Abstract
Early Infantile Epileptic Encephalopathy (EIEE) presents shortly after birth with frequent, severe seizures and progressive disturbance of cerebral function. This study was to investigate a cohort of Chinese children with unexplained EIEE, infants with previous genetic diagnoses, causative brain malformations, or inborn errors of metabolism were excluded. We used targeted next-generation sequencing to identify potential pathogenic variants of 308 genes in 68 Han Chinese patients with unexplained EIEE. A filter process was performed to prioritize rare variants of potential functional significance. In all cases where parental testing was accessible, Sanger sequencing confirmed the variants and determined the parental origin. In 15% of patients (n = 10/68), we identified nine de novo pathogenic variants, and one assumed de novo pathogenic variant in the following genes: CDKL5 (n = 2), STXBP1 (n = 2), SCN1A (n = 3), KCNQ2 (n = 2), SCN8A (n = 1), four of the variants are novel variants. In 4% patients (n = 3/68), we identified three likely pathogenic variants; two assumed de novo and one X-linked in the following genes: SCN1A (n = 2) and ARX (n = 1), two of these variants are novel. Variants were assumed de novo when parental testing was not available. Our findings were first reported in Han Chinese patients with unexplained EIEE, enriching the EIEE mutation spectrum bank.
Epilepsy is one of the most common neurologic disorders, with a prevalence of 5–10 per 1,000/year1. Early infantile epileptic encephalopathies (EIEEs) are a heterogeneous group of disorders characterized by intractable seizures and unremitting interictal paroxysmal epileptiform activity that consequently impair neurodevelopmental outcomes during the first year of life2,3. It is one of the most severe and earliest form of epilepsy4. Genetic causes should be considered in the absence of structural brain abnormalities or inborn errors of metabolism5. A genetic cause for an epileptic encephalopathy was first recognized in 2001, when all seven children who were recruited in a study of Dravet syndrome had a de-novo SCN1A mutation6. Now molecular techniques, such as chromosomal microarray and next generation sequencing (NGS) of multiple genes, have contributed to today’s rapid growth in gene discovery for epileptic encephalopathies7,8,9, Copy number variants (CNVs) are important molecular causes of epileptic encephalopathy, with up to 8% of cases showing a causative or potentially contributing CNVs10.
As molecular diagnostics evolve, and with the ease of using them in some advanced facilities besides the underlying burdens of epilepsy especially in infancy, there is always a need to demonstrate the various clinical and research approaches. Profound understanding of the broader clinical spectrum and interpretation of genotype correlations requires accurate phenotyping. In this study we describe a cohort of previously investigated infants with unexplained sporadic EIEE and report the use of targeted NGS, followed by analysis of selected epilepsy genes in the probands.
Methods
Patients
Sixty eight infants with EIEEs were recruited for our study from Department of Pediatrics Neurology, Central South University, Xiangya first hospital, from 2012 to 2015 by a pediatric neurologist. Standardized clinical information was collected using a pre-test questionnaire completed by the recruiting clinician. Lymphocyte DNA was collected in all cases and their parents (as possible as paternal testing was accessible) using standard procedures. All clinical, neurophysiology, and imaging data were checked carefully to clearly define phenotypes. Phenotypes were classified into known electroclinical syndromes according to International League Against Epilepsy (ILAE) classification, where possible, or the electroclinical evolution defined as “unclassified”.
All patients were enrolled according to the following criteria; seizures onset within a year of age; severe electroencephalography EEG findings; Intellectual disabilities (ID); and no pinpointed cause. Patients who were examined previously for inborn errors of metabolism, structural brain malformation and common causes of EIEEs were excluded by detailed histories and routine examinations including blood glucose, blood ammonia, lactic acid, serum electrolytes, cranial magnetic resonance imaging (MRI), brain Computed Tomography (CT), amino acid and organic analyses, urinary metabolic screening, chromosome karyotype analysis and copy number variations. In addition, patients with monogenic disorders such as tuberous sclerosis complex, typical Rett syndrome, and MMPSI with KCNT1 ([Online Mendelian Inheritance in Man, http://www.omim.org/] OMIM: 608167) variants were not enrolled in our cohort. All patients were ethnically Han Chinese. Our study included 44 cases of West syndrome, four cases of Dravet syndrome, two cases of Ohtahara syndrome and eighteen cases were unclassified EIEEs. Clinical information, including clinical manifestation, EEG, MRI, CT and family history were collected and patients were followed up either by phone calls or inpatient/outpatient visits.
To assess intellectual disabilities in our patients we used the diagnostic criteria of the DSM-5 for Intellectual disabilities (Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, American Psychiatric Association, 2013), we assessed our patients adaptive functioning through observations at home and school, clinical interviews and standardized age-related rating scales as follow; for patients younger than 2–4 years of age we used Gesell Developmental Schedules, for patients between 4–6 years we used the Wechsler Preschool and Primary Scales of Intelligence Fourth Edition (WPPSI-IV), and for patients who are 6 years old or older 6 we used the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV). Patients with deficits in their adaptive and intellectual functioning with an onset during their developmental period were classified into mild ID when their development quotient (DQ) or intelligence quotient (IQ) values were between 50 to 69, moderate ID when DQ/IQ values were between 35 to 49 and severe ID when DQ/IQ values were less than 35.
Written informed consent was obtained from each patient and their participating parents. Methods were carried out in accordance with the relevant guidelines and regulations. This study was approved by the Central South University First Hospital Medical Ethics Committee.
Targeted Next-Generation Sequencing
We selected 308 genes for analysis in the panel including 16 known epilepsy-associated genes; genes analyzed were ARX, CDKL5, SLC25A22, STXBP1, SPTAN1, SCN1A, KCNQ2, ARHGEF9, PNKP, SCN2A, PLCB1, SCN8A, KCNT1, TBC1D24, GABRA1 and SYNGAP1 (see Supplementary Table S1). A custom-designed panel capturing the Exon regions of 308 genes associated with early infantile epileptic encephalopathy was synthetized using the Agilent Sure-Select Target Enrichment technique. Targeted next generation sequencing (NGS) was subsequently performed on an Illumina Hiseq 2000 platform (Illumina, San Diego, CA, USA) using a paired-end sequencing of 100 bp to screen for variants. Multiple sequence alignments of the affected amino acids were performed using a sequence alignment (Clustal W; The Biology Workbench, San Diego, CA, U.S.A.). Image analysis and base calling were performed by RTA software (real-time analysis, Illumina) and CASAVA software v1.8.2 (Illumina). After marking duplicate reads and filtering out reads of low base quality score using the Genome Analysis Tool kit (GATK), Sequence reads in FASTQ format were aligned to the reference human genome (hg19) using BWA (0.6.1-r104) and default settings, using BWA software (Pittsburgh Supercomputing Center, Pittsburgh, PA, USA)11. In addition to insertion-deletions (indels) and single-nucleotide polymorphisms (SNPs) identified using the GATK, variants were annotated using ANNOVAR (www.openbioinformatics.org/annovar/annovar_download.html#credit). The average sequencing depth was 140×.
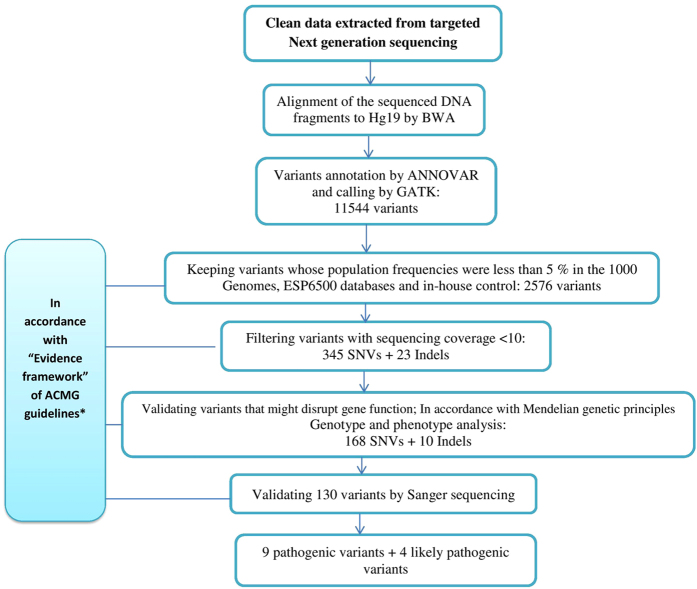
In accordance with ACMG Standards and Guidelines12, we performed several steps of filtering data to identify possible pathogenic variants: (a) Obtaining the frequencies of variants in population databases; Exom Aggregation Consortium, 1000 Genomes Project and ESP6500 databases and in-house control (200 healthy controls were used by the company which performed NGS for our team); (b) Assessment of variants pathogenicity in disease databases; OMIM, Human Gene Mutation database and ClinVar; (c) Determination of the effect of the variant on the primary and alternative gene transcripts, other genomic elements, as well as the potential impact of the variant on the protein through computational (in silico) predictive programs, PlyPhen-2 and SIFT. Variants validated after the above noted steps were then checked in the published literature where possible and considered to be a candidate for pathogenic variants and were picked out for further investigation. In accordance with Mendelian genetic principles (the inheritance pattern of the involved genes) we chose variations which to validate by Sanger sequencing to identify the segregate status in these families and indicate the candidate pathogenic variants according to parental origin of the variations and clinical features of the patients. Candidate pathogenic variants were then assessed in accordance with ACMG standards and guidelines’ “Evidence framework’ to be classified into pathogenic and likely pathogenic variants (see Fig. 1).
Figure 1. Screening of Potentially Pathogenic and Likely Pathogenic Variants in Our Study of 68 Patients with Unexplained EIEE.
*Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–423 (2015)12.
Results
Clinical Characteristics
We recruited 68 infants with unexplained early infantile epileptic encephalopathy, all were less than a year old; male to female ratio was 1:0.45. We had 44 cases of West syndrome (n = 44), four cases of Dravet syndrome (n = 4), two cases of Ohtahara syndrome (n = 2) and eighteen cases were unclassified EIEEs (n = 18). Seizures onset was as follow; 24 cases within three months of life, 29 cases from three to six months of life and 15 cases from seven to twelve months of life, average age of seizures onset was 4.65 ± 2.37 months, thus 78% (n = 53) of cases developed epileptic encephalopathy within six months of life. Assessment of patients’ intellectual disability revealed 18 patients with mild ID (26.4%), 20 patients with moderate ID (29.4%) and 30 patients with severe ID (44.2%). In our patients, family history was positive in nine patients where their first degree relatives have had epilepsy or intellectual disability and mother’s pregnancy history also was positive in nine patients. Table 1.
Table 1. Summary of the Clinical Features of Patients.
| Clinical Characteristics | N |
|---|---|
| Age of Seizure Onset | |
| <3 month | 24 |
| 3~6 month | 29 |
| 7~12 month | 15 |
| Sex | |
| Male:female | 47:21 |
| Family History | |
| Positive family history* | 9 |
| Mother’s Pregnancy History | |
| Spontaneous abortion | 3 |
| Threatened abortion | 3 |
| Pregnancy hypertension | 1 |
| Premature labour | 2 |
| EIEE Classification | |
| West syndrome | 44 |
| Dravet’s syndrome | 4 |
| Ohtahara syndrome | 2 |
| uEEEs | 18 |
| Seizure Types | |
| Spasms | 33 |
| Spasms-tonic | 4 |
| Tonic | 3 |
| Tonic clonic | 12 |
| Partial | 9 |
| Multiple seizure types | 7 |
| Head MRI or CT Scan | |
| Normal | 39 |
| Ventriculomegaly | 18 |
| Arachnoid cyst | 3 |
| Myelination delay | 1 |
| Formation of CSP | 1 |
| Cases where MRI or CT was not checked | 6 |
| EEG | |
| Hypsarrhythmia | 48 |
| Sharp waves | 3 |
| Slow waves | 2 |
| Slow-spike-and-wave complexes | 2 |
| Slow basic background activity | 1 |
| Burst-suppresion | 4 |
| Multiple epileptiform discharges | 8 |
| Intellectual Disabilities (ID) | |
| Mild | 18 |
| Moderate | 20 |
| Severe | 30 |
| Response to AEDs Therapy | |
| Seizures-free cases | 13 |
| Seizures-controlled cases** | 16 |
| AEDs resistant cases*** | 18 |
| Deceased cases | 3 |
| Cases we lost contact with | 8 |
*First-degree relative had history of epilepsy or intellectual disability.
**Seizures were considered controlled if the reduction of frequency of seizures attacks became more than 50% after treatment.
***Patients were considered resistant to treatment if the reduction of seizures attacks became less than 50% after taking AEDs.
In West syndrome cases (n = 44), seizures onset was as follow; 12 cases before three month of life, 22 cases from three to six months and ten cases from seven to twelve months of life, the seizures types were as follow; 31 patients had infantile spasms, 4 had spasms and tonic seizures, 5 had tonic-clonic seizures, and 4 with partial, tonic or tonic-clonic seizures. Their EEG showed Hypsarrhythmia including five patients with 50% of their epileptiform discharges happened during the non-rapid eye movement sleep cycle. Assessment of their ID showed 12 patients with mild ID, 10 patients with moderate ID and 22 patients with severe ID.
As noted above, the other cases of unexplained EIEE (n = 24) included four cases of Dravet syndrome, two cases of Ohtahara syndrome and eighteen cases were unclassified EIEEs (n = 18), in these cases seizures onset was as follow: 12 cases before three month of life, 7 cases from three to six months and 5 cases from seven to twelve months; the seizures types were as follow: 2 patients had infantile spasms, 7 had tonic-clonic seizures, 9 with partial seizure, 1 with tonic seizure and 5 had multiple types of seizures. EEG findings in these cases are described as follow: intermittent burst-suppression during sleep cycle in four cases; spikes, sharp waves and polyspikes in four cases; spikes, slow-spike-and-wave and polyspike-and-slow-wave complexes in five patients; intermittent hypsarrhythmia in four cases; sharp waves in three cases; slow waves with high amplitude in two cases; widely spread slow-spike-and-wave complexes in one case with 100% of these epileptiform discharge happened during the non-rapid eye movement sleep cycle and one case of slow basic background activity rhythms. Assessment of ID in these cases showed 6 patients with mild ID, 10 patients with moderate ID and 8 patients with severe ID.
Among the 68 patients recruited in our cohort, the efficacy of antiepileptic drugs (AEDs) was illustrated as follow; clinical seizure freedom was achieved in 13 patients, 16 patients had their seizures controlled, 28 patients were resistant to treatment, three patients have died (one case probably due to nocturnal asphyxia, one case of probable sudden unexpected death in epilepsy (SUDEP) and one case of unknown cause of death). We lost contact with eight patients. Patients’ response to AEDs had been followed up from one month to four years. Patients were treated with a single or poly AEDs, adrenocorticotropic hormone (ACTH) and/or ketogenic diet. AEDs were chosen according to patients’ response to treatment. AEDs options were; Oxcarbazepine (OXC), Carbamazepine (CBZ), Levetiracetam (LEV), Phenobarbital (PB), Topiramate (TPM) and Sodium valproate (VPA). Clinical features of the 68 patients in our cohort including MRI and CT scan findings are summarized in Table 1.
Identification of Variants
Of the 68 patients with unexplained EIEEs, variants were detected in 13 patients (19%). Nine de novo pathogenic variants including four novel variants, and one assumed de novo pathogenic variant were identified in 15% of patients (n = 10/68). Variants in these patients and associated phenotypes are described as follow: two variants of CDKL5 (c.278dupA/p.E93fs, c.1110delC/p.N370fs) and one variant of STXBP1 (c.1216C > T/p.R406C) were identified in three West syndrome patients; Three variants of SCN1A (c.225G > T/p.E75D, c.2134C > T/p.R712X, c.4811G > A/p.W1604X) were associated with three Dravet syndrome patients; two KCNQ2 variants (c.1574G > A/p.R525Q, c.1574G > A/p.R525Q) and one SCN8A variant (c.5615G > A/p.R1872Q) were identified in three patients with unclassified EIEEs; one STXBP1 variant (c.1216C > T/p.R406C) was identified in a patient with Ohtahara syndrome.
Three likely pathogenic variants; two assumed de novo and one X-linked were identified in 4% patients (n = 3/68) in the following genes: SCN1A (n = 2) and ARX (n = 1), two of these variants are novel. Variants in these patients and associated phenotypes are described as follow; two variants of SCN1A (c.1703G > A/p.R568Q, c.4176T > A/p.N1392K) were identified in two patients of unclassified EEES and one variant of ARX (c.1600G > C/p.A534P) in a West syndrome patient. Variants were assumed de novo when parental testing was not available. In accordance with ACMG Standards and Guidelines and its rules for combining criteria to classify sequence variants, variants were classified into pathogenic and likely pathogenic variants12. Among the 13 cases with detected variants, SCN1A was the most frequently affected gene in our study, accounting for 38.5% (5/13), followed by STXBP1, CDKL5, KCNQ2, ARX and SCN8A of 15.4% (2/13), 15.4% (2/13), 15.4% (2/13), 7.7% (1/13) and 7.7% (1/13) respectively (see Fig. 2). Phenotypes, inheritance, and molecular characteristics for all patients with variants are described in Table 2.
Figure 2. Percentage of Cases with Variants Among All the Variants Identified in Our Study.
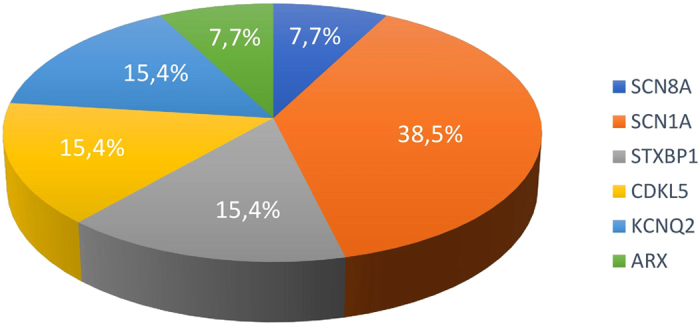
Among the 13 cases with detected variants, SCN1A was the most frequently affected gene in our study, accounting for 38.5%, followed by STXBP1, CDKL5, KCNQ2, ARX and SCN8A of 15.4%, 15.4%, 15.4%, 7.7% and 7.7% respectively.
Table 2. Summary of the 13 Cases with Variants Detected in Our Study.
| Patient | Age of Onset | Clinical Diagnosis | Seizures types | EEG (age) | Neuroimaging | Response to AEDs | DQ/IQ results & Age at evaluation | ID | Mutant Gene, Inheritance | HGMD Reported or Novel | SIFT/Polyphen 2 Prediction | Mutation | Location of Variant (Protein) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pathogenic Variants | |||||||||||||
| C0106 | 1 month (M) | WS | Partial then epileptic spasms | Hypsarrhythmia | Ventriculomegaly | Resistant to AEDs and ketogenic diet | (30) 2 y and 8 mo | Severe | CDKL5 Xp22.13, de novo | Novel | / | (NM_003159) chrX :18593605–18593606(insA) c.278dupA/p.E93fs | Protein kinase domain |
| S553 | 3 months (F) | WS | Partial then epileptic spasms | Hypsarrhythmia | Normal | Resistant to AEDs and ketogenic diet | (27) 1 y and 5 mo | Severe | CDKL5 Xp22.13, de novo | (Zhao 2014)1 | / | (NM_003159) chrX :18622154–18622155(delC) c.1110delC/p.N370fs | Cytoplasmic domain |
| S559 | 2 days (F) | uEEEs | Tonic | Sharp waves | Arachnoid cyst | PB then lev and VPA (not controlled) then monotherapy LEV (seizure-free) | (60) 4mo & (55) 4 y | Mild | KCNQ2 20q13.33, de novo | (Moulard 2001)2,**** | Deleterious/probably damaging | (NM_004518) chr20:62044908(G > A) c.1574G > A/p.R525Q | C-terminal |
| C0107 | 1 months (F) | uEEEs | Partial | Spikes, slow-spike-and-wave and polyspike-and-wave complexes | Normal | LEV then LEV and VPA (seizures-controlled) | (42) 1 y and 6 mo | Moderate | KCNQ2 20q13.33, NT | (Moulard 2001)2,**** | Deleterious/probably damaging | (NM_004518) chr20:62044908(G > A) c.1574G > A/p.R525Q | C-terminal |
| S557 | 4 months (M) | uEEEs | Tonic | Hypsarrhythmia, Spike wave (11 months) | Ventriculomegaly | LEV then LEVPB and CBZ, then VPA (refractory seizures) | (36) 2 y | Moderate | SCN8A 12q13.13, de novo | Novel | Deleterious/probably damaging | (NM_014191) chr12:52200885(G > A) c.5615G > A/p.R1872Q | Calmodulin-binding motif |
| C0125 | 6 months (F)) | DS | Partial, myoclonic Then tonic | Normal, then spikes,sharp waves and polyspikes (7 months) Slow wave and abnormal background (4 years) | Formation of CSP | OXC, LEV, TPM, then ketogenic diet (slightly-controlled) | (40) 4 y and 2 mo | Moderate | SCN1A 2q24.3, de novo | (Sugawara 2002)3 | Tolerated/probably damaging | (NM_001202435) chr2:166898844(C > T) c.2134C > T/p.R712X | Cytoplasmic domain |
| C0129 | 7 months (M) | DS | Tonic clonic, Febrile convulsions (13 months), tonic, tonic clonic and partial (2y 7months) | Slow-spike-and- wave complexes | Normal | VPA controlled for 1 year, then LEV (controlled, but induced by fever) | (55) 3 y & (52) 5 y | Mild | SCN1A 2q24.3, de novo | Novel | Tolerated/probably damaging | (NM_001202435) chr2:166929907(G > T) c.225G > T/p.E75D | Cytoplasmic domain |
| R1014 | 6 months (M) | DS | Fever-induced tonic or tonic clonic | Normal then spikes, sharp waves and polyspikes (10 months) | Normal | PB, LEV and TPM (slightly-controlled) | (38) 2 y and 6 mo | Moderate | SCN1A 2q24.3, de novo | Novel | Deleterious/probably damaging | (NM_001202435) chr2:166850697(G > A) c.4811G > A/p.W1604X | Ion transport domain |
| C0108 | 3 months (M) | WS | spasms | Hypsarrhythmia | Arachnoid cyst | ACTH,TPM and VPA (refractory seizures) Then TPM, VPA and ketogenic diet (refractory seizures) | (19) 3 y and 10 mo | Severe | STXBP1 9q34.11, de novo | (Allen 2016)4 | Deleterious/probably damaging | (NM_003165) chr9:130438188(C > T) c.1216C > T/p.R406C | Syntaxin-binding protein 1 chain |
| R1007 | 3 months (M) | OS | Spasms | Intermittent burst-suppression during sleep cycle | Ventriculomegaly | VPA and ACTH (refractory seizures) | (36) 1 y | Moderate | STXBP1 9q34.11, de novo | (Allen 2016)4 | Deleterious/probably damaging | (NM_003165) chr9:130438188(C > T) c.1216 C > T/p.R406C | Syntaxin-binding protein 1 chain |
| Likely Pathogenic Variants | |||||||||||||
| C0117 | 7 months (M) | uEEEs | Partial | Slow waves with high amplitude | Ventriculomegaly | VPA, TPM and LEV (refractory seizures) | (44) 1 y and 6 mo | Moderate | SCN1A 2q24.3, NT | Novel | Deleterious/probably damaging | (NM_001202435) chr2:166859090(T > A) c.4176T > A/p.N1392K | Ion transport domain |
| S560 | 1 year (F) | uEEEs | Tonic | Slow-spike-and-wave complexes 100% during NREM sleep cycle | Myelination delay in the cerebral white matter | VPA then LEV and VPA (refractory seizures) | (37) 2 y and 4 mo | Moderate | SCN1A 2q24.3, NT | *** | Deleterious/probably damaging | (NM_001202435) chr2:166900519(G > A) c.1703G > A/p.R568Q | Cytoplasmic domain |
| S569 | 53 days (M) | OS developed to WS | Spasms | Intermittent burst-suppression during sleep cycle | Normal | ACTH and LEV (seizure controlled) then refractory seizures after 4 months. Then LEV and VPA (refractory seizures) SUDEP | (21) 2 y and 6 mo | Severe | ARX Xp21.3, maternal | Novel | Deleterious/probably damaging | (NM_139058) chrX:25022876 (G > C) c.1600G > C/p.A534P | Aristaless domain |
(M) Male; (F) Female; (OS) Ohtarah syndrome; (WS) West syndrome; (DS) Dravet syndrome; (uEEEs) unknown type of early epileptic encephalopathy; (NT) not tested; (SUDEP) sudden unexpected death in epilepsy; (y) year; (mo) month; (ACTH) adrenocorticotropic hormone; (OXC) Oxcarbazepine; (CBZ) Carbamazepine; (LEV) Levetiracetam; (PB) Phenobarbital; (TPM) Topiramate (VPA); Sodium valproate (VPA); (NREM) non-rapid eye movement.
1(Zhao 2014): The variant was reported in variants of CDKL5 and early-onset epileptic encephalopathies or Hanefeld variants of RTT(Rett syndrome); Zhao, Y. et al. Clinical features and gene mutational spectrum of CDKL5-related diseases in a cohort of Chinese patients. BMC Med. Genet. 25, 15:24 (2014)50.
2(Moulard 2001): The variant was reported in the association between the benign neonatal epilepsy and variants in genes coding for potassium channel subunit KCNQ2; Moulard, B. et al. Ion channel variation causes epilepsies. Brain Res. Brain Res. Rev. 36, 275–284 (2001)51.
3(Sugawara 2002) : The variant was reported in Myoclonic epilepsy of infancy; Sugawara, T. et al. Frequent variants of SCN1A in severe myoclonic epilepsy in infancy. Neurology 58, 1122–1124 (2002)16.
4(Allen 2016): The variant was reported in Unexplained early onset epileptic encephalopathy; Allen, N. M. et al. Unexplained early onset epileptic encephalopathy: Exome screening and phenotype expansion. Epilepsia 57, e12–7 (2016)31.
***The variant is not reported in HGMD and the ExAC_MAF = 0, but it is reported in dbSNP build 146 rs794727025 dbSNP: rs794727025 Position: chr2:166900519 Band: 2q24.3.
****The variant is not reported in the ExAC, but it is reported in dbSNP build 146 rs118192234 dbSNP: rs118192234 Position: chr20:62044908 Band: 20q13.33.
*****Although parental testing was not available, but since (S559 and C0107) share the same variant (c.1574G > A; p.R525Q) in KCNQ2, since this variant is pathogenic in patient S559, the same variant of patient C0107 was considered pathogenic according to the ACMG Standards and guidelines for the interpretation of sequence variants12.
Discussion
Wide range of genotype and phenotype heterogeneity makes it difficult to predict with certainty the potentially responsible gene for many EIEEs. Our group has conducted this study on Chinese Han infants. In our study the total detection rate of variants was 19% (13/68) including pathogenic variants in 15% of the cases (10/68), and likely pathogenic variants in 4% of the cases (3/68). Variants were found in patients with a broad range of phenotypes (see Table 2). Perinatal Sodium channelopathies were identified in six patients with variants in SCN1A and SCN8A, five and one respectively.
De novo variants in SCN1A are an increasingly recognized cause of an early-onset seizure and developmental delay. Roughly 80% of Dravet syndrome patients carry a mutation in the SCN1A gene13,14,15. Variants in SCN1A identified in our study are described as follow: Three de novo pathogenic variants of SCN1A including two novel variants were detected in three cases of Dravet syndrome (C0125, C0129 and R1014), several studies have supported the association of SCN1A gene mutation and Dravet syndrome13,14,15. In case (C0125), no remarkable ID was noticed till the age of one year, at the age of two years old she could walk and talk, at the age of four years she would face some difficulties climbing up and down stairs, her DQ at the age of four years and two months indicates moderate ID. (C0129) was delivered at the age of 32 weeks. Till the age of two years no remarkable ID was noticed and at the age of three years and five years his DQ indicates mild ID. (3). In case (R1014) no remarkable ID was noticed before the age of two years. but then he started to lag behind his peers and his DQ indicates moderate ID at the age of 2 years and six months. For the above mentioned cases parental testing was available. Two likely pathogenic variants of SCN1A including one novel variant were detected in two unclassified EEEs (C0117, S560) parental testing was not possible. (C0117) was delivered at the age of 28 weeks and was diagnosed with hyperbilirubinemia, at the age of one year and one month his DQ indicates moderate ID. (2). (S560) female, at the age of two years and four months her DQ indicates moderate ID. The variant we identified in (C0125) was previously reported and led to protein change (Arg712X)16. Although these observations provide further support for the proposed association of SCN1A variants and Dravet syndrome17,18, further studies are needed to confirm the linkage between SCN1A variants and other unclassified EEEs. It worth mentioning that most of reported SCN1A variants in patients with seizures onset within the first year of life were associated with severe developmental delay, while in our study, SCN1A carrying cases were shown mild to moderate ID, this finding could be due to the small size of our cohort or population diversity and different inclusion criteria. De novo variants in SCN8A are a recently recognized cause of early-onset seizures with moderate to severe developmental delay18,19,20,21,22,23. We identified in one case (S557) with unclassified EEE, two de novo novel variants; SCN8A (NM_014191) c.5615G > A/p.R1872Q and KCNMA1 (NM_001014797) c.3488A > G/p.N1163S. This case was a male patient with irrelevant perinatal and family histories; now at the age of two, he suffers severe epilepsy, he cannot speak or walk, and his DQ shows moderate ID. Previous studies have proposed that de novo variants may often be pathogenic variants in a child with severe epilepsy and negative family history21. De novo SCN8A heterozygous variants also have been proven to be pathogenic18,19,20,21,22, especially in patients with seizures onset within the first year of life23, which matches our findings. To our knowledge, there is only two reported cases of KCNMA1 variants; first study, Tomas M et al.24, when he reported a relation between KCNMA1 and severe essential hypertension and myocardial infarction24, second, Du W et al.25, when they reported that KCNMA1 mutation would result in generalized epilepsy and paroxysmal dyskinesia25. We are here the first to report the possible association between KCNMA1 gene variant and unclassified EEEs. We did not discuss this finding in details for the possibility that the phenotype of the patient was mainly due to the pathogenic variant of SCN8A.
Heterozygous variants in KCNQ2 are a well-understood cause of early-onset seizures. Reported phenotypes differ from benign familial neonatal seizures to a progressive pharmacoresistant EIEE26,27. We identified two KCNQ2 variants, in two cases of EIEE (S559, C0107), both carried the same mutation in KCNQ2 gene: NM_004518, c.1574G > A/p.R525Q. The variant in the first case (S559) was de novo, while for the other one (C0107) parental testing was not possible. (S559) had refractory seizures till the age of a year and five months, after she was given monotherapy of LEV, she was declared seizure-free. Mild ID was detected as early as four months of age, now at the age of four years her DQ indicates mild ID. (2). (C0107) had refractory seizures till she was given VPA and LEV at the age of one year and one month. Now at the age of one year and six months seizures are controlled and her DQ indicates moderate ID. Although several studies have reported that variants with KCNQ2 are usually associated with severe developmental delay17,28, our cases showed mild and moderate ID, we refer this to early control of seizures and the efficacy of AEDs. A recent study has reported the efficacy of VPA, LEV and TPA with patients who carry KCNQ2 variants28. Formerly reported genotype-phenotype studies have emphasized that truncating variants of KCNQ2 are associated with benign, inherited phenotype (benign familial neonatal seizures1)26, while missense variants of KCNQ2 are the causative variants of severe, sporadic phenotypes27,29 (EIEE), cellular experiments point out that these last-mentioned variations may have a dominant negative effect on the function at a cellular level27,29, thus our findings are adding momentum to the fact that The KCNQ2 gene, is responsible for about 10% of EIEEs with neonatal onset30.
De novo missense variant in STXBP1 was identified in two cases (C0108, R1007), the seizures in both cases were refractory. (C0108) was diagnosed with West syndrome, now at the age of three years and ten months he cannot sit alone or call a person and his DQ indicates severe ID. (R1007) was diagnosed with Ohtahara syndrome, now at the age of one year old his DQ indicates moderate ID. Both of these patients carried the same missense variant in STXBP1 gene: (NM_003165), c.1216C > T; p.R406C, this variant was reported as a pathogenic variant in a case of Ohtahara syndrome with profound ID31, which matches our result and support the findings of previously reported studies that mutation in STXBP1 is extensively associated with severe early-onset epileptic encephalopathies including Ohtahara syndrome, West syndrome and other epileptic phenotypes with moderate to severe ID32,33,34,35.
An ARX gene variant was identified in our study in a male case (S569) with West syndrome; it revealed maternally inherited in the ARX gene (NM_139058, c.1600G > C/p.A534P) of a non-consanguineous marriage and irrelevant perinatal and family histories, it was deemed as a likely pathogenic variant. Seizures were refractory to AEDs, sadly we lost this patient when he was 2 years and six months old due to probable SUDEP, when no severe respiratory or cardiovascular disorders could be linked to his death. Prior to his death, his DQ indicated severe ID, which firmly associated ARX gene variants and severe ID/DD35. The linkage between ARX gene variants and SUDEP was previously considered as a potential cause of SUDEP36, but further studies are needed to emphasize this hypothesis, which firmly associated ARX gene variants and severe ID35, variants in this gene have been associated with X-linked severe ID, lissencephaly with abnormal genitalia36,37,38,39,40,41. Previous case control studies have suggested that epilepsy onset, AED polytherapy and poor seizure control are major risk factors for SUDEP42.
De novo variants in CDKL5 are a well-recognized cause of EIEE and severe, Rett-like developmental delay43,44, we identified two de novo CDKL5 gene variants in two case (S553 female, C0106 male) NM_003159, c.1110delC/p.N370fs and NM_003159 c.278dupA/p.E93fs respectively, now at the age of one year and five months (S553), is not able to sit independently, she cannot talk and sporadically shows random involuntary movements, (C0106) is two years and eight months old and cannot talk or walk, they both have severe ID, and their seizures are resistant to AEDs and/or ketogenic diet. CDKL5 gene is located on the X chromosome, and the majority of reports describe de novo X-linked variants in females43,45, here, we identified a variant in a male patient, which spots the lights on the potential under-recognition of CDKL5 gene mutation as a pathogenic variant in males, which has been recently emphasized28,30. Previously reported studies have shown that patients with CDKL5 variants are mainly presented as early onset epileptic encephalopathy (EOEE) with epileptic spasms and severe ID, and suggested that CDKL5 variants should be kept in consideration first in patients showing EOEE with involuntary movements which is being advised by our results which is being enforced by our results28,30,46.
In variants carrying cases, severe ID was found in 31% (4/13), moderate ID in 54% (7/13) and mild ID was found in 15% (2/13), this result demonstrates the well-established relation between unexplained EIEE and intellectual disabilities and enriches their genotype-phenotype correlations.
Comparison with Other Cohort Studies of EE Tested by NGS
Zhang Y et al.17, used targeted next-generation sequencing to detect variants within 300 genes related to epilepsy and ID/DD in 253 Chinese children with unexplained epilepsy and ID/DD. The detection rate was 18% (46/253) in the whole group and 26% (17/65) in the early-onset (before three months after birth) epilepsy group, in their cohort, patients with an SCN1A variants accounted for the largest proportion, 17% (8/46), which matches our results when we found that SCN1A was the most frequently mutated gene in our study, accounting for 5 (38%) of 13 variants, emphasizing on the linkage between SCN1A variants and EIEE. Gokben S et al.47, reported a cohort of 30 patients of early-onset EE and identified twelve definite or potential causal variants using targeted next generation sequencing analysis. The detection rate in our study was 19% while in their study was 40%; this inconsistency could be due to that parental consanguinity was found in 40%of the cases and perinatal asphyxia was reported in 27% of the patients. Thus different inclusion criteria may account for inconsistent rate between similar studies. Recently, Zhang Q et al.28, reported a cohort of 175 Chinese patients with EOEEs, the author identified variants is 56 patients de novo heterozygous variants, unlike our study CDKL5 gene mutation accounted for the largest proportion 13.1% (23/175). In their study, the majority of cases (68%, 119/175) remained unexplained which we found quite similar to our findings that (81%, 55/68) of our cases continued to be unexplained, this observation spots the light on the additional candidate pathogenic genes that still need to be unraveled in the future, ARX was a candidate gene in their cohort of 175 patients, but no detected variant was found in this gene, unlike our study. The author recommended VPA, LEV and TPM for patients with KCNQ2 variants, which matches our observation, as VPA and LEV were effective in our patients with KCNQ2 variants. Kong et al.23, reported a cohort of Chinese patients and identified five de novo SCN8A variants that stated to be the first reported in Chinese patients with epilepsy and ID/DD, in our cohort we identified a novel de novo SCN8A mutation associated with unclassified EEEs and moderate ID, thus enriching the relation between SCN8A variants and EIEE. Mercimek-Mahmutoglu et al.48, conducted a retrospective cohort study of 110 patients with intractable epilepsy, global developmental delay, and cognitive dysfunction, Detection rate by targeted next-generation sequencing was 12.7% and SCN1A was the most frequently mutated gene accounting for 29% of 14 variants, which we found similar to our results. Hardies k et al.49, reviewed 35 NGS studies that focused on patients with epilepsy, and cited that genetic factors are thought to have a role in 70% of all epilepsy; also the author reported that NGS findings have additionally increased the recognition of phenotypical and genetic heterogeneity which was demonstrated in our study.
Summary
Through next-generation sequencing in 68 Han Chinese patients with unexplained EIEEs we were able to detect pathogenic variants and likely pathogenic variants in 15% and 4% of the cases, respectively. Six of these variants are novel. A total detection rate of 19% of variants adds weight to the known efficacy of next-generation sequencing in detecting variants in patients with unexplained EIEE. Moderate to severe ID were presented in eleven patients of the thirteen variants-carrying patients which augments the recognized relation between EIEE and moderate to severe ID, especially in patients with seizures onset within the first year of life. We advise that further attention should be paid to EIEEs patients with ARX gene variants, especially those who are on AED poly therapy and with poor seizure control, as we lost one patient with a variant in ARX gene due to SUDEP.
Our study not only helped to improve our understanding of the clinical characteristics and the possible etiology of EIEEs, but also enriched the EIEEs genes bank and enlightened our comprehension of EIEE-related concerns and would potentially serve as a valuable reference for further studies.
Study Limitations
Parental testing was not available in three cases. Without results of segregation studies and in vitro/in vivo analyses, a low probability of pathogenicity should be considered, and translation into clinical practice should be implemented with caution. The difference in socio-cultural backgrounds and the small size of our cohort (68 patients) may have resulted in a different percentage of variants with mild and moderate ID compared to some previously reported studies.
Additional Information
How to cite this article: Arafat, A. et al. Unexplained Early Infantile Epileptic Encephalopathy in Han Chinese Children: Next-Generation Sequencing and Phenotype Enriching. Sci. Rep. 7, 46227; doi: 10.1038/srep46227 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We would like to thank the patients and their parents for their participation in this study, and the entire team who worked on this study as well as our mentors for their continuous help and advice. This work was supported by The National Natural Science Foundation of China (Fund Project 81370771) and The Key Research Project of the Ministry of Science and Technology of China (Grant No. 2016YFC0904400).
Footnotes
The authors declare no competing financial interests.
Author Contributions The original data upon which this work is based is preserved and retrievable for reanalysis. All of the information and data here are representative of the original data, and we will do our utmost best to minimize obstacles to the sharing of data, materials, algorithms or reagents described in this work. Authors that participated in this work are as follows: Ahmed Arafat, the main author, participated in the entire process of our project and helped to analyze all of the data in addition to writing the main body of the manuscript text. Prof. Peng Jing, the first co-author, designed the gene panel of this study and helped to analyze the data and review the manuscript after a complete revision was advised by one of the reviewers. Yuping Ma, the second co-author, helped in the process of analyzing patients’ data and the results, and helped in comparing our results to the already published literature. Miao Pu, the third co-author, helped in the process of analyzing the clinical features of the patients and in analyzing their EEG and MRI results. Gai Nan, the fourth co-author, was responsible for gene analysis and analyzing the results according to ACMG standards and guidelines. He Fang, and Chen Chen, the fifth and sixth co-authors, respectively, were responsible for diagnosis and treatment of the patients enrolled in our study. Prof. Yin Fei, the corresponding author, is the head of the team, this work was his idea and he funded it and supervised each and every step of this study. All of the authors have reviewed the manuscript.
References
- Sander J. W. The epidemiology of epilepsy revisited. Curr. Opin. Neurol. 16, 165–170 (2003). [DOI] [PubMed] [Google Scholar]
- Berg A. T. et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia 51, 676–685 (2010). [DOI] [PubMed] [Google Scholar]
- Sharma S. & Prasad A. N. Genetic testing of epileptic encephalopathies of infancy: an approach. Can. J. Neurol. Sci. 40, 10–16 (2013). [DOI] [PubMed] [Google Scholar]
- Saitsu H. et al. De novo variants in the gene encoding STXBP1 (MUNC18-1) cause early infantile epileptic encephalopathy. Nat. Genet. 40, 782–788 (2008). [DOI] [PubMed] [Google Scholar]
- Mastrangelo M. & Leuzzi V. Genes of early-onset epileptic encephalopathies: from genotype to phenotype. Pediatr. Neurol. 46, 24–41 (2012). [DOI] [PubMed] [Google Scholar]
- Claes L. et al. De novo variants in the sodium-channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am. J. Hum. Genet. 68, 1327–32 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvill G. L. et al. GRIN2A variants cause epilepsy-aphasia spectrum disorders. Nat. Genet. 45, 1073–1076 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A. S. et al. De novo variants in epileptic encephalopathies. Nature 501, 217–221 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcia G. et al. De novo gain-of-function KCNT1 channel variants cause malignant migrating partial seizures of infancy. Nat. Genet. 44, 1255–1259 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mefford H. C. et al. Rare copy number variants are an important cause of epileptic encephalopathies. Ann. Neurol. 70, 974–985 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. & Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–423 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini C. et al. The genetics of Dravet syndrome. Epilepsia 52, (Suppl. 2), 24–29 (2011). [DOI] [PubMed] [Google Scholar]
- Xu X. et al. Early clinical features and diagnosis of Dravet syndrome in 138 Chinese patients with SCN1A variants. Brain Dev. 36, 676–681 (2014). [DOI] [PubMed] [Google Scholar]
- Hirose S. et al. SCN1A testing for epilepsy: application in clinical practice. Epilepsia 54, 946–952 (2013). [DOI] [PubMed] [Google Scholar]
- Sugawara T. et al. Frequent variants of SCN1A in severe myoclonic epilepsy in infancy. Neurology 58, 1122–1124 (2002). [DOI] [PubMed] [Google Scholar]
- Zhang Y. et al. Gene Mutation Analysis in 253 Chinese Children with Unexplained Epilepsy and Intellectual/Developmental Disabilities. PLoS One 10, e0141782 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly M. B. Dravet Syndrome: Diagnosis and Long-Term Course. Can. J. Neurol. Sci. 43, (Suppl. 3), 3–8 (2016). [DOI] [PubMed] [Google Scholar]
- Ohba C. et al. Early onset epileptic encephalopathy caused by de novo SCN8A variants. Epilepsia 55, 994–1000 (2014). [DOI] [PubMed] [Google Scholar]
- Larsen J. et al. The phenotypic spectrum of SCN8A encephalopathy. Neurology 84, 480–489 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer I. E. Genetic testing in epilepsy: what should you be doing? Epilepsy Curr. 11, 107–111 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagnon J. L. et al. Convulsive seizures and SUDEP in a mouse model of SCN8A related epileptic encephalopathy. Hum. Mol. Genet. 24, 506–515 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W. et al. SCN8A variants in Chinese children with early onset epilepsy and intellectual disability. Epilepsia 56, 431–438 (2015). [DOI] [PubMed] [Google Scholar]
- Tomás M. et al. Genetic variation in the KCNMA1 potassium channel alpha subunit as risk factor for severe essential hypertension and myocardial infarction. J. Hypertens. 26, 2147–2153 (2008). [DOI] [PubMed] [Google Scholar]
- Du W. et al. Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat. Genet. 37, 733–738 (2005). [DOI] [PubMed] [Google Scholar]
- Singh N. A. et al. A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Na. Genet. 18, 25–29 (1998). [DOI] [PubMed] [Google Scholar]
- Miceli F. et al. Genotype-phenotype correlations in neonatal epilepsies caused by variants in the voltage sensor of K(v)7.2 potassium channel subunits. Proc. Natl. Acad. Sci. USA 110, 4386–4391(2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q. et al. Gene mutation analysis of 175 Chinese patients with early-onset epileptic encephalopathy. Clin. Genet., doi: 10.1111/cge.12901. (2016). [DOI] [PubMed] [Google Scholar]
- Trump N. et al. Improving diagnosis and broadening the phenotypes in early-onset seizure and severe developmental delay disorders through gene panel analysis. J. Med. Genet. 53, 310–317 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milh M. et al. Similar early characteristics but variable neurological outcome of patients with a denovo mutation of KCNQ2. Orphanet J. Rare Dis. 8, 80 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen N. M. et al. Unexplained early onset epileptic encephalopathy: Exome screening and phenotype expansion. Epilepsia 57, e12–7 (2016). [DOI] [PubMed] [Google Scholar]
- Otsuka M. et al. STXBP1 variants cause not only Ohtahara syndrome but also West syndrome–result of Japanese cohort study. Epilepsia 51, 2449–2452 (2010). [DOI] [PubMed] [Google Scholar]
- Mastrangelo M. et al. Neonatal suppression-burst without epileptic seizures: expanding the electroclinical phenotype of STXBP1-related, early-onset encephalopathy. Epileptic Disord. 15, 55–61 (2013). [DOI] [PubMed] [Google Scholar]
- Carvill G. L. et al. GABRA1 and STXBP1: novel genetic causes of Dravet syndrome. Neurology 82, 1245–1253 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud J. L., Lachance M., Hamdan F. F. et al. The genetic landscape of infantile spasms. Hum. Mol. Genet. 23, 4846–4858 (2014). [DOI] [PubMed] [Google Scholar]
- Coll M. et al. Genetic investigation of sudden unexpected death in epilepsy cohort by panel target resequencing, Int. J. Legal Med. 130, 331–339 (2016). [DOI] [PubMed] [Google Scholar]
- Kitamura K. et al. Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nat. Genet. 32, 359–369 (2002). [DOI] [PubMed] [Google Scholar]
- Bienvenu T. et al. ARX, a novel Prd-class-homeobox gene highly expressed in the telencephalon, is mutated in X-linked mental retardation. Hum. Mol. Genet. 11, 981–991 (2002). [DOI] [PubMed] [Google Scholar]
- Kato M. et al. A longer polyalanine expansion mutation in the ARX gene causes early infantile epileptic encephalopathy with suppression-burst pattern (Ohtahara syndrome). Am. J. Hum. Genet. 81, 361–366 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer I. E. et al. X-linked myoclonic epilepsy with spasticity and intellectual disability: mutation in the homeobox gene ARX. Neurology 59, 348–356 (2002). [DOI] [PubMed] [Google Scholar]
- Sherr E. H. The ARX story (epilepsy, mental retardation, autism, and cerebral malformations): one gene leads to many phenotypes. Curr. Opin. Pediatr. 15, 567–571 (2003). [DOI] [PubMed] [Google Scholar]
- Tomson T., Walczak T., Sillanpaa M. & Sander J. W. Sudden unexpected death in epilepsy: a review of incidence and risk factors. Epilepsia 46, (Suppl. 11), 54–61 (2005). [DOI] [PubMed] [Google Scholar]
- Kalscheuer V. M. et al. Disruption of the serine/threonine kinase 9 gene causes severe X-linked infantile spasms and mental retardation. Am. J. Hum. Genet. 72, 1401–1411 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J. et al. Variants in the X-linked cyclin-dependent kinase-like 5 (CDKL5/STK9) gene are associated with severe neurodevelopmental retardation. Am. J. Hum. Genet. 75, 1149–1154 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr S. et al. The CDKL5 disorder is an independent clinical entity associated with early-onset encephalopathy. Eur. J. Hum. Genet. 21, 266–273 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y. et al. High prevalence of genetic alterations in early-onset epileptic encephalopathies associated with infantile movement disorders. Brain Dev. 38, 285–292 (2016). [DOI] [PubMed] [Google Scholar]
- Gokben S. et al. Targeted next generation sequencing: the diagnostic value in early-onset epileptic encephalopathy. Acta. Neurol. Belg. doi: 10.1007/s13760-016-0709-z (2016). [DOI] [PubMed] [Google Scholar]
- Mercimek-Mahmutoglu S. et al. Diagnostic yield of genetic testing in epileptic encephalopathy in childhood. Epilepsia 56, 707–716 (2015). [DOI] [PubMed] [Google Scholar]
- Hardies K., Weckhuysen S., De Jonghe P. & Suls A. Lessons learned from gene identification studies in Mendelian epilepsy disorders. Eur. J. Hum. Genet. 24, 961–967 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y. et al. Clinical features and gene mutational spectrum of CDKL5-related diseases in a cohort of Chinese patients. BMC Med. Genet. 25, 15:24 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulard B. et al. Ion channel variation causes epilepsies. Brain Res. Brain Res. Rev. 36, 275–284 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.