Abstract
The composition of leukocytes in the liver is highly distinct from that of the blood and lymphoid organs. In particular, the liver is highly enriched in non-conventional T cells such as natural killer T (NKT) cells, γδ T cells and mucosal-associated invariant T cells. In addition, there are significant populations of tissue-resident NK cells (or innate lymphoid cells (ILC1)) and memory CD8+ T cells. These cells are joined in conditions of inflammation by neutrophils, monocytes and macrophages. In recent years a multitude of studies have generated insights into how these cells arrest, move and remain resident in the liver. This new understanding has largely been due to the use of intra-vital microscopy to track immune cells in the liver, coupled with gene expression profiling and parabiosis techniques. These studies have revealed that leukocyte recruitment in the liver does not correspond to the classical paradigm of the leukocyte adhesion cascade. Rather, both lymphoid and myeloid cells have been found to adhere in the liver sinusoids in a platelet-dependent manner. Leukocytes have also been observed to patrol the hepatic sinusoids using a characteristic crawling motility. Moreover, T cells have been observed surveying hepatocytes for antigen through the unique fenestrated endothelium of the liver sinusoids, potentially negating the need for extravasation. In this review we highlight some of these recent discoveries and examine the different molecular interactions required for the recruitment, retention and—in some cases—residence of diverse leukocyte populations within the liver.
Immunosurveillance by the liver
The liver has a unique role in defense against blood borne pathogens. It is the largest internal organ, and every minute ~30% of the total blood volume of the body passes through it.1 Blood enters the liver via the hepatic artery (~20%) and the portal vein (~80%), which enables screening for systemic and gut-based pathogens.2 Once blood enters the liver it circulates through a complex vascular network comprised of capillary-like vessels, called sinusoids. Within the sinusoids blood flow is reduced, flowing at a rate of ~100–400 μm s−1;3 this, coupled with the sinusoids' unique endothelial structure, maximizes the opportunity for pathogen detection by immune cells within the liver.
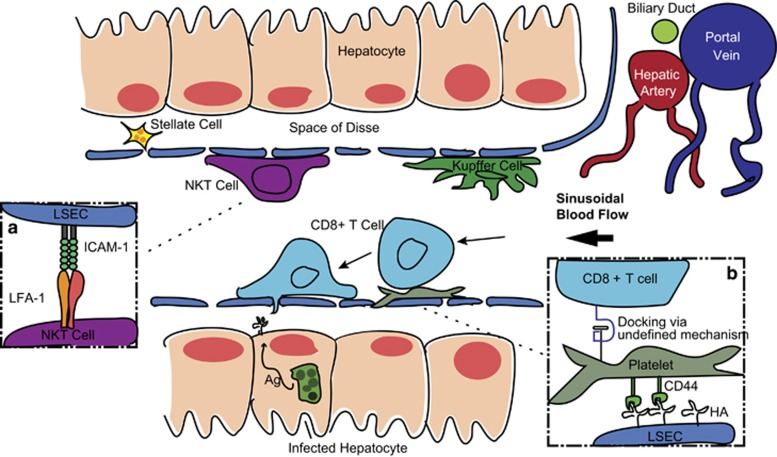
The structure of the liver contains several cell types, almost all of which have immune functions that have been reviewed in more detail previously.2 The dominant parenchymal cells of the liver are the hepatocytes, whose main functions involve protein synthesis, neutralization of toxic compounds and nutrient metabolism.2 Each hepatocyte is separated from the blood flow solely by a unique fenestrated endothelium that contains sieve-like open pores, which allow the ready exchange of large macromolecules and even direct contact between hepatocytes and cells within the sinusoids.4, 5 This fenestrated endothelium is formed by specialized liver sinusoidal endothelial cells (LSECs) that can also have a variety of immune functions and have the capacity to act as antigen-presenting cells.6 LSECs constitutively express adhesion molecules including intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 and vascular adhesion protein-1 (VAP-1) at levels usually found in inflamed tissue.7 In addition to LSECs, the liver harbors a large population of tissue-resident macrophages called Kupffer cells that adhere to LSECs and remain stationary in the vasculature. This localization allows Kupffer cells to capture bacteria as they flow through the blood, unlike other macrophages that do not take up pathogens under flowing conditions.2 One study has found that depletion of Kupffer cells resulted in 100% mortality following infection with a normally sub-lethal dose of Listeria monocytogenes,8 demonstrating the critical role they can have in controlling bacteraemia. Finally, hepatic stellate cells are cells that reside between LSECs and hepatocytes in an extracellular region called the space of Disse. These cells are primarily involved in lipid storage, though there is evidence that they may have a role in presenting antigen to lymphocytes within the liver, particularly natural killer T (NKT) cells and CD8+ T cells.9
In addition to these cells that comprise the liver structure, the liver also harbors large numbers of migratory leukocytes. Even in baseline conditions, the livers of both humans and mice are highly enriched in NKT cells, γδ T cells and CD8+ T cells, particularly memory CD8+ T cells compared with the peripheral blood.10, 11, 12 The human liver in particular, is also highly enriched in mucosal-associated invariant T cells whose role is reviewed elsewhere in this issue.13 In inflammatory conditions, these cells are joined by neutrophils, CD4+ T cells and monocytes. Interestingly, however, the recruitment of cells to the liver does not follow the classical leukocyte adhesion cascade seen in other endothelial tissues.4, 14, 15, 16 The leukocyte adhesion cascade is a stepwise process in which cells are initially captured from circulation by selectin-mediated interactions, after which their speed is decreased further as they roll along the endothelial surface via tethering to selectins, and once arrested they begin the process of extravasation into the parenchyma via integrin-mediated interactions.17 As selectins have been determined to be dispensable for trapping immune cells within the liver,15, 16 it appears that a combination of low blood flow, and in many cases platelet-mediated interactions, seems sufficient for leukocyte arrest.4, 18 Once recruited even ‘resident' cells such as NKT cells and CD8+ tissue-resident memory T cells (TRM) often remain in the hepatic sinusoids rather than extravasating into the parenchyma.19, 20 These cells may nonetheless be able to efficiently survey hepatocytes for cognate antigen across the sinusoidal endothelium and thus may not need to extravasate.4, 5 In this review we will examine the behaviors of these various motile leukocyte subsets within the liver, in particular the molecular interactions that facilitate their recruitment to the liver and their motility and residence once inside this organ. As words such as ‘motility' and ‘residence' are typically used quite loosely in the literature, we define what we mean by such terms and others in Table 1.
Table 1. Definitions of terms used for leukocyte migration.
| Term | Definition |
|---|---|
| Motility | The movement behavior of cells within the liver parenchyma/sinusoids. |
| Homing | The organ-specific recruitment of leukocytes, often mediated by specific expression of homing receptors on leukocytes (that is, adhesion molecules) and proteins on the vascular endothelium. However, as most cells arrive in the liver sinusoids passively via the circulation, they cannot strictly be said to ‘home' to the liver. A notable exception to this is the homing of macrophages to sites of inflammation in the liver from the peritoneal cavity. |
| Recruitment | The process by which leukocytes are retained in the liver following the upregulation of adhesion molecules by hepatic stromal cells or leukocytes already present in the liver. |
| Retention | Any process by which cells cease to circulate freely and thus remain in liver either temporarily or permanently (see residence). Retained cells may be arrested in the sinusoids, crawling in the sinusoids or may extravasate and migrate to sites of inflammation or infection. |
| Residency | Residency of cells in a particular organ is defined by the inability of cells to recirculate (as often demonstrated with parabiosis experiments). |
| Crawling | Manner of leukocyte motility characterized by movement along endothelial cells independent of blood flow, elongation of the leukocyte and pseudopod protrusions. |
| Patrolling | Crawling behavior that is not directed, rather it is characterized by a type of random walk as the cells search for antigen. |
| Arrest | Process of causing leukocytes to arrest on the endothelial wells via specific molecular interactions to be retained in the sinusoids. |
Residence and behavior of lymphocyte populations in the liver
NKT Cells
One of the most notable components of the intrahepatic lymphocyte population is the significant enrichment of non-conventional T cells including NKT cells and γδ T cells.10, 11, 12 Though relatively little work has been done on the motility and residence of γδ T cells, NKT cells have been extensively investigated, particularly in murine models.20, 21, 22, 23 NKT cells are lymphocytes that express a restricted set of αβ T-cell receptors as well as NK cell markers. Notably they recognize antigens presented on CD1d, which enables them to directly recognize foreign lipids and glycoproteins that are not presented via MHC Class I. There are two main classes of NKT cells; Type 1 NKT cells, which express an invariant TCR α chain (Vα14- Jα18 in mice, Vα24-Jα18 in humans),24 and Type 2 NKT cells that possess a more varied TCR repertoire. Type 1 NKT cells are the dominant subtype within mice, while humans have a markedly increased proportion of Type 2 NKT cells.25, 26 Despite comprising fewer than <1% of lymphocytes in peripheral blood and various other organs, NKT cells comprise up to 30% of intrahepatic lymphocytes in mice.27 In humans the frequency of NKT cells within the liver is lower, comprising ~1% of intrahepatic lymphocytes; however, this is still one of the highest organ-specific frequencies of NKT cells, which only account for 0.01–0.1% of human peripheral blood leukocytes.28 Importantly, the recruitment of NKT cells to the liver is not a transient effect due to inflammation or exposure to microbial products (for example, lipopolysaccharide (LPS)), as they are retained even within the livers of germ-free mice in the absence of pathogen exposure.29
Direct evidence of liver residency can be established using parabiosis experiments, in which congenic mice are surgically conjoined in order to share the same circulatory system. A seminal NKT parabiosis study revealed that although most lymphocyte populations recirculated within the blood and are exchanged between the parabionts, liver NKT cell populations remain within the liver of their mouse of origins, suggesting that these are long-term residents of the liver.23 This parabiosis data are not inconsistent with an older hypothesis that NKT cells (and other lymphocyte populations enriched in the liver) accumulate there because they are retained due to interactions between specific adhesion molecules.10, 30 In this model the entry rate of liver-tropic lymphocytes far exceeds their exit rate, leading to their accumulation. These cells are then lost from the liver mainly as a result of cell death, rather than recirculation to other tissues.10, 30
NKT cells have recently been found to express many of the same genes found in populations of TRM CD8+ T cells, further supporting the hypothesis that these are a bona fide-resident population.31 These genes have been suggested to constitute a ‘core signature' of tissue residency—a universal transcriptional program responsible for the retention of tissue-resident lymphocyte populations—which is under the control of two related transcription factors, Hobit and Blimp1.31 Despite this, the mechanism of residence in the liver for NKT cells appears to differ from the mechanism of retention of many tissue-resident CD8+ T-cell populations. Although many TRM require the integrin CD103 for maintaining residence,32, 33 NKT cell residency in the liver appears to be dependent upon interactions between lymphocyte functional antigen-1 (LFA-1) and ICAM-1.23 In parabiotic pairs of mice there is very limited exchange of NKT liver cells between naive mice, but simultaneous treatment with blocking antibodies anti-ICAM-1 and anti-LFA-1 disengaged these cells from the livers and allowed them to freely move into the partner parabiont.23 This data complements older data suggesting that high levels of LFA-1 are required for the homing of thymus-derived NKT cells to the liver.21 Furthermore, NKTs characteristically express very high levels of LFA-1, which appears to be under the control of the transcription factor promyelocytic leukemia zinc finger protein.23 Collectively, these studies provide evidence to support the classification of NKT cells as true liver-resident population.
In addition to their residency, another notable feature of liver NKT cells is their characteristic crawling behavior within the hepatic sinusoids as distinct from how cells move in the classical leukocyte adhesion cascade.20 In a seminal imaging study, the behavior of NKT cells was followed in mice in which the CXCR6 gene had been replaced by GFP.20 In the livers of heterozygous Cxcr6+/gfp mice almost 100% of NKT cells expressed GFP, and 75% of GFP+ cells were NKT cells. In the absence of antigen these crawling GFP+ cells appeared to ‘patrol' the sinusoids, as they moved independently of blood flow, frequently changing their direction of movement. However, upon injection of the NKT antigen α-galactosylceramide, the cells ceased patrolling and arrested in the sinusoids. This α-galactosylceramide treatment confirmed the cells being tracked were NKT cells, and not the small percentage of other GFP+ cells. Analysis of Cxcr6gfp/gfp mice (which lack CXCR6 altogether) showed that CXCR6 itself is involved in the recruitment and survival of NKT cells in the liver, but does not influence their sinusoidal migration.20 The mechanism of crawling used by these cells has not been investigated; however, it may be dependent upon LFA-1: ICAM-1 interactions as in vitro studies have shown how lymphocytes can crawl on ICAM-1-coated surfaces by cytoskeletal rearrangement entirely induced by LFA-1: ICAM-1 binding.34, 35
NK Cells
In addition to the population of tissue-resident NKT cells, the liver also harbors a substantial population of NK cells in both mice and humans.30 Of these cells a population of tissue-resident NK cells (trNK), which are distinct from conventional NK cells (cNK) found in the blood and spleen, have been defined within the livers of mice.31, 36 An equivalent population has also been identified in humans, defined as CD56hiCD16− cells.37, 38 These liver trNK cells (a type of group 1 innate lymphoid cell, (ILC)) can be distinguished from cNK by their expression of the molecule CD49a, which associates with CD29 to form the β1 integrin very late antigen.36 Mouse liver trNK have also been found to share the same core gene transcriptional signature as NKT cells and many CD8+ TRM.31 As with NKT cells, murine parabiosis experiments have shown that liver trNK cells do not recirculate between parabionts, whereas their cNKs counterparts readily equilibrate between the congenic counterparts.36
The molecular mechanism for trNK cell liver-specific residency is undefined. However, given that like NKT cells, trNK cells—but not cNK cells—express the transcription factor promyelocytic leukemia zinc-finger protein, LFA-1-mediated retention could also have a role in their residency in the same manner as for NKT cells.39, 40 In humans the CD56hiCD16−, but not CD56lo cNK, liver populations express high levels of CXCR6 and CCR5, and it has been suggested that these molecules may help hold these cells in the sinusoidal niche perhaps by providing key survival signals.37, 41 Interestingly, although trNK are partly defined by their expression of the integrin very late antigen-1 upon their surface, the role of this molecule in residence and migration has not been investigated. Very late antigen-1 on lymphocytes has previously been implicated in binding collagen and the extracellular matrix;42, 43 as such future intra-vital imaging studies may be required to examine the motility of this subset of cells.
CD8+ T cells
Like NKT cells, CD8+ T cells are highly enriched within the liver compared with the blood or lymphoid organs of both humans and mice.10, 11 The accumulation of CD8+ T cells within the liver was traditionally thought to be due to the liver functioning as a T-cell ‘graveyard', for the final fate of a cell following an immune response.44 However, from recent studies it is becoming clear that viable CD8+ T cells not only migrate through the liver, but form long-lasting resident populations, especially against hepatic pathogens.19, 31, 45 Utilizing the lymphocytic choriomeningitis virus infection model and transgenic lymphocytic choriomeningitis virus-specific P14 CD8+ T cells, Steinert et al.45 studied the CD8+ T-cell memory response in various non-lymphoid organs including the liver. Prior to killing, 4–5 months post-infection mice were injected i.v. with an α-CD8α antibody in order to differentiate memory CD8+ T cells located within the vasculature and the parenchyma of different non-lymphoid organs.45 TRM are conventionally considered to be located within the non-lymphoid organs parenchyma, while effector memory cells (TEM) circulate throughout the blood. Other factors used to differentiate the memory subsets were markers such as CD69+ and CD103+ (though this study found them to be imperfect indicators of residency), and the anatomic location of cells via quantitative immunofluorescence microscopy. Strikingly, it was observed using parabiosis that within the liver more than 35% of vascular, α-CD8α-labeled cells, were TRM. Collectively these findings demonstrated that there are resident memory CD8+ T cells in the liver, but that these cells did not have to extravasate from the circulation to remain in this organ.45
Complementing the lymphocytic choriomeningitis virus studies, the residency of CD8+ T cells in the liver has also been established and explored in a murine model following Plasmodium (malaria) infection. Utilizing PbT-I transgenic T cells, which recognize an antigen expressed by the Plasmodium parasite at multiple life stages, a population of liver TRM was found after immunization of mice with attenuated parasites. PbT-I liver TRM were defined as CD69+KLRG1lo cells to distinguish them from circulating PbT-1 TEM that were CD69−KLRG1hi.19 Crucially, parabiosis studies confirmed that the CD69+KLRG1− population of cells was a bona fide TRM populations. These TRM were also found to be retained in the liver upon adoptive transfer to naive hosts.19 Finally, microarray analysis confirmed that the CD69+KLRG1lo TRM shared the core gene signature of many other well-established TRM populations in other organs.19, 31, 46 Although there has been interest in the commonalities between different tissue-resident populations (the so-called 'core signature' of residency), it will be of interest to examine the different characteristics of resident populations within the liver (including NKT and trNK cells) compared with resident populations found in other organs.
In addition to residency, the motility of both effector and memory CD8+ T cells within the liver has been well studied in recent intra-vital imaging studies.4, 19, 47 In vitro-activated effector CD8+ T cells were recently shown to move throughout the liver by patroling the luminal walls of the sinusoids in a manner akin to NKT cells and distinct again from the classical leukocyte adhesion cascade.4 This sinusoidal migration occurred at speeds of ~10 μm min−1 and was completely independent of blood flow, though upon recognition of cognate antigen presented by hepatocytes, the CD8+ T cells ceased crawling and became arrested.4 In agreement with previous work5 the CD8+ T cells appeared to be capable of recognizing antigen presented by the hepatocyte itself by putting protrusions through the fenestrated endothelium of the liver.4 The behavior of effector CD8+ T cells in the presence of a hepatic pathogen has been imaged in real-time in the case of Plasmodium infection.47 In this case effector CD8+ T cells were similarly found to be arrested around infected hepatocytes; interestingly, once a CD8+ T cell had located an infected hepatocyte it appears to recruit other effector CD8+ T cells within the liver to the local region to form T-cell ‘clusters' around the infected cell.47 This process is directed by a positive-feedback loop, with each CD8+ T cell entering the cluster recruiting more T cells.47 This feedback loop is likely to involve specific chemokines, as it could be inhibited by blockade of G-protein coupled receptor signaling using pertussis toxin.47
In addition to the behavior of effector CD8+ T cells, the motility of CD8+ TEM and CD8+ TRM cells in the liver was compared following Plasmodium sporozoite immunization.19 In these experiments PbT-I mice were crossed either to Cx3cr1gfp/+ mice to label TEM populations or Cxcr6gfp/+ mice to label TRM populations; these GFP+-expressing mice were selected, as the respective markers had previously been established to be differentially expressed on CD8+ TEM and CD8+ TRM within the liver.19 Interestingly, CD8+ TEM cells were rounded up and transiently trapped in the liver sinusoids, or were flowing through the sinusoids with the blood flow. On the other hand, the TRM cells appeared elongated and crawled similarly to NKT cells.19, 20 While we are learning increasing amounts about CD8+ T-cell behavior in the liver, the exact mechanism for how CD8+ T cells migrate throughout the sinusoids independently from the blood flow still remains to be defined.
The role of chemokines in the recruitment of CD8+ T cells to the liver has also been studied. In particular, CXCR6 has been suggested to be important for the homing of CD8+ T cells to the liver, as CXCR6 is expressed on many CD8+ T cells in the liver, in particular TRM. Moreover the chemokine CXCL16 is expressed abundantly on LSECs.20 In humans, lymphocytes infiltrating the liver in Hepatitis C infection were found to express high levels of CXCR6, along with CXCR3 and CCR5.48 In agreement with this hypothesis CXCR6-deficient lymphocytes were found not to be retained in the liver in a model of graft versus host disease.49 However a subsequent study using the Plasmodium infection found that the defect in CXCR6-deficient CD8+ T cells was not in their ability to home to the liver, rather CXCR6 was found to be a crucial factor for maintaining memory CD8+ T cells within the liver.50 This is perhaps not surprisingly as in most cases leukocytes do not need to specifically 'home' to the liver, as circulating cells will pass through the liver multiple times per hour. Rather the key interactions may be those that facilitate the retention, survival and residence of CD8+ T cells.
The mechanism for the recruitment and retention of CD8+ T cells within the sinusoids is still unclear. Early studies suggested that activated, but not naive, lymphocytes might be retained in the liver as a result of the exposure of asioglycoconjugates on the surface of these cells, resulting in interactions with the Ashwell–Morel lectin, which is highly expressed on hepatocytes.51 More recent studies have shown that CD8+ T-cell binding to LSECs appears to be independent of the selectin-mediated rolling that is essential for the initial slowing down of T cells from the blood flow in the classical leukocyte adhesion cascade (Figure 1b).4, 52 Rather, it has been found that CD8+ T cells are initially arrested in the liver by binding (via an unknown mechanism) to platelets, which use CD44 to adhere to hyaluronic acid (HA) on LSECs (Figure 1).4 Activated CD8+ T cells appear to be retained within the liver via interactions with ICAM-1 when there is local antigen presentation, while vascular cell adhesion molecule-1 has been implicated in retaining CD8+ T cells in the absence of antigen presentation.7 However, surprisingly other studies have found blockade of β2 integrins, which bind to ICAM-1, does not appear to affect effector CD8+ T cell recruitment to the liver;4 it has also been observed that LFA-1-deficient mice have normal numbers of liver CD8+ T cells.22 Thus, the role of β2 integrins in the retention and residence of CD8+ T cells in the liver is unclear.
Figure 1.
Lymphocytes within the sinusoidal microenvironment. The sinusoids are lined by specialized liver sinusoidal endothelial cells (LSEC), which are fenestrated and allow interactions to occur between lymphocytes in the sinusoidal blood and hepatocytes. The largest subsets of lymphocytes within the liver are NKT cells and CD8+ T cells, which both migrate along the luminal surface of LSECs independent of the blood flow. NKT cells are retained within the liver via LFA-1:ICAM-1 interactions (a), whereas CD8+ T cells are initially captured from circulation via platelets (b; HA, hyaluronic acid) and interact with a variety of adhesion molecules on LSECs. Other liver resident cells include Kupffer macrophages, which are primarily situated on LSECs in the sinusoids, and hepatic stellate cells that reside within the space of Disse.
CD4+ T cells
CD4+ T cells are present in the liver at markedly reduced numbers compared with their CD8+ counterparts,10, 30 nevertheless, in inflammatory conditions the discrepancy between T-cell subsets diminishes in the liver, as the proportion of CD4+ T cells increases.53 This difference in retention of CD4+ T cells and CD8+ T cells suggested that different molecular interactions might be important for the recruitment of these different T-cell types. In agreement with this many studies have highlighted a role for VAP-1 in the retention of CD4+, but not CD8+ T in the liver.4, 52, 54, 55, 56 VAP-1 is already constitutively expressed in the liver sinusoids at elevated levels, but its expression is increased in various inflammatory liver conditions in humans and in mouse models of inflammation.54, 56 In these inflammatory conditions CD4+ T cells that enter the liver are retained in the sinusoids and post-sinusoidal venules. Intriguingly, the molecular mechanism for adhesion differs between the Th1 and Th2 subtypes of CD4+ T cells. It was determined that Th1 cell retention is mediated by the α4β1 integrin, whereas Th2 cells adhere via interactions with VAP-1.54 Notably, all CD4+ T cells were retained independently of interactions with P-selectin, which has been found to be critical in the trafficking of Th1 cells to inflamed skin, and is expressed highly in the inflamed liver.54
Given the roles of VAP-1 in mediating CD4+ T-cell recruitment to the liver, and roles of these cells in liver inflammation there is intense interest in developing inhibitors of this molecule.57 However, this is complicated by the fact that the mechanism of action of VAP-1 is unclear.56 In addition to being present on the surface of hepatic stromal cells, VAP-1 can exist in a soluble form, which is present in elevated amounts in various inflammatory liver conditions.58, 59 VAP-1 can also act as an enzyme with monoamine oxidase activity, which may allow soluble VAP-1 to facilitate leukocyte migration in the liver through the catalytic generation of reactive oxygen species. To test this mice were generated with an enzymatically inactive form of VAP-1; in these mice CD4+ T-cell infiltration in the liver was significantly reduced in a mouse model of non-alcoholic steatohepatitis, demonstrating that targeting the enzymatic activity of VAP-1 may be useful therapeutically.56 However, antibody blockade of VAP-1 away from the catalytic site also reduced leukocyte infiltration (including CD4+ T-cell infiltration) in various mouse models of liver inflammation.56 Collectively these data suggest that VAP-1 may facilitate leukocyte recruitment and retention by multiple mechanisms.
Migration and recruitment of myeloid cells in the liver
In addition to the range of resident lymphocyte populations that patrol the liver, small numbers of myeloid cells are present in the hepatic circulation in the steady state.60 However, during infection or sterile inflammation these numbers can increase substantially. In many cases there are similarities between the motility of lymphocyte and myeloid cell populations. Both neutrophils and monocytes can crawl within the hepatic sinusoids in a process mediated by interactions with platelets and β2 integrins.61, 62 However, neutrophils can display a range of other migratory behaviors in the liver, utilizing a variety of different molecular interactions. Monocytes also use a variety of different molecules for crawling, chemotaxis and arrest in the liver. Perhaps most surprisingly, it has recently been shown that incoming myeloid populations do not have to come from the blood stream but can home to the liver from the peritoneal cavity (Figure 2a).63
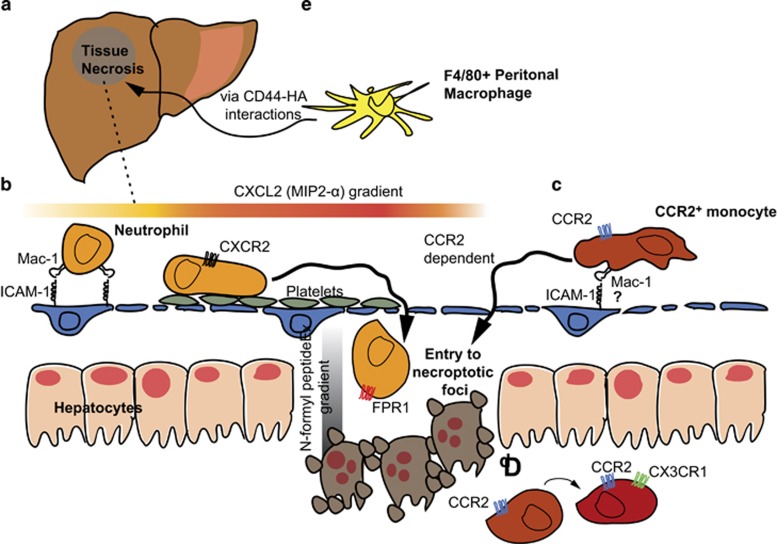
Figure 2.
Migration of myeloid populations in the liver following sterile injury. (a) In a well-established model of sterile injury a small, defined inflammatory focus can be induced by thermal injury. (b) Platelets are then recruited that pave the fenestrated endothelium to allow the migration of neutrophils; these neutrophils that initially follow a CXCL2 gradient to the edge of the site of injury and are subsequently recruited into the site of injury following a N-formyl peptide gradient. (c) CCR2+ monocytes also display patrolling behavior in the sinusoids and require CCR2 to enter the site of necrosis. (d) Having entered the site of injury CCR2+ monocytes are observed to upregulate CX3CR1 potentially to facilitate tissue repair. (e) F4/80 macrophages from the peritoneal cavity can also migrate in a CD44-dependent manner into the liver.
Recruitment and retention of neutrophils
Although neutrophils have distinct roles in clearing both viruses and bacteria from the circulation,64, 65, 66 they can also be mediators of organ failure and immunopathology.67, 68 They have also been implicated in tissue damage following sterile insults such as drug-induced toxicity and ischemic injuries.69, 70, 71 By studying the motility of neutrophils in the different conditions of infection and inflammation, it has become clear that they can interact with LSECs and other immune cells, especially platelets, in diverse ways.18, 66, 72 Depending on the exact conditions of inflammation, different molecules are up and downregulated on the surface of the endothelium and the neutrophils themselves, resulting in different patterns of motility. In conditions of sterile inflammation neutrophils crawl toward inflammatory stimuli, in some cases leading to extravasation, which can cause tissue damage and pathology.62, 73 On the other hand during infection neutrophils can arrest within the sinusoids where they remain sessile and participate in the trapping of microbes.15, 66, 72
To study the behavior of neutrophils during sterile injury in the liver, a model was developed in which a necrotic focus is created by thermal injury to the organ surface.62 This results in a core of necrotic cells, surrounded by a ring of largely intact tissue that nonetheless has low blood flow (Figure 2b).62 In this model, the number of neutrophils increased by at least 10-fold from baseline, 4 h after injury.62 The neutrophils crawled through the sinusoids in a highly directed manner toward the site of injury.62 This crawling was dependent upon the β2 integrin Mac-1, as blockade of this integrin—but not LFA-1—stopped the motility of the cells, reducing their velocity from around 6 μm per minute to 2 μm per minute. The guidance of neutrophils toward injury appears to be an exquisitely tightly controlled process. Mac-1-mediated neutrophil crawling was found to be aided by IL-1β-mediated upregulation of ICAM-1.62 The initial directionality of neutrophils toward the site of inflammation was driven by CXCR2 expression allowing the cells to follow a MIP-2 gradient to the edge of the low blood flow area. At this point, formylated peptides direct neutrophil migration toward the necrotic focus via the formyl peptide receptor.62 These signals overrule the CXCR2 signals that would otherwise hold the neutrophils just outside the area of injury.74 Accordingly, blockade or knockout of this receptor results in the cells being unable to enter the site of injury.62 More recently it has been shown that this entry of neutrophils into the necroptotic foci is preceded by platelets, which accumulate in the unperfused region surrounding the tissue damage itself. The platelets are not responsible for vessel obstruction; rather they carpet the surfaces of the vessels and facilitate neutrophil crawling. In the absence of platelets neutrophils are unable to traverse the unperfused zone and enter the actual site of injury.18
In contrast, the migratory behavior of neutrophils and platelets in the liver is very different in conditions of infection or LPS-induced inflammation.15 In LPS-treated mice, neutrophils arrest in the sinusoids though they do not apparently crawl.73 Although neutrophils undergo classical selectin-mediated rolling and β2-integrin-mediated arrest in post-sinusoidal venules, the arrest of neutrophils in the hepatic sinusoids has long been known to be independent of selectins.14, 15, 16 In the absence of other data it was initially hypothesized that the neutrophils were merely physically trapped in these vessels.75, 76 However, a systematic examination of several candidate molecules revealed that CD44−/− mice lack neutrophil accumulation in the sinusoids following LPS challenge.15 Moreover, anti-CD44 treatment can flush neutrophils from these vessels, reversing neutrophil-induced pathology.15 CD44 mediates binding of neutrophils to the sinusoids but not the post-sinusoidal vessel, as only the sinusoidal endothelium is coated with its HA ligand. Although CD44 can cycle between high- and low-binding conformations,77 LPS treatment did not affect the ability of neutrophil CD44 to bind HA. Rather, systemic LPS treatment appeared to increase the affinity of HA on the endothelial surface for CD44 by inducing the binding of serum-derived HA-binding protein,15 which enzymatically alters HA, increasing its avidity for CD44 allowing the recruitment of neutrophils.78
Like LPS treatment or bacteraemia, delivery of blood-borne virus results in the arrest of neutrophils in the liver.66 As with LPS treatment, in a poxvirus infection model the neutrophils similarly arrested on the sinusoidal walls without displaying patroling behavior, though in this instance the arrest was mediated by Mac-1, not CD44, on the neutrophils.66 In conditions of both bacterial sepsis and viraemia, neutrophils arrested in the sinusoids produce neutrophil extracellular traps in order to facilitate the clearance of circulating bacteria or virus.66, 72 During Gram negative bacterial infection the induction of neutrophil extracellular traps appears to be critically dependent upon the binding of platelets to the arrested neutrophils, which appears to occur via interactions with LFA-1.72 Inhibition of neutrophil extracellular traps formation either by platelet depletion or using LFA-1-deficient mice resulted in enhanced Escherichia coli bacteraemia, but decreased tissue pathology.72
The need to maintain a balance between control of pathogens and immunopathology implies a requirement that neutrophil arrest and migration in the liver is tightly regulated. Clearly neutrophils display different patterns of behaviors depending on the role they are playing in the liver. Notably, when protecting the liver itself from damage (as in sterile injury models) they crawl in a manner akin to the NKT and tissue-resident CD8+ T cells described above. Similarly to T-cell migration, the migration of neutrophils when crawling appears to be dependent upon β2 integrin interactions following platelet-mediated trapping.18, 62 On the other hand, when acting as blood filters for systemic infections, they arrest on the sinusoidal endothelium, either via CD44 or Mac-1 and trap bacteria or virus by producing neutrophil extracellular traps.15, 66, 72 This bifurcation of behavior is incompletely understood, however, it has been shown that LPS treatment results in a variety of changes to both the neutrophils and the endothelial surfaces that favor arrest rather than crawling. Not only does LPS drive neutrophil arrest in the sinusoids by enhancing the levels of high-binding HA, but also by decreasing levels of ICAM-1 in the sinusoids thereby reducing the probability of β2 integrin interactions.73 Furthermore, LPS also induces IL-10 production, which downregulates Mac-1 expression on neutrophils.73 By downregulating Mac-1 expression, IL-10 may limit pathology by preventing neutrophils from extravasating, where they can cause damage through excessive inflammation, and are of little use in removing pathogens from the blood.73
Recruitment and migration of monocytes
In addition to neutrophils, monocytes are also recruited to the liver in conditions of both sterile inflammation and infection.61, 79 In addition to their well-known roles in the control of infection and tissue repair, monocyte populations have recently been shown to form a niche for the proliferation of CD8+ T cells in the liver by providing co-stimulatory signals via OX40.80 Murine blood monocytes are typically divided into CX3CR1hiCCR2−GR1− and CXCR3loCCR2+GR1− populations, which respectively correspond to CD14loCD16+ monocytes and CD14+CD16− monocytes in humans.81 CXC3CR1hi cells have been shown to patrol the luminal surface of blood vessels in the skin and gut in an LFA-1-dependent manner,82 whereas CCR2+ monocytes—often referred to as inflammatory monocytes—are usually sequestered in the bone marrow but rapidly migrate to sites of inflammation.83, 84 Although CX3CR1hi monocytes are not seen at baseline in the liver, small populations of CCR2+ cells are present in mice.61 The role of CCR2+ monocytes has been well studied in infection and inflammation; in particular, CCR2+ monocytes have been known to have a role in clearing the Gram negative bacterium L. monocytogenes from the blood stream and liver.85 Early studies in mice showed that antibody blockade of Mac-1 prevented monocyte accumulation in the liver, and allowed bacterial titers to increase resulting in significant mortality.86
Closer investigation of the key molecules required for the recruitment of CCR2+ monocytes to the murine liver following Listeria infection revealed that CCR2 itself is not required.79 In this study monocytes in the liver migrated very rapidly (~20 μm min−1) compared with neutrophils (~6 μm min−1) or T cells (~10 μm min−1) and both anti-CD44 and anti-Mac-1 antibodies could block this motility within the liver. A role for Mac-1:ICAM-1 interactions was further supported by the fact that ICAM-1 is highly upregulated in the sinusoids surrounding foci of infection.79 Once at the site of infection these CCR2+ monocytes converted to TNF and iNOS producing DCs, which may control the infection directly.79 It has subsequently been shown that this early pro-inflammatory response gives way to an IL-33-mediated response that favors tissue repair by inducing the inflammatory monocytes to differentiate into tissue-resident macrophages.87 These macrophages ostensibly replace the sessile fetal-derived Kupffer cells that die in large numbers during Listeria infection in the liver.87
Migration of CCR2+ monocytes into the liver has also been seen in sterile inflammation models. The retention of GR1+CCR2+ monocytes in the liver was observed after following carbon tetrachloride-induced liver injury.88 CCR2−/− mice had greatly reduced infiltrates and mice did not display the same degree of fibrosis as wild-type mice. In this situation, as in Listeria infection, CCR2 was found to be critical for the egress of these monocytes into out of the bone marrow rather than their accumulation in the liver.79, 88 The motility of CC2+ monocytes was directly imaged in CCR2-RFP mice in the thermal injury model. In this case large numbers of CCR2+ cells were seen even at baseline migrating within the sinusoids, though few of these were monocytes.61 Upon injury the CCR2+ monocytes accumulated around the edge of the injury (in contrast to neutrophils that rapidly entered the injury site62). Subsequently, they were able to enter the necroptotic focus in a manner that was CCR2-dependent (Figure 2c). Further studies using dual reporter mice revealed that the CCR2+ monocytes differentiated into CX3CR1hi monocytes once inside the inflammatory focus (Figure 2d).61 CX3CR1hi monocytes are believed to have an alternative activation phenotype characterized by the production of IL-10, which would be consistent with a role in tissue repair during tissue injury.82, 89 These data are paralleled in a human study, in which it was found that inflammatory monocytes expressing (CD14hiCD16−) could differentiate into CD14hiCD16+ monocytes, which were more immunoregulatory, leading to their accumulation in the livers of humans with various disease etiologies,90 However, it was also suggested that CD16+ monocytes could be recruited directly into the inflamed human liver from the circulation in a manner dependent on CX3CR1 and VAP-1.91
Classically, monocytes in the liver are considered to be recruited from the circulation, however, a recent study using the thermal injury model has shown that macrophages may also enter the liver directly from the peritoneal cavity (Figure 2e).63 Although CCR2+ monocytes were recruited to sites of injury over a period of several hours, a population of F4/80 macrophages was seen to directly enter the site of injury within an hour of the insult being delivered. These cells were not derived from Kupffer cells, which remained sessile even after injury; rather they were derived from a population of large peritoneal macrophages that express GATA6. These cells eschewed a chemokine/β2 integrin mode of migration, instead migrating in response to pyrogens in a CD44-dependent fashion.63
Concluding remarks
It has long been known that leukocytes adhere in the liver in unique ways, resulting in a distinct immune cell composition within this organ. However, until recently it was unclear if cells such as NKT cells and memory CD8+ T cells were truly resident in the liver, and how they behave in the hepatic environment. The application of intra-vital microscopy has led to a new understanding of how these cells migrate within the liver, whereas parabiosis experiments suggest that these cells are truly resident in the liver.19, 23, 45 Finally, gene expression profiling has shown that these cell populations share a core signature of 'tissue residency' not only with each other but with tissue-resident cell populations in other tissues.19, 31
What is particularly remarkable about the residency of these cells is that they appear to be mostly present in the sinusoids, that is, within the vasculature. For NKT cells this vascular residency is apparently dependent upon LFA-1,23 though for the other cell populations the key adhesion molecules for residency have not been determined. The fact that lymphocytes do not appear to extravasate in large numbers may be a consequence of the liver architecture that allows cells to survey hepatocytes through the fenestrated endothelium (that is, without the need to enter the parenchyma). As such, the liver architecture itself facilitates immune surveillance, and allows highly motile cells to efficiently scan an enormous number of hepatocytes to find potentially rare pathogens.
In inflammatory conditions these resident cells can be joined by inflammatory monocytes and neutrophils. Again recent publications have provided new insights into how these populations patrol the sinusoids, and the key molecular interactions involved. These studies reveal several common features in the trapping of these myeloid cells and the retention of various resident lymphocytes. For both cell types selectins appear to be dispensable, instead in most conditions interactions between CD44 and HA appear to facilitate binding, either directly as in the case of neutrophils, or by allowing platelets to bind to the endothelium.4, 15, 63 Migration upon platelets also appears to be a common feature of both neutrophil and CD8+ T-cell migration in the liver.4, 18 Understanding the key molecules for leukocyte adhesion and retention in the liver will be important in clinical settings: increasing numbers of beneficial liver TRM may be crucial to vaccination against liver pathogens, whereas conversely blocking pathogenic infiltrations of neutrophils can be important in protecting against immune pathology during inflammation.
Footnotes
The authors declare no conflict of interest.
References
- Sheth K, Bankey P. The liver as an immune organ. Curr Opin Crit Care 2001; 7: 99–104. [DOI] [PubMed] [Google Scholar]
- Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol 2013; 14: 996–1006. [DOI] [PubMed] [Google Scholar]
- Sironi L, Bouzin M, Inverso D, D'Alfonso L, Pozzi P, Cotelli F et al. In vivo flow mapping in complex vessel networks by single image correlation. Sci Rep 2014; 4: 7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti LG, Inverso D, Sironi L, Di Lucia P, Fioravanti J, Ganzer L et al. Immunosurveillance of the liver by intravascular effector CD8(+) T cells. Cell 2015; 161: 486–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren A, Le Couteur DG, Fraser R, Bowen DG, McCaughan GW, Bertolino P. T lymphocytes interact with hepatocytes through fenestrations in murine liver sinusoidal endothelial cells. Hepatology 2006; 44: 1182–1190. [DOI] [PubMed] [Google Scholar]
- Knolle PA, Wohlleber D. Immunological functions of liver sinusoidal endothelial cells. Cell Mol Immunol 2016; 13: 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John B, Crispe IN. Passive and active mechanisms trap activated CD8+ T cells in the liver. J Immunol 2004; 172: 5222–5229. [DOI] [PubMed] [Google Scholar]
- Ebe Y, Hasegawa G, Takatsuka H, Umezu H, Mitsuyama M, Arakawa M et al. The role of Kupffer cells and regulation of neutrophil migration into the liver by macrophage inflammatory protein-2 in primary listeriosis in mice. Pathol Int 1999; 49: 519–532. [DOI] [PubMed] [Google Scholar]
- Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, Sieling PA et al. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity 2007; 26: 117–129. [DOI] [PubMed] [Google Scholar]
- Klugewitz K, Blumenthal-Barby F, Eulenburg K, Emoto M, Hamann A. The spectrum of lymphoid subsets preferentially recruited into the liver reflects that of resident populations. Immunol Lett 2004; 93: 159–162. [DOI] [PubMed] [Google Scholar]
- Norris S, Collins C, Doherty DG, Smith F, McEntee G, Traynor O et al. Resident human hepatic lymphocytes are phenotypically different from circulating lymphocytes. J Hepatol 1998; 28: 84–90. [DOI] [PubMed] [Google Scholar]
- Norris S, Doherty DG, Collins C, McEntee G, Traynor O, Hegarty JE et al. Natural T cells in the human liver: cytotoxic lymphocytes with dual T cell and natural killer cell phenotype and function are phenotypically heterogenous and include Valpha24-JalphaQ and gammadelta T cell receptor bearing cells. Hum Immunol 1999; 60: 20–31. [DOI] [PubMed] [Google Scholar]
- Kurioka A, Walker LJ, Klenerman P, Willberg CB. MAIT cells: new guardians of the liver. Clin Transl Immunology 2016; 5: e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Smith CW. Cell adhesion and migration. III. Leukocyte adhesion and transmigration in the liver vasculature. Am J Physiol 1997; 273: G1169–G1173. [DOI] [PubMed] [Google Scholar]
- McDonald B, McAvoy EF, Lam F, Gill V, de la Motte C, Savani RC et al. Interaction of CD44 and hyaluronan is the dominant mechanism for neutrophil sequestration in inflamed liver sinusoids. J Exp Med 2008; 205: 915–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J, Johnston B, Lee SS, Bullard DC, Smith CW, Beaudet AL et al. A minimal role for selectins in the recruitment of leukocytes into the inflamed liver microvasculature. J Clin Invest 1997; 99: 2782–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 2007; 7: 678–689. [DOI] [PubMed] [Google Scholar]
- Slaba I, Wang J, Kolaczkowska E, McDonald B, Lee WY, Kubes P. Imaging the dynamic platelet-neutrophil response in sterile liver injury and repair in mice. Hepatology 2015; 62: 1593–1605. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz D, Ng WY, Holz LE, Ma JZ, Zaid A, Wong YC et al. Liver-resident memory CD8(+) T cells form a front-line defense against malaria liver-stage infection. Immunity 2016; 45: 889–902. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol 2005; 3: e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emoto M, Mittrucker HW, Schmits R, Mak TW, Kaufmann SH. Critical role of leukocyte function-associated antigen-1 in liver accumulation of CD4+NKT cells. J Immunol 1999; 162: 5094–5098. [PubMed] [Google Scholar]
- Ohteki T, Maki C, Koyasu S, Mak TW, Ohashi PS. Cutting edge: LFA-1 is required for liver NK1.1+TCR alpha beta+ cell development: evidence that liver NK1.1+TCR alpha beta+ cells originate from multiple pathways. J Immunol 1999; 162: 3753–3756. [PubMed] [Google Scholar]
- Thomas SY, Scanlon ST, Griewank KG, Constantinides MG, Savage AK, Barr KA et al. PLZF induces an intravascular surveillance program mediated by long-lived LFA-1-ICAM-1 interactions. J Exp Med 2011; 208: 1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Ann Rev of Immunol 1997; 15: 535–562. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay K, Marrero I, Kumar V. NKT cell subsets as key participants in liver physiology and pathology. Cell Mol Immunol 2016; 13: 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley MA, Tahir SMA, Cheng O, Shaulov A, Joyce R, Avigan D et al. Cutting edge: a major fraction of human bone marrow lymphocytes are Th2-like CD1d-reactive T cells that can suppress mixed lymphocyte responses. J Immunol 2001; 167: 5531–5534. [DOI] [PubMed] [Google Scholar]
- Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR et al. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med 2000; 192: 741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzins SP, Smyth MJ, Baxter AG. Presumed guilty: natural killer T cell defects and human disease. Nat Rev Immunol 2011; 11: 131–142. [DOI] [PubMed] [Google Scholar]
- Park SH, Benlagha K, Lee D, Balish E, Bendelac A. Unaltered phenotype, tissue distribution and function of Valpha14(+) NKT cells in germ-free mice. Eur J Immunol 2000; 30: 620–625. [DOI] [PubMed] [Google Scholar]
- Klugewitz K, Adams DH, Emoto M, Eulenburg K, Hamann A. The composition of intrahepatic lymphocytes: shaped by selective recruitment? Trends Immunol 2004; 25: 590–594. [DOI] [PubMed] [Google Scholar]
- Mackay LK, Minnich M, Kragten NA, Liao Y, Nota B, Seillet C et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 2016; 352: 459–463. [DOI] [PubMed] [Google Scholar]
- Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol 2012; 188: 4866–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol 2013; 14: 1294–1301. [DOI] [PubMed] [Google Scholar]
- Smith A, Bracke M, Leitinger B, Porter JC, Hogg N. LFA-1-induced T cell migration on ICAM-1 involves regulation of MLCK-mediated attachment and ROCK-dependent detachment. J Cell Sci 2003; 116: 3123–3133. [DOI] [PubMed] [Google Scholar]
- Smith A, Carrasco YR, Stanley P, Kieffer N, Batista FD, Hogg N. A talin-dependent LFA-1 focal zone is formed by rapidly migrating T lymphocytes. J Cell Biol 2005; 170: 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Jiang X, Chen Y, Sojka DK, Wei H, Gao X et al. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J Clin Invest 2013; 123: 1444–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth K, Donadon M, Cimino M, Pontarini E, Tentorio P, Preti M et al. Human liver-resident CD56(bright)/CD16(neg) NK cells are retained within hepatic sinusoids via the engagement of CCR5 and CXCR6 pathways. J Autoimmun 2016; 66: 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt N, Beziat V, Nystrom S, Hengst J, Ivarsson MA, Kekalainen E et al. Cutting edge: identification and characterization of human intrahepatic CD49a(+) NK Cells. J Immunol 2015; 194: 2467–2471. [DOI] [PubMed] [Google Scholar]
- Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. A committed precursor to innate lymphoid cells. Nature 2014; 508: 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose CS, Flach M, Mohle L, Rogell L, Hoyler T, Ebert K et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell 2014; 157: 340–356. [DOI] [PubMed] [Google Scholar]
- Stegmann KA, Robertson F, Hansi N, Gill U, Pallant C, Christophides T et al. CXCR6 marks a novel subset of T-bet(lo)Eomes(hi) natural killer cells residing in human liver. Sci Rep 2016; 6: 26157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray SJ, Franki SN, Pierce RH, Dimitrova S, Koteliansky V, Sprague AG et al. The collagen binding alpha 1 beta 1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity 2004; 20: 167–179. [DOI] [PubMed] [Google Scholar]
- Richter M, Ray SJ, Chapman TJ, Austin SJ, Rebhahn J, Mosmann TR et al. Collagen distribution and expression of collagen-binding alpha(1)beta(1) (VLA-1) and alpha(2)beta(1) (VLA-2) Integrins on CD4 and CD8 T cells during influenza infection. J Immunol 2007; 178: 4506–4516. [DOI] [PubMed] [Google Scholar]
- Crispe IN, Dao T, Klugewitz K, Mehal WZ, Metz DP. The liver as a site of T-cell apoptosis: graveyard, or killing field? Immunol Rev 2000; 174: 47–62. [DOI] [PubMed] [Google Scholar]
- Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, Igyarto BZ et al. Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell 2015; 161: 737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse SW, Cockburn IA, Zhang H, Scott AL, Zavala F. Unique transcriptional profile of liver-resident memory CD8+ T cells induced by immunization with malaria sporozoites. Genes Immun 2013; 14: 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn IA, Amino R, Kelemen RK, Kuo SC, Tse SW, Radtke A et al. In vivo imaging of CD8+ T cell-mediated elimination of malaria liver stages. Proc Natl Acad Sci USA 2013; 110: 9090–9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisvert J, Kunkel EJ, Campbell JJ, Keeffe EB, Butcher EC, Greenberg HB. Liver-infiltrating lymphocytes in end-stage hepatitis C virus: subsets, activation status, and chemokine receptor phenotypes. J Hepatol 2003; 38: 67–75. [DOI] [PubMed] [Google Scholar]
- Sato T, Thorlacius H, Johnston B, Staton TL, Xiang W, Littman DR et al. Role for CXCR6 in recruitment of activated CD8+ lymphocytes to inflamed liver. J Immunol 2005; 174: 277–283. [DOI] [PubMed] [Google Scholar]
- Tse SW, Radtke AJ, Espinosa DA, Cockburn IA, Zavala F. The chemokine receptor CXCR6 is required for the maintenance of liver memory CD8+ T cells specific for infectious pathogens. J Infect Dis 2014; 210: 1508–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samlowski WE, Spangrude GJ, Daynes RA. Studies on the liver sequestration of lymphocytes bearing membrane-associated galactose-terminal glycoconjugates: reversal with agents that effectively compete for the asialoglycoprotein receptor. Cell Immunol 1984; 88: 309–322. [DOI] [PubMed] [Google Scholar]
- Bertolino P, Schrage A, Bowen DG, Klugewitz K, Ghani S, Eulenburg K et al. Early intrahepatic antigen-specific retention of naive CD8+ T cells is predominantly ICAM-1/LFA-1 dependent in mice. Hepatology 2005; 42: 1063–1071. [DOI] [PubMed] [Google Scholar]
- Hata K, Van Thiel DH, Herberman RB, Whiteside TL. Natural killer activity of human liver-derived lymphocytes in various liver diseases. Hepatology 1991; 14: 495–503. [PubMed] [Google Scholar]
- Bonder CS, Norman MU, Swain MG, Zbytnuik LD, Yamanouchi J, Santamaria P et al. Rules of recruitment for Th1 and Th2 lymphocytes in inflamed liver: a role for alpha-4 integrin and vascular adhesion protein-1. Immunity 2005; 23: 153–163. [DOI] [PubMed] [Google Scholar]
- Lalor PF, Edwards S, McNab G, Salmi M, Jalkanen S, Adams DH. Vascular adhesion protein-1 mediates adhesion and transmigration of lymphocytes on human hepatic endothelial cells. J Immunol 2002; 169: 983–992. [DOI] [PubMed] [Google Scholar]
- Weston CJ, Shepherd EL, Claridge LC, Rantakari P, Curbishley SM, Tomlinson JW et al. Vascular adhesion protein-1 promotes liver inflammation and drives hepatic. J Clin Invest 2015; 125: 501–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DJ, Vainio PJ. Targeting vascular adhesion protein-1 to treat autoimmune and inflammatory diseases. Ann NY Acad Sci 2007; 1110: 382–388. [DOI] [PubMed] [Google Scholar]
- Kurkijarvi R, Adams DH, Leino R, Mottonen T, Jalkanen S, Salmi M. Circulating form of human vascular adhesion protein-1 (VAP-1): increased serum levels in inflammatory liver diseases. J Immunol 1998; 161: 1549–1557. [PubMed] [Google Scholar]
- Kurkijarvi R, Yegutkin GG, Gunson BK, Jalkanen S, Salmi M, Adams DH. Circulating soluble vascular adhesion protein 1 accounts fear the increased serum monoamine oxidase activity in chronic liver disease. Gastroenterology 2000; 119: 1096–1103. [DOI] [PubMed] [Google Scholar]
- David BA, Rezende RM, Antunes MM, Santos MM, Freitas Lopes MA, Diniz AB et al. Combination of mass cytometry and imaging analysis reveals origin, location, and functional repopulation of liver myeloid cells in mice. Gastroenterology 2016; 151: 1176–1191. [DOI] [PubMed] [Google Scholar]
- Dal-Secco D, Wang J, Zeng Z, Kolaczkowska E, Wong CH, Petri B et al. A dynamic spectrum of monocytes arising from the in situ reprogramming of CCR2+ monocytes at a site of sterile injury. J Exp Med 2015; 212: 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 2010; 330: 362–366. [DOI] [PubMed] [Google Scholar]
- Wang J, Kubes P. A reservoir of mature cavity macrophages that can rapidly invade visceral organs to affect tissue repair. Cell 2016; 165: 668–678. [DOI] [PubMed] [Google Scholar]
- Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med 2007; 13: 463–469. [DOI] [PubMed] [Google Scholar]
- Gregory SH, Sagnimeni AJ, Wing EJ. Bacteria in the bloodstream are trapped in the liver and killed by immigrating neutrophils. J Immunol 1996; 157: 2514–2520. [PubMed] [Google Scholar]
- Jenne CN, Wong CH, Zemp FJ, McDonald B, Rahman MM, Forsyth PA et al. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host Microbe 2013; 13: 169–180. [DOI] [PubMed] [Google Scholar]
- Brown KA, Brain SD, Pearson JD, Edgeworth JD, Lewis SM, Treacher DF. Neutrophils in development of multiple organ failure in sepsis. Lancet 2006; 368: 157–169. [DOI] [PubMed] [Google Scholar]
- Dhainaut JF, Marin N, Mignon A, Vinsonneau C. Hepatic response to sepsis: interaction between coagulation and inflammatory processes. Crit Care Med 2001; 29: S42–S47. [DOI] [PubMed] [Google Scholar]
- Hyman MC, Petrovic-Djergovic D, Visovatti SH, Liao H, Yanamadala S, Bouis D et al. Self-regulation of inflammatory cell trafficking in mice by the leukocyte surface apyrase CD39. J Clin Invest 2009; 119: 1136–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest 2009; 119: 305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010; 464: 104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe 2012; 12: 324–333. [DOI] [PubMed] [Google Scholar]
- Menezes GB, Lee WY, Zhou H, Waterhouse CC, Cara DC, Kubes P. Selective down-regulation of neutrophil Mac-1 in endotoxemic hepatic microcirculation via IL-10. J Immunol 2009; 183: 7557–7568. [DOI] [PubMed] [Google Scholar]
- Heit B, Robbins SM, Downey CM, Guan Z, Colarusso P, Miller BJ et al. PTEN functions to 'prioritize' chemotactic cues and prevent 'distraction' in migrating neutrophils. Nat Immunol 2008; 9: 743–752. [DOI] [PubMed] [Google Scholar]
- Fox-Robichaud A, Kubes P. Molecular mechanisms of tumor necrosis factor alpha-stimulated leukocyte recruitment into the murine hepatic circulation. Hepatology 2000; 31: 1123–1127. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Farhood A, Fisher MA, Smith CW. Sequestration of neutrophils in the hepatic vasculature during endotoxemia is independent of beta 2 integrins and intercellular adhesion molecule-1. Shock 1996; 6: 351–356. [DOI] [PubMed] [Google Scholar]
- Lesley J, Hascall VC, Tammi M, Hyman R. Hyaluronan binding by cell surface CD44. J Biol Chem 2000; 275: 26967–26975. [DOI] [PubMed] [Google Scholar]
- Zhuo L, Kanamori A, Kannagi R, Itano N, Wu J, Hamaguchi M et al. SHAP potentiates the CD44-mediated leukocyte adhesion to the hyaluronan substratum. J Biol Chem 2006; 281: 20303–20314. [DOI] [PubMed] [Google Scholar]
- Shi C, Velazquez P, Hohl TM, Leiner I, Dustin ML, Pamer EG. Monocyte trafficking to hepatic sites of bacterial infection is chemokine independent and directed by focal intercellular adhesion molecule-1 expression. J Immunol 2010; 184: 6266–6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LR, Wohlleber D, Reisinger F, Jenne CN, Cheng RL, Abdullah Z et al. Intrahepatic myeloid-cell aggregates enable local proliferation of CD8(+) T cells and successful immunotherapy against chronic viral liver infection. Nat Immunol 2013; 14: 574–583. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003; 19: 71–82. [DOI] [PubMed] [Google Scholar]
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 2007; 317: 666–670. [DOI] [PubMed] [Google Scholar]
- Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol 2008; 26: 421–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R et al. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med 2007; 204: 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara T, Warr G, Loy J, Bravo R. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J Exp Med 1997; 186: 1757–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H, Gordon S, North RJ. Exacerbation of murine listeriosis by a monoclonal antibody specific for the type 3 complement receptor of myelomonocytic cells. Absence of monocytes at infective foci allows Listeria to multiply in nonphagocytic cells. J Exp Med 1989; 170: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleriot C, Dupuis T, Jouvion G, Eberl G, Disson O, Lecuit M. Liver-resident macrophage necroptosis orchestrates type 1 microbicidal inflammation and type-2-mediated tissue repair during bacterial infection. Immunity 2015; 42: 145–158. [DOI] [PubMed] [Google Scholar]
- Karlmark KR, Weiskirchen R, Zimmermann HW, Gasssler N, Ginhoux F, Weber C et al. Hepatic recruitment of the inflammatory Gr1(+) monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology 2009; 50: 261–274. [DOI] [PubMed] [Google Scholar]
- Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med 2007; 204: 1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaskou E, Zimmermann HW, Li KK, Oo YH, Suresh S, Stamataki Z et al. Monocyte subsets in human liver disease show distinct phenotypic and functional characteristics. Hepatology 2013; 57: 385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspinall AI, Curbishley SM, Lalor PF, Weston CJ, Blahova M, Liaskou E et al. CX(3)CR1 and vascular adhesion protein-1-dependent recruitment of CD16(+) monocytes across human liver sinusoidal endothelium. Hepatology 2010; 51: 2030–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]