAI12 was more sensitive to SA compared with that of WT and C-FgLAI12 (SA treatment in Fig. 4A,B). These results indicated that FgLAI12 plays important roles in mycelial growth and fungal response to SA.
Figure 4. Effect of FgLAI12 on fungal biology.
Mycelial growth of the WT, ΔFgLAI12 and C-FgLAI12 on mSNA-1 (A) and PDA plates (B) under treatment with LA and SA on the 4th, 6th and 8th days after inoculation. (C) Microscopic observation of mycelia growth within mSNA-1 plates supplemented with LA and SA. The depth of hyphae is marked with green lines. Agar plugs (0.5 cm2) cut from the edge of growing mycelia (Fig. 4A) were photographed with an Olympus SZ51 (Japan). (D) Comparison of conidial length, width and the number of septa of the WT, ΔFgLAI12 and C-FgLAI12. Different letters above each column indicate a significant difference (P < 0.05; n = 1000). The same rule as mentioned in the legend of Fig. 3 is used for indicating the significance within treatment, when there are more than one treatments in the same chart.
To clarify the influence of FgLAI12 on fungal growth within agar, a 0.5-cm2 agar plug that excluded aerial mycelia was cut from the edge of 6-d-old mSNA-1 plates, and was observed using a stereomicroscope at 100× (Fig. 4C). The hyphae of ΔFgLAI12 grew more deeply on mSNA-1 plates than hyphae of WT and C-FgLAI12. In the presence of LA or SA, the hyphae of WT, ΔFgLAI12 and C-FgLAI12 grew more shallowly than those of the controls. Interestingly, the hyphae of ΔFgLAI12 grew more shallowly under LA treatment than those of WT and C-FgLAI12. No notable difference between the strains under SA treatment.
To clarify whether FgLAI12 affected conidial morphology, we compared the length, width and number of septa of 1 × 103 randomly selected conidia. No significant difference among WT, ΔFgLAI12 and C-FgLAI12 was observed for each parameter (Fig. 4D).
Subcellular localisation of FgLAI12 protein
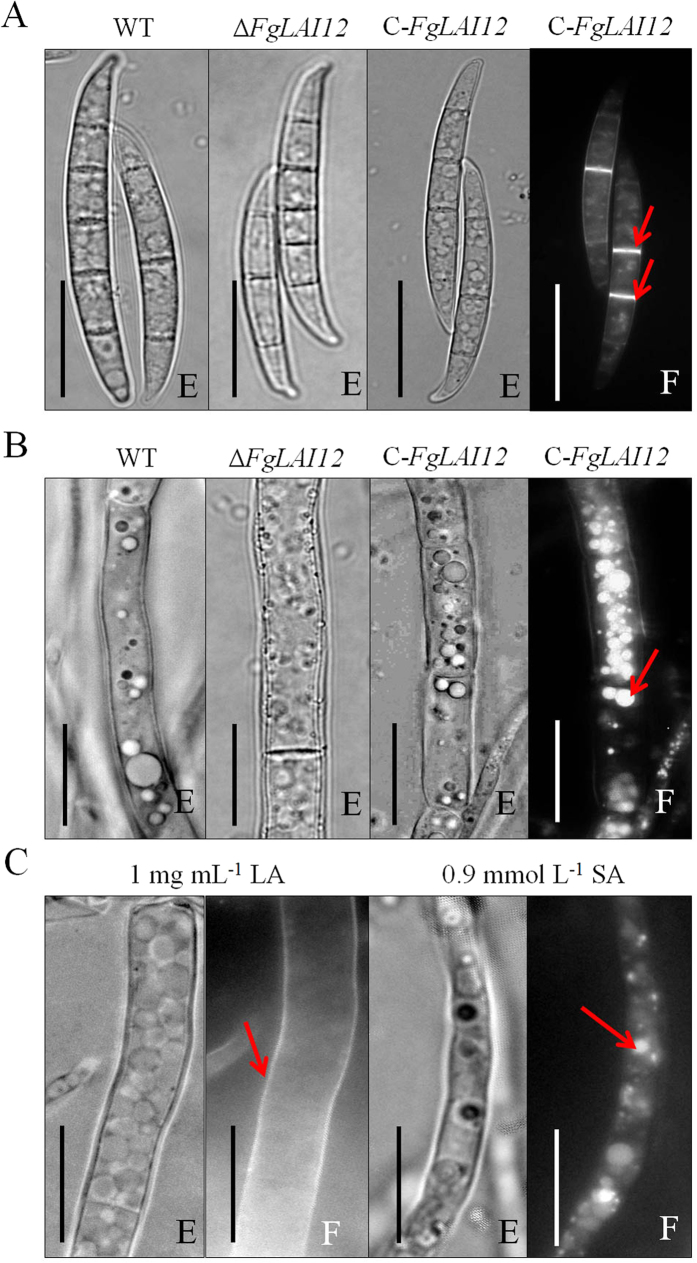
The C-FgLAI12 strain harbouring a green fluorescent protein gene (GFP) tag was used to examine the subcellular localisation of FgLAI12 protein. Conidia and hyphae at different vegetative growth stages were used to detect GFP fluorescence. As expected, no morphological differences among conidia of the WT, ΔFgLAI12 and C-FgLAI12 strains were observed (Fig. 5A). FgLAI12 protein was predominantly localised on septa of conidia (Fig. 5A), and in vacuoles within hyphae (Fig. 5B). In response to LA treatment, FgLAI12 protein was mainly localised on the cell membrane (Fig. 5C). In the presence of exogenous SA, FgLAI12 protein remained localised in vacuoles but the fluorescence intensity was reduced, reflecting the inhibitory effect of SA on FgLAI12 expression (Fig. 5C).
Figure 5. Subcellular localisation of FgLAI12 protein.
Conidia (A) and mycelia (B and C) of the WT, ΔFgLAI12 and C-FgLAI12 strains photographed with a Nikon-80i fluorescence microscope (Japan). The fluorescent protein signal is marked with red arrows. (E), Optical microscope; (F), fluorescence microscope. Scale bar, 10 μm.
FgLAI12 affects pathogenicity in wheat
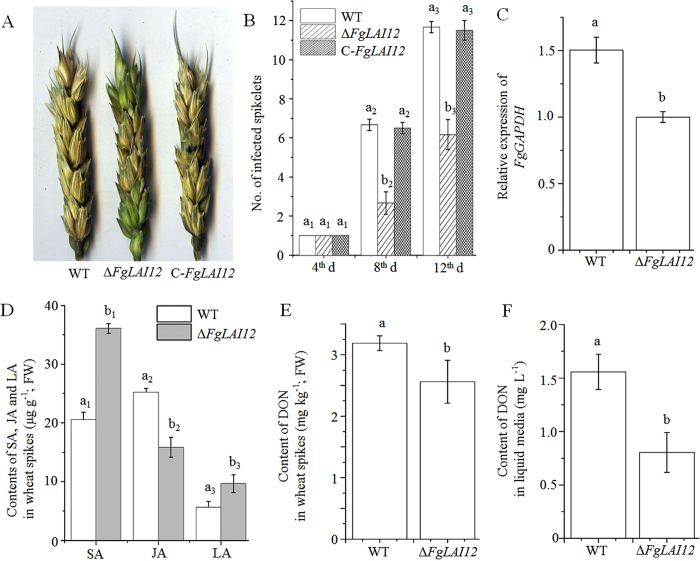
To clarify whether FgLAI12 was involved in the pathogenicity of F. graminearum, we point-inoculated two fully developed florets of a central spikelet within a spike with conidial suspensions of WT, ΔFgLAI12 and C-FgLAI12. ΔFgLAI12 spikes showed much lower severity of disease compared with those of WT and C-FgLAI12 (Fig. 6A,B). FgLAI12 was associated with variation in fungal biomass in wheat spikes, which was indicated by the relative expression level of the F. graminearum GAPDH gene. The spikes inoculated with ΔFgLAI12 conidia showed a significantly lower GAPDH expression level than spikes inoculated with WT conidia (Fig. 6C). In addition, the concentration of DON in the liquid culture medium and in wheat spikes was compared. ΔFgLAI12 showed much lower DON production than that of WT (Fig. 6E,F).
Figure 6. Effect of FgLAI12 on pathogenicity of F. graminearum in wheat.
(A) Symptoms of Fusarium head blight in spikes of the WT, ΔFgLAI12 and C-FgLAI12 strains on the 12th day after inoculation. (B) Numbers of infected and bleached spikelets on the 4th, 8th and 12th days after inoculation. (C) Relative expression level of FgGAPDH in wheat spikes. (D) Concentrations of salicylic acid (SA), jasmonic acid (JA) and linoleic acid (LA) in spikes inoculated with WT and ΔFgLAI12. (E) Concentration of deoxynivalenol (DON) in wheat spikes. (F) Measurement of DON in liquid medium (the same amount of mycelia was used). Values are the mean ± standard deviation of three biological replicates per treatment. Different letters above each column indicate a significant difference (P < 0.05; n = 3). FW, fresh weight. The same rule as mentioned in the legend of Fig. 3 is used for indicating the significance within treatment, when there are more than one treatments in the same chart.
Both SA, jasmonic acid (JA) and LA are important components in the wheat defence response against F. graminearum, and infection of spikes by F. graminearum leads to a significant increase in their accumulation6,21. To further understand the reduced disease severity of ΔFgLAI12 spikes compared with WT spikes, we compared the amount of SA, JA and LA in spikes inoculated with WT and ΔFgLAI12 conidia. Spikes infected with ΔFgLAI12 accumulated higher concentrations of SA and LA, and a lower concentration of JA (Fig. 6D).
Discussion
Fusarium graminearum is a fungal pathogen that causes severe damage to wheat crops worldwide. The genetics and biology of F. graminearum have been well studied. However, the metabolism of LA and its role in fungal biology and pathogenicity has attracted little attention. The FgLAI12 gene of F. graminearum encodes a cis-12 LAI. Our results demonstrated that this cis-12 LAI was responsible for transformation of LA to cis-9,trans-11 CLA. In addition, FgLAI12 was necessary for fungal growth, fungal response to SA, aerial mycelium development, synthesis of a mycotoxin and pathogenicity in wheat.
Both LDS8,9 and LAI metabolise LA in fungi (Fig. 1). We previously reported that SA downregulates expression of FgLAI12, which encodes a predicted LDS enzyme20. Surprisingly, GC-MS analysis demonstrated that FgLAI12 functioned as a LAI in vivo. Deletion of FgLAI12 had no significant effect on conidial production (data not shown) and morphology (Fig. 4D), even though FgLAI12 played a major role in the isomerism of LA in F. graminearum (Fig. 3A). Disruption of LDS expression in Emericella nidulans and Neosartorya fumigata results in a decrease in ascospore production8, because one product of LDS activity, (8 R)-hydroperoxylinoleate, is a sexual sporulation hormone22. These results indicate the functional difference of LDS and LAI in fungal biology. Additional studies of LDS genes would help to understand the role of LA in F. graminearum.
LA plays an important role in wheat and barley in defence against F. graminearum and has been established to be a FHB resistance-related metabolite6,7,23. Higher concentrations of LA accumulate in FHB-resistant wheat lines than in susceptible lines7, which suggests that LA contributes to FHB resistance. LA is a precursor in the biosynthesis of the phytohormone JA, which is a critical signalling molecule for wheat resistance against FHB21,24. Spikes inoculated with ΔFgLAI12 conidia accumulated greater quantities of LA than those inoculated with WT conidia (Fig. 6D), which was consistent with the hypothesised function of FgLAI12. Unexpectedly, spikes inoculated with ΔFgLAI12 accumulated lower concentrations of JA, possibly because of the lower fungal biomass (Fig. 6C). Besides being a chemical inhibitor, LA can reinforce the cuticle, which acts as a barrier to pathogen entry7. In this regard, the lower disease severity of ΔFgLAI12-inoculated spikes compared with spikes inoculated with WT is understandable.
Fluorescent microscopic observation showed that FgLAI12 protein was usually localised in the septa of conidia and the vacuoles of hyphae. However, exogenous LA treatment resulted in localisation of FgLAI12 in the cell membrane of hyphae, which might reflect the mechanism by which FgLAI12 protein metabolises LA synthesised by the host plant.
SA and JA are important defence phytohormones, for which both antagonistic and synergistic interactions are reported25,26,27,28. Both SA and JA accumulate in wheat spikes infected by F. graminearum, which indicates that synergistic interactions between the two phytohormones may contribute to FHB resistance21. Expression of FgLAI12 was downregulated by SA20, and degradation of LA was reduced in response to exogenous SA (Fig. 3C). Spikes inoculated with ΔFgLAI12 accumulated higher quantities of SA and reduced amounts of JA compared with spikes inoculated with the WT strain (Fig. 6D), indicating that FgLAI12 plays a role in manipulating biosynthesis of SA and JA in planta. Therefore, we speculate that FgLAI12 represents a link between the SA and JA defence-response pathways during the host–pathogen interaction, which warrants future investigation.
Mycelial growth of ΔFgLAI12 was reduced on mSNA-1 and PDA plates compared with that of WT (Fig. 4A). This finding suggests that FgLAI12 is essential for normal growth of F. graminearum. Polyunsaturated fatty acids (PUFAs), such as γ-linolenic, arachidonic and eicosapentaenoic acids, can modify membrane-bound proteins, ATPase and the histocompatibility complex, and control the reaction of fatty acid binding proteins29,30. As a PUFA, LA may perform a similar function to other PUFAs. Disruption of FgLAI12 is likely to have disturbed the balance of PUFAs and negatively affected growth of fungal mycelia.
The mycotoxin DON is a threat to human and animal health1. Many countries have adopted a maximum allowable level for DON in grains for human consumption and animal feedstuffs. We previously showed that SA significantly reduced DON production20 and SA also significantly inhibited expression of FgLAI12. In the present study, we demonstrated that disruption of FgLAI12 reduced accumulation of DON in wheat spikes and in the culture medium. Therefore, FgLAI12 may be a regulatory target for SA to downregulate DON production in F. graminearum.
FgLAI12 catalyses the transformation of LA to cis-9,trans-11 CLA. CLA has been shown to lower cancer risk, enhance immunity and reduce body fat while increasing lean body mass in animals31,32. The cis-9,trans-11 CLA and trans-10,cis-12 CLA isomers show the highest biological activities and are transformed by cis-12 and cis-9 isomerases, respectively (Fig. 1). CLA is currently marketed as a dietary supplement, which is produced by alkaline isomerization of LA33 or vegetable oils containing triglyceride esters of LA34. However, chemically synthesised CLA contains a variety of other isomers and is unsuitable for nutritional purposes35. Industrial-scale production of CLA by biotransformation from LA is increasingly attractive. Therefore, identification of the genes encoding LAI enzymes is warranted. The cis-12 LAI gene characterised in this study is potentially valuable for commercial use.
Methods
Experimental materials and growth conditions
Plants of Triticum aestivum ‘Roblin’ were grown in greenhouses under 16/8 h (day/night) cycles at 23/18 °C. Plants were watered as needed and fertilised before planting with 15-15-15 (N-P-K) fertiliser. ‘Roblin’ is highly susceptible to F. graminearum infection.
The F. graminearum isolate DAOM180378 (Canadian Fungal Culture Collection, AAFC, Ottawa, ON, Canada), which is highly virulent on wheat, was used in all experiments. This isolate was also used for measurement of the expression level of FGSG_02668 (FgLAI12) under SA treatment20. The isolate was cultured on modified mSNA-1 (1 g KH2PO4, 1 g KNO3, 0.5 g MgSO4, 0.5 g KCl, 1 g glucose, 1 g sucrose and 20 g agar per litre) plates at 25 °C. Conidia were produced in carboxymethyl cellulose liquid medium at 28 °C, with shaking (180 rpm) for 5 d36. For mycelial growth studies, PDA (BD Difco, Sparks, MD, USA) and mSNA-1 plates were used. LA and SA were added to media after autoclaving. Each plate was inoculated with 1 × 103 conidia of F. graminearum and all inoculated plates were incubated in a dark cabinet at 28 °C. There were 10 replicates per treatment and the growth experiments were repeated three times. For deletion and complementation of the FGSG_02668 gene, Agrobacterium tumefaciens strain AGL-1, which was used for transformation of F. graminearum, was grown at 28 °C in yeast extract broth (5 g nutrient broth, 1 g yeast extract, 5 g peptone, 5 g sucrose and 0.5 g MgSO4 per litre; pH 7.4). Escherichia coli strain DH5α (Tiangen, Beijing, China) was cultured in Luria–Bertani broth at 37 °C37. Unless specifically noted, all chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA).
Sequence analysis and primer design
The nucleotide sequence of FGSG_02668 was downloaded from the Ensembl Fungi database (http://fungi.ensembl.org/index.html). Primer Premier 5.0 software (Premier Biosoft, Palo Alto, Canada) was used to design PCR primers (Table 1).
Table 1. Primers used in this study.
| Primer | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| FgLAI12-LB | GGAAGCTTCAAAGCGGCAACAC | GGACTAGTTTCCCATCAACAACATAT |
| FgLAI12-RB | GCGGGCCCGGCTTATCTCAACGACA | GCGAGCTCTACCCTGAATCCAACAT |
| P145 | CTTTTCTCTTAGGTTTACCCG | TAATGCAGGAGTCGCATAAG |
| P345 | CCCAAAAAGTGCTCCTTCAA | TGTGCTGCAAGGCGATTAA |
| P545 | TTGTCACCGCCAAAGC | ACCTACTACTGGGCTGCTT |
| P745 | CTGACCAGTTGCCTAA | ACAGTTCATTCCGAGAC |
| R-FgLAI12 | GGACTAGTTGCTCAACTTACGCCACTG | GGCACGTGTCGACGCAGAAGCT |
| RJ-FgLAI12 | ATCCCAAGCCACCAG | GCCAAGTTCTCCGTCA |
| Fg-GAPDH20 | TGACTTGACTGTTCGCCTCGAGAA | ATGGAGGAGTTGGTGTTGCCGTTA |
| w-GAPDH20 | AACTGTTCATGCCATCACTGCCAC | AGGACATACCAGTGAGCTTGCCAT |
| Aox20 | GACTTGTCATGGTAGATGCCTG | CAGGACGAGCATAACCATTCTC |
| hn-RNP-Q20 | TCACCTTCGCCAAGCTCAGAACTA | AGTTGAACTTGCCCGAAACATGCC |
| Fg-β-tubulin20 | GTTGATCTCCAAGATCCGTG | CATGCAAATGTCGTAGAGGG |
| Fg-Factor120 | CCTCCAGGATGTCTACAAGA | CTCAACGGACTTGACTTCAG |
| HPH | CGATCTTAGCCAGACGAGCG | TTGCCCTCGGACGAGTGCTG |
Horizontal lines under nucleotides indicate the cutting sites for restriction enzymes.
Disruption and complementation of FGSG_02668
Genomic DNA of F. graminearum was extracted from mycelia, which were cultured on mSNA-1 plates for 5 d at 25 °C, by the CTAB method38. Disruption of FGSG_02668 in F. graminearum is schematically shown in Fig. 2A. The pRF-HU2 vector was used for targeted gene replacement in F. graminearum through A. tumefaciens-mediated transformation39,40. The left border (LB) and right border (RB) of FGSG_02668 targeted for homologous recombination were amplified with the primer pairs FgLAI12-LB forward + FgLAI12-LB reverse and FgLAI12-RB forward + FgLAI12-RB reverse, respectively. The resulting recombinant plasmids were verified by PCR and sequencing (Invitrogen, Shanghai, China) with the primer pairs P1 forward + P1 reverse and P3 forward + P3 reverse (Fig. 2A). Agrobacterium-mediated transformation of F. graminearum was performed as described previously41. The resulting mutants were verified by PCR and sequencing (Invitrogen) (Fig. 2A,C). The full open reading frame of FGSG_02668 was inserted into the pCAMBIA1302 vector. The 3′ terminus of FGSG_02668 was fused with a GFP tag under the control of the 35 S promoter. The recombinant pCAMBIA1302 vector was transformed into ΔFgLAI12 to create complementation mutants. The complementation mutants were verified by PCR and sequencing (Fig. 2B,D). Five knockout mutants and four complementation mutants were used throughout the research.
To confirm the single integration of the FGSG_02668 knockout construct into the genome, quantitative real-time PCR (qPCR) was performed to determine the copy number of T-DNA inserts in fungal transformants as described previously42, with some modification. Genomic DNA was used as the qPCR template. The HPH gene was used to determine the copy number of the integrated T-DNA construct for gene knockout. The primer pairs Fg-GAPDH forward + Fg-GAPDH reverse, Fg-β-tubulin forward + Fg-β-tubulin reverse, and Fg-Factor1 forward + Fg-Factor1 forward, which target the single-copy house-keeping gene FgGAPDH (FGSG_06257), β-tubulin (FGSG_09530) and elongation factor 1 (FGSG_08811) of F. graminearum, respectively, were used as references for normalisation of data. The primer pair HPH-F + HPH-R was used to amplify the HPH gene in qPCR reactions. The qPCR analyses were carried out as described previously43 using a MyiQ Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA).
RT-PCR analyses
Total RNA was extracted from 100 mg (fresh weight) of mycelia grown on mSNA-1 plates using the E.Z.N.A.® Total RNA Kit I (Omega Bio-Tek, Norcross, GA, USA) in accordance with the manufacturer’s instructions. RNA was reverse transcribed using the PrimeScript™ RT Reagent Kit with genomic DNA Eraser (Takara, Dalian, China) following the protocol of the manufacturer. The primer pair RJ-FgLAI12 forward + RJ-FgLAI12 reverse was used to measure the expression level of FGSG_02668 by RT-PCR. The FgGAPDH gene was used as a reference.
LAI enzyme activity assay
The crude enzyme was extracted as described previously13, which was utilised for extacting recombinant LDS enzyme. Mycelia of the WT, ΔFgLAI12 and C-FgLAI12 strains were cultured and collected on liquid mSNA-2 (1 g KH2PO4, 1 g KNO3, 0.5 g MgSO4, 0.5 g KCl, 6 g glucose and 6 g sucrose per litre) medium, and incubated at 28 °C on an orbital shaker for 6 d in darkness at 120 rpm. The collected mycelia (100 mg fresh weight) were supplemented with 0.1 mol L−1 sodium borate and 10 mg LA, then incubated with shaking (90 rpm) for 4 h at 28 °C. Subsequently, mycelia were collected by filtration and dried with filter paper, before being ground in liquid nitrogen. For each strain, three biological replicates were performed.
One millilitre of enzyme extraction solution (0.2 mol L−1 sucrose, 0.1 mol L−1 Tris, 10 mmol L−1 ethylene diamine tetraacetic acid, 0.1 mol L−1 sodium borate and 20% [v/v] glycerin; pH 8.0) was added to each 50 mg of mycelia powder. The supernatant was collected by centrifugation at 1 × 104 rpm (Eppendorf centrifuge 5417 R, Hamburg, Germany) for 10 min at 4 °C, and then immediately mixed with 10 mg LA for 1 h at 28 °C. Finally, the targeted chemicals were enriched with SepPak/C18, eluted with 0.5 mL methyl alcohol, and dried over Na2SO4. The enriched samples were analysed by preparative GC-MS. A GC system (6890 N Network, Agilent, China) with a non-polar column (30 m, DB-5; J&W Scientific, Folsom, CA, USA; film, 0.25 μm; diameter, 0.25 mm; carrier gas He, 15 psi; flow rate, 1 mL min−1) and MS detector (5973 Network; Agilent, USA) were used. After splitless injections of samples in heptane, the GC was programmed from 80 °C for 4 min, to 200 °C at a rate of 30 °C min−1, to 233 °C at 3 °C min−1 and then to 285 °C at 10 °C min−1. The split ratio was 1:25 and the injected sample size was 1 μL. The standard chemicals for GC-MS (LA, stearic acid and cis-9,trans-11 CLA) were purchased from Sigma-Aldrich (St Louis, MO, USA).
Virulence assay
To determine whether FGSG_02668 affected the severity of FHB disease symptoms in wheat spikes, two florets of a single central spikelet per spike were each point-inoculated with a micropipette at the mid-anthesis stage with 1 × 103 conidial suspension of F. graminearum. The spikes were enclosed in plastic wrap, and placed in a room for 48 h at 25 °C under 16/8 h (day/night) cycles. Blight symptoms were assessed on the 4th, 8th and 12th days after inoculation. Five to ten plants were used per treatment.
To examine whether disruption of FGSG_02668 impacted on DON synthesis, the accumulation of DON in liquid media and wheat spikes were tested. Measurement of DON content in liquid media was carried out following a two-stage protocol as described previously20,44. Two fully deveoped florets of each spikelet of one head were each point inoculated with a micropipette at the mid-anthesis stage with 1 × 103 conidia of F. graminearum. The inoculated spikes were treated as above to induce FHB disease. On the 4th day after inocultion, each spikelet was harvested and ground in liquid nitrogen. Three biological replicates were conducted with at least six heads per treatment. Measurement of DON accumulation in wheat spikes was performed as described preciously45. The DON concentrations were measured using the DON ELISA kit (Beacon, Hebei, China) and Multiskan Spectrum (Thermo Scientific, Finland).
Measurement of SA, JA, LA and fungal biomass in wheat spikes
To prepare spike samples, two florets of each fully developed spikelet in an entire spike at mid-anthesis were inoculated with 1 × 103 conidia. After inoculation, the wheat plants were placed in a room as described above. At 48 h after initial inoculation, the spikes were harvested and ground to fine powder in liquid nitrogen for chemical measurement and RNA extraction. Three biological replicates were conducted with at least six heads per treatment. Quantification of SA, JA and LA was performed as described previously46.
RNA was extracted as described above. The relative amount of F. graminearum was estimated by measuring the expression level of FgGAPDH in the RNA samples by qPCR, with normalisation to the three wheat reference genes (w-GAPDH, NCBI UniGene Ta.66461; Aox, Ta.6172; hn-RNP-Q, Ta.10105)20.
Microscopic assay
For optical microscopic and fluorescence microscopic assays, conidia were produced in carboxymethyl cellulose liquid medium at 28 °C with shaking (180 rpm) for 5 d. Conidia (1 × 103) were inoculated into 3 mL mSNA-2 liquid media, incubated at 28 °C on an orbital shaker for 6 d in darkness at 120 rpm, and then 20 mg LA was added 1 d before microscopic observation. The conidia and hyphae were observed with a Nikon-80i fluorescence microscope (Nikon, Japan).
Statistical analysis
Student’s t-test (implemented in DPS version 12.01 software) was used to test the significance of differences in the means for LA metabolism, conidial morphology, DON production and disease severity between treatments.
Additional Information
How to cite this article: Zhang, Y.-Z. et al. Linoleic acid isomerase gene FgLAI12 affects sensitivity to salicylic acid, mycelial growth and virulence of Fusarium graminearum. Sci. Rep. 7, 46129; doi: 10.1038/srep46129 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (31230053) and International S&T Cooperation program of China (2015DFA30600). We thank Prof. Wei Wu of Sichuan Agricultural University and Dr Kai Zhong of Sichuan University for technical assistance. We thank Dr Ji-Rui Wang of the Triticeae Research Institute for kind advice. The authors offer special thanks to Dr Therese Ouellet of Agriculture and Agri-Food Canada for providing research materials.
Footnotes
The authors declare no competing financial interests.
Author Contributions P.F.Q. and Y.M.W. designed the experiments. P.F.Q., Y.Z.Z. and Q.C. wrote the manuscript and analysed the data. P.F.Q. and Y.Z.Z. prepared the figures. P.F.Q., Y.Z.Z., Z.Z.W., Q.C., C.H.L., Z.R.G., Y.L.C., L.J.Z., Y.N.H., C.C., Y.W., Y.Y.Q., X.F., T.Z., D.M. and B.J.X. performed the experiments. W.L., Q.T.J. and Y.L.Z. provided key reagents and advice. All authors reviewed the results and approved the final version of the manuscript.
References
- Fernando W. G., Paulitz T. C., Seaman W. L., Dutilleul P. & Miller J. D. Head blight gradients caused by Gibberella zeae from area sources of inoculum in wheat field plots. Phytopathology 87, 414–421 (1997). [DOI] [PubMed] [Google Scholar]
- Chandler E. A. & Simpson D. R. Development of PCR assays to TRI7 and TRI13 trichothecene biosynthetic genes, and characterization of chemotypes of Fuarium graminearum, Fuarium culomrum and Fuarium cerealis. Physiol. Mol. Plant P. 62, 355–367 (2003). [Google Scholar]
- Goswami R. S. & Kistler H. C. Heading for disaster: Fusariun graminearum on cereal crops. Mol. Plant Pathol. 5, 515–525 (2004). [DOI] [PubMed] [Google Scholar]
- Anand A. et al. Greenhouse and field testing of transgenic wheat plants stably expressing genes for thaumatin-like protein, chitinase and glucanase against Fusarium graminearum. J. Exp. Bot. 54, 1101–1111 (2003). [DOI] [PubMed] [Google Scholar]
- Mesterhazy A. Types and components of resistance to Fusarium head blight. Plant Breeding 114, 377–386 (2006). [Google Scholar]
- Ellinger D., Sode B., Falter C. & Voigt C. A. Resistance of callose synthase activity to free fatty acid inhibition as an indicator of Fusarium head blight resistance in wheat. Plant Signal Behav. 9, e28982 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blümke A. et al. Secreted fungal effector lipase releases free fatty acids to inhibit innate immunity-related callose formation during wheat head infection. Plant Physiol. 165, 346–358 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garscha U. et al. Identification of dioxygenases required for Aspergillus development. J. Biol. Chem. 282, 34707–34718 (2007). [DOI] [PubMed] [Google Scholar]
- Hoffmann I. & Oliw E. H. 7,8- and 5,8-Linoleate diol synthases support the heterolytic scission of oxygen-oxygen bonds by different amide residues. Arch. Biochem. Biophys. 539, 87–91 (2013). [DOI] [PubMed] [Google Scholar]
- Hao G. F. et al. Role of malic enzyme during fatty acid synthesis in the oleaginous fungus Mortierella alpina. Appl. Environ. Microb. 80, 2672–2678 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao D. et al. Production of conjugated linoleic acid by heterologous expression of linoleic acid isomerase in oleaginous fungus Mortierella alpina. Biotechnol. Lett. 37, 1983–1992 (2015). [DOI] [PubMed] [Google Scholar]
- Tsitsigiannis D. I., Zarnowski R. & Keller N. P. The lipid body protein, PpoA, coordinates sexual and asexual sporulation in Aspergillus nidulans. J. Biol. Chem. 279, 11344–11353 (2004). [DOI] [PubMed] [Google Scholar]
- Sooman L. & Oliw E. H. Discovery of a novel linoleate dioxygenase of Fusarium oxysporum and linoleate diol synthase of Colletotrichum graminicola. Lipids 50, 1243–1252 (2015). [DOI] [PubMed] [Google Scholar]
- Kepler C. R., Hirons K. P., McNeill J. J. & Tove S. B. Intermediates and products of the biohydrogenation of linoleic acid by Butyrinvibrio fibrisolvens. J. Biol. Chem. 241, 1350–1354 (1966). [PubMed] [Google Scholar]
- Wise M. L., Hamberg M. & Gerwick W. H. Biosynthesis of conjugated triene-containing fatty acids by a novel isomerase from the red marine alga Ptilota filicina. Biochemistry 33, 15223–15232 (1994). [DOI] [PubMed] [Google Scholar]
- Destaillats F., Trottier J. P., Galvez J. M. & Angers P. Analysis of alpha-linolenic acid biohydrogenation intermediates in milk fat with emphasis on conjugated linoleic acids. J. Dairy Sci. 88, 3231–3239 (2005). [DOI] [PubMed] [Google Scholar]
- Churruca I., Fernández-Quintela A. & Portillo M. P. Conjugated linoleic acid isomers: differences in metabolism and biological effects. Biofactors 35, 105–111 (2009). [DOI] [PubMed] [Google Scholar]
- Rosberg-Cody E., Johnson M. C., Fitzgerald G. F., Ross P. R. & Stanton C. Heterologous expression of linoleic acid isomerase from Propionibacterium acnes and anti-proliferative activity of recombinant trans-10, cis-12 conjugated linoleic acid. Microbiology 153, 2483–2490 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S. S., Deng M. D., Grund A. D. & Rosson R. A. Purification and characterization of a membrane-bound linoleic acid isomerase from Clostridium sporogenes. Enzyme Microb. Tech. 40, 831–839 (2007). [Google Scholar]
- Qi P. F. et al. Effect of salicylic acid on Fusarium graminearum, the major causal agent of fusarium head blight in wheat. Fungal Biol. 116, 413–426 (2012). [DOI] [PubMed] [Google Scholar]
- Qi P. F. et al. Jasmonic acid and abscisic acid play important roles in host-pathogen interaction between Fusarium graminearum and wheat during the early stages of fusarium head blight. Physiol. Mol. Plant P. 93, 39–48 (2016). [Google Scholar]
- Champe S. P. & El-zayat A. A. E. Isolation of a sexual sporulation hormone from Aspergillus nidulans. J. Bacteriol. 171, 3982–3988 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaraswamy K. G., Kushalappa A. C., Choo T. M., Dion Y. & Rioux S. Mass spectrometry based metabolomics to identify potential biomarkers for resistance in barley against fusarium head blight (Fusarium graminearum). J. Chem. Ecol. 37, 846–856 (2011). [DOI] [PubMed] [Google Scholar]
- Li G. & Yen Y. Jasmonate and ethylene signaling pathway may mediate fusarium head blight resistance in wheat, Crop Sci. 48, 1888–1896 (2008). [Google Scholar]
- Robert-Seilaniantz A., Navarro L., Bari R. & Jones J. D. G. Pathological hormone imbalances. Curr. Opin. Plant Biol. 10, 372–379 (2007). [DOI] [PubMed] [Google Scholar]
- Koornneef A. & Pieterse C. M. J. Cross talk in defense signalling. Plant Physiol. 146, 839–844 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon-Reyes A. et al. Ethylene signaling renders the jasmonate response of Arabidopsis insensitive to future suppression by salicylic acid. Mol. Plant Microbe Interact. 23, 187–197 (2010). [DOI] [PubMed] [Google Scholar]
- Mika A., Boenisch M. J., Hopff D. & Lüthje S. Membrane-bound guaiacol peroxidases from maize (Zea mays L.) roots are regulated by methyl jasmonate, salicylic acid, and pathogen elicitors. J. Exp. Bot. 61, 831–841 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman P., Turk J., Jakschik B. A., Morrison A. R. & Lefkowith J. B. Arachidonic acid metabolism. Annu. Rev. Biochem. 55, 69–102 (1986). [DOI] [PubMed] [Google Scholar]
- Zhang X. et al. Disruption of the fatty acid Delta6-desaturase gene in the oil-producing fungus Mortierella isabellina by homologous recombination. Curr. Microbiol. 55, 128–134 (2007). [DOI] [PubMed] [Google Scholar]
- Pariza M., Park Y. & Cook M. The biologically active isomers of conjugated linoleic acid. Prog. Lipid Res. 40, 293–298 (2001). [DOI] [PubMed] [Google Scholar]
- Pariza M. W. Perspective on the safety and effectiveness of conjugated linoleic acid. Am. J. Clin. Nutr. 79, 1132S–1136S (2004). [DOI] [PubMed] [Google Scholar]
- Zheng G., Gengwang Z. & Yan S. Preparation of conjugated linoleic acid and indentification of its isomers. Chinese J. Chem. Eng. 11, 130–135 (2003). [Google Scholar]
- Deng M. D. et al. Linoleic acid isomerase from Propionibacterium acnes: purification, characterization, molecular cloning, and heterologous expression. Appl. Biochem. Biotechnol. 143, 199–211 (2007). [DOI] [PubMed] [Google Scholar]
- Sehat N. et al. Silver-ion high-performance liquid chromatographic separation and identification of conjugated linoleic acid isomers. Lipids 33, 217–221 (1998). [DOI] [PubMed] [Google Scholar]
- Capellini R. A. & Peterson J. L. Macroconidium formation in submerged cultures by a non-sporulating strain of Gibberella zeae. Mycologia 57, 962–966 (1965). [Google Scholar]
- Bertani G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 62, 293–300 (1951). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodhi M. A., Ye G. N., Weeden N. F. & Reisch B. I. A simple and efficient method for DNA extraction from grapevine cultivars and Vitis species. Plant Mol. Biol. Rep. 12, 6–13 (1994). [Google Scholar]
- Frandsen R. J., Andersson J. A., Kristensen M. B. & Giese H. Efficient four fragment cloning for the construction of vectors for targeted gene replacement in filamentous fungi. BMC Mol. Biol. 9, 1–11 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsen R. J., Frandsen M. & Giese H. Targeted gene replacement in fungal pathogens via Agrobacterium tumefaciens-mediated transformation. Methods Mol. Biol. 835, 17–45 (2012). [DOI] [PubMed] [Google Scholar]
- Maier F. J., Malz S., Lösch A. P., Lacour T. & Schäfer W. Development of a highly efficient gene targeting system for Fusarium graminearum using the disruption of a polyketide synthase gene as a visible marker. FEMS Yeast Res. 5, 653–662 (2005). [DOI] [PubMed] [Google Scholar]
- Solomon P. S., Ipcho S. V. S., Hane J. K., Tan K. C. & Oliver R. P. A quantitative PCR approach to determine gene copy number. Fungal Genet. Rep. 55, 5–8 (2008). [Google Scholar]
- Wang J. R. et al. RNA profiling of fusarium head blight-resistant wheat addition lines containing the Thinopyrum elongtum chromosome 7E. Can. J. Plant Pathol. 32, 188–214 (2010). [Google Scholar]
- Miller D. & Blackwell B. A. Biosynthesis of 3-acetyldeoxynivalenol and other metabolites by Fusarium culmorum HLK 1503 in a stirred jar fermentor. Can. J. Bot. 64 (1986). [Google Scholar]
- Zhang Y. Z. et al. Chitin synthase gene FgCHS8 affects virulence and fungal cell wall sensitivity to environmental stress in Fusarium graminearum. Fungal Biol. 120, 764–774 (2016). [DOI] [PubMed] [Google Scholar]
- Siciliano I., Amaral Carneiro G., Spadaro D., Garibaldi A. & Gullino M. L. Jasmonic acid, abscisic acid, and salicylic acid are involved in the phytoalexin responses of rice to Fusarium fujikuroi, a high gibberellin producer pathogen. J. Agric. Food Chem. 63, 8134–8142 (2015). [DOI] [PubMed] [Google Scholar]