MCU is the pore-forming subunit of the mitochondrial inner membrane Ca2+ uniporter ion channel that mediates Ca2+ uptake into the matrix to regulate metabolism, cell death, and cytoplasmic Ca2+ signaling. We previously identified MCUR1 (Mitochondrial Calcium Uniporter Regulator 1) as an important regulator of MCU activity and showed that MCUR1 biochemically interacted with MCU (Mallilankaraman et al., 2012). MCUR1 regulated MCU-dependent mitochondrial Ca2+ uptake driven by the inner membrane voltage (ψm) generated by the electron transport chain, and MCUR1 knockdown abrogated Ca2+ uptake by mitochondria in intact and permeabilized cells, and disrupted oxidative phosphorylation (OXPHOS). Paupe et al. recently challenged our conclusion that MCUR1 directly regulates MCU (Paupe et al., 2015). While both teams agreed regarding the effects of MCUR1 knockdown on cell metabolism and mitochondrial Ca2+ uptake, Paupe et al. (2015) proposed that CCDC90A is a cytochrome c oxidase (COX) assembly factor and that the Ca2+ transport observations in Mallilankaraman et al. (2012) were secondary effects due to a reduced ψm as a consequence of defective COX assembly.
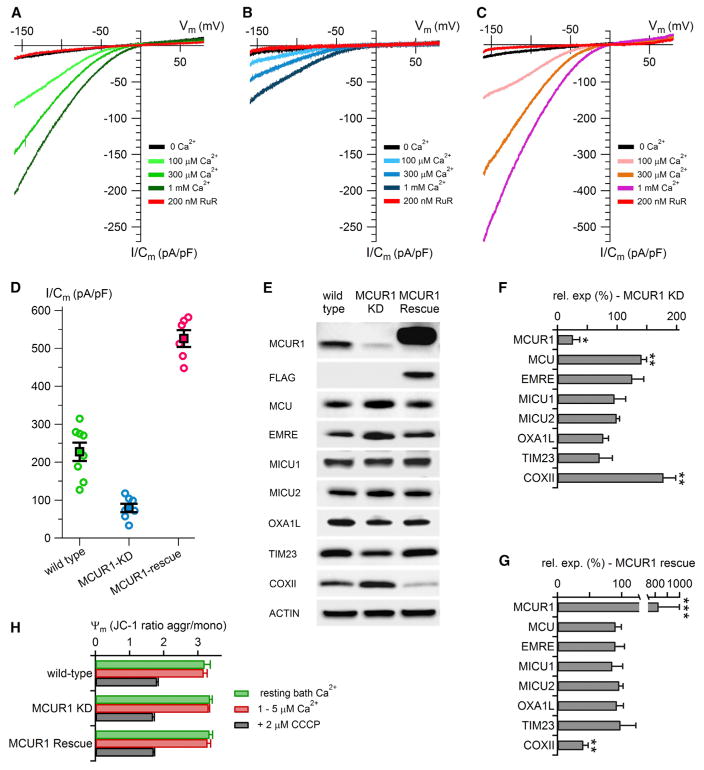
To determine whether a reduction of ψm could fully account for the effects of MCUR1 on MCU-mediated Ca2+ uptake, we have directly measured MCU activity by recording MCU-mediated Ca2+ currents by patch clamp electrophysiology of mitoplasts (Fieni et al., 2012; Kirichok et al., 2004) isolated from wild-type HEK293 cells and HEK cells with MCUR1 knocked down (Figure 1). Here, the membrane potential is precisely controlled by the voltage-clamp apparatus. Ruthenium red-sensitive, inwardly rectifying, Ca2+ concentration-dependent Ca2+ currents were recorded in control cells with amplitudes and characteristics similar to those previously described for the mitochondrial Ca2+ uniporter (Fieni et al., 2012; Kirichok et al., 2004) (Figure 1A). Stable knockdown of MCUR1 (by ~75%, Figures 1E and 1F) diminished mitochondrial Ca2+ uniporter Ca2+ currents by ~65% (Figures 1B and 1D), which were rescued by expression of an shRNAi-insensitive MCUR1 (Figures 1C and 1D). Notably, there was a correlation between the level of MCUR1 expression and the magnitude of MCU-mediated Ca2+ currents (Figures 1D–1G). Knockdown of MCUR1 in HeLa cells caused a small upregulation of MCU expression (Mallilankaraman et al., 2012) that was also observed here in HEK cells (Figures 1E and 1F). However, western blot analyses indicated that MCUR1 knockdown or rescue was without effect on the expression of other components (MICU1, MICU2, EMRE) of the uniporter channel complex (Figures 1E–1G) or of other mitochondrial membrane proteins (Figures 1E and 1F) in HEK293 cells. Thus, our new data suggest that a reduction of membrane potential cannot be the sole mechanism by which MCUR1 knockdown reduces mitochondrial Ca2+ uptake, as proposed by Paupe et al. (2015), and they confirm our previous conclusion that MCUR1 is a direct regulator of MCU Ca2+ channel activity.
Figure 1. MCUR1 Regulates MCU Ca2+ Channel Activity.
(A) Family of Ca2+ currents measured in different bath [Ca2+] over a range of voltages (1 s ramp) obtained from isolated wild-type HEK cell mitoplasts in whole-mitoplast patch clamp configuration, normalized to membrane capacitance, in the absence and presence of ruthenium red (RuR).
(B) Similar to (A) measured in mitoplasts isolated from cells with MCUR1 protein knocked down by ~75%.
(C) Similar to (A) in cells with MCUR1 overexpressed in MCUR1-knockdown cells.
(D) Scatter plot summary of uniporter Ca2+ current densities measured at −160 mV in 1 mM bath Ca2+ in wild-type and MCUR1-knockdown and MCUR1-rescued cell mitoplasts. Each symbol is the current density measured from an individual mitoplast. Bars represent SEM.
(E) Western blot analysis of expression levels of uniporter channel components and mitochondrial proteins OXA1L, TIM23, and cytochrome oxidase subunit II (COXII).
(F) Summary of β-actin normalized protein levels in MCUR1-knockdown cells, normalized to levels in wild-type cells (n = 3–7 western blots; mean ± SE; ***p < 0.001; **p < 0.01; *p < 0.05).
(G) As in (F), but for MCUR1-knockdown cells in which a MCUR1 FLAG-tagged rescue construct was overexpressed.
(H) Ratiometric JC-1 fluorescence of mitochondrial inner membrane potential in control and MCUR1-knockdown cells, before and after voltage dissipation by CCCP (n = 4).
Error bars represent means ± SE in all panels.
Paupe et al. (2015) demonstrated that reduced expression of MCUR1 impaired COX assembly that resulted in impaired OXPHOX that reduced ψm and the driving force for Ca2+ uptake, and suggested that MCUR1 does not directly regulate MCU. The effects of MCUR1 that we observe are unlikely to depend on an intact respiratory chain since the single mitoplasts used for patch-clamp electro-physiology have likely lost water-soluble cytochrome c. Furthermore, we did not observe an effect of MCUR1 knockdown or overexpression on ψm here (Figure 1H) or previously (Mallilankaraman et al., 2012). Notably, we observed increased COXII expression in our MCUR1 knockdown cells that was rescued by re-expression of MCUR1 (Figures 1E–1G; n = 4). While this could be the result of cell-type differences in our and the studies of Paupe et al. (2015), it supports our conclusion that changes in COXII expression do not account for the effects of MCUR1 on MCU activity. We note that in our previous study (Mallilankaraman et al., 2012) and in Paupe et al. (2015), most MCUR1 resided in a membrane fraction where it could not participate in COX chaperone activity. Finally, as the yeast homolog lacks the first half of MCUR1, including one of two trans-membrane domains present in MCUR1, and it has only 25% sequence identity in the remaining sequence, it is unclear if MCUR1 has the same functions in yeast and mammalian cells.
Our results here and previously (Mallilankaraman et al., 2012) suggest that MCUR1 functions as a direct regulator of the mitochondrial Ca2+ uniporter. Whereas MCUR1 was not identified in a proteomics analysis of MCU immunoprecipitation-associated proteins (Sancak et al., 2013), we (Mallilankaraman et al., 2012) and others (Lee et al., 2015) have reported that MCUR1 can be immunoprecipitated with MCU. Future studies are required to reconcile these discrepancies as well as to fully establish the mechanisms by which MCU activity is regulated by MCUR1 and by other uniporter-associated proteins, including EMRE, MICU1, and MICU2 (Foskett and Philipson, 2015).
Acknowledgments
We thank F. Fieni and Y. Kirichok for advice and training in mitoplast electrophysiology. Supported by R37GM56328 to J.K.F.
Footnotes
AUTHOR CONTRIBUTIONS
H.V. performed the electrophysiology. K.M. created the cell lines. J.E.T. and M.M. performed western blot analyses. R.P. made the membrane potential measurements. J.K.F. helped with experimental design and supervision and co-wrote the manuscript with all authors.
References
- Fieni F, Lee SB, Jan YN, Kirichok Y. Nat Commun. 2012;3:1317. doi: 10.1038/ncomms2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foskett JK, Philipson B. J Mol Cell Cardiol. 2015;78:3–8. doi: 10.1016/j.yjmcc.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirichok Y, Krapivinsky G, Clapham DE. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- Lee Y, Min CK, Kim TG, Song HK, Lim Y, Kim D, Shin K, Kang M, Kang JY, Youn HS, et al. EMBO Rep. 2015:e201540436. doi: 10.15252/embr.201540436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallilankaraman K, Cárdenas C, Doonan PJ, Chandramoorthy HC, Irrinki KM, Golenár T, Csordás G, Madireddi P, Yang J, Müller M, et al. Nat Cell Biol. 2012;14:1336–1343. doi: 10.1038/ncb2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paupe V, Prudent J, Dassa EP, Rendon OZ, Shoubridge EA. Cell Metab. 2015;21:109–116. doi: 10.1016/j.cmet.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Sancak Y, Markhard AL, Kitami T, Kovács-Bogdán E, Kamer KJ, Udeshi ND, Carr SA, Chaudhuri D, Clapham DE, Li AA, et al. Science. 2013;342:1379–1382. doi: 10.1126/science.1242993. [DOI] [PMC free article] [PubMed] [Google Scholar]