Abstract
Activating transcription factor 4 (ATF4) is a translationally activated protein that plays a role in cellular adaptation to several stresses. Because these stresses are associated with various diseases, the translational control of ATF4 needs to be evaluated from the physiological and pathological points of view. We have developed a transgenic mouse model to monitor the translational activation of ATF4 in response to cellular stress. By using this mouse model, we were able to detect nutrient starvation response, antivirus response, endoplasmic reticulum (ER) stress response, and oxidative stress in vitro and ex vivo, as well as in vivo. The reporter system introduced into our mouse model was also shown to work in a stress intensity-dependent manner and a stress duration-dependent manner. The mouse model is therefore a useful tool for imaging ATF4 translational activation at various levels, from cell cultures to whole bodies, and it has a range of useful applications in investigations on the physiological and pathological roles of ATF4-related stress and in the development of clinical drugs for treating ATF4-associated diseases.
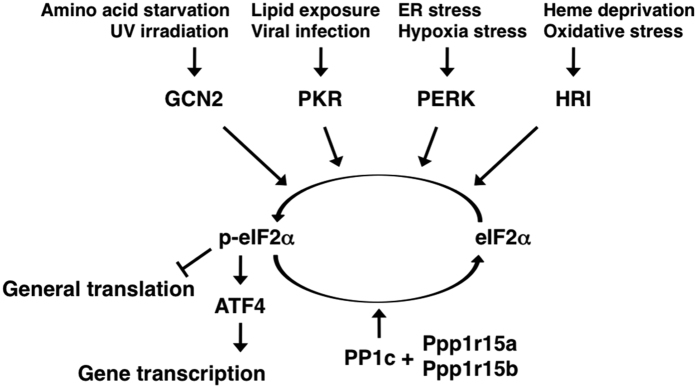
ATF4 (also known as C/ATF, CREB-2, mTR67, or TAXCREB67) belongs to the activating transcription factor (ATF)/cyclic AMP responsive element binding (CREB) family of proteins, which contains a basic leucine zipper domain and recognizes the consensus DNA sequences TGACGT(C/A)(G/A)1. The molecular function of mammalian ATF4 is mainly in controlling the expression of several specific genes associated with stress response, homeostasis, development, fertility, and memory2. ATF4 mRNA is expressed in all tissues examined so far3,4,5; however, its expression levels are regulated by a variety of extracellular signals in different cell types6,7,8,9,10,11,12. The expression level of ATF4 protein is also regulated by two mechanisms: translational control and stability control. ATF4 stability is modulated by the SCFβTrCP class of ubiquitin ligase13 and by histone acetyl-transferase p30014. ATF4 translation is induced by phosphorylation of the eukaryotic initiation factor 2α (eIF2α) as follows. ATF4 mRNA contains two or three upstream open reading frames (uORFs), so that a low level of phosphorylated eIF2α leads to translational initiation at the first uORF (uORF1) and reinitiation at the second uORF (uORF2) or third uORF (uORF3). Translational initiation and reinitiation at these uORFs also inhibit the production of ATF4 protein, because the last uORF overlaps with the coding region. However, a high level of phosphorylated eIF2α leads to slow formation of the translational initiation complex and scans through uORF2 or uORF3, resulting in reinitiation at the ORF encoding ATF415,16. Phosphorylation of eIF2α is catalysed by at least four kinases: general control nonderepressible 2 (GCN2), protein kinase RNA-activated (PKR), PKR-like ER kinase (PERK), and heme-regulated inhibitor kinase (HRI)17. Each kinase responds to a distinct type of stress, as shown in Fig. 1. For this reason, the eIF2α-ATF4 signalling pathway is referred to as the integrated stress response (ISR)18. A high level of phosphorylated eIF2α leads to not only translational induction of ATF4, but also to global repression of protein synthesis19. On the other hand, dephosphorylation of eIF2α is regulated by protein phosphatase 1 regulatory subunits 15a and 15b20,21.
Figure 1. Schematic of the integrated stress response (ISR).
Four eIF2α kinases (GCN2, PKR, PERK, and/or HRI) are specifically activated under certain stress conditions. A high level of phosphorylated eIF2α leads to global repression of protein synthesis and to translational induction of ATF4. These responses alleviate the burden of the stress factor and activate genes for stress resistance. On the other hand, dephosphorylation of eIF2α is regulated by Ppp1r15a and/or Ppp1r15b.
Many researchers have attempted to elucidate the functions of eIF2α and ATF4 at the cellular level, as described above, and they have suggested an association between the ISR and various human diseases22. However, the physiological and pathological roles of the ISR remain to be fully understood in mammals, because there is no suitable in vivo model for studying the response at the whole-body level. Here, we reported on our development of a transgenic mouse model for imaging ATF4-related cellular stress response. This mouse model is transduced with a transgene that contains an ATF4 uORF region and a luciferase-encoding region. Under conditions of stress that cause phosphorylation of eIF2α, luminescence signals are generated in tissues or cells collected from the transgenic mice, as well as in the whole bodies of the mice. Consequently, this mouse model is a useful tool for easily monitoring the ISR in vivo. In the future, by using this mouse model, we might be able to investigate the physiological and pathological roles of the ISR and to develop clinical drugs for treating ISR-associated diseases.
Results
Design and construction of UMAI reporter gene
As described above, cellular stress activates specific eIF2α kinases, leading to translational induction of ATF4. To permit the detection of such ISRs, we designed a gene construct in which the coding region of ATF4 is replaced with that of luciferase; this construct is also activated transcriptionally by a constitutive promoter/enhancer and regulated translationally by uORFs. We named this gene construct ‘UMAI’, from uORFs mediated ATF4 indicator. The mRNA transcribed from the UMAI construct should behave similarly to endogenous ATF4 mRNA during cellular stress. Thus, uORFs of the UMAI construct are dominantly translated under normal conditions, whereas luciferase is alternatively translated under conditions of stress. We should therefore be able to monitor the ISR in vivo by measurement of luminescence signals from animals or culture cells introduced with the UMAI construct (Fig. 2). By developing the UMAI construct on the basis of human ATF4 gene and introducing the construct into HeLa or NIH3T3, we were able to confirm that luciferase activity increased markedly in response to deprivation of a specific amino acid (Leu) as well as to treatments with poly(I)poly(C) nucleotide (pIC), which mimics virus infection; tunicamycin (Tun), an endoplasmic reticulum (ER) stress inducer; and sodium arsenite (ASN), an oxidative stress inducer (Fig. 3a). Whereas mRNA transcribed from the UMAI construct was expressed at the same level under both normal and stress conditions, the indicator protein translated from the construct was expressed at a higher level under stress conditions than under normal conditions, in a similar manner to endogenous ATF4 protein and phosphorylated eIF2α protein (Fig. 3b).
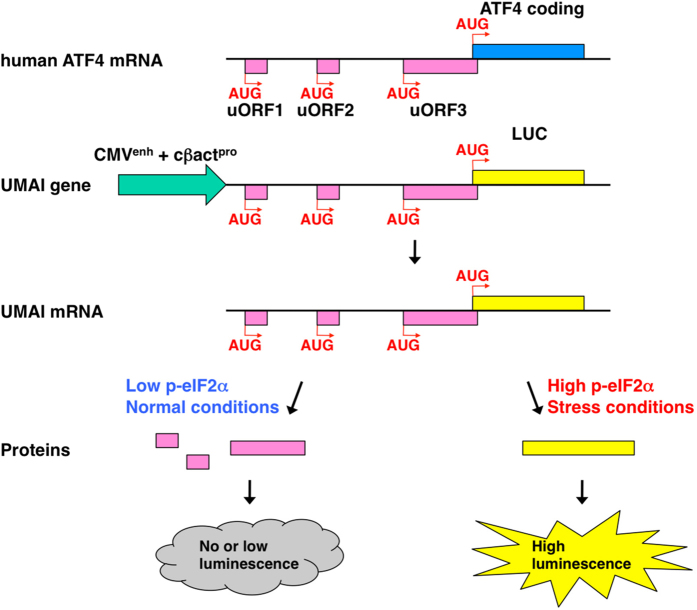
Figure 2. Schematic of the UMAI function.
To detect the ISR, the UMAI gene was designed as follows; uORFs (pink)-containing human ATF4 gene whose coding region (blue) is replaced with that of luciferase (yellow) is regulated by a promoter/enhancer (green) activated constitutively. The mRNA transcribed from the UMAI gene should perform similarly to endogenous ATF4 mRNA during cellular stress. Thus, uORFs of the UMAI gene are dominantly translated under normal conditions, although luciferase is alternatively translated under stress conditions. We should therefore be able to monitor the ISR in vivo by measurement of luminescence signals from animals and cultured cells introduced with the UMAI gene.
Figure 3. Characterization of UMAI in vitro.
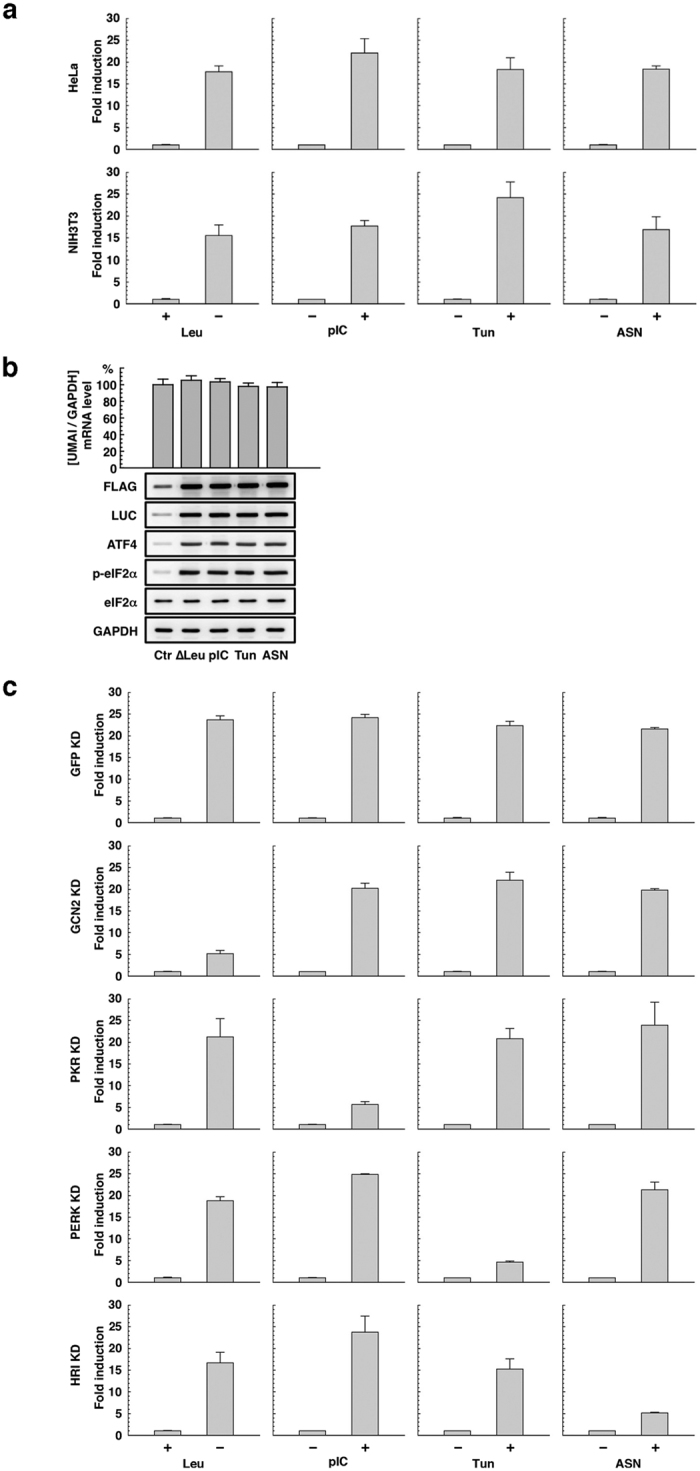
(a) Luciferase activity in HeLa and NIH3T3 cells transfected with the UMAI plasmid and treated with various stressors to induce the ISR. (b, upper) Quantitative PCR analysis of UMAI mRNA expression in NIH3T3 cells transfected with the UMAI plasmid and treated with various stressors to induce the ISR by using a specific probe/primer set. The expression level of endogenous GAPDH mRNA was measured as an internal standard. This histogram is shown as mean (column) ± S.E.M (error bar) from triplicate experiments. (b, lower) Western blot analysis, performed by using an anti-FLAG antibody and an anti-luciferase antibody, of UMAI protein expression in NIH3T3 cells transfected with the UMAI plasmid and treated with the various stressors to induce the ISR: expression levels of total ATF4 protein, phosphorylated eIF2α protein, total eIF2α protein, and total GAPDH protein were examined as comparative and internal standards. (c) Luciferase activity in NIH3T3 cells transfected with the UMAI plasmid and the appropriate eIF2α kinase KD plasmid and treated with the various stressors to induce the ISR. Luciferase activity in NIH3T3 cells transfected with the UMAI plasmid and GFP KD plasmid was examined as a control experiment. These histograms are shown as mean (column) ± S.E.M (error bar) from triplicate experiments.
The uORFs of the ATF4 mRNA are conserved among vertebrates15,16. Consequently, the UMAI can also be constructed on the basis of the mouse ATF4 gene (Fig. S1). As a trial, we also constructed the mouse-derived UMAI, and we compared the stress responsivity in NIH3T3 of the human and mouse indicators. The human-type UMAI showed a slightly higher responsivity than the mouse type did, although both indicators were robustly activated under all the stress conditions that we examined (Fig. S2). This result persuaded us to generate transgenic mice by using the human type of UMAI construct.
By introducing the human type of UMAI construct into NIH3T3 cells that were impaired in a specific eIF2α kinase, we confirmed that responsivity of the UMAI to stress is dependent on each eIF2α kinase as follows. Knockdown (KD) of GCN2, PKR, PERK, and HRI obviously diminished the responsivity of the UMAI to Leu (−), pIC (+), Tun (+), and ASN (+), respectively (Fig. 3c and Fig. S3). These results indicated that the UMAI construct is a functional tool for easily monitoring the ISR.
Generation and characterization of UMAI transgenic mice
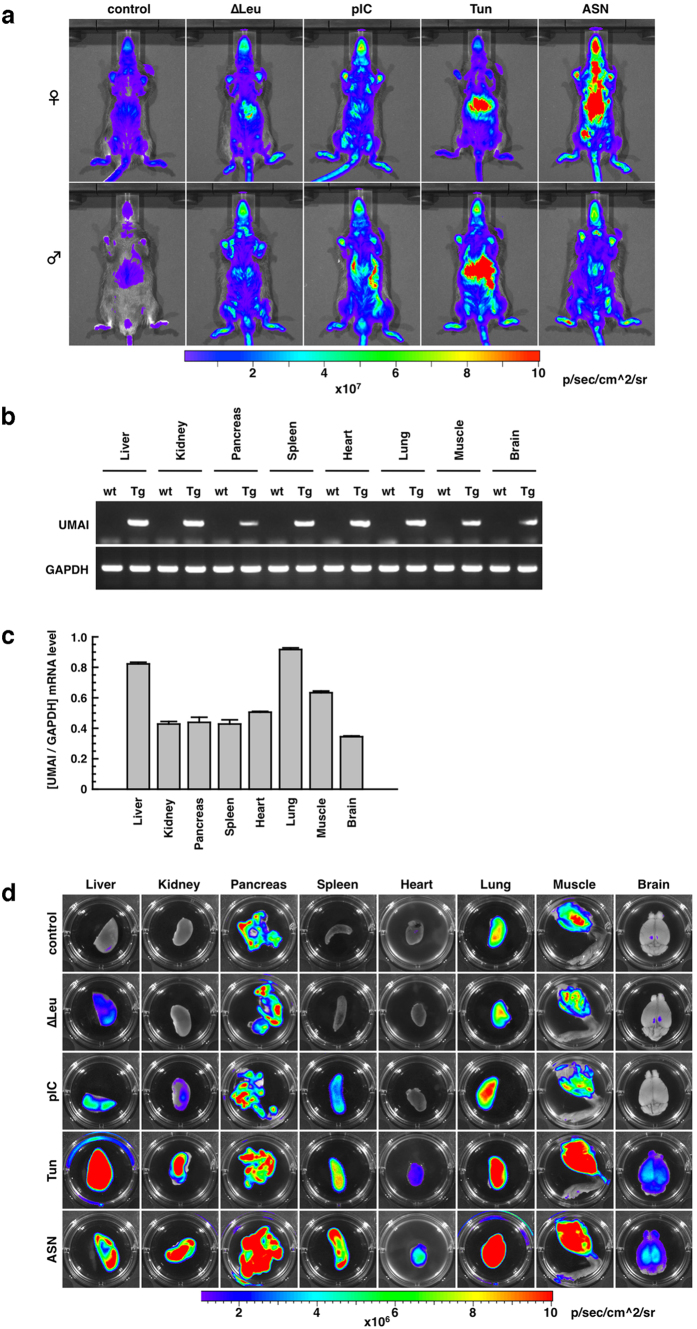
By microinjection of the UMAI construct into approximately 400 fertilized mouse eggs, we generated 27 UMAI transgenic mice (F0). Seven of these F0 mice had transgenic-positive offspring, but the other line did not. Offspring derived from the seven F0 mice were maintained as individual lines and analysed for in vivo imaging of the ISR. Six lines of these unexpectedly showed stronger luminescence signals under normal condition or weaker luminescence signals under stress conditions. One line, however, showed lower luminescence signals under normal conditions and higher luminescence signals under stress conditions (Fig. 4a). Expression of the UMAI mRNA in this mouse line was evaluated by RT-PCR analysis and by quantitative PCR analysis; these showed that the UMAI mRNA was expressed in all the examined organs, even under normal conditions (Fig. 4b), and that the expression level of the UMAI mRNA remained within a factor of three among these organs (Fig. 4c). From these results, we consider that this UMAI mouse line is a potential animal resource for investigating the ISR in vivo. Ex vivo analysis of the transgenic line also revealed significant UMAI activity under normal conditions, and stress-dependent differences in UMAI activity. In particular, under normal conditions, the pancreas, muscle, and lung collected from UMAI transgenic mice showed bright signals compared with the other organs. A Leu (−) diet modestly increased the UMAI activity of the liver, but had little effect on other organs. Administration of pIC also had moderately increased the UMAI activity of the liver, kidney, spleen, and lung, but had little effect on the pancreas, heart, muscle, or brain. On treatment with Tun and ASN, UMAI activity was robustly displayed in all the examined organs (Fig. 4d). These features of UMAI activity in vivo and ex vivo were also sustained in other generations (Figs S4 and S5), and were well correlated with endogenous ATF4 protein level and endogenous eIF2α phosphorylation level (Fig. S6).
Figure 4. Characterization of UMAI in vivo.
(a) Luminescence signals obtained from UMAI mice (12-week-old) at the F2 generation by in vivo imaging analysis at the whole-body level. (b) RT-PCR analysis of UMAI transgene expression in various tissues. GAPDH was used as an internal standard. Wild type, wt; transgenic, Tg. The tested RNA was extracted from UMAI mice (12-week-old, female) at the F2 generation. (c) Quantitative PCR analysis of the transgene expression in various tissues derived from UMAI mice. The tested RNA was extracted from UMAI mice (12-week-old, female) at the F2, F3, and F6 generations. This histogram is shown as mean (column) ± S.E.M (error bar) from 3 samples (F2, F3, and F6). GAPDH was used as an internal standard. (d) Luminescence signals obtained from various tissues of UMAI mice (12-week-old, female) at the F2 generation by ex vivo imaging analysis. The tissues for ex vivo imaging analysis were collected from UMAI mice treated with the various stressors to induce the ISR.
Some F0 mice of unestablished lines were introduced with multiple copies of the transgene and other F0 mice were introduced with the transgene(s) in an unexpected manner. However, the F0 mouse of the line established as the UMAI mouse was introduced with a single copy of the transgene in the expected manner (Fig. S7a). The copy number of the transgene was sustained in subsequent generations of the established UMAI line (Fig. S7b). In addition CpG methylation was examined in the CMV enhancer region of the transgene introduced into the established UMAI mice. The CMV enhancer region was found to have low methylation level in various tissues of both young (10-week-old) and old (42-week-old) UMAI mice (Supplementary Table). The transgene was expressed with the same pattern in both male and female UMAI mice, and in both young and old UMAI mice (Fig. S8).
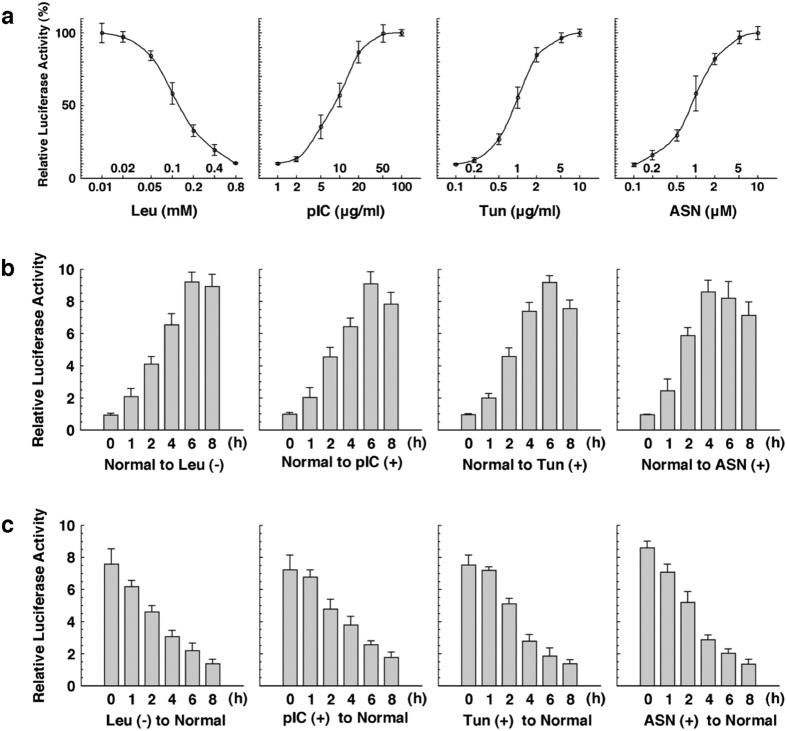
By using mouse embryonic fibroblasts (MEFs) derived from this UMAI line, we were able to obtain data on how UMAI activity varied with the intensity and duration of stress treatment. UMAI activity was found to change widely in the ranges of the following stress intensity: Leu (0.01–0.8 mM), pIC (1–100 μg/mL), Tun (0.1–10 μg/mL), and ASN (0.1–10 μM) (Fig. 5a). Under all conditions [Leu (−), pIC (+), Tun (+), and ASN (+)], the UMAI activity reached a peak 4–6 hours after the initiation of stress treatment (Fig. 5b), and reverted to the basal line 8 hours after removal of the stress (Fig. 5c). These UMAI activities were well correlated with both the expression level of endogenous ATF4 protein and the phosphorylation level of endogenous eIF2α (Figs S9 and S10).
Figure 5. Evaluation of the ISR by using UMAI MEFs.
(a) Luciferase activity in UMAI MEFs treated with the indicated concentration of leucine (Leu), poly(I)poly(C) nucleotide (pIC), tunicamycin (Tun), or sodium arsenite (ASN) for 6 h. (b) Luciferase activity in UMAI MEFs treated with Leu (−), pIC, Tun, and ASN for the indicated time. (c) Luciferase activity in UMAI MEFs treated with Leu (−), pIC, Tun, and ASN for 6 h and then cultured in a normal medium for the indicated time. Each graph is shown as mean (plot or column) ± S.E.M (error bar) from triplicate experiments.
Discussion
It has been reported that the ISR is associated with various diseases, and this association needs to be investigated by using sophisticated mouse models. Here, we developed UMAI constructs and UMAI mice. Under conditions stimulating eIF2α-ATF4 pathway, cultured cell lines introduced with UMAI construct expectedly induce translation of reporter protein and show high reporter activity (Fig. 3a,b). The UMAI mice also constitutively expressed the transgene-derived mRNA in all the examined organs and they showed the expected increase in reporter activity under conditions stimulating eIF2α-ATF4 pathway (Fig. 4). These features indicate that the UMAI construct and UMAI mice are useful biological resources for the investigation of the ISR in vitro and in vivo. In addition, UMAI activity is finely regulated in a stress-intensity-dependent manner and a stress-duration-dependent manner (Fig. 5), and it is profoundly influenced by the function of eIF2α kinases (Fig. 3c). These findings suggest that UMAI might contribute to research on the roles of GCN2, PKR, PERK, and HRI in detail. The performance demonstrated by our construct and the model mouse is reasonable, because UMAI-like constructs have previously been reported to function as indicators for the ISR23. The specificity of the response of the UMAI to the various stresses shown in our KD experiments is coincided with that of previous reports on the disruption of eIF2α kinases15,24,25,26. We therefore believe that UMAI is a reliable experimental tool.
A variety of model mice with transgene constructs consisting of transcriptional regulatory elements and a luminescent/fluorescent reporter have been developed for imaging of biological response in vivo. Among these, the CARE-LUC mouse model is designed to express luciferase by transcriptional regulation with ATF4 and its binding elements, and this model is used for the evaluation of eIF2α-ATF4 signalling under stress conditions that activate GCN2 or PERK27. However, there is concern that the signals detected from CARE-LUC mice might reflect factors other than the ISR. This is because the expression level of ATF4 is regulated not only by ISR-related translation, but also by promoter-related transcription and ubiquitin/proteasome system-related protein degradation, as described in the introduction, and a variation in the expression level of ATF4 has a transcriptional effect on the CARE-LUC gene. On the other hand, the reporter expression of UMAI is regulated by translational control of uORFs, and not by the transcriptional activating function of ATF4. Consequently, UMAI should be used in monitoring the ISR in vivo to avoid the effects of the expression level of endogenous ATF4.
UMAI mice show a high luciferase activity in the pancreas, muscle, and lung, even under normal conditions. We do not have a clear answer to the question of what stress or which eIF2α kinase gives rise to the signal from those tissues. However, we can obtain some hints from other model mice previously developed for imaging of cellular stress. OKD48 mice have a reporter gene regulated by Keap1 and Nrf2, and they have been developed for imaging oxidative stress in vivo28,29. These mice show hardly reporter signal in any tissue under normal conditions. ERAI mice have a reporter gene that is regulated by IRE1α and XBP1, and they have been developed for imaging ER stress in vivo30,31. These mice show a high reporter activity in the pancreas and the muscle, even under normal conditions. The data from other mouse model suggest that the signals in the pancreas and the muscle tissue of UMAI mice might be caused by PERK activated by the physiological ER stress in these tissues.
UMAI mice have a unique reporter gene translationally controlled by uORFs of ATF4 and they are useful in low-invasiveness monitoring of eIF2α-ATF4 signalling under stress conditions that activate GCN2, PKR, PERK, and HRI. Consequently, by using UMAI mice, we might be able to address various problems associated with the ISR in diseases and in pharmaceutical development. For example, by mating UMAI mice with a disease mouse model, we might be able to examine temporal change in the ISR and ATF4 translational activation with the progression of the disease in identical mice. By using UMAI mice treated with a certain drug, we might easily examine the effects of that drug on the ISR and ATF4 translational activation at the whole-body level of mice. Thus, UMAI mice might be useful in developing new diagnostic and therapeutic methods for ATF4-related diseases. We therefore expect that UMAI mice will form a valuable resource for medical science.
Methods
Cell culture, transfection, and treatment
MEFs were collected in accordance with a previously described procedure32. NIH3T3 cells, HeLa cells, and MEFs were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum at 37 °C under 5% CO2. The calcium phosphate–DNA precipitation method was used to introduce plasmids into NIH3T3 cells and HeLa cells. To induce an amino acid-deprivation response, cells were treated in the absence or presence of the indicated concentration of l-leucine for 6 h or the indicated time. To induce an antivirus response, cells were treated with 100 μg/mL or the indicated concentration of poly(I)poly(C) nucleotide (#27-4732-01; GE Healthcare Life Sciences, Chicago, IL) for 6 h or the indicated time. To induce an ER stress response, cells were treated with 10 μg/mL or the indicated concentration of tunicamycin (#T7765; Sigma-Aldrich, Saint Louis, MO) for 6 h or the indicated time. To induce an oxidative stress response, cells were treated with 10 μM or the indicated concentration of sodium arsenite (#106277; Merck Millipore, Billerica, MA) for 6 h or the indicated time. Leucine (−) medium was obtained commercially (#1642149; MP Biomedicals, Santa Ana, CA).
Gene constructs
pCAX-LUC-F(XhoI) was made by insertion of a LUC-F fragment into the HindIII/BamHI sites of pCAX. The LUC-F fragment encoding luciferase with Flag-tag on its 3′ terminal was produced by PCR using 5′-ccc aag ctt cca cca tgc tcg agg aag acg cca aaa aca taa ag-3′ as the forward primer, 5′-cgc gga tcc tta ctt gtc atc gtc gtc ctt gta gtc cac ggc gat ctt tcc gcc ctt c-3′ as the reverse primer, and pGL3-basic (#E1751; Promega, Fitchburg, WI) as the template.
The pCAX-hATF4(uORFs)-LUC-F, human type of UMAI construct was made by insertion of an hATF4(uORFs) fragment into the HindIII/XhoI sites of pCAX-LUC-F(XhoI). The hATF4(uORFs) fragment was produced by PCR using 5′-ccc aag ctt gtt ttc tac ttt gcc cgc cca cag atg tag ttt tct ctg c-3′ as the forward primer, 5′-ccg ctc gag cat ggt gca gtg ctt tgc tgg aat caa cg-3′ as the reverse primer, and human ATF4 cDNA as the template.
The pCAX-mATF4(uORFs)-LUC-F, mouse type of UMAI construct was made by insertion of mATF4(uORFs) fragment into the HindIII/XhoI sites of pCAX-LUC-F(XhoI). The mATF4(uORFs) fragment was produced by PCR by using 5′-ccc aag ctt ttt tct gct tgc tgt ctg ccg gtt tga gtt gtg c-3′ as the forward primer, 5′-ccg ctc gag cat gtt gtg ggg ctt tgc tgg att cca gg-3′ as the reverse primer, and mouse ATF4 cDNA as the template.
To produce pSUPER-mGCN2, pSUPER-mPKR, pSUPER-mPERK, and pSUPER-mHRI for KD of the genes coding the corresponding eIF2α kinases, gta tac gtg caa gtg gaa c, ctt agt act tcg gga cct c, tca tca gca ctt tag atg g, and cac ttg agc cat gtg cac g, respectively, were selected as target sequences and cloned into pSUPER (#VEC-PBS-0002; Oligoengine, Seattle, WA) in accordance with supplier’s instructions. As a control experiment, pSUPER-GFP was produced in the same manner with gca agc tga ccc tga agt t as the target sequence.
Luciferase reporter assay
NIH3T3 cells and HeLa cells were seeded in 12-well plates at 5 × 104 cells/well, then transfected with plasmid DNA (2 μg/well). 30 h after transfection and 6 h or the indicated time after the appropriate stress treatment, the cells were lysed for luciferase assay. phRL-TK (#E6241; Promega) was used as an internal control in this assay. UMAI MEFs were also seeded in 12-well plates at 5 × 104 cells/well, treated with the indicated stress, and then lysed for luciferase assay. Luciferase activity was measured by using a luciferase assay system (#E1910; Promega) and a luminometer (#LB960; Berthold Technologies, Bad Wildbad, Germany).
Transgenic mice and stress treatment
The 4.5-kb SpeI-PvuII fragment of pCAX-hATF4(uORFs)-LUC-F was microinjected as a transgene into fertilized mouse eggs (C57BL/6), and the transgenic offspring were screened by PCR using the following primers: 5′-ggc cac cat ggc gta tta gg-3′ and 5′-tct tcc agc gga tag aat gg-3′. To induce an amino acid-deprivation response, mice were fed with a leucine-free diet (#A08051503; Research Diets, New Brunswick, NJ) for 24 hours. An amino acid-complete diet (#A08051501; Research Diets) was given to mice as a control. To induce an antivirus response, mice were injected intraperitoneally with poly(I)poly(C) nucleotide (5 μg per gram body weight) 6 h before imaging analysis. To induce an ER stress response, mice were injected intraperitoneally with tunicamycin (500 ng per gram body weight) 6 h before imaging analysis. To induce an oxidative stress response, mice were injected intraperitoneally with sodium arsenite (15 μg per gram body weight) 6 h before imaging analysis. Poly(I)poly(C) nucleotide (#27-4732-01; GE Healthcare Life Sciences), tunicamycin (#T7765; Sigma-Aldrich), and sodium arsenite (#106277; Merck Millipore) were obtained commercially. Heterozygous UMAI mice were used in all the experiments of this research. The experimental protocols that involved animals were approved by the Animal Studies Committees at Kumamoto University, TransGenic Inc., and Kanazawa Medical University (A25-051, 2016J03, and 2016-107, respectively); all experiments were performed in accordance with the appropriate institutional guidelines.
RT-PCR and quantitative PCR
Total RNA derived from plasmid-transfected culture cells and various tissues of wild-type and UMAI mice was prepared by using Isogen reagent (#311-02501; Nippon Gene, Tokyo, Japan). The cDNA was synthesized by using the SuperScript first-strand synthesis system (#11904-018; Invitrogen, Waltham, MA) in accordance with the manufacturer’s instructions. UMAI cDNA was amplified by 35 cycles of PCR with 5′-ggc cac cat ggc gta tta gg-3′ and 5′-act cag cgt aag tga tgt cc-3′ as primers. GAPDH cDNA was also amplified by 35 cycles of PCR with 5′-ctg aac ggg aag ctc act gg-3′ and 5′-cac cac cct gtt gct gta gc-3′ as primers. Quantitative PCR analysis of each transcript was performed by using TaqMan probe and StepOnePlus (#4376592; Applied Biosystems, Waltham, MA), in accordance with the manufacturer’s instructions. Probe/primer sets: Mm00469222_m1, Mm01235643_m1, Mm00438700_m1, Mm01202300_m1, and 4352339E (Applied Biosystems) were used for quantification of GCN2, PKR, PERK, HRI, and GAPDH transcripts, respectively. UMAI transcripts were quantified by using 5′-tgc aca tat cga ggt gga cat c-3′ as the forward primer, 5′-tgc caa ccg aac gga cat-3′ as the reverse primer, and 5′-FAM-ctt acg ctg agt act tcg-MGB-3′ as the probe.
Western blot analysis
The cells were lysed in SDS sample buffer (50 mM Tris-HCl, pH 6.8, 2% SDS, 50 mM DTT, 10% glycerol, and 1 mg/mL bromophenol blue). The lysate was heated to 98 °C for 10 min, and SDS-PAGE was performed to separate the proteins in the lysate. After electrophoresis, the proteins were electrotransferred onto a PVDF microporous membrane and immunodetected with the appropriate antibody. The following antibodies were used for Western blot analysis: anti-FLAG polyclonal antibody (#F7425; Sigma-Aldrich), anti-luciferase polyclonal antibody (#G7541; Promega), anti-ATF4 monoclonal antibody (#11815; Cell Signaling Technology, Beverly, MA), anti-eIF2α monoclonal antibody (#5324; Cell Signaling Technology), anti-phospho-eIF2α (Ser51) monoclonal antibody (#3398; Cell Signaling Technology), and anti-GAPDH monoclonal antibody (#2118; Cell Signaling Technology).
Imaging of luminescence signals in vivo and ex vivo
UMAI mice were injected intraperitoneally with d-luciferin (150 mg/g body weight) in PBS 10 min before imaging analysis. Tissues were collected surgically from the UMAI mice 10 min after luciferin injection and were immersed in a 300 mg/mL solution of d-luciferin. Imaging analyses were performed by using IVIS (#Lumina; Perkin Elmer, Waltham, MA). After a grey-scale photograph had been acquired, luminescence images were obtained under the following conditions. For in vivo analysis: exposure, 60 s; field of view, 12.5 cm; binning (resolution) factor 2; open filter. For ex vivo analysis: exposure, 60 s; field of view, 5 cm, binning (resolution) factor 2; open filter.
Additional Information
How to cite this article: Iwawaki, T. et al. Transgenic mouse model for imaging of ATF4 translational activation-related cellular stress responses in vivo. Sci. Rep. 7, 46230; doi: 10.1038/srep46230 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We are grateful to the Research Support Center (Medical Research Institute, Kanazawa Medical University) for breeding the mice. This work was supported by grants from Kanazawa Medical University (to T.I.), Research Foundation for Opto-Science and Technology (to T.I.), Nakatani Foundation (to T.I.), Toray Science Foundation (to T.I.), and Scientific Support Programs for Cancer Research, Grant-in-Aid for Scientific Research on Innovative Areas, Ministry of Education, Culture, Sports, Science and Technology (to K.Y.).
Footnotes
The authors declare no competing financial interests.
Author Contributions T.I. contributed to the study design, research data, and preparation of the manuscript. R.A. contributed to the research data in vivo. T.T. contributed to the gene constructs and the research data in vitro. N.T., T.O.I. and K.Y. contributed to the creation of the transgenic mice. T.I. is the guarantor of this work and, as such, had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- Hai T. & Hartman M. G. The molecular biology and nomenclature of the activating transcription factor/cAMP responsive element binding family of transcription factors: activating transcription factor proteins and homeostasis. Gene 273, 1–11 (2001). [DOI] [PubMed] [Google Scholar]
- Ameri K. & Harris A. L., Activating transcription factor 4. Int. J. Biochem. Cell Biol. 40, 14–21 (2008). [DOI] [PubMed] [Google Scholar]
- Karpinski B. A., Morle G. D., Huggenvik J., Uhler M. D. & Leiden J. M. Molecular cloning of human CREB-2: an ATF/CREB transcription factor that can negatively regulate transcription from the cAMP response element. Proc. Natl. Acad. Sci. USA 89, 4820–4824 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo M., Ron D., Miller C. P. & Habener J. F. C/ATF, a member of the activating transcription factor family of DNA-binding proteins, dimerizes with CAAT/enhancer-binding proteins and directs their binding to cAMP response elements. Proc. Natl. Acad. Sci. USA 90, 4679–4683 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y. et al. Presence of activating transcription factor 4 (ATF4) in the porcine anterior pituitary. Mol. Cell. Endocrinol. 154, 151–159 (1999). [DOI] [PubMed] [Google Scholar]
- Tsujimoto A., Nyunoya H., Morita T., Sato T. & Shimotohno K. Isolation of cDNAs for DNA-binding proteins which specifically bind to a tax-responsive enhancer element in the long terminal repeat of human T-cell leukemia virus type I. J. Virol. 65, 1420–1426 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes S. D., Stoler D. L. & Anderson G. R. Normal fibroblasts induce the C/EBPβ and ATF-4 bZIP transcription factors in response to anoxia. Exp. Cell Res. 220, 47–54 (1995). [DOI] [PubMed] [Google Scholar]
- Kokame K., Kato H. & Miyata T. Homocysteine-respondent genes in vascular endothelial cells identified by differential display analysis. GRP78/BiP and novel genes. J. Biol. Chem. 271, 29659–29665 (1996). [DOI] [PubMed] [Google Scholar]
- Sato N., Kokame K., Shimokado K., Kato H. & Miyata T. Changes of gene expression by lysophosphatidylcholine in vascular endothelial cells: 12 up-regulated distinct genes including 5 cell growth-related, 3 thrombosis-related, and 4 others. J. Biochem. 123, 1119–1126 (1998). [DOI] [PubMed] [Google Scholar]
- Talukder A. H., Vadlamudi R. Mandal M. & Kumar R. Heregulin induces expression, DNA binding activity, and transactivating functions of basic leucine zipper activating transcription factor 4. Cancer Res. 60, 276–281 (2000). [PubMed] [Google Scholar]
- Levenson V. V., Davidovich I. A. & Roninson I. B. Pleiotropic resistance to DNA-interactive drugs is associated with increased expression of genes involved in DNA replication, repair, and stress response. Cancer Res. 60, 5027–5030 (2000). [PubMed] [Google Scholar]
- Siu F., Bain P. J., LeBlanc-Chaffin R., Chen H. & Kilberg M. S. ATF4 is a mediator of the nutrient-sensing response pathway that activates the human asparagine synthetase gene. J. Biol. Chem. 277, 24120–24127 (2002). [DOI] [PubMed] [Google Scholar]
- Lassot I. et al. ATF4 degradation relies on a phosphorylation-dependent interaction with the SCFβTrCP ubiquitin ligase. Mol. Cell. Biol. 21, 2192–2202 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassot I. et al. p300 modulates ATF4 stability and transcriptional activity independently of its acetyltransferase domain. J. Biol. Chem. 280, 42537–42545 (2005). [DOI] [PubMed] [Google Scholar]
- Harding H. P. et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell. 6, 1099–1108 (2000). [DOI] [PubMed] [Google Scholar]
- Vattem K. M. & Wek R. C. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl. Acad. Sci. USA 101, 11269–11274 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly N., Gorman A. M., Gupta S. & Samali A. The eIF2α kinases: their structures and functions. Cell. Mol. Life Sci. 70, 3493–3511 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding H. P. et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell. 11, 619–633 (2003). [DOI] [PubMed] [Google Scholar]
- Holcik M. & Sonenberg N. Translational control in stress and apoptosis. Nat. Rev. Mol. Cell Biol. 6, 318–327 (2005). [DOI] [PubMed] [Google Scholar]
- Novoa I., Zeng H., Harding H. P. & Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2α. J. Cell Biol. 153, 1011–1021 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jousse C. et al. Inhibition of a constitutive translation initiation factor 2α phosphatase, CReP, promotes survival of stressed cells. J. Cell Biol. 163, 767–775 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird T. D. & Wek R. C. Eukaryotic initiation factor 2 phosphorylation and translational control in metabolism. Adv. Nutr. 3, 307–321 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter F., Schmid J., Düssmann H., Concannon C. G. & Prehn J. H. Imaging of single cell responses to ER stress indicates that the relative dynamics of IRE1/XBP1 and PERK/ATF4 signalling rather than a switch between signalling branches determine cell survival. Cell Death Differ. 20, 1502–1516 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Han A. P. & Chen J. J. Translation initiation control by heme-regulated eukaryotic initiation factor 2α kinase in erythroid cells under cytoplasmic stresses. Mol. Cell. Biol. 21, 7971–7980 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P. et al. The GCN2 eIF2α kinase is required for adaptation to amino acid deprivation in mice. Mol. Cell. Biol. 22, 6681–6688 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran S. et al. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13, 129–141 (2000). [DOI] [PubMed] [Google Scholar]
- Chaveroux C. et al. In vivo imaging of the spatiotemporal activity of the eIF2α-ATF4 signaling pathway: Insights into stress and related disorders. Sci. Signal. 8, rs5 (2015). [DOI] [PubMed] [Google Scholar]
- Oikawa D., Akai R., Tokuda M. & Iwawaki T. A transgenic mouse model for monitoring oxidative stress. Sci. Rep. 2, 229 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi T. et al. Temporal activation of Nrf2 in the penumbra and Nrf2 activator-mediated neuroprotection in ischemia-reperfusion injury. Free Radic. Biol. Med. 72, 124–133 (2014). [DOI] [PubMed] [Google Scholar]
- Iwawaki T., Akai R., Kohno K. & Miura M. A transgenic mouse model for monitoring endoplasmic reticulum stress. Nat. Med. 10, 98–102 (2004). [DOI] [PubMed] [Google Scholar]
- Iwawaki T., Akai R., Yamanaka S. & Kohno K. Function of IRE1 alpha in the placenta is essential for placental development and embryonic viability. Proc. Natl. Acad. Sci. USA 106, 16657–16662 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A. Gertsenstein M., Vintersten K. & Behringer R. Manipulating the Mouse Embryo: A Laboratory Manual (third edition, 2003) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.