Abstract
Despite major advances in our understanding of the biology of HIV-1 infection, and advances in antiretroviral therapy to treat the disease, there were 2.1 million new cases of HIV-1 infection in 2015, and 36.7 million people living with AIDS (http://www.unaids.org/en/resources/fact-sheet). Thus, a vaccine that can prevent HIV-infection remains a global priority. Thirty-three years after the discovery of HIV-1(1), and the demonstration it was the cause of AIDS(2) and after 6 HIV-1 vaccine efficacy trials (3–8), no HIV-1 candidate vaccine has shown enough efficacy to be approved for clinical use. Of several vaccine concepts tested in efficacy trials, only one, the RV144 pox virus prime, protein boost (ALVAC/AIDSVAX B/E) vaccine, showed a low level of vaccine protection with an estimated 31% vaccine efficacy (8). Candidate vaccines have sought to elicit both antibody and T-cell responses, but to fully prevent the acquisition of infection, a major focus has been on the induction of protective antibody responses (9, 10). Hence, the focus of this issue of Immunologic Reviews is “Antibodies and Immunity to HIV”. Animal models have demonstrated that passive administration of HIV-1-- neutralizing antibodies can fully protect against infection, but the induction of such antibodies via immunization remains a major scientific challenge. With recent advances in the isolation and characterization of broadly neutralizing antibodies (bnAbs) from HIV-1-infected subjects, in elucidating structures of the HIV-1 envelope glycoprotein (Env), in defining novel approaches to immunogen design, and in improved understanding of the immunological pathways leading to bNAb elicitation, the challenge developing an HIV-1 vaccine appears to be more tractable. The articles in this issue highlight both major areas of HIV-1 vaccine development progress and remaining obstacles, and provide context for the renewed optimism that a highly effective vaccine, while not imminent, is possible.
Main Text
The HIV-1 vaccine field has now realized that development of a safe and globally effective HIV-1 vaccine will require a greater level of understanding of host-virus interactions than has ever been required or achieved for any previous vaccine, (10–12). This is necessitated by a unique combination of virological and immunological characteristics that make protection against HIV-1 infection particularly difficult. Upon infection, HIV-1 genes are incorporated into the host genome, leading to a latent pool of infected CD4+ T cells that are resistant to removal by either anti-retroviral treatment (ART) or by the host immune system. Chronic ongoing replication outpaces the ability of the immune system to control HIV-1 infection and outfits the virus with unfavorable characteristics, including extraordinary antigenic diversity, heavy glycosylation and the shielding of potentially vulnerable Env epitopes. The induction of effective antibodies to HIV-1 Env is further complicated by the high level of somatic hypermutation (SHM) associated with potent neutralization, and the limitation of bnAb B cell lineage development by host immune tolerance mechanisms.
The only HIV-1 vaccine efficacy trial that showed some protection (estimated 31% vaccine efficacy at 42 months) was carried out by the US Army and the government of Thailand using a canarypox vector (ALVAC) and a bivalent gp120 boost (AIDSVAX B/E) (8). The trial, called RV144, was conducted in Thailand where the predominant HIV-1 strain was CRF01_AE. Studies of the immune correlates of decreased transmission risk in RV144 demonstrated that antibodies to the second variable (V2) loop were associated with lower risk of infection. These antibodies were not able to neutralize (tier 2) HIV-1 primary isolate strains, but were able to mediate antibody dependent cellular cytotoxicity (ADCC). To follow up on the RV144 trial, a new trial using a similar design but with clade C envelope inserts (ALVAC-C prime, ALVAC + C/C gp120 boost) is now planned for South Africa where the epidemic is most pressing (13). In parallel with these plans, vaccine researchers continue to focus on the difficult task of developing a vaccine that is able to induce broadly neutralizing antibodies (bnAbs), i.e. antibodies that can potently neutralize the majority of diverse circulating strains of HIV-1 (9, 10). Such bnAbs can be made, but thus far, only in the setting of HIV infection, and only years after HIV transmission.
Thus, the HIV-1 vaccine field is pursuing a two-pronged approach for vaccine development—first to design immunogens and adjuvants that will result in increased vaccine efficacy from that seen in RV144, and second to design immunogens and immunization strategies that will result in induction of the difficult-to-induce antibodies that can neutralize tier 2 HIV-1 primary isolates. Critical questions addressed in this volume of Immunological Reviews are: 1) Why do bnAbs develop in HIV-infected individuals but not in the setting of vaccination? 2) How are bnAbs regulated compared to easily-induced non-neutralizing or tier 1-virus neutralizing antibodies? 3) Are there characteristics of HIV-infected individuals who make bnAbs that can help define strategies to induce bnAbs? 4) What are preferred structures or forms of immunogens that are needed to induce bnAbs? 5) Are there additional strategies for use of antibodies to prevent HIV transmission other than via vaccine induction of bnAbs? and 6) What are strategies for improvement on the vaccine efficacy seen in the RV144 trial? The reviews in this volume provide an in depth series of discussions on these questions.
Broadly neutralizing antibodies: structure and function
While there was initial enthusiasm in 1988–90 for identification of the third variable loop (V3) of gp120 as the principle neutralization determinant of HIV-1 (14–16), the exposure of the V3 loops was soon found to be unusually characteristic of viruses adapted to growth in T-cell lines. Data emerged from 1990–1994 that HIV-1 primary isolates grown in CD4 T-cells were much more resistant to soluble CD4 and to gp120-induced neutralizing antibodies, including easy-to-induce gp120 V3 loop and CD4 binding site antibodies (17–20). Over the last 10 years, progress has been made both in defining the vulnerable and relatively conserved neutralizing antibody sites on the HIV-1 envelope (Env), and in isolating large numbers of bnAbs from HIV-1 infected individuals. Initial progress involved recruiting large numbers of HIV-1 infected individuals, identifying those with plasma bnAb activity, and defining the specificities of bnAbs in plasma (9, 10). Laura McCoy and Dennis Burton open the volume with a review of the five major specificities of bnAbs, and discuss the techniques used to isolate them from HIV-1 infected individuals (21). Critical to HIV-1 vaccine development has been the observation that bnAbs can indeed be made by the human immune system in the setting of infection, and the elucidation of their structural mode of recognition. Andrew Ward and Ian Wilson review the history of structural analysis of the HIV-1 Env and discuss the remarkable journey over the past 10 years resulting in an understanding of the structural mode of recognition of bnAbs, culminating with co-crystal and cryo-electron microscopy structures of a soluble native Env trimer and a membrane bound native trimers (22).
The immunobiology of broadly neutralizing antibodies
It has been known for over 30 years that HIV-1 infection perturbs the immune system, with profound effects on antibody responses (23). Susan Moir and Anthony Fauci review their work on the effects of HIV-1 infection on the human immune system, and in particular on B cell function (24). They provide a brief review of normal B cell development and then an in depth review of the effects of HIV-1 on B cell functions. In particular, they note the dramatic changes in HIV-1 infection in memory B and plasmablast populations, and the profound dysfunction that can occur in specific antigen responses. Nonetheless, in ~50% of HIV-1 infected individuals, cross-reactive neutralizing antibodies do develop in the setting of the profound perturbations caused by HIV-1 (25). T follicular helper cells (Tfh) are key CD4+ T cells in germinal centers for induction of high affinity antibodies. Colin Havenar-Daughton, Jeong Hyun Lee and Shane Crotty review their work on changes in Tfh levels in those that make bnAbs and the role of Tfh in the germinal center in generation of bnAbs.(26). Moreover they discuss their work on development of fine needle aspiration as a tool for study of Tfh over time in the setting of vaccination (26). Persephone Borrow and Anthony Moody discuss work characterizing Tfh and T regulatory cells (Treg) in the large CHAVI cohort of HIV-1 infected individuals who make bnAbs versus those that do not. The immunologic phenotype they discuss of elevated Tfh, low Treg, exhausted Treg and high frequency of plasma autoantibodies is similar to findings in autoimmune disease patients such as systemic lupus erythematosus (27–29). They go on to discuss the relevance of their findings to bnAb regulation (30).
Critical to our understanding how to elicit bnAbs via vaccination has been the understanding of the host controls limiting bnAb induction. In 2005 it was found that three of the known bnAbs were polyreactive for host antigens (29). This generated the hypothesis that, due to bnAb unusual traits such as long HCDR3 regions and polyreactivity, they were controlled by immune tolerance mechanisms. Tolerance control of bnAb lineages implies that bnAb precursors have been culled from bone marrow and the periphery or made anergic at tolerance checkpoints, and therefore are subdominant and disfavored. Here, Garnett Kelsoe and one of us (BFH) review evidence for host immune tolerance control of bnAb development, describe host molecules that cross-react with bnAbs, and describe the rationale and methods for enhancing bnAb development with immune checkpoint inhibitors (31).
Understanding bnAb development and targeting naïve B cell receptors by vaccine candidates has required the development of new computational biology methods and programs for inferring germline antibody sequences and constructing evolutionary trees of bnAbs. Peter Kwong, Lawrence Shapiro and colleagues outline their multiple “antibodyome” approaches to computational analysis of the antibody response to HIV-1 (32). Next, Thomas Kepler and Kevin Wiehe discuss their programs for immunoglobulin gene annotation, and strategies for inference of bnAb lineage intermediate and germline antibodies (33). Moreover, they go on to discuss progress in the genetic and structural analysis of HIV-1 neutralizing antibody affinity maturation.
One technology that has propelled the study of HIV-1 vaccine development forward has been the use of human gene knock-in (KI) mice to study both control of bnAb development by host control mechanisms and the ability of immunogens to drive bnAb lineage development. Laurent Verkoczy, Ming Tian and Fred Alt review work done by them and the field with bnAb KI mice, including bnAb KI models expressing deduced precursor V(D)J rearrangements of mature bnAbs or unrearranged germline V, D, J segments (that can be assembled into variable region exons that encode bnAb precursors). These new mouse models allow the evaluation of vaccine immunogens and regimens for inducing bnAbs. They also discuss mouse models that have revealed immune tolerance control of bnAb development and as well, review progress made in using immunogens to activate bnAb germline B cells in KI mice (34).
Immunogen design for induction of bnAb B cell lineages
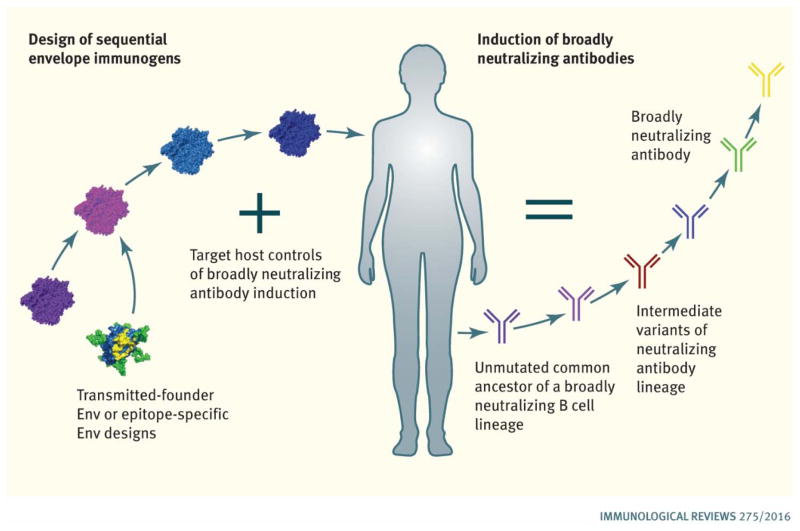
For over 20 years the HIV-1 vaccine development field has been trying to induce protective antibodies with a variety of individual Env immunogens, or with mixtures of unrelated Envs, but without consistent success (9, 10). As noted above, the realization that immune tolerance can control bnAb lineages, suggests such lineages are disfavored and may require unique approaches to stimulate and induce affinity maturation (12, 35–37). Over the past 7 years the concept has arisen that specific Env immunogens will be needed to target the appropriate naïve B-cells that expresses the unmutated ancestor (UA) of a neutralizing antibody lineage. Furthermore, sequential immunogens may be needed to mature such lineages and to drive subdominant and disfavored B cell lineages toward effective virus neutralization (Fig. 1). Mattia Bonsignori and colleagues describe the strategy of B cell lineage design, wherein the co-evolution of HIV-1 and bnAb B cell lineages are mapped and specific Envs are chosen from an individual that can be used in sequence for inducing similar bnAb B cell lineages (38).
Figure 1.
Strategies for Induction of HIV-1 Broadly Neutralizing Antibodies. Envs are being selected or designed to bind to unmutated common ancestors of bnAb B cell linages. Host controls limiting bnAb induction are being targeted by design of sequential immunogens, and by using adjuvants that target molecules that enhance bnAb B cell lineage affinity maturation.
They describe the results of mapping the co-evolution of CD4 binding site, V1V2-glycan and V3-glycan targeted bnAbs in HIV-1 infected individuals, and describe vaccine strategies that derive from this work. Penny Moore, Jason Gorman, Nicole Doria-Rose and Lynn Morris describe elegant studies in an African HIV-1 infected individual that made V1V2-glycan bnAbs and describe strategies for recapitulating bnAb induction in the setting of vaccination from their work (39).
Rogier Sanders and John Moore describe the design of the soluble recombinant Env trimeric proteins that include a disulfide bond between gp120 and gp41, and additional stabilizing mutations (SOSIP Env). Such SOSIP trimers have antigenic characteristics similar to native Env trimers., Sanders and Moore review both technical aspects of their purification, and data that these trimers can induce tier 2 autologous neutralizing antibodies better than Env monomers or non-SOSIP oligomers (40). The review by Gunilla Karlsson Hedenstam, Javier Guenaga, Martin Corcoran and Richard Wyatt describes an additional approach to constructing stable recombinant Env trimers by replacing the native gp120–41 cleavage site with a native flexible linker (NFL). They further describe new and evolving tools to evaluate B-cell vaccine response, including approaches to use next generation sequencing for antibody lineage tracing (41).
Leonidas Stamatatos, Andrew McGuire and Marie Pancera discuss in depth the issue of necessity of targeting B cells expressing the unmutated ancestor (sometimes called germline) of a bnAb lineages. It was originally observed that germline reverted variants of bnAb did not bind HIV-1 Env and thus speculated that this was a prime reason for the inability of HIV Env to induce bnAbs (42, 43). With studies of bnAb co-evolution beginning with the transmitted/founder (TF) virus Envs from the time of transmission, we now know that for some bnAb specificities, TF Envs can initiate bnAb lineages (44, 45). Nonetheless, structurally designed immunogens, particularly those for the VRC01 class of CD4 binding-site bnAbs, have proven highly immunogenic for initiation of UA BCR-bearing B cell activation and beginning of affinity maturation in bnAb knockin mice (46–52). Thus, the review by Stamatatos, McGuire and Pancera review the need to target bnAb germlines, and strategies of immunogen design that can meet this purpose (53)
For many years, the Los Alamos National Laboratory HIV-1 Sequence Database has served the HIV vaccine field by collecting now over 500,000 HIV sequences from all over the world (http://www.hiv.lanl.gov/content/sequence/HIV/mainpage.html). Bette Korber, Peter Hraber, Kshitij Wagh and Beatrice Hahn review how use of the LANL database, structural biology data sets and new sets of HIV-1 Env sequences from HIV-1/bnAb co-evolution studies can inform polyvalent Env vaccine design (54).
Non-neutralizing antibody-dependent cellular cytotoxicity (ADCC) and other FcR-mediated effector functions
As noted above, the only HIV-1 vaccine trial to show any vaccine efficacy (RV144) utilized a canarypox vector (ALVAC-AE) and a bivalent B/E gp120 + ALVAC boost (8). Phase I trials with a new ALVAC-C prime, ALVAC-C/ gp120 C/C boost vaccine have been completed and a new vaccine efficacy trial in Africa is planned. An immune correlates analysis of the RV144 trial showed that the decreased risk of transmission was associated with high IgG antibodies to the V2 region of Env (55). Furthermore, such antibodies displayed FcR-mediated ADCC as an effector function (55). Stylianos Bournazos and Jeffrey Ravetch provide an in depth review of anti-retroviral antibody FcγR-mediated effector functions and explain in how antibodies can control and/or prevent HIV-1 infection (56). Georgia Tomaras and Stanley Plotkin review immune correlate principles in general and discuss the immune correlates work on the RV144 trial over the past 7 years (57). This work reveals the complexity of protectively immunity - with the suggestion that multiple types of immune responses together, comprise an immune correlate — thus implicating polyfunctional immune control of HIV-1 transmission. Margaret Ackerman, Dan Barouch and Galit Alter describe new techniques for system serology analysis of vaccine trials for more comprehensive analysis of vaccine trial immune correlates that incorporates multiple antibody characteristic and effector functions (58). George Lewis, Marzena Pazgier and Anthony DeVico describe an interesting new hypothesis of how to find targets of FcR-mediated effector function on HIV-1 Env that can directly impact vaccine design. They review examples of protection in animal models of ADCC and other non-neutralizing effector functions (59).
BnAbs for prevention and treatment
Finally, one of us (JRM) and coauthors Amarendra Pegu, Ann Hessell and Nancy Haigwood (60), discuss our current understanding of the role of bnAbs in mediating protection against infection. The newest generation of natural and engineered bnAbs demonstrate remarkable potency and breadth – providing the opportunity to use such products clinically, as pre-exposure prophylaxis, to prevent HIV infection in high risk individuals. Jacqueline Brady, David Baltimore and Alejandro Balazs (61), discuss an alternative approach to antibody immunoprophylaxis involving vector-mediated gene transfer by adeno-associated virus (AAV). They provide insights into the potential advantages, and obstacles to overcome, for the gene-based delivery of antibodies. Finally, David Margolis, Richard Koup and Guido Ferrari review the role and potential advantages of antibodies to treat HIV infection. They highlight antibody functions such as the ability to opsonize virus-infected cells and mediate ADCC, and ultimately, the potential to clear infected cells. In particular, they discuss the construction of bispecific antibodies as well as other antibody designs that may be superior to naturally occurring bnAbs, and as well, may be able to target the latently infected pool of CD4 T cells (62).
Summary
In summary, this volume of Immunological Reviews brings together an extraordinary group of investigators who are conducting research on antibody-based immunity. It is fascinating to see the fields of virology, structural biology, humoral immunology and vaccinology converge to provide new insights into vaccine design and immunization strategies that have the potential to induce protective antibody reposes against HIV-1. There is a major focus on understanding how the immune system generates bnAbs during natural infection and learning from both the isolation and structural characterization of such bnAbs, and the dissection of the immune pathways leading to the evolution of broad and potent antibodies, including virus-host coevolution and host tolerance mechanisms that limit the induction and maturation of bnAbs. Substantial progress has been made in eliciting neutralizing antibodies in humanized mouse models, and this provides insights into how to design vaccines for human trials. In addition to antibody-based prevention, there is recent interest in the use of bnAbs to treat, or eradicate, HIV-1 infection. To verify and potentially improve on the RV144 vaccine trial, the strategy has centered on understanding immune correlates of protection and increasing the breadth of ADCC and other FcR mediated responses to strains of HIV-1 circulating in South Africa. New approaches to understanding the quality of the antibody response, including IgG isotype, subclass, glycosylation patterns and Fc-mediated effector functions are adding a new level of analysis, and potentially understanding, to in vivo protection. For induction of broadly neutralizing antibodies, the field is taking advantage of rapid progress in the structural understanding of the HIV-1 Env and its neutralization epitopes, and recent data on immune pathways of bnABs, and is focused on design of sequential immunogens as well as recreating the immunologic milieu of HIV-1 infection in the setting of vaccination such that bnAbs will be allowed by the immune system to be made (Figure 1). This work represents the interface between basic and translational research to evaluate vaccine and therapeutic candidates in the context of the human immune system. No other vaccine has required such a detailed understanding about fundamental aspects of the human immune system and its interaction with novel antigens. Several years ago, there was little optimism that a broadly neutralizing antibody-based HIV-1 vaccine could be designed, or would be feasible. The reviews in this issue provide a rational basis for optimism. We have observed how the immune system generates bNAbs in response to vial evolution, and we understand the critical nature of the initial interaction between antigen and the naïve B-cells that will generate bnAb lineages. These are the first steps toward the elicitation of broadly protective antibodies, and ultimately to development of an HIV-1 vaccine that will be globally effective.
Acknowledgments
This work was funded through an NIH, NIAID Division of AIDS UM1 grant AI100645 to BFH for the Duke Center for HIV/AIDS Vaccine Immunology-Immunogen Discovery. JRM was supported by NIH intramural funds to the NIAID Vaccine Research Center. We thank Brenda Hartman for graphical assistance.
References
- 1.Barre-Sinoussi F, Chermann JC, Rey F, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 2.Gallo RC, Salahuddin SZ, Popovic M, et al. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984;224:500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- 3.Buchbinder SP, Mehrotra DV, Duerr A, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert P, Wang M, Wrin T, et al. Magnitude and breadth of a nonprotective neutralizing antibody response in an efficacy trial of a candidate HIV-1 gp120 vaccine. The Journal of infectious diseases. 2010;202:595–605. doi: 10.1086/654816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gray GE, Allen M, Moodie Z, et al. Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: a double-blind, randomised, placebo-controlled test-of-concept phase 2b study. The Lancet Infectious diseases. 2011;11:507–515. doi: 10.1016/S1473-3099(11)70098-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammer SM, Sobieszczyk ME, Janes H, et al. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. The New England journal of medicine. 2013;369:2083–2092. doi: 10.1056/NEJMoa1310566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pitisuttithum P, Gilbert P, Gurwith M, et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. The Journal of infectious diseases. 2006;194:1661–1671. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 8.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. The New England journal of medicine. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 9.Burton DR, Mascola JR. Antibody responses to envelope glycoproteins in HIV-1 infection. Nature immunology. 2015;16:571–576. doi: 10.1038/ni.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mascola JR, Haynes BF. HIV-1 neutralizing antibodies: understanding nature’s pathways. Immunological reviews. 2013;254:225–244. doi: 10.1111/imr.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fauci AS. An HIV Vaccine: Mapping Uncharted Territory. Jama. 2016;316:143–144. doi: 10.1001/jama.2016.7538. [DOI] [PubMed] [Google Scholar]
- 12.Haynes BF, Shaw GM, Korber B, et al. HIV-Host Interactions: Implications for Vaccine Design. Cell host & microbe. 2016;19:292–303. doi: 10.1016/j.chom.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corey L, Gilbert PB, Tomaras GD, Haynes BF, Pantaleo G, Fauci AS. Immune correlates of vaccine protection against HIV-1 acquisition. Science translational medicine. 2015;7:310rv317. doi: 10.1126/scitranslmed.aac7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goudsmit J, Debouck C, Meloen RH, et al. Human immunodeficiency virus type 1 neutralization epitope with conserved architecture elicits early type-specific antibodies in experimentally infected chimpanzees. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:4478–4482. doi: 10.1073/pnas.85.12.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Javaherian K, Langlois AJ, McDanal C, et al. Principal neutralizing domain of the human immunodeficiency virus type 1 envelope protein. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:6768–6772. doi: 10.1073/pnas.86.17.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palker TJ, Clark ME, Langlois AJ, et al. Type-specific neutralization of the human immunodeficiency virus with antibodies to env-encoded synthetic peptides. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:1932–1936. doi: 10.1073/pnas.85.6.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daar ES, Li XL, Moudgil T, Ho DD. High concentrations of recombinant soluble CD4 are required to neutralize primary human immunodeficiency virus type 1 isolates. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:6574–6578. doi: 10.1073/pnas.87.17.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golding H, D’Souza MP, Bradac J, Mathieson B, Fast P. Neutralization of HIV-1. AIDS research and human retroviruses. 1994;10:633–643. doi: 10.1089/aid.1994.10.633. [DOI] [PubMed] [Google Scholar]
- 19.Hanson CV. Measuring vaccine-induced HIV neutralization: report of a workshop. AIDS research and human retroviruses. 1994;10:645–648. doi: 10.1089/aid.1994.10.645. [DOI] [PubMed] [Google Scholar]
- 20.Matthews TJ. Dilemma of neutralization resistance of HIV-1 field isolates and vaccine development. AIDS research and human retroviruses. 1994;10:631–632. doi: 10.1089/aid.1994.10.631. [DOI] [PubMed] [Google Scholar]
- 21.McCoy LE, Burton D. Identification and Specificity of Broadly Neutralizing Antibodies Against HIV. Antibodies and Immunity to HIV. 2016:275. doi: 10.1111/imr.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward AB, Wilson I. The HIV-1 Envelope Glycoprotein Structure: Nailing Down a Moving Target Antibodies and Immunity to HIV. Immunological Reviews. 2017:275. doi: 10.1111/imr.12507. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lane HC, Masur H, Edgar LC, Whalen G, Rook AH, Fauci AS. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. The New England journal of medicine. 1983;309:453–458. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- 24.Moir S, Facui AS. B Cell Responses to HIV Infection. Antibodies and Immunity to HIV. Immunological Reviews. 2017:275. doi: 10.1111/imr.12502. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hraber P, Seaman MS, Bailer RT, Mascola JR, Montefiori DC, Korber BT. Prevalence of broadly neutralizing antibody responses during chronic HIV-1 infection. Aids. 2014;28:163–169. doi: 10.1097/QAD.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Havenar-Daughton C, Lee JH, Crotty S. TFH Cells and HIV bnAbs, an Immunodominance Model of the HIV Neutralizing Antibody Generation Problem. Antibodies and Immunity to HIV. Immunological Reviews. 2017:275. doi: 10.1111/imr.12512. In Press. [DOI] [PubMed] [Google Scholar]
- 27.Bonsignori M, Wiehe K, Grimm SK, et al. An autoreactive antibody from an SLE/HIV-1 individual broadly neutralizes HIV-1. The Journal of clinical investigation. 2014;124:1835–1843. doi: 10.1172/JCI73441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moody MA, Pedroza-Pacheco I, Vandergrift N, Chui C, Lloyd KE, Parks R, Soderberg KA, Ogbe AT, Cohen MS, Liao HX, Gao F, McMichael AJ, Montefiori DC, Verkoczy L, Kelsoe G, Huang J, Shea PR, Connors M, Borrow P, Haynes BF. Immune perturbations in HIV-1–infected individuals who make broadly neutralizing antibodies. Science Immunology. 2016;1:aag0851. doi: 10.1126/sciimmunol.aag0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haynes BF, Fleming J, St Clair EW, et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- 30.Borrow P, Mood MA. Immunologic Characteristics of HIV-Infected Individuals Who make Broadly Neutralizing Antibodies. Antibodies and Immunity to HIV. Immunological Reviews. 2017:275. doi: 10.1111/imr.12504. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelsoe G, Haynes BF. Host Controls of HIV Broadly Neutralizing Antibody Development. Antibodies and Immunity to HIV. Immunological Reviews. 2017:275. doi: 10.1111/imr.12508. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwong PD, Chuang GY, DeKosky BJ, et al. Antibodyomics: Bioinformatics Technologies for Understanding B Cell Immunity to HIV-1. Antibodies and Immunity to HIV. Immunological Reviews. 2017:275. doi: 10.1111/imr.12480. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kepler TB, Wiehe K. Genetic and Structural Analyses of Affinity Maturation in the Humoral Responses to HIV-1. Antibodies and Immunity to HIV. Immunological Reviews. 2017:275. doi: 10.1111/imr.12513. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verkoczy L, Alt F, Tian M. Human Ig Knock In Mice to Study the Development and Regulation of HIV-1 Broadly Neutralizing Antibodies. antibodies and Immunity to HIV. Immunological Reviews. 2017:275. doi: 10.1111/imr.12505. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haynes BF, Kelsoe G, Harrison SC, Kepler TB. B-cell-lineage immunogen design in vaccine development with HIV-1 as a case study. Nature biotechnology. 2012;30:423–433. doi: 10.1038/nbt.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haynes BF, Moody MA, Verkoczy L, Kelsoe G, Alam SM. Antibody polyspecificity and neutralization of HIV-1: a hypothesis. Human antibodies. 2005;14:59–67. [PMC free article] [PubMed] [Google Scholar]
- 37.Haynes BF, Verkoczy L. AIDS/HIV. Host controls of HIV neutralizing antibodies. Science. 2014;344:588–589. doi: 10.1126/science.1254990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonsignori M, Liao HX, Gao F, et al. Antibody-Virus Co-Evolution in HIV Infection: Paths for HIV Vaccine Development. Antibodies and Immunity to HIV. Immunological Reviews. 2017:275. doi: 10.1111/imr.12509. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore PL, Gorman J, Doria-Rose N, Morris L. Ontogeny-Based Immunogens for the Induction of V2-Directed HIV Broadly Neutralizing Antibodies. Antibodies and Immunity to HIV. Immunological Reviews. 2017:275. doi: 10.1111/imr.12501. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanders RW, Moore J. Native-Like Env Trimers as a Platform for HIV-1 Vaccine Design. Antibodies and Immunity to HIV. Immunological Reviews. 2017:275. doi: 10.1111/imr.12481. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hedestam GBK, Guenaga J, Corcoran M, Wyatt RT. Evolution of B Cell Analysis and Env Trimer Redesign. Antibodies and Immunity to HIV. Immunological Reviews. 2017:275. doi: 10.1111/imr.12515. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoot S, McGuire AT, Cohen KW, et al. Recombinant HIV envelope proteins fail to engage germline versions of anti-CD4bs bNAbs. PLoS pathogens. 2013;9:e1003106. doi: 10.1371/journal.ppat.1003106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao X, Chen W, Feng Y, et al. Germline-like predecessors of broadly neutralizing antibodies lack measurable binding to HIV-1 envelope glycoproteins: implications for evasion of immune responses and design of vaccine immunogens. Biochemical and biophysical research communications. 2009;390:404–409. doi: 10.1016/j.bbrc.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doria-Rose NA, Schramm CA, Gorman J, et al. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature. 2014;509:55–62. doi: 10.1038/nature13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liao HX, Lynch R, Zhou T, et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013;496:469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Briney B, Sok D, Jardine JG, et al. Tailored Immunogens Direct Affinity Maturation toward HIV Neutralizing Antibodies. Cell. 2016;166:1459–1470 e1411. doi: 10.1016/j.cell.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dosenovic P, von Boehmer L, Escolano A, et al. Immunization for HIV-1 Broadly Neutralizing Antibodies in Human Ig Knockin Mice. Cell. 2015;161:1505–1515. doi: 10.1016/j.cell.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Escolano A, Steichen JM, Dosenovic P, et al. Sequential Immunization Elicits Broadly Neutralizing Anti-HIV-1 Antibodies in Ig Knockin Mice. Cell. 2016;166:1445–1458 e1412. doi: 10.1016/j.cell.2016.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jardine J, Julien JP, Menis S, et al. Rational HIV immunogen design to target specific germline B cell receptors. Science. 2013;340:711–716. doi: 10.1126/science.1234150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jardine JG, Kulp DW, Havenar-Daughton C, et al. HIV-1 broadly neutralizing antibody precursor B cells revealed by germline-targeting immunogen. Science. 2016;351:1458–1463. doi: 10.1126/science.aad9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jardine JG, Ota T, Sok D, et al. HIV-1 VACCINES. Priming a broadly neutralizing antibody response to HIV-1 using a germline-targeting immunogen. Science. 2015;349:156–161. doi: 10.1126/science.aac5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tian M, Cheng C, Chen X, et al. Induction of HIV Neutralizing Antibody Lineages in Mice with Diverse Precursor Repertoires. Cell. 2016;166:1471–1484 e1418. doi: 10.1016/j.cell.2016.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stamatatos L, Pancera M, McGuire AT. Germline Targeting Immunogens. Antibodies and Immunity to HIV. Immunological Reviews. 2017:275. doi: 10.1111/imr.12483. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Korber B, Hraber P, Wagh K, Hahn B. Polyvalent Vaccine Approaches to Combat HIV-1 Diversity. Antibodies and Immunity to HIV. Immunological Reviews. 2017:275. doi: 10.1111/imr.12516. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haynes BF, Gilbert PB, McElrath MJ, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. The New England journal of medicine. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bournazos S, Ravetch JV. Anti-Retroviral FcyR-mediated Effector Functions. Antibodies and Immunity to HIV. Immunological Reviews. 2017:275. doi: 10.1111/imr.12482. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tomaras GT, Plotkin S. Complex Immune Correlates of Protection in HIV-1 Vaccine Efficiacy Trials. Antibodies and Immunity to HIV. Immunological Reviews. 2017:275. doi: 10.1111/imr.12514. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ackerman ME, Barouch D, Alter G. Systems Serology for Evaluation of HIV Vaccine Trials. Antibodies and Immunity to HIV. Immunological Reviews. 2017:275. doi: 10.1111/imr.12503. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lewis G, DeVico A, Pazgier M. Induction of Non-Neutralizing Antibodies for Protection. antibodies and Immunity to HIV. Immunological Reviews. 2017:275. In Press. [Google Scholar]
- 60.Pegu A, Hessell AJ, Mascola JR, Haigwood NL. Use of Broadly Neutralizing Antibodies for HIV-1 Prevention. Antibodies and Immunity to HIV. Immunological Reviews. 2017:275. doi: 10.1111/imr.12511. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brady JM, Baltimore D, Balazs AB. Antbody Gene Transfer with Adeno-Associated Viral Vectors as a Method for HIV Prevention. antibodies and Immunity to HIV. Immunological Reviews. 2017:275. doi: 10.1111/imr.12478. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Margolis DM, Koup R, Ferrari G. HIV Antibodies for Treatment of HIV Infection. Antibodies and Immunity to HIV. Immunological Reviews. 2017:275. doi: 10.1111/imr.12506. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]