Abstract
Rationale
Adverse early life experiences are risk factors for drug abuse and addiction. Changes in brain opioid systems have been demonstrated in response to neonatal visceral pain (NVP), but the impact of these changes on abuse-related effects of morphine are unknown. The NVP procedure used models chronic visceral hyperalgesia persisting across development.
Objectives
Intravenous self-administration, drug discrimination, and locomotor activity were used to compare the abuse-related effects of morphine in NVP and control rats.
Methods
Rats self-administered 0.3 mg/kg/inj morphine under an FR1 schedule, and dose–effect functions for morphine were then established. Separate rats were trained to discriminate 3.2 mg/kg morphine from saline under an FR20 schedule, and morphine dose–effect functions were then determined in the absence and presence of 0.1 mg/kg naltrexone. A third group of rats was tested with a range of morphine doses in an assay of locomotor activity, then injected daily with 10 mg/kg morphine to assess locomotor sensitization.
Results
NVP rats self-administered more morphine than controls at reinforcing doses. Discriminative stimulus effects of morphine were similar between groups, but in the presence of naltrexone, the ED50 for morphine was more than 12× greater in control rats than in NVP animals. Morphine did not stimulate locomotor activity at any tested dose in NVP rats, although significant effects were observed in controls. Finally, significant locomotor sensitization was observed only in NVP rats.
Conclusions
NVP-induced changes in brain opioid systems have persistent pharmacological consequences into adulthood and may increase sensitivity to abuse-related effects of opioids across development.
Keywords: Morphine, Visceral pain, Self-administration, Drug discrimination, Locomotor sensitization
Introduction
Major theories of drug addiction suggest that early life stress is a predisposing factor in adolescent and adult substance abuse (Sinha 2001). Studies have shown that early life stressors, such as child abuse, significantly increase the likelihood of substance abuse later in life (Huang et al. 2011). Perhaps the most common animal model of early life stress is neonatal maternal separation, where studies in rodents consistently demonstrate increased rates of acquisition of drug self-administration (Kosten et al. 2000), sensitivity to reinstatement of previously drug-reinforced responding (Ewing Corcoran and Howell 2010; Goeders 2002; Goeders and Guerin 1994, 1996), greater amphetamine-induced dopamine release (Hall et al. 1999; Kehoe et al. 1996), and alterations in opioid-induced place conditioning (Michaels and Holtzman 2008).
In humans, it has also been shown that early life trauma can increase the vulnerability to substance abuse and lead to earlier initiation of heroin use (Li et al. 2012). Therefore, it may be the case that early life pain could also predispose individuals to drug addiction later in life. In support of this notion, it has been shown that rats subjected to somatic injury (spinal cord contusion) (Woller et al. 2012) or electrical pain (shock) (Ferguson et al. 2004) exhibited greater conditioned place preference in response to morphine than controls. However, inflammatory pain (adjuvant-induced arthritis) (Lyness et al. 1989) and neuropathic pain (L5 and L6 spinal nerve ligation) (Martin and Saleeby 2007) each blunted the apparent reinforcing effects of morphine in rats. Thus, the relationship between pain and abuse-related effects of morphine may critically depend on the pain modality employed. Similarly, the timing of the pain manipulation may also modulate its impact on drug effects, as developing opioid systems may be particularly sensitive to nociceptive stimulation (Bhutta et al. 2001; Lidow 2002; Ren et al. 2005; Walker et al. 2003; Wang et al. 2004). Whether early life visceral pain may render individuals more vulnerable to opioid abuse later in life has not previously been investigated in humans or in laboratory species.
In this regard, pain affects a large number of newborns each year. Due to advances in neonatal care, most of the hundreds of thousands of infants that are born prematurely in the USA now survive to adulthood (Qiu 2006). However, the medical procedures employed in a neonatal intensive care unit are often invasive in nature (Anand 1998; Grunau et al. 2005; Simons et al. 2003), and are performed during a time when neurodevelopmental plasticity is especially pronounced (De Jonckheere et al. 2011). Studies using animal models of early life pain demonstrate that lasting changes in neurophysiology and behavior that are applicable to the actions of opioids are induced by these procedures. For example, in rats, inflammatory pain experienced on the day of birth increases adult levels of beta-endorphin and met/leu-enkephalin in the CNS, and concurrently decreases expression of μ opioid receptors (Laprairie and Murphy 2009). Similarly, adult rats with a history of neonatal somatic pain display a generalized decrease in nociceptive sensitivity and a blunted analgesic response to morphine (Bhutta et al. 2001; Shimada et al. 1990). In a neonatal visceral pain (NVP) model in the rat, persistent visceral pain has been shown to affect the developing nervous system by impacting early life pain experiences, and these alterations may be long-lasting (Al-Chaer et al. 2000). Thus, while this model of NVP has been shown to increase visceral pain sensitivity across development, no changes in somatic pain perception have been detected at any age (Al-Chaer et al. 2000).
The fact that early life pain alters developing opioid systems may suggest that individuals with a history of NVP may be more sensitive to the abuse-related effects of opioids later in life. Any identification of any early life factors that may potentially increase the relative risk of opioid abuse or addiction in later life would be advantageous to the health care system as a whole. In these studies, we compared the reinforcing effects, discriminative stimulus effects, and loco-motor sensitizing effects of the prototypical opioid morphine in rats with and without a history of NVP. The model of NVP used in this study was achieved by administering an intracolonic irritant during postnatal development (Al-Chaer et al. 2000; Wang et al. 2008). The central theme of these studies is that the persistent effects of neonatal visceral pain are not limited to later pain perception, but have a broader impact on neuroplasticity that extends from birth to adolescence and into adulthood, conferring sensitivity to abuse-related opioid effects across development.
Methods
Animals
All experiments were conducted in adult male Sprague Dawley rats (Harlan Sprague Dawley Inc., Indianapolis, IN) housed in the University of Arkansas for Medical Sciences (UAMS) vivarium. Pregnant females were received, and neonates were separated by sex at 8 days after birth and housed eight per cage with one adult female until they were 25 days old. Culled female neonates were housed with an adult female and used for studies not related to this manuscript. The adult female had access to food and water ad libitum. After 25 days, the male rats were housed four per cage with access to food and water ad libitum until the behavioral studies began. When rats grew to 250 g, they were pair housed (if used in drug discrimination experiments) or singly housed (if used in other assays). On average it took 8–10 weeks for the rats to reach 250 g. All behavioral experiments took place during the light phase of the circadian cycle.
Neonatal visceral pain
The model of NVP used in this study was achieved by administering an intracolonic irritant during postnatal development. Rats (8 days old) were divided into two groups for purposes of different treatments. The NVP neonates received an infusion of allyl isothiocyanate and mustard oil (0.2 ml, 5 %) into the colon via PE90 tubing inserted 2 cm past the anus without the use of anesthesia. Mustard oil was administered on postnatal days 8, 10, and 12. Control neonates were handled similarly to NVP rats with the exception that no colonic insertion was made with the PE90 tubing. Instead, rats in the control group were gently held and touched on the perineal area on a schedule similar to that described for the NVP rats. Regardless of group, the amount of time the animals were separated from the home cage during administration of mustard oil or performance of control procedures was standardized at ~5 min per treatment. After the third and final colonic irritation or control procedure, no further intervention (with the exception of standard animal husbandry procedures) was performed with either group until rats reached 250 g and experimental procedures began (Al-Chaer et al. 2000; Wang et al. 2008).
Surgery
Intravenous catheters
Anesthesia was induced with inhaled isoflurane and maintained throughout the procedure. The scruff of the neck and mid-scapular region of each rat was shaved and sanitized. A small incision was made along the lateral aspect of the neck, and a blunt dissection with sterile forceps was performed to expose the external jugular vein. After isolating the vessel, the distal end of the vein was tied off with silk suture, and a small cut opened the vein. A pre-measured, heparin-coated polyurethane round tip catheter was then introduced into the vessel and passed to the approximate level of the atrium. The catheter was secured to the vein with silk suture, and then passed subcutaneously using a sterile trocar. The catheter exited in the midscapular region and terminated at an injection port attached to the rat with a silastic harness. Surgeries were performed 10 days before initiation of experiments, allowing time for incisions to heal and for rats to recover normal body weights. Following surgery, all catheterized rats were individually housed in Plexiglas cages for the duration of intravenous drug self-administration experiments.
Biotelemetry probes
Following anesthesia with ketamine (100 mg/kg, ip) and xylazine (3 mg/kg, ip), the abdominal area of each rat was shaved and sanitized with iodine and alcohol. A rostro-caudal incision was made with a sterile scalpel, providing access to the intraperitoneal cavity. A cylindrical glass-encapsulated radiotelemetry probe (model ER-4000 E-Mitter, Mini Mitter, Bend, OR, USA) was inserted and stitched to the abdominal muscle with absorbable 5-0 chromic gut suture. The incision was then closed in two layers, using the same absorbable suture to close the muscle and 5-0 vicryl to close the skin. Surgeries were performed 10 days before initiation of experiments, allowing time for incisions to heal and for rats to recover normal body weights. Following surgery, all implanted rats were individually housed in Plexiglas cages for the duration of telemetry experiments.
Intravenous drug self-administration
Rats were trained to lever press during daily 1-h training sessions using food reinforcement (45 mg chocolate flavor Dustless Precision Pellets no. F0299, Bio-Serv, Frenchtown, NJ). All training and testing sessions were conducted in custom-built, operant conditioning chambers equipped with response levers, auditory and visual feedback stimuli, a food pellet dispenser, and an infusion pump. Sessions began at approximately 0700 h. At the start of each session, the house light was illuminated, a retractable lever extended into the chamber, and lever pressing behavior was reinforced on an FR1 schedule of reinforcement. Every 20th reinforcer that was earned increased the FR value by two responses, and in this manner, rats were gradually shaped to a terminal FR20 schedule of reinforcement, commensurate with individual performance, in order to compare acquisition of food-maintained responding and response rates under the terminal schedule. All sessions terminated automatically once 60 reinforcers were delivered or 1 h had elapsed. Once food-maintained response rates were stably maintained under the FR20 schedule, rats were outfitted with a silastic harness and allowed five to seven sessions to habituate. Heparin-coated polyurethane round tip catheters were then implanted as previously described.
Morphine self-administration was initially engendered using a unit dose of 0.3 mg/kg/inj under an FR1 schedule in an NVP group (n=5) and a control group (n=6). Each response activated an infusion pump that delivered 0.110 ml of either morphine or saline over a 2-s duration. Concurrent with the start of each infusion, the house and stimulus lights were turned off, and a tone was initiated to signal a 10-s post-infusion time-out. After 10 s, the house and stimulus lights were illuminated, and morphine or saline was again available for self-injection. All sessions terminated after 60 min or after 60 infusions were self-administered, whichever came first.
After seven sessions of morphine-reinforced responding at the 0.3 mg/kg/inj maintenance dose, substitutions with various doses of morphine were performed to establish a dose–effect function. Testing conditions were identical to those used during training and were conducted with 0.01, 0.03, 0.1, and 0.3 mg/kg/infusion of morphine as well as with 1.0 ml/kg/infusion saline. Doses were tested in an irregular order with the stipulation that no more than two ascending or descending doses could be tested in a row. Given the long-lasting effects of the NVP procedure on pain processing and visceral hypersensitivity, we were initially concerned that maintenance of catheter patency in these animals might prove problematic. However, patency was maintained in all animals for more than 10 weeks, which was more than sufficient to generate the dose–effect functions presented.
Drug discrimination
After initial shaping of operant responding, identical to the procedure used above to engender food-maintained responding under a terminal FR20 schedule, adult rats with a history of NVP (n=6) and controls (n=6) were trained to discriminate 1.5 mg/kg morphine from saline. Daily training sessions consisted of a 10-min timeout period before the session began followed by a 60-min response period. During the response period, the house light and stimulus lights were illuminated and 60 food pellets (Bio-Serv 45 mg dustless precision pellets) were available under a FR 20 food-maintained schedule of reinforcement. When saline was administered, completion of the response requirement on the left lever (saline-appropriate lever) resulted in food delivery. When the training dose of morphine was administered, completion of the response requirement on the right lever (morphine-appropriate lever) resulted in food delivery. If all 60 food pellets were delivered before the end of the 60-min response period, the house light and stimulus lights were turned off, and the session ended. Training sessions were conducted 5 days per week. Training continued until the following three criteria were met for three consecutive sessions: (1) percentage of injection appropriate responding before delivery of the first reinforcer was >85 %; (2) percentage of injection-appropriate responding for the entire session was >90 %; and (3) all available food pellets were earned during saline training sessions. These criteria were in effect for the duration of the study, such that all animals were required to pass 3 days of training prior to each substitution test. If a rat did not pass all three of the criteria previously stated, then the same training (saline or 1.5 mg/kg morphine) was repeated for the next training session. Test sessions were identical to training sessions except that 20 consecutive responses on either lever delivered food, and the sessions ended after delivery of a single pellet. Dose–effect functions for morphine were subsequently re-determined following a 10-min pre-session injection of 0.1 mg/kg naltrexone.
Locomotor activity and sensitization
Locomotor activity was used to evaluate the sensitivity to the direct effects of acute morphine administration in adult rats subjected to NVP (n=6) and in controls (n=6). Implanted transmitters (see above) produced activity-modulated signals which were sent to a receiver (model ER-4000 Receiver, Mini Mitter Co., Inc.) underneath each cage. Receivers were affixed inside standard light- and sound-attenuating chambers (Model ENV-022M, Med Associates, St. Albans, VT, USA) to minimize environmental variability. Each chamber was equipped with a house light (to establish a photoperiod) and to an exhaust fan which partially masked ambient laboratory noise. After at least 60 min of baseline data collection, subjects were removed from the chambers, injected with saline, 1.0, 3.2, or 10.0 mg/kg morphine (in ascending order), returned to the home cage, then returned to the chambers for 8 h of data collection. Behavioral sensitization experiments were conducted in the same subjects, using the same equipment and procedures as described above, with the exception that rats were repeatedly injected with 10.0 mg/kg morphine every day for nine consecutive days.
Drugs
Allyl isothiocyanate (Sigma-Aldrich), morphine sulfate salt pentahydrate, and naltrexone hydrochloride (Sigma-Aldrich) were dissolved in sterile 0.9 % physiological saline. Injections were administered in a volume of 1.0 ml/kg via 28 gauge needles (for drug discrimination and locomotor activity studies) or via 3 French (0.036 in.) heparin-coated polyurethane round tip catheters (for self-administration experiments). All injections were administered SC in drug discrimination and locomotor studies, while in self-administration experiments, infusions were delivered IV by a single-speed infusion pump at a flow rate of 0.055 ml/s for a duration of 2 s.
Data analysis
Sessions to criteria and response rates under the terminal FR20 schedule of food-reinforced responding were quantified for all NVP and control rats prior to initiation of intravenous self-administration or drug discrimination experiments. These values were compared across groups using a Mann–Whitney U test since the data were not normally distributed. For self-administration experiments, the numbers of infusions earned at each unit dose of morphine were compared across groups, and to contingent saline within-group, using a one-way repeated measures analysis of variance (ANOVA), and post hoc comparisons were performed using Tukey's HSD test. For drug discrimination studies, ED50 values for NVP and control rats were compared using a t test, and the fold shifts produced by naltrexone pretreatment were compared across groups using a Mann–Whitney U test since the data were not normally distributed. Total locomotor counts were collected in 5-m intervals and binned into 30-m averages. The acute effects of morphine dose were compared across groups, and to the locomotor effects of saline administration within-group, using a one-way repeated measures ANOVA, and post hoc comparisons were performed using Tukey's HSD test. Locomotor sensitization was assessed by comparing the summed 30-m averages for 8 h after drug administration across successive treatments, within and between groups, using a two-way repeated measures ANOVA and the Holm–Sidak multiple comparisons test.
Results
Operant responding
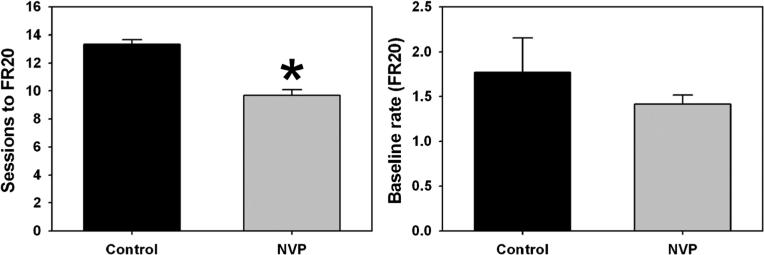
Food-maintained responding was acquired by both control (black bars) and NVP (gray bars) rats, although the number of sessions required to attain the terminal FR20 schedule was significantly fewer for NVP animals (T=57.00, p<0.05) (Fig. 1, left). However, once maintained under the FR20 schedule, response rates engendered by contingent food pellet delivery for control and NVP rats were statistically indistinguishable (Fig. 1, right). The NVP procedure therefore did not appear to impact operant responding in adulthood.
Fig. 1.
Mean (±SEM) sessions to criteria during shaping of food-maintained responding to a terminal FR20 schedule (left) and mean (±SEM) response rates engendered under that schedule (right) for control (black) and NVP (gray) rats subsequently used in morphine self-administration and drug discrimination experiments. Abscissae, groups of rats. Ordinates, sessions required to advance from FR1 to FR20 (left) or rate (responses/second) under the terminal FR20 schedule. Asterisks indicate significant differences from control, as determined by the Mann–Whitney rank sum test for abnormally distributed data (p<0.05)
Drug self-administration
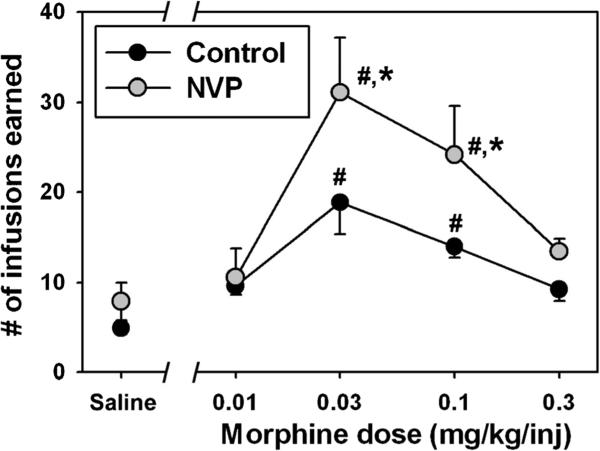
The overall ANOVA for number of infusions earned was significant (F=15.99, df=9, p<0.001). Few infusions of contingent saline were self-administered by either group, and there was no statistical difference in saline-maintained responding (Q=1.36, p=0.99) between NVP (gray circles) and control (black circles) rats (Fig. 2). Self-administration of morphine generated classic biphasic dose–effect curves in each group, where unit doses of 0.03 and 0.1 mg/kg/inj morphine engendered significantly more infusions than contingent saline in NVP (Q=11.19 for 0.03 mg/kg/inj morphine, Q=7.84, for 0.01 mg/kg/inj morphine, p<0.05 for both doses) and control rats (Q=7.37 for 0.03 mg/kg/inj morphine, Q=4.80 for 0.01 mg/kg/inj morphine, p<0.05 for both doses). Importantly, significant differences in morphine reinforcement were apparent between groups, as NVP rats self-administered significantly more infusions of morphine at 0.03 (Q=6.02, p<0.05) and 0.1 (Q=4.97, p=0.05)mg/kg/inj unit doses than controls.
Fig. 2.
Reinforcing effects of morphine in control (black) and NVP (gray) rats trained to respond for food pellets under an FR20 schedule, then maintained on intravenous morphine under continuous reinforcement. Abscissa, injection condition, where Saline represents substitution sessions where intravenous saline was delivered contingently, or where various doses of morphine were available for self-administration. Ordinate, mean (±SEM) number of reinforcers earned. Hash marks indicate significant differences from the appropriate saline control condition, while asterisks indicate significant differences from control at that dose, as determined by one-way repeated measures ANOVA and Tukey's HSD test (p<0.05)
Drug discrimination
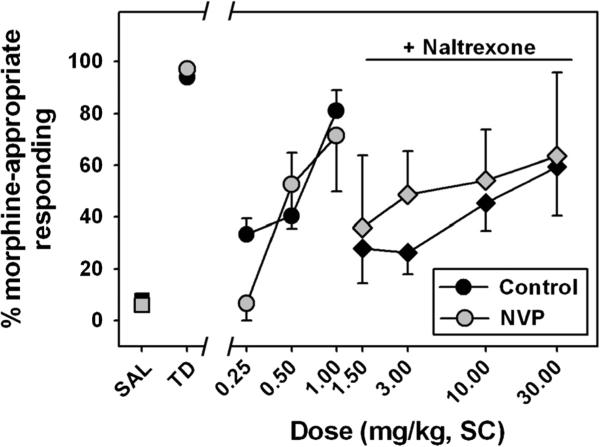
All subjects successfully acquired the morphine discrimination with a similar rate of acquisition across groups (26±5 sessions to criteria for controls, 25±3 sessions to criteria for NVP rats, T=0.80, df=8, p=0.45), and with similar response rates during training sessions. Once trained, discriminative performance was similar when saline (squares) or the morphine training dose (circles) was administered (Fig. 3, “Saline” and “Training dose,” respectively), with almost exclusive responding on the injection-appropriate lever being the norm. The overall Kruskal–Wallis ANOVA for morphine-appropriate responding was significant (H=35.28, df=9, p<0.05). During testing, morphine dose-dependently and fully substituted for its training dose in NVP (gray circles) and control (black circles) rats, and discriminative performance when the training dose was administered was significantly different than observed following saline administration for control (Q=3.28) and NVP (Q=3.18) rats (p<0.05 for both comparisons.) No significant differences in morphine-appropriate responding were observed between groups, at any dose. The average ED50 values for morphine substitution were 0.42±0.09 and 0.74±0.08 mg/kg for the control and NVP rats, respectively (Table 1), and there was no significant difference in these values between groups.
Fig. 3.
Discriminative stimulus effects of morphine in control (black) and NVP (gray) rats trained to discriminate 1.5 mg/kg morphine from saline. Circles represent substitution tests with discrete morphine doses, while diamonds represent assessment of interoceptive effects of morphine following a pre-session injection of 0.1 mg/kg naltrexone. Abscissa, injection condition, where SAL and TD represent training sessions where saline or the morphine training dose, respectively, were administered, or where various doses of morphine were substituted. Ordinate, mean (±SEM) percent of total responses emitted on the morphine-appropriate lever. No between-group statistical comparisons were significant
Table 1.
Mean dose (±SEM) required to engender 50 % responding on the morphine lever (ED50) in drug discrimination studies conducted in the absence (“Morphine ED50 (baseline)”) or presence (“Morphine ED50 (naltrexone)”) of a pre-session injection of 0.1 mg/kg naltrexone, and the mean (±SEM) shifts in the corresponding dose-effect curves as a function of naltrexone treatment
| Group | Morphine ED50 (baseline) | Morphine ED50 (naltrexone) | Fold shift |
|---|---|---|---|
| Control | 0.42±0.09 | 12.35±2.11 | 35.56±8.48 |
| NVP | 0.74±0.08 | 2.13±0.22* | 2.89±0.03* |
p<0.05 (significant differences from control values, as determined by t test (ED50 data) or Mann–Whitney rank sum test for abnormally distributed data (fold shifts))
When the discriminative stimulus effects of morphine were redetermined in the presence of 0.1 mg/kg naltrexone, rightward shifts in dose–effect curves were observed in both groups of rats (Table 1). In the presence of naltrexone, the ED50 for morphine in control rats (Fig. 3, black diamonds) was 12.35±2.11 mg/kg, which represents a greater than 35-fold rightward shift. In contrast, morphine substitution following naltrexone pretreatment in NVP rats (gray diamonds) resulted in an ED50 of 2.13±0.22 mg/kg, a shift of only ~3-fold to the right. The between-group differences in morphine ED50 values in the presence of naltrexone (T=4.26, df=7, p<0.05) and the fold shifts in dose–effect functions elicited by naltrexone pretreatment (T=10.00, df=7, p<0.05) were both significant. While the morphine dose–effect functions generated in the presence of naltrexone were not parallel to the mean dose–effect curves, fold shifts were based on ED50 values, and all animals met or exceeded 50 % morphine-lever selection at some dose.
Locomotor activity and sensitization
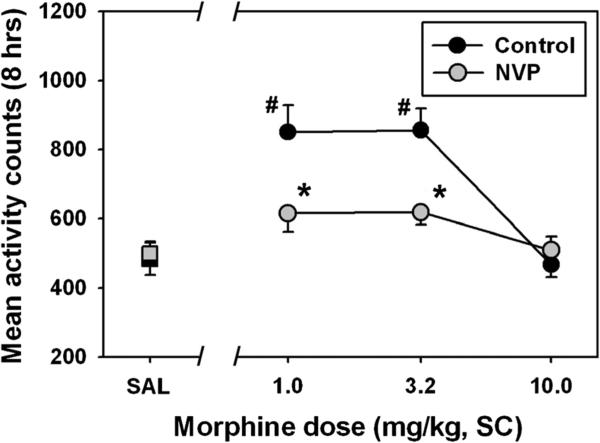
Mean locomotor activity summed over 8 h following saline administration was similar for control and NVP rats (Fig. 4, black and gray squares, respectively.) The overall ANOVA for locomotor activity was significant (F=13.58, df=7, p<0.05). Administration of morphine to control rats (black circles) significantly increased motor activity above that observed following saline administration at doses of 1.0 (Q=8.53, p<0.05) and 3.2 mg/kg (Q=8.62, p<0.05), but non-significantly suppressed locomotor behavior at 10.0 mg/kg. For NVP rats (gray circles), no dose of morphine significantly increased motor activity above that observed following saline administration, and the 10.0 mg/kg dose trended towards suppressing locomotor behavior. Importantly, significant differences in morphine-elicited locomotor activity were apparent between groups, as control rats emitted significantly more motor behavior following morphine doses of 1.0 (Q=5.71, p<0.05) and 3.2 mg/kg (q=5.61, p<0.05), as compared to NVP rats.
Fig. 4.
Acute effects of morphine on locomotor activity (summed over 8 h after injection) in control (black) and NVP (gray) rats, as measured in a home cage setting using radiotelemetry. Abscissa, injection condition, where SAL represents a control session where saline was administered, or where various doses of morphine were injected. Ordinate, mean (±SEM) locomotor counts recorded. Hash marks indicate significant differences from the appropriate saline control condition, while asterisks indicate significant differences from control at that dose, as determined by twoway repeated measures ANOVA and Holm–Sidak's multiple comparisons test (p<0.05)
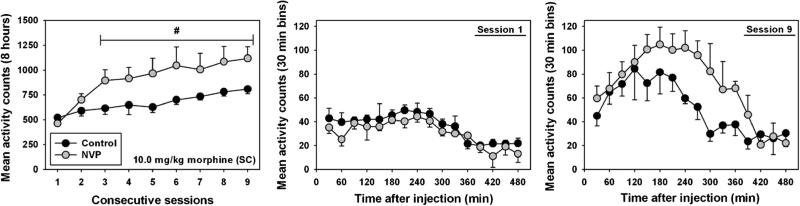
Locomotor activity elicited by daily administration of 10.0 mg/kg morphine (Fig. 5, left panel) followed an increasing trend across the treatment period for control (black circles) and NVP (gray circles) rats, consistent with psychomotor sensitization. No significant effect of repeated dosing was observed in control animals, but NVP rats emitted significantly more motor activity than observed during the first session on sessions 3 (Q=5.71), 4 (Q=5.97), 5 (Q=6.65), 6 (Q=7.71), 7 (Q=7.17), 8 (Q=8.20), and 9 (Q=8.64, p<0.05 for all comparisons). Analysis of locomotor time–activity curves during the first session (Fig. 5, middle panel) shows similar time courses of locomotor behavior upon the initial dose, and no statistical differences were observed between groups (F=0.029, p=0.868). Similarly, no statistical difference in morphine-elicited motor activity was observed during the final session (Fig. 5, right panel, F=0.56, p=0.47), although the NVP animals trended towards more locomotor behavior than controls from 150 to 390 min after injection, suggesting a longer duration of locomotor stimulant action.
Fig. 5.
Locomotor sensitization elicited by daily administration of 10 mg/kg morphine in control (black) and NVP (gray) rats. Left panel, mean (±SEM) locomotor counts summed over 8 h after injection, across nine consecutive days of morphine administration. Hash marks indicate significant differences from relevant control on session 1, as determined by ANOVA and Tukey's HSD test (p<0.05). Middle panel, time–activity curves for 8 h of locomotor activity on the first day of 10 mg/kg morphine administration. Right panel, time–activity curves for 8 h of locomotor activity on the final day of 10 mg/kg morphine administration
Discussion
Opioid abuse places a large burden on the US health care system. In 2005 dollars, this economic burden was more than $9.5 billion (White et al. 2005a, b). Compared to healthy individuals, opioid abusers consume more medical services and more prescription drugs, and are many times more likely to be diagnosed with physiological and psychiatric ailments (Ness et al. 2001). Therefore, possible identification of any early life factors that may predispose an individual to opioid abuse and addiction may pave the way for development of therapeutic interventions which would be of considerable benefit to the public health system. The present studies suggest that early life experiences of visceral pain may be one such predisposing factor.
In the self-administration experiments, the reinforcing effects of intermediate doses of morphine were apparently greater in NVP rats as compared to controls. This difference is not likely due to simple behavioral variables such as motivation to engage in operant tasks because no differences in food-maintained responding were observed between the NVP and control groups under the terminal FR20 schedule, and because self-injection of contingent saline, as well as the lowest and highest unit doses of morphine, was similar for both groups. Indeed, the same unit doses of morphine elicited reinforcing effects in both control and NVP rats, but NVP animals self-administered more injections at those doses than controls. Previous research aimed at understanding the impact of prenatal stress on subsequent changes in drug self-administration also suggest that such changes are dose-dependent, with lower unit doses of cocaine unaffected by adverse early life events, but an increased rate of acquisition and overall drug intake at higher unit doses (Thomas et al. 2009). The present pattern of a “vertical shift” in the morphine dose–effect function observed in NVP animals is distinct from these previous results. Thus, it may be the case that early life experience with visceral pain sensitizes rats to the reinforcing effects of morphine in a manner different from that observed in other models of early life pain and stress.
The relationship between increased drug self-administration and locomotor sensitization is poorly understood, and studies with similar designs and methods report conflicting results of escalation of drug self-administration producing (Ferrario et al. 2005) or not producing (Ahmed and Cador 2006; Knackstedt and Kalivas 2007) behavioral sensitization to locomotor stimulant effects of cocaine. The present results suggest that rats subjected to NVP were less sensitive to locomotor stimulant effects of acute morphine administration across the dose range tested. Interestingly, rats with a history of escalation of heroin self-administration were also less sensitive to heroin-induced locomotor stimulant effects as compared to control subjects (Lenoir and Ahmed 2008). However, NVP rats exhibited locomotor sensitization in response to a chronic dose regimen of morphine, while control rats did not. It might be the case that a longer treatment period, or treatment with a different morphine dose, would have also elicited psychomotor sensitization in the control rats, as a clear trend in that direction was observed in these animals under the present conditions. But based upon these experiments, it appears that NVP renders animals more vulnerable to locomotor sensitization than controls.
Unlike reinforcing and locomotor effects, the discriminative stimulus effects of morphine were not significantly impacted by the NVP procedure. All rats acquired the initial discrimination at a similar rate, and the dose–effect curves for substitution doses of morphine were superimposeable across NVP and control rats. However, the capacity of the opioid antagonist naltrexone to antagonize the discriminative stimulus effects of morphine was at least 10-fold greater in control rats than in rats with early life exposure to visceral pain. It has previously been shown in clinical and animal research that introduction of noxious stimuli during the neonatal period may result in lasting changes in sensory processing across development (Laprairie and Murphy 2009), and it may be the case that the NVP procedure used in the present studies also impacts development of central opioid receptors, with persistent effects into adulthood. However, a simple alteration of receptor number would be expected to influence sensitivity to both agonists and antagonists, but here we only observed changes in responsiveness to naltrexone challenge. Future studies to characterize the behavioral phenotype of opioid antagonist treatment in NVP rats, as well as studies to examine the central effects of NVP on opioid receptor expression and function, are clearly warranted.
To our knowledge, this study is the first evaluation of the predisposing effects of neonatal visceral pain on the abuse-related effects of morphine. Changes in brain neurophysiology, such as increased adult levels of beta-endorphin and met/leuenkephalin in the CNS, have been demonstrated in response to early life inflammatory pain (Laprairie and Murphy 2009). Adult rats with a history of neonatal somatic pain are known to display a decreased sensitivity to nociception and a reduced analgesic response to morphine (Bhutta et al. 2001; Shimada et al. 1990). More specifically related to the present experiments, persistent visceral pain has been shown to affect the developing nervous system by impacting early life pain experiences, and these alterations are evident throughout development and into adulthood (Al-Chaer et al. 2000). The present data suggest that these visceral pain-elicited changes have persistent functional pharmacological consequences relevant to opioid abuse and addiction, and identify early life visceral pain as a possible risk factor for drug abuse later in life. A thorough understanding of the impact of neonatal visceral pain on susceptibility to opioid abuse will help to clarify the clinical consequences of neonatal pain and may identify important variables predicting misuse and abuse of opioids to guide treatment options for patients with a history of neonatal visceral pain.
Contributor Information
Andrew P. Norwood, Interdisciplanary Biomedical Sciences Program, University of Arkansas for Medical Sciences, Little Rock, AR, USA Department of Pharmacology and Toxicology, University of Arkansas for Medical Sciences College of Medicine, 4301 West Markham, Slot 638, Little Rock, AR 72205, USA.
Elie D. Al-Chaer, Center for Pain Research, University of Arkansas for Medical Sciences, Little Rock, AR, USA
William E. Fantegrossi, Department of Pharmacology and Toxicology, University of Arkansas for Medical Sciences College of Medicine, 4301 West Markham, Slot 638, Little Rock, AR 72205, USA
References
- Ahmed SH, Cador M. Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2006;31:563–571. doi: 10.1038/sj.npp.1300834. [DOI] [PubMed] [Google Scholar]
- Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- Anand KJ. Clinical importance of pain and stress in preterm neonates. Biol Neonate. 1998;73:1–9. doi: 10.1159/000013953. [DOI] [PubMed] [Google Scholar]
- Bhutta AT, Rovnaghi C, Simpson PM, Gossett JM, Scalzo FM, Anand KJ. Interactions of inflammatory pain and morphine in infant rats: long-term behavioral effects. Physiol Behav. 2001;73:51–58. doi: 10.1016/s0031-9384(01)00432-2. [DOI] [PubMed] [Google Scholar]
- De Jonckheere J, Rakza T, Logier R, Jeanne M, Jounwaz R, Storme L. Heart rate variability analysis for newborn infants prolonged pain assessment. Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference. 2011;2011:7747–50. doi: 10.1109/IEMBS.2011.6091909. [DOI] [PubMed] [Google Scholar]
- Ewing Corcoran SB, Howell LL. Impact of early life stress on the reinforcing and behavioral-stimulant effects of psychostimulants in rhesus monkeys. Behav Pharmacol. 2010;21:69–76. doi: 10.1097/FBP.0b013e3283359f53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson AR, Patton BC, Bopp AC, Meagher MW, Grau JW. Brief exposure to a mild stressor enhances morphine-conditioned place preference in male rats. Psychopharmacology. 2004;175:47–52. doi: 10.1007/s00213-004-1780-3. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry. 2005;58:751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Goeders NE. Stress and cocaine addiction. J Pharmacol Exp Ther. 2002;301:785–789. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF. Non-contingent electric footshock facilitates the acquisition of intravenous cocaine self-administration in rats. Psychopharmacology. 1994;114:63–70. doi: 10.1007/BF02245445. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF. Effects of surgical and pharmacological adrenalectomy on the initiation and maintenance of intravenous cocaine self-administration in rats. Brain Res. 1996;722:145–152. doi: 10.1016/0006-8993(96)00206-5. [DOI] [PubMed] [Google Scholar]
- Grunau RE, Holsti L, Haley DW, Oberlander T, Weinberg J, Solimano A, Whitfield MF, Fitzgerald C, Yu W. Neonatal procedural pain exposure predicts lower cortisol and behavioral reactivity in preterm infants in the NICU. Pain. 2005;113:293–300. doi: 10.1016/j.pain.2004.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS, Wilkinson LS, Humby T, Robbins TW. Maternal deprivation of neonatal rats produces enduring changes in dopamine function. Synapse. 1999;32:37–43. doi: 10.1002/(SICI)1098-2396(199904)32:1<37::AID-SYN5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Huang S, Trapido E, Fleming L, Arheart K, Crandall L, French M, Malcolm S, Prado G. The long-term effects of childhood maltreatment experiences on subsequent illicit drug use and drug-related problems in young adulthood. Addict Behav. 2011;36:95–102. doi: 10.1016/j.addbeh.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe P, Shoemaker WJ, Triano L, Hoffman J, Arons C. Repeated isolation in the neonatal rat produces alterations in behavior and ventral striatal dopamine release in the juvenile after amphetamine challenge. Behav Neurosci. 1996;110:1435–1444. doi: 10.1037//0735-7044.110.6.1435. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. Extended access to cocaine self-administration enhances drug-primed reinstatement but not behavioral sensitization. J Pharmacol Exp Ther. 2007;322:1103–1109. doi: 10.1124/jpet.107.122861. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Kehoe P. Enhanced acquisition of cocaine self-administration in adult rats with neonatal isolation stress experience. Brain Res. 2000;875:44–50. doi: 10.1016/s0006-8993(00)02595-6. [DOI] [PubMed] [Google Scholar]
- Laprairie JL, Murphy AZ. Neonatal injury alters adult pain sensitivity by increasing opioid tone in the periaqueductal gray. Front Behav Neurosci. 2009;3:31. doi: 10.3389/neuro.08.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Ahmed SH. Supply of a nondrug substitute reduces escalated heroin consumption. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2008;33:2272–2282. doi: 10.1038/sj.npp.1301602. [DOI] [PubMed] [Google Scholar]
- Li T, Du J, Yu S, Jiang H, Fu Y, Wang D, Sun H, Chen H, Zhao M. Pathways to age of onset of heroin use: a structural model approach exploring the relationship of the COMT gene, impulsivity and childhood trauma. PLoS One. 2012;7:e48735. doi: 10.1371/journal.pone.0048735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidow MS. Long-term effects of neonatal pain on nociceptive systems. Pain. 2002;99:377–383. doi: 10.1016/S0304-3959(02)00258-0. [DOI] [PubMed] [Google Scholar]
- Lyness WH, Smith FL, Heavner JE, Iacono CU, Garvin RD. Morphine self-administration in the rat during adjuvant-induced arthritis. Life Sci. 1989;45:2217–2224. doi: 10.1016/0024-3205(89)90062-3. [DOI] [PubMed] [Google Scholar]
- Martin CM, Saleeby LG. All pain is not the same: an overview of neuropathic pain in the elderly. Consult Pharm J Am Soc Consult Pharm. 2007;22:283–294. doi: 10.4140/tcp.n.2007.283. [DOI] [PubMed] [Google Scholar]
- Michaels CC, Holtzman SG. Early postnatal stress alters place conditioning to both mu- and kappa-opioid agonists. J Pharmacol Exp Ther. 2008;325:313–318. doi: 10.1124/jpet.107.129908. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Lewis-Sides A, Castroman P. Characterization of pressor and visceromotor reflex responses to bladder distention in rats: sources of variability and effect of analgesics. J Urol. 2001;165:968–974. [PubMed] [Google Scholar]
- Qiu J. Infant pain: does it hurt? Nature. 2006;444:143–145. doi: 10.1038/444143a. [DOI] [PubMed] [Google Scholar]
- Ren Y, Zou X, Fang L, Lin Q. Sympathetic modulation of activity in Adelta- and C-primary nociceptive afferents after intradermal injection of capsaicin in rats. J Neurophysiol. 2005;93:365–377. doi: 10.1152/jn.00804.2004. [DOI] [PubMed] [Google Scholar]
- Shimada C, Kurumiya S, Noguchi Y, Umemoto M. The effect of neonatal exposure to chronic footshock on pain-responsiveness and sensitivity to morphine after maturation in the rat. Behav Brain Res. 1990;36:105–111. doi: 10.1016/0166-4328(90)90165-b. [DOI] [PubMed] [Google Scholar]
- Simons SH, van Dijk M, Anand KS, Roofthooft D, van Lingen RA, Tibboel D. Do we still hurt newborn babies? A prospective study of procedural pain and analgesia in neonates. Arch Pediatr Adolesc Med. 2003;157:1058–1064. doi: 10.1001/archpedi.157.11.1058. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Thomas MB, Hu M, Lee TM, Bhatnagar S, Becker JB. Sex-specific susceptibility to cocaine in rats with a history of prenatal stress. Physiol Behav. 2009;97:270–277. doi: 10.1016/j.physbeh.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Walker JR, Chen SA, Moffitt H, Inturrisi CE, Koob GF. Chronic opioid exposure produces increased heroin self-administration in rats. Pharmacol Biochem Behav. 2003;75:349–354. doi: 10.1016/s0091-3057(03)00094-7. [DOI] [PubMed] [Google Scholar]
- Wang JY, Zhang HT, Han JS, Chang JY, Woodward DJ, Luo F. Differential modulation of nociceptive neural responses in medial and lateral pain pathways by peripheral electrical stimulation: a multichannel recording study. Brain Res. 2004;1014:197–208. doi: 10.1016/j.brainres.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Wang J, Gu C, Al-Chaer ED. Altered behavior and digestive outcomes in adult male rats primed with minimal colon pain as neonates. Behav Brain Funct: BBF. 2008;4:28. doi: 10.1186/1744-9081-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AG, Birnbaum HG, Mareva MN, Daher M, Vallow S, Schein J, Katz N. Direct costs of opioid abuse in an insured population in the United States. J Manag Care Pharm: JMCP. 2005a;11:469–479. doi: 10.18553/jmcp.2005.11.6.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AG, Birnbaum HG, Mareva MN, Henckler AE, Grossman P, Mallett DA. Economic burden of illness for employees with painful conditions. J Occup Environ Med Am Coll Occup Environ Med. 2005b;47:884–892. doi: 10.1097/01.jom.0000172867.29041.eb. [DOI] [PubMed] [Google Scholar]
- Woller SA, Moreno GL, Hart N, Wellman PJ, Grau JW, Hook MA. Analgesia or addiction?: implications for morphine use after spinal cord injury. J Neurotrauma. 2012;29:1650–1662. doi: 10.1089/neu.2011.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]