Abstract
Down syndrome (DS), present in nearly six million people, is associated with an extremely high risk to develop Alzheimer's disease (AD). Amyloid-β and tau pathology are omnipresent from age 40 years onward, but clinical symptoms do not appear in all DS individuals. Dementia diagnostics is complex in this population, illustrating the great need for predictive biomarkers. Although blood biomarkers have not yet proven useful, cerebrospinal fluid (CSF) biomarkers (low amyloid-β42, high t-tau, and high p-tau) effectively contribute to AD diagnoses in the general population and are increasingly used in clinical practice. Surprisingly, CSF biomarkers have been barely evaluated in DS. Breaking the taboo on CSF analyses would finally allow for the elucidation of its utility in (differential) diagnoses and staging of disease severity. A sensitive and specific biomarker profile for AD in DS would be of paramount importance to daily care, adaptive caregiving, and specific therapeutic interventions.
Keywords: Alzheimer's disease, Biomarkers, Cerebrospinal fluid, Dementia, Down syndrome
1. Introduction: Down syndrome at high risk for Alzheimer's disease
Down syndrome (DS), present in nearly six million people worldwide, is the main genetic cause of intellectual disability in humans with a live birth prevalence of approximately one in 650 to 1000 [1], [2]. DS is caused by the triplication of chromosome 21, hence trisomy 21. In addition to the intellectual disability, people with DS face an extremely high risk to develop dementia because of Alzheimer's disease (AD) later in life. By the age of 65, 68% to 80% of DS individuals develop AD [3] compared with about 11% of those aged >65 years in the general (nonintellectually disabled) population [4]. Because of improved medical care, DS life expectancy has increased tremendously in the last century: from 9 years in 1929 to an actual average life expectancy of 61.1 years for men and 57.8 years for women [5]. Consequently, dementia has become evident in the aging DS population, being a major challenge in current daily care.
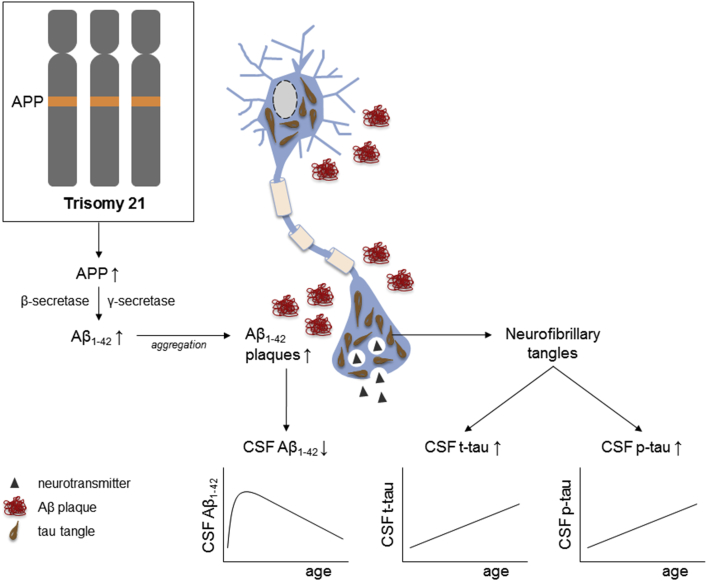
The high risk for AD in DS is generally attributed to the triplication of the amyloid precursor protein (APP) gene, encoded on chromosome 21. The APP protein is cleaved by β- and γ-secretase into amyloid-β (Aβ) peptides, the main constituent of the amyloid plaques found in AD. Overproduction of the APP protein, and thus increased formation of its splicing product Aβ, is present from birth onward, resulting in early Aβ accumulation and deposition in the brain (Fig. 1). Plaque formation has been reported to start with deposition of the longer Aβ1–42 fragments, already observed in a 12-year-old child with DS, later followed by formation of more compacted fibrillary plaques that contain Aβ1–40 as well [6]. Neuropathologic studies showed that the abundance of amyloid plaques and neurofibrillary tangles—the second hallmark of AD pathology—increases strongly in the third and fourth decade of life. By the age of 40 years, pathology is omnipresent in virtually all persons with DS, meeting the neuropathologic criteria for AD [7], [8]. Interestingly, a 78-year-old DS woman with a partial trisomy 21 lacking the third copy of the APP gene was found to display neither symptoms of dementia nor evident AD pathology [9], illustrating the central role of the triplication of the APP gene.
Fig. 1.
Schematic illustration of AD neuropathology and related changes in CSF biomarkers in DS. DS is caused by trisomy 21. The APP gene is encoded on chromosome 21, causing an overproduction of the APP protein in DS from birth onward. The enzymes β- and γ-secretase cleave the APP protein into Aβ peptides, which aggregate into plaques. The longer Aβ1–42 fragments are most prone to aggregate. Extensive neuropathology, that is, extracellular plaques, but also intracellular neurofibrillary tangles consisting of p-tau and t-tau, increases strongly in the third and fourth decade of life in virtually all DS individuals. These neuropathologic hallmarks are reflected by altered levels of CSF biomarkers. The CSF AD profile (low levels of Aβ42, and high levels of p-tau and t-tau) demonstrates high sensitivity and specificity in the general population. Whether a similar biomarker profile is useful for AD in DS remains to be elucidated. The very limited number of small-sized CSF studies in DS suggests that CSF Aβ1–42 increases in early childhood when the aggregation of Aβ1–42 into plaques is still relatively low. Once the deposition of Aβ1–42 into plaques augments (i.e., reduced clearance from the brain), CSF Aβ1–42 gradually decreases. In contrast, CSF t-tau and p-tau both correlate positively with age in DS. Abbreviations: Aβ, amyloid-β; APP, amyloid precursor protein; CSF, cerebrospinal fluid; DS, Down syndrome; p-tau, phosphorylated tau; t-tau, total tau.
Strikingly, despite the presence of pathology from midlife, not all DS individuals develop clinical dementia symptoms, thus complicating the prediction and monitoring of (the course to) dementia [10]. Indeed, DS individuals may reach their 70s free of dementia symptoms [11]. Therefore, diagnosing AD in DS is relatively difficult compared with the general population considering the (variable extent of) intellectual disability, pre-existing behavior, and comorbidities that might be misinterpreted as dementia symptoms. Differentiating among low(er) cognitive capacities because of the intellectual disability, cognitive decline because of normal aging, and deterioration because of AD is a fairly complex endeavor, heavily relying on clinical observations and caregiver reports [10], [12].
Consequently, an objective biomarker profile for AD in DS would greatly aid the diagnostic procedure and contribute to more sensitive and earlier diagnoses. In fact, predicting the onset and monitoring the progression of AD in DS is of paramount importance to daily care. It would contribute to awareness and understanding among caregivers and relatives, leading to increased acceptance—the starting point for adaptive caregiving: allocating additional time, adjusting the living environment, and optimizing management and support. Furthermore, it would enable specific therapeutic interventions. Currently, treatment options are limited: in the few randomized controlled trials in the DS population, effectiveness of donepezil and memantine was not proven [13]. Alternatively, contemporary intellectual disability care focuses on timely (non-)pharmacologic therapy to reduce behavioral and psychological symptoms of dementia—the major cause of referral [14]—to improve quality of life and reduce caregiver burden [10], [15]. Although the process of neurodegeneration cannot yet be prevented or stopped, a recently completed clinical trial (phase Ib) using immunotherapy with aducanumab reported a dose- and time-dependent reduction of Aβ plaques in the brain of patients with prodromal or mild AD, indicating the potential of this human monoclonal antibody as disease-modifying strategy for AD [16].
2. Cerebrospinal fluid biomarkers for AD in the general population
In the general population, mounting evidence demonstrates the high sensitivity and specificity of cerebrospinal fluid (CSF) biomarkers for AD, contributing to accurate and earlier diagnosis, and aiding the differential diagnosis of AD from other dementia etiologies. Because CSF is in direct contact with the extracellular space in the brain, CSF biomarkers are considered to reflect biochemical changes in the brain better than other biological fluids such as plasma/serum or urine [17]. In recent years, a number of studies confirmed the diagnostic value of the so-called “AD signature” or “AD profile” in CSF: low levels of Aβ42 and high levels of total tau (t-tau) and phosphorylated tau at threonine181 (p-tau) typically found in patients with AD compared with cognitively normal control subjects, individuals with subjective memory complaints, and patients with other non-AD dementias [18], [19], [20], [21], [22], [23]. Low Aβ1–42, high t-tau, and high p-tau levels in CSF, respectively, reflect the deposition of Aβ1–42 into plaques, neuronal damage and degeneration, and neocortical neurofibrillary pathology [18].
As a consequence, this panel of CSF biomarkers receives vast attention in research and in clinical practice. CSF biomarkers have been included in the revised diagnostic criteria for AD by the National Institute on Aging and the Alzheimer's Association [24], [25] and are increasingly used in memory clinics [26]. Routine CSF analysis has been recommended by the European Federation of Neurological Societies with respect to the differential diagnosis of atypical AD [27]. In most of the European Federation of Neurological Societies member countries Aβ1–42, t-tau, and p-tau are frequently evaluated, although specific cutoff values for these three biomarkers may differ per country or per center. Although the frequency of lumbar punctures was traditionally low in Canada and the United States in comparison with northern European countries, clinical research initiatives such as the Alzheimer's Disease Neuroimaging Initiative positively impacted the attitude toward lumbar punctures in recent years and contributed to the implementation of CSF biomarkers in clinical practice [18]. Indeed, lumbar punctures, regularly offered to patients as option in the standard clinical procedure, may aid neurologists in their diagnostic workup: CSF biomarkers were found to change diagnoses, increase diagnostic confidence, and affect patient management [28].
More recently, Skillbäck et al. [20] demonstrated that low Aβ1–42, high t-tau, and high p-tau correlated with cognitive decline in patients with AD represented by the Mini-Mental State Examination scores, thus indicating that these CSF biomarkers might be useful for staging of disease severity. Given that AD neuropathology is omnipresent in DS brains from the age of 40 years onward, but not all individuals develop clinical dementia symptoms, it is of utmost importance to monitor clinical symptoms. Biomarkers that would not only reflect the deposition of neuropathology but also correlate with cognitive decline would be a major advantage. After all, people cope with the symptoms rather than neuropathology, that is, the presence of neuropathology without clinical symptoms does not affect daily care.
Although the importance of CSF biomarkers in AD diagnosis has long been recognized in the general population and efforts are made to standardize (pre-)analytical procedures [18], [29], CSF biomarkers for AD in DS have been largely neglected so far. Despite their high risk on AD, not more than a mere handful of small-sized studies evaluated Aβ42, t-tau, and p-tau in CSF of DS individuals. Key study information and CSF results from the five published studies are listed in Table 1.
Table 1.
CSF levels of Aβ1–42, t-tau, and p-tau in DS
| Study reference | Study population | Clinical dementia diagnosis | CSF Aβ1–42 (pg/mL) | CSF t-tau (pg/mL) | CSF p-tau (pg/mL) |
|---|---|---|---|---|---|
| [30]∗ | 5 DS (55.3 ± 3.4 y) | Unknown | 817† ± 496 | — | — |
| 34 Diseased control subjects (67.9 ± 10.4 y) | No AD | 1457† ± 745 | — | — | |
| [8] | 12 DS (41 ± 11 y) | 3 Clinical history of dementia | 572‡ ± 160 (<40 y, n = 6) 370‡§ ± 105 (>40 y, n = 6) |
144¶ ± 101 (<40 y, n = 6) 500¶ ± 341 (>40 y, n = 6) |
— |
| 19 Control subjects (53 ± 5 y) | Nondemented | 578§ ± 129 | 246 ± 109 | — | |
| [31] | DS children, longitudinal: 8 mo (n = 9), 20–40 mo (n = 11), and 54 mo (n = 4) | n/a | 1200 (8 mo), 1800 (20–40 mo), and 1800 (54 mo) | n.s. | n.s. |
| [32] | 12 DS (41 ± 11 y) | 3 Clinical history of dementia | 637 ± 201 | 431 ± 369 | 52 ± 31 |
| 20 Healthy control subjects (40 ± 15 y) | n/a | 674 ± 145 | 210 ± 87 | 34 ± 8.8 | |
| [35] | 12 DS (41 ± 11 y) | 3 Clinical history of dementia | Relative abundance (HI-MS) lowest in DS compared with control subjects|| | — | — |
| 20 Healthy control subjects (40 ± 15 y) | n/a | — | — |
Abbreviations: AD, Alzheimer's disease; Aβ, amyloid-β; CSF, cerebrospinal fluid; DS, Down syndrome; HI-MS, hybrid immunoaffinity mass spectrometry; n.s., not specified in the article; p-tau, phosphorylated tau; t-tau, total tau.
CSF Aβ1–42 is expressed in picomolars in the original publication. Concentrations have been converted into picograms per milliliter using 4512.21 g/mol as molecular mass of Aβ1–42 in CSF [33].
P < .05.
P < .05.
P < .01.
P < .01.
P < .0001.
3. Few CSF studies in DS
To our knowledge, the first study measuring CSF levels of Aβ species was conducted by Tamaoka et al. in 1999 [30]. Aβ1–40, Aβ1–42, and the truncated species Aβx–40 and Aβx–42 were measured in CSF of five DS individuals in their 50s (55.3 ± 3.4 years) and compared with 34 non-DS patients (67.9 ± 10.4 years) diagnosed with neurologic diseases not associated with dementia (diseased control group). Although mean CSF levels of Aβ1–40 and the truncated species did not significantly differ between both groups, Aβ1–42 was significantly lower in DS than in diseased control subjects, which corresponds to lower CSF Aβ1–42 reported in patients with AD in the general population. Considering the mean age of the DS group, reduced Aβ1–42 likely reflects the deposition of AD pathology in the DS brain that is omnipresent from the age of 40 years onward. Whether DS individuals were clinically diagnosed with dementia was not described [30].
Two years later, a Finnish group determined Aβ1–42 and t-tau in CSF of 12 DS individuals (including three subjects with a clinical dementia diagnosis) and 19 non-DS, nondemented control subjects [8]. CSF tau did not differ significantly between both groups, although CSF Aβ1–42 was significantly lower in older DS individuals (aged >40 years) compared with control subjects (P < .01). Apolipoprotein E genotype was not related to either Aβ1–42 or tau. To explore a possible age-related change in CSF values, the authors recruited people in a wide age range (21–61 years). In the DS group, CSF Aβ1–42 correlated negatively with age (r = −0.785; P < .005), whereas t-tau demonstrated a positive age correlation (r = 0.718; P < .05). Alternatively, the DS study population was divided into a younger and older age group with the age of 40 years as cutoff: CSF levels of Aβ1–42 and t-tau were, respectively, lower and higher in older (>40 years, n = 6) than in younger DS individuals (<40 years, n = 6). Interestingly, the highest level of CSF Aβ1–42 was found in the youngest participant with DS (aged 21 years), leading the authors to suggest that CSF Aβ1–42 increases because of the trisomy 21–related overproduction of the APP protein, and thus of Aβ fragments, whereas the Aβ accumulation into plaques is still low [8], [34].
Increased CSF Aβ in young DS individuals is further supported by findings of Englund et al. [31]. They measured a series of Aβ species (Aβ1–37, Aβ1–38, Aβ1–39, Aβ1–40, and Aβ1–42) and t-tau and p-tau in longitudinal CSF samples from a small group of very young DS children at 8 months (n = 9), 20 to 40 months (n = 11), and 54 months (n = 4). Indeed, increasing levels of all Aβ species (Western blot) were observed from age 8 to 54 months, although only Aβ1–37 and Aβ1–38 reached significance. The ratio Aβ1–42/Aβ1–40 did not change over time. CSF t-tau and p-tau levels did not change significantly over the months, which would correspond to the likely absence of tau pathology at this young age.
Recently, Portelius et al. [32] retested the Finnish CSF aliquots [8] (n = 12 DS) to determine whether the aforementioned results could be replicated. In this new study, other Aβ species (Aβx–38, Aβx–40, and Aβx–42) and soluble APP fragments (sAPPα and sAPPβ) were measured in addition to Aβ1–42, t-tau, and p-tau. Compared with a healthy age- and gender-matched control group (n = 20), CSF levels of Aβx–40, sAPPα, and sAPPβ were significantly higher in DS. Aβx–38, Aβx–42, Aβ1–42, t-tau, and p-tau did not differ significantly between both groups. Resembling the previous study, a negative correlation was observed between the Aβ1–42 concentration and age (r = −0.69, P = .015). T-tau was positively correlated with age (r = 0.76, P = .0062). No correlations with age were found for the other measured markers. Subsequently, the DS group was divided again into a younger (<40 years, n = 6 DS) and an older subgroup (>40 years, n = 6 DS), revealing that older DS individuals had significantly higher t-tau and p-tau concentrations than their younger counterparts. Lower Aβ1–42 levels in the older DS group, as reported in the first study of this cohort [8], could not be replicated here [32].
In the same year, Portelius et al. elaborated on their findings by performing hybrid immunoaffinity enrichment of the CSF Aβ peptides followed by matrix-assisted laser desorption/ionization time-of-flight/time-of-flight (MALDI-TOF/TOF) mass spectrometry, demonstrating that the relative abundance of Aβ1–42 was significantly decreased in DS (n = 12) compared with control subjects (n = 20, age- and gender-matched). This relative abundance is based on the relative peak heights/areas of the various CSF Aβ peptides and therefore does not directly reflect the absolute abundance, but rather indicates relative changes in the peptides. Relative Aβ1–42 levels were also found to correlate negatively with age in DS [35], which resembles previous (absolute) findings [8], [32] and likely relates to the increased deposition into plaques/reduced clearance.
In summary, the previously described studies suggest that CSF Aβ1–42 levels are lower in DS than in non-DS control subjects and correlate negatively with age in the DS population. In early childhood, Aβ levels tend to increase in DS, followed by a gradual decrease (reduced clearance from the brain) once the deposition of Aβ1–42 into plaques augments. Tau, on the other hand, does not differ between DS and control subjects but appears to correlate positively with age in DS individuals (schematically illustrated in Fig. 1). The aforementioned results should be interpreted with caution, however, given the very small study sizes (not exceeding 12 DS participants, thus being hugely underpowered), groups that were not age-matched, and the fact that three of five studies analyzed CSF from the same participants [8], [32], [35]. Importantly, clinical diagnoses and the severity of dementia were not taken into account, leaving the question open whether these biomarkers would only reflect neuropathology or correlate with disease status and severity as well. Although it was originally thought that CSF biomarkers were not useful for staging of disease severity [18], low Aβ1–42, high t-tau, and high p-tau were recently found to correlate with the Mini-Mental State Examination scores, that is, disease severity, in the general population [20]. Therefore, future DS biomarker studies need high-quality clinical documentation with standardized and validated cognitive, behavioral, and functional testing to establish whether CSF biomarkers in DS correlate with disease severity and progression as well.
4. Need for CSF analyses in DS
The importance of research on AD in DS and the true need for biomarkers reflecting clinical symptoms are now justly recognized in the field [1], [3], [36]. Indeed, various international working groups on AD in DS have been established in recent years, such as the Committee for Clinical Research of the Trisomy 21 Research Society, the Professional Interest Area on Down Syndrome and Alzheimer's Disease by the American Alzheimer's Association, and the Down Syndrome and Other Genetic Developmental Disorders Network initiated by the European College of Neuropsychopharmacology. Despite all efforts, CSF analyses evidently remain a problematic avenue.
The question arises what hampers the implementation of CSF biomarkers in DS. First of all, one could argue that an etiologic diagnosis of dementia is of limited added value in DS considering their evident genetic predisposition to dementia of the Alzheimer type. Such a view, however, ignores the complex nature of dementia diagnostics in this population. Disentangling dementia from normal aging and distinguishing dementia from depression—a particular vulnerability in DS—are major challenges for clinicians. Symptoms of depression and dementia overlap considerably and may occur concomitantly [10], [37]. In particular, depressive symptoms can mimic dementia by negatively affecting cognition and daily functioning. Misdiagnosis of depression as dementia, or vice versa, may strongly impact the selected therapeutic strategy. An established and validated CSF AD biomarker profile in DS would enhance sensitivity and specificity of diagnosis of AD in DS, that is, to confirm the AD diagnosis. Furthermore, CSF biomarkers might identify those individuals at risk for conversion to clinical AD, which would strongly aid adaptive caregiving.
Moreover, lumbar punctures are regarded a relatively invasive procedure, raising ethical issues especially in this vulnerable intellectually disabled population. It has been reported that patients in the general population may fear adverse effects and pain, whereas clinicians may have a negative attitude toward lumbar punctures with respect to time constraints and lack of training [18]. The most common complication concerns the postlumbar puncture headache (PLPH). Although the incidence of PLPH differs markedly per site and procedure [26], the frequency and severity of PLPH has been reported low in demented patients [38]. The use of an atraumatic needle, for instance, is associated with a lower incidence of PLPH and a favorable safety profile in patients with AD [39], [40]. Interestingly, Peskind et al. reported <2% PLPH [39], which approaches the risk of headache related to positron emission tomography (PET) imaging of amyloid deposition (1.8%) [18], a technique that has become popular in DS research. In that respect, lumbar punctures convey a relatively low risk on complications, also shown by the increasing popularity of CSF biomarker analyses in memory clinics throughout the world [26].
With respect to DS, the implementation of CSF sampling is further complicated by the fact that most DS individuals are not capable of weighing the benefits and risks and make a deliberated decision about consent and participation. Consequently, one relies on informed consent by proxy from the legal representative, often the parents. Although a substantial body of evidence points at the relatively low risk on complications, good safety profile and high sensitivity and specificity of the CSF AD profile in the general population, such data are not broadly available for DS. More recently, we have shown the safety of lumbar punctures in the largest cohort of DS individuals to have undergone CSF sampling, the Down Alzheimer Barcelona Neuroimaging Initiative (DABNI), using a validated protocol with a semistructured telephone interview [41]. Among the participants, 90% did not report any complication. Headache (6.25%) was found to be the most frequent complication, with only one participant suffering from a typical PLPH with moderate severity. The incidence of reported complications was found to be lower than in the general population. Given the relatively early onset of AD in DS, it is of interest to mention that younger age and female gender appear as risk factors for PLPH in the general population [26]. Unfortunately, the aforementioned safety study in DS was not powered to assess this [41], nor were these risk factors addressed in the few CSF studies in DS so far [8], [30], [31], [32], [35]. Therefore, balancing the pros and cons in the context of DS is more complicated and will be highly individual. In addition to the personal decision on whether to participate, approval of the Institutional Review Board might be a substantial hurdle. From the American perspective, Hartley et al. state that DS individuals form “a health disparity population” with an extremely high risk for AD who “should have access to the latest interventions and clinical trials,” a strategical argument that would contribute to approval by the Institutional Review Board [36].
Many researchers have circumvented this issue in advance by designing studies with less invasive procedures, mainly collection of plasma/serum. Because blood examination is part of routine clinical workup, including tests for hypothyroidism and vitamin B12 deficiency with respect to the dementia diagnosis in DS, drawing additional plasma/serum for research purposes has been widely accepted. For instance, several DS studies have analyzed Aβ1–42 and Aβ1–40 in serum or plasma in relation to the status of dementia. Results summarized in Coppus et al. [42] have not been concordant, however. Notably, it remains indistinct whether plasma/serum Aβ1–42 reflects pathologic changes in the brain, as peripheral cells may also produce Aβ. In the general population, plasma Aβ1–42 correlated neither with CSF Aβ1–42 nor with amyloid burden in frontal and temporal neocortex [43], [44].
Accordingly, CSF is the preferred source of AD biomarkers: it is in direct contact with the extracellular space, and confounding influences from the periphery are diminished. It has been argued that lumbar punctures might be difficult in DS because they may not directly benefit the individual [36]. However, this runs the risk of circular reasoning: if the aforementioned CSF AD profile established in the general population is not thoroughly validated in DS, evaluation of CSF Aβ1–42, t-tau, and p-tau will not benefit the diagnostic process in DS. An apparent discrepancy exists between large CSF biomarker studies in the general population and (very) small CSF studies in DS. Clearly, different standards are applied in the general population compared with people with intellectual disabilities, as shown by the fact that DS studies with only 12 individuals have still been accepted for publication in recent years. On the basis of the very few available studies and regular discussions with colleagues in the field, a kind of taboo emerges on lumbar punctures in DS. Despite a broad consensus on the need to perform CSF studies in DS [36], CSF sampling has been hardly incorporated in study protocols so far.
For instance, consideration of ongoing larger scale clinical initiatives confirms the limited focus on CSF biomarkers in DS adults. In the ClinicalTrials.gov registry, a search for “Down syndrome + CSF” or “Trisomy 21 + CSF” yielded only two results: a phase I dose escalation study of the ACI-24 liposome vaccine (developed to evoke an antibody response against aggregated Aβ peptides) in 24 DS individuals (aged 35–45 years) mentioning “change from baseline over 25 months in plasma/CSF amyloid [and] tau” as a secondary outcome measure [45], and the start of a new biobank, planning to include CSF sampling as well [46]. Moreover, the Down Syndrome Biomarker Initiative, one of the major DS initiatives in the United States, primarily focuses on neuroimaging and does not mention CSF biomarkers in their pilot study publication [47]. Indeed, amyloid PET receives substantial attention in DS. PET neuroimaging, however, is not likely to replace CSF AD biomarkers: a recent study in the general population revealed that CSF Aβ1–42 becomes abnormal before amyloid PET, that is, abnormal Aβ accumulation is detected earlier through CSF analysis [48]. Clearly, CSF analyses in DS remain marginally available and lumbar punctures are far from being a common procedure.
In the general population, however, CSF biomarkers are increasingly being included in clinical trials of disease-modifying therapies for AD. The use of CSF biomarkers in clinical trials serves different purposes—reviewed in [49]: (1) to evaluate and quantify the pharmacodynamic effects of AD-modifying therapies, for example, CSF Aβ1–42 levels as an indicator of the effect of a new β-secretase inhibitor, (2) to contribute to a more homogenous and accurate selection of patients (aiding the clinical diagnosis on which patient enrollment is generally based), and (3) to identify prodromal subjects or those at risk to convert to AD. Moreover, CSF biomarkers might serve as surrogate end points of disease progression [49]. Importantly, clinical trials for preventive or curative treatment of AD in DS are warranted. Because the diagnosis of dementia and the clinical evaluation of disease progression in this high-risk population are rather complex, the availability of valid CSF biomarkers would be crucial in such trials.
Are researchers limiting themselves in advance by not including CSF measures in study protocols, afraid of long delays in Institutional Review Board procedures or negative responses from families? Taking the extremely high risk for AD into account, the authors are convinced that people with DS should get the same opportunities and high-quality research as patients with AD in the general population. Rather than deciding for DS individuals and their caregivers and relatives beforehand, researchers and clinicians could apply a “you never know, until you ask” approach. In our experience, caregivers and relatives are very open to scientific research and willing to consider participation requests as long as the information is crystal clear and the procedure is adapted to people with DS with staff that is familiar with DS-specific circumstances. Indeed, the CSF studies described previously (listed in Table 1) and three other studies measuring concentrations of monoamine neurotransmitters and metabolites [50], [51], and proteins [52], demonstrate that lumbar punctures in DS are within the bounds of possibility. The CSF production rate and the caudorostral gradients of total CSF protein have been reported normal in DS compared with age-matched non-DS healthy control subjects [53], thus not constituting any medical barrier herein. Importantly, within the DABNI study—currently the only DS cohort study in Europe to offer CSF sampling on a large scale—more than 20% of DS individuals and their families consented to a lumbar puncture [41], [54], which is a substantial proportion given the aforementioned ethical considerations. To the best of our knowledge, no studies so far have further assessed the reasons to refuse a lumbar puncture in DS. In our clinical experience, however, these reasons do not differ from the general population (i.e., protection of the individual, fear of adverse effects and pain, and a lack of interest in the etiologic diagnosis), although informed consent by proxy is a complicating factor. Evidently, not all eligible individuals will participate in such studies, but in multicenter approach sufficiently large numbers are feasible.
5. Future avenues
CSF biomarker studies in DS are still in its infancy, but the results on the feasibility, safety, and acceptance of lumbar punctures are promising. Accordingly, which approach should be considered to move the field further? First, most studies in general population have been performed within the setting of specialized memory clinics in which patient care and research converge, whereas only a very few clinics have specialized in DS. Reinforcing this infrastructure for DS by fostering collaboration between memory clinics and intellectual disability experts is warranted, for example, the DABNI study has proven the success of such a partnership [41]. Nevertheless, not all individuals will undergo a lumbar puncture, pointing at the need for a multicenter approach to achieve sufficiently large and relevant sample sizes. Together with the key DS clinical research centers in Europe we are working on harmonizing clinical protocols and have initiated the first steps toward an European collaborative biobanking initiative for DS, resembling the concept of the renowned Dutch Parelsnoer Institute for Neurodegenerative Diseases—a prospective, standardized, multicenter cohort study strongly focusing on biomarkers for early and differential diagnosis of dementia and disease monitoring in the general population [55].
Consequently, this would allow to determine the diagnostic value of the CSF AD profile (low Aβ42, high t-tau, and high p-tau) and investigate alternative CSF biomarkers for AD in DS. Candidate biomarkers have been primarily identified in blood so far, such as the major (nor)adrenergic metabolite 3-methoxy-4-hydroxyphenylglycol (MHPG). Compared with DS individuals without dementia, MHPG levels in serum were found to be significantly lower in demented persons with DS (P < .0001) but also in the nondemented individuals who converted to dementia during clinical follow-up (P < .0001), pointing at the predictive potential of MHPG [56]. Measuring MHPG in CSF would be the logical next step, particularly after the combination of CSF MHPG with CSF Aβ42, t-tau, and p-tau was found to improve the discrimination of AD versus dementia with Lewy bodies in the general population [57]. Next to amyloid and tau pathology, AD in DS is characterized by neuroinflammation [58]. Portelius et al. [32] measured the microglial activation marker YKL-40 and showed that DS subjects aged >40 years (n = 6) had significantly higher levels of YKL-40 in CSF compared with their younger counterparts (<40 years, n = 6). Whether YKL-40 or other inflammatory markers would serve useful as biomarker remains to be elucidated. Plasma/serum studies determining different proinflammatory factors in DS have not been decisive yet [59], [60]. Finally, chromosome 21-encoded proteins that relate to AD pathology—summarized in Wiseman et al. [3]—should be studied more extensively in the context of biomarker identification, such as the DYRK1A kinase [61], [62] and the synapse-associated protein synaptojanin-1 [63].
6. Conclusions
The discrepancy between the presence of extensive AD neuropathology from the age of 40 years and the highly variable onset of clinical dementia symptoms in DS illustrates the need for objective AD biomarkers in this population. CSF Aβ42, t-tau, and p-tau, contributing to the so-called CSF AD profile, have proven useful in aiding AD diagnoses in the general population and demonstrated high sensitivity and specificity, and good safety profiles. Surprisingly, lumbar punctures are rarely performed in DS, a population at high risk to develop AD dementia. As little as five studies evaluated CSF Aβ42, t-tau, and p-tau in DS. Being underpowered and without considering the clinical status of dementia, these studies, nonetheless, indicate that CSF Aβ1–42 negatively correlates with age and is lower than in non-DS control subjects, whereas tau in DS correlates positively with age but does not differ between DS and control subjects. Breaking the taboo on lumbar punctures in DS would finally enable the scientific community to investigate the utility of CSF Aβ42, t-tau, and p-tau in diagnosing AD in DS, and if so, to what extent this panel of biomarkers would aid differential diagnosis and staging of disease severity. Because blood biomarkers have not yet proven useful in clinical practice, a validated, sensitive and specific CSF “AD in DS profile” would be of paramount importance to daily care. It would contribute to understanding and acceptance among caregivers and relatives, enable adaptive caregiving, allow for specific therapeutic interventions, and might serve useful in future clinical trials for preventive or curative treatment of AD in DS.
Research in context.
-
1.
Systematic review: Because of the limited number of cerebrospinal fluid (CSF) studies in Down syndrome (DS), we conducted a broad, unrestricted search strategy to identify all relevant articles. Non-English articles were not considered.
-
2.
Interpretation: Amyloid-β42, total tau, and phosphorylated tau in CSF may strongly aid the diagnosis of Alzheimer's disease (AD) in the general population. DS individuals are at very high risk to develop AD and the diagnostic procedure in this population is complicated by the pre-existing intellectual disability and behavior. Because blood biomarkers have not proven useful in clinical practice, it is surprising that the large potential of CSF AD biomarkers is barely studied in DS.
-
3.
Future directions: Investigate the utility of CSF amyloid-β42, total tau, and phosphorylated tau, as well as potential alternative CSF biomarkers in DS. A sensitive and specific CSF biomarker profile for AD in DS would allow for earlier and more accurate diagnoses, aiding daily care and therapy.
Acknowledgments
This work was supported by the Alzheimer Research Center of the University Medical Center Groningen (UMCG), the Research School for Behavioural and Cognitive Neurosciences of the University of Groningen (RUG), and a subsidy from the Gratama Stichting/Stichting Groninger Universiteitsfonds (2015-04). These public sponsors had no further role in collecting and interpreting the data and writing the manuscript. A.D.D. and P.P.D.D. further acknowledge the Down Syndrome and Other Genetic Developmental Disorders (DSG2D) Network of the European College of Neuropsychopharmacology (ECNP) and the Trisomy 21 Research Society (T21RS). All authors contributed to the interpretation and critical evaluation of the data and participated in writing and editing the text. The authors have approved the final manuscript.
Conflicts of interest: The authors have no conflicts of interest to disclose.
References
- 1.Ballard C., Mobley W.C., Hardy J., Williams G., Corbett A. Dementia in Down's syndrome. Lancet Neurol. 2016;15:622–636. doi: 10.1016/S1474-4422(16)00063-6. [DOI] [PubMed] [Google Scholar]
- 2.Bittles A.H., Bower C., Hussain R., Glasson E.J. The four ages of Down syndrome. Eur J Public Health. 2007;17:221–225. doi: 10.1093/eurpub/ckl103. [DOI] [PubMed] [Google Scholar]
- 3.Wiseman F.K., Al-Janabi T., Hardy J., Karmiloff-Smith A., Nizetic D., Tybulewicz V.L. A genetic cause of Alzheimer disease: mechanistic insights from Down syndrome. Nat Rev Neurosci. 2015;16:564–574. doi: 10.1038/nrn3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alzheimer's Assocation 2016 Alzheimer's disease facts and figures. Alzheimers Dement. 2016;12:459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Glasson E.J., Sullivan S.G., Hussain R., Petterson B.A., Montgomery P.D., Bittles A.H. Comparative survival advantage of males with Down syndrome. Am J Hum Biol. 2003;15:192–195. doi: 10.1002/ajhb.10132. [DOI] [PubMed] [Google Scholar]
- 6.Lemere C.A., Blusztajn J.K., Yamaguchi H., Wisniewski T.M., Saido T.C., Selkoe D.J. Sequence of deposition of heterogeneous amyloid beta-peptides and APO E in Down syndrome: implications for initial events in amyloid plaque formation. Neurobiol Dis. 1996;3:16–32. doi: 10.1006/nbdi.1996.0003. [DOI] [PubMed] [Google Scholar]
- 7.Mann D.M. Alzheimer's disease and Down's syndrome. Histopathology. 1988;13:125–137. doi: 10.1111/j.1365-2559.1988.tb02018.x. [DOI] [PubMed] [Google Scholar]
- 8.Tapiola T., Soininen H., Pirttilä T. CSF tau and Abeta42 levels in patients with Down's syndrome. Neurology. 2001;56:979–980. doi: 10.1212/wnl.56.7.979. [DOI] [PubMed] [Google Scholar]
- 9.Prasher V.P., Farrer M.J., Kessling A.M., Fisher E.M., West R.J., Barber P.C. Molecular mapping of Alzheimer-type dementia in Down's syndrome. Ann Neurol. 1998;43:380–383. doi: 10.1002/ana.410430316. [DOI] [PubMed] [Google Scholar]
- 10.Dekker A.D., Strydom A., Coppus A.M., Nizetic D., Vermeiren Y., Naude P.J. Behavioural and psychological symptoms of dementia in Down syndrome: early indicators of clinical Alzheimer's disease? Cortex. 2015;73:36–61. doi: 10.1016/j.cortex.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 11.Krinsky-McHale S.J., Devenny D.A., Gu H., Jenkins E.C., Kittler P., Murty V.V. Successful aging in a 70-year-old man with Down syndrome: a case study. Intellect Dev Disabil. 2008;46:215–228. doi: 10.1352/2008.46:215-228. [DOI] [PubMed] [Google Scholar]
- 12.Devenny D.A., Krinsky-McHale S.J., Sersen G., Silverman W.P. Sequence of cognitive decline in dementia in adults with Down's syndrome. J Intellect Disabil Res. 2000;44:654–665. doi: 10.1046/j.1365-2788.2000.00305.x. [DOI] [PubMed] [Google Scholar]
- 13.Livingstone N., Hanratty J., McShane R., Macdonald G. Pharmacological interventions for cognitive decline in people with Down syndrome. Cochrane Database Syst Rev. 2015:CD011546. doi: 10.1002/14651858.CD011546.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams D., Oliver C., Kalsy S., Peters S., Broquard M., Basra T. Behavioural characteristics associated with dementia assessment referrals in adults with Down syndrome. J Intellect Disabil Res. 2008;52:358–368. doi: 10.1111/j.1365-2788.2007.01036.x. [DOI] [PubMed] [Google Scholar]
- 15.Nowrangi M.A., Lyketsos C.G., Rosenberg P.B. Principles and management of neuropsychiatric symptoms in Alzheimer's dementia. Alzheimers Res Ther. 2015;7:1–10. doi: 10.1186/s13195-015-0096-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sevigny J., Chiao P., Bussière T., Weinreb P.H., Williams L., Maier M. The antibody aducanumab reduces Aβ plaques in Alzheimer's disease. Nature. 2016;537:50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 17.Hampel H., Lista S., Khachaturian Z.S. Development of biomarkers to chart all Alzheimer's disease stages: the royal road to cutting the therapeutic Gordian Knot. Alzheimers Dement. 2012;8:312–336. doi: 10.1016/j.jalz.2012.05.2116. [DOI] [PubMed] [Google Scholar]
- 18.Blennow K., Dubois B., Fagan A.M., Lewczuk P., de Leon M.J., Hampel H. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer's disease. Alzheimers Dement. 2015;11:58–69. doi: 10.1016/j.jalz.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Deyn P.P. Dementia: cerebrospinal fluid biomarkers in dementias. Nat Rev Neurol. 2015;11:549–550. doi: 10.1038/nrneurol.2015.175. [DOI] [PubMed] [Google Scholar]
- 20.Skillbäck T., Farahmand B.Y., Rosén C., Mattsson N., Nägga K., Kilander L. Cerebrospinal fluid tau and amyloid-β1-42 in patients with dementia. Brain. 2015;138:2716–2731. doi: 10.1093/brain/awv181. [DOI] [PubMed] [Google Scholar]
- 21.Engelborghs S., De Vreese K., Van de Casteele T., Vanderstichele H., Van Everbroeck B., Cras P. Diagnostic performance of a CSF-biomarker panel in autopsy-confirmed dementia. Neurobiol Aging. 2008;29:1143–1159. doi: 10.1016/j.neurobiolaging.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Schoonenboom N.S., Reesink F.E., Verwey N.A., Kester M.I., Teunissen C.E., van de Ven P.M. Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology. 2012;78:47–54. doi: 10.1212/WNL.0b013e31823ed0f0. [DOI] [PubMed] [Google Scholar]
- 23.Alcolea D.A., Carmona-Iragui M., Suárez-Calvet M., Sánchez-Saudinós M.B., Sala I., Antón-Aguirre S. Relationship between β-secretase, inflammation and core cerebrospinal fluid biomarkers for Alzheimer's disease. J Alzheimers Dis. 2014;42:157–167. doi: 10.3233/JAD-140240. [DOI] [PubMed] [Google Scholar]
- 24.Jack C.R., Albert M.S., Knopman D.S., McKhann G.M., Sperling R.A., Carrillo M.C. Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duits F.H., Martinez-Lage P., Paquet C., Engelborghs S., Lleó A., Hausner L. Performance and complications of lumbar puncture in memory clinics: results of the multicenter lumbar puncture feasibility study. Alzheimers Dement. 2016;12:154–163. doi: 10.1016/j.jalz.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Hort J., O'Brien J.T., Gainotti G., Pirttila T., Popescu B.O., Rektorova I. EFNS guidelines for the diagnosis and management of Alzheimer's disease. Eur J Neurol. 2010;17:1236–1248. doi: 10.1111/j.1468-1331.2010.03040.x. [DOI] [PubMed] [Google Scholar]
- 28.Duits F.H., Prins N.D., Lemstra A.W., Pijnenburg Y.A., Bouwman F.H., Teunissen C.E. Diagnostic impact of CSF biomarkers for Alzheimer's disease in a tertiary memory clinic. Alzheimers Dement. 2015;11:523–532. doi: 10.1016/j.jalz.2014.05.1753. [DOI] [PubMed] [Google Scholar]
- 29.Le Bastard N., De Deyn P.P., Engelborghs S. Importance and impact of preanalytical variables on Alzheimer disease biomarker concentrations in cerebrospinal fluid. Clin Chem. 2015;61:734–743. doi: 10.1373/clinchem.2014.236679. [DOI] [PubMed] [Google Scholar]
- 30.Tamaoka A., Sekijima Y., Matsuno S., Tokuda T., Shoji S., Ikeda S.I. Amyloid beta protein species in cerebrospinal fluid and in brain from patients with Down's syndrome. Ann Neurol. 1999;46:933. doi: 10.1002/1531-8249(199912)46:6<933::aid-ana20>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 31.Englund H., Annerén G., Gustafsson J., Wester U., Wiltfang J., Lannfelt L. Increase in beta-amyloid levels in cerebrospinal fluid of children with Down syndrome. Dement Geriatr Cogn Disord. 2007;24:369–374. doi: 10.1159/000109215. [DOI] [PubMed] [Google Scholar]
- 32.Portelius E., Soininen H., Andreasson U., Zetterberg H., Persson R., Karlsson G. Exploring Alzheimer molecular pathology in Down's syndrome cerebrospinal fluid. Neurodegener Dis. 2014;14:98–106. doi: 10.1159/000358800. [DOI] [PubMed] [Google Scholar]
- 33.Portelius E., Westman-Brinkmalm A., Zetterberg H., Blennow K. Determination of beta-amyloid peptide signatures in cerebrospinal fluid using immunoprecipitation-mass spectrometry. J Proteome Res. 2006;5:1010–1016. doi: 10.1021/pr050475v. [DOI] [PubMed] [Google Scholar]
- 34.Tapiola T., Lehtovirta M., Ramberg J., Helisalmi S., Linnaranta K., Riekkinen P. CSF tau is related to apolipoprotein E genotype in early Alzheimer's disease. Neurology. 1998;50:169–174. doi: 10.1212/wnl.50.1.169. [DOI] [PubMed] [Google Scholar]
- 35.Portelius E., Hölttä M., Soininen H., Bjerke M., Zetterberg H., Westerlund A. Altered cerebrospinal fluid levels of amyloid β and amyloid precursor-like protein 1 peptides in Down's syndrome. Neuromolecular Med. 2014;16:510–516. doi: 10.1007/s12017-014-8302-1. [DOI] [PubMed] [Google Scholar]
- 36.Hartley D., Blumenthal T., Carrillo M.C., DiPaolo G., Esralew L., Gardiner K.J. Down syndrome and Alzheimer's disease: common pathways, common goals. Alzheimers Dement. 2015;11:700–709. doi: 10.1016/j.jalz.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wark S., Hussain R., Parmenter T. Down syndrome and dementia: is depression a confounder for accurate diagnosis and treatment? J Intellect Disabil. 2014;18:305–314. doi: 10.1177/1744629514552152. [DOI] [PubMed] [Google Scholar]
- 38.Andreasen N., Blennow K. CSF biomarkers for mild cognitive impairment and early Alzheimer's disease. Clin Neurol Neurosurg. 2005;107:165–173. doi: 10.1016/j.clineuro.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 39.Peskind E., Nordberg A., Darreh-Shori T., Soininen H. Safety of lumbar puncture procedures in patients with Alzheimer's disease. Curr Alzheimer Res. 2009;6:290–292. doi: 10.2174/156720509788486509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alcolea D.A., Martínez-Lage P., Izagirre A., Clerigué M., Carmona-Iragui M., Alvarez R.M. Feasibility of lumbar puncture in the study of cerebrospinal fluid biomarkers for Alzheimer's disease: a multicenter study in Spain. J Alzheimers Dis. 2014;39:719–726. doi: 10.3233/JAD-131334. [DOI] [PubMed] [Google Scholar]
- 41.Carmona-Iragui M., Santos T., Videla S., Fernández S., Benejam B., Videla L. Feasibility of lumbar puncture in the study of cerebrospinal fluid biomarkers for Alzheimer's disease in subjects with Down syndrome. J Alzheimers Dis. 2017;55:1489–1496. doi: 10.3233/JAD-160827. [DOI] [PubMed] [Google Scholar]
- 42.Coppus A.M., Schuur M., Vergeer J., Janssens A.C., Oostra B.A., Verbeek M.M. Plasma beta amyloid and the risk of Alzheimer's disease in Down syndrome. Neurobiol Aging. 2012;33:1988–1994. doi: 10.1016/j.neurobiolaging.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Freeman S.H., Raju S., Hyman B.T., Frosch M.P., Irizarry M.C. Plasma Abeta levels do not reflect brain Abeta levels. J Neuropathol Exp Neurol. 2007;66:264–271. doi: 10.1097/NEN.0b013e31803d3ae4. [DOI] [PubMed] [Google Scholar]
- 44.Le Bastard N., Aerts L., Leurs J., Blomme W., De Deyn P.P., Engelborghs S. No correlation between time-linked plasma and CSF Abeta levels. Neurochem Int. 2009;55:820–825. doi: 10.1016/j.neuint.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 45.AC Immune SA. A Phase Ib Multi-Center, Double-Blind, Randomized, Placebo-Controlled Dose Escalation Study of the safety, tolerability and immunogenicity of ACI-24 in adults with Down syndrome. Clin Identifier NCT02738450 2016. Available at: https://clinicaltrials.gov/ct2/show/study/NCT02738450?term=Trisomy+21+AND+CSF&rank=1. Accessed January 5, 2017.
- 46.University of Colorado Denver. Rocky Mountain Alzheimer's Disease Center at the University of Colorado School of Medicine (RMADC at UCSOM) Longitudinal Biomarker and Clinical Phenotyping Study. Clin Identifier NCT02612376 2015. Available at: https://clinicaltrials.gov/ct2/show/study/NCT02612376?term=Trisomy+21+AND+CSF&rank=2. Accessed January 5, 2017.
- 47.Rafii M.S., Wishnek H., Brewer J.B., Donohue M.C., Ness S., Mobley W.C. The Down syndrome biomarker initiative (DSBI) pilot: proof of concept for deep phenotyping of Alzheimer's disease biomarkers in Down syndrome. Front Behav Neurosci. 2015;9:239. doi: 10.3389/fnbeh.2015.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palmqvist S., Mattsson N., Hansson O. Cerebrospinal fluid analysis detects cerebral amyloid-β accumulation earlier than positron emission tomography. Brain. 2016;139:1226–1236. doi: 10.1093/brain/aww015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim D., Kim Y.S., Shin D.W., Park C.S., Kang J.H. Harnessing cerebrospinal fluid biomarkers in Clinical Trials for treating Alzheimer's and Parkinson's diseases: potential and challenges. J Clin Neurol. 2016;12:381–392. doi: 10.3988/jcn.2016.12.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kay A.D., Schapiro M.B., Riker A.K., Haxby J.V., Rapoport S.I., Cutler N.R. Cerebrospinal fluid monoaminergic metabolites are elevated in adults with Down's syndrome. Ann Neurol. 1987;21:408–411. doi: 10.1002/ana.410210416. [DOI] [PubMed] [Google Scholar]
- 51.Schapiro M.B., Kay A.D., May C., Ryker A.K., Haxby J.V., Kaufman S. Cerebrospinal fluid monoamines in Down's syndrome adults at different ages. J Ment Defic Res. 1987;31:259–269. doi: 10.1111/j.1365-2788.1987.tb01369.x. [DOI] [PubMed] [Google Scholar]
- 52.Elovaara I. Proteins in serum and cerebrospinal fluid in demented patients with Down's syndrome. Acta Neurol Scand. 1984;69:302–305. doi: 10.1111/j.1600-0404.1984.tb07817.x. [DOI] [PubMed] [Google Scholar]
- 53.Atack J.R., Rapoport S.I., Schapiro M.B. Cerebrospinal fluid production is normal in Down syndrome. Neurobiol Aging. 1998;19:307–309. doi: 10.1016/s0197-4580(98)00069-4. [DOI] [PubMed] [Google Scholar]
- 54.Fortea J., Carmona-Iragui M., Fernandez S., Benejam B., Videla L., Alcolea D. Down Alzheimer Barcelona Neuroimaging Initiative (DABNI): a prospective longitudinal biomarker cohort to study Alzheimer's disease in Down syndrome. Alzheimers Dement. 2016;12:P380–P381. [Google Scholar]
- 55.Aalten P., Ramakers I.H., Biessels G.J., De Deyn P.P., Koek H.L., OldeRikkert M.G. The Dutch Parelsnoer Institute—neurodegenerative diseases; methods, design and baseline results. BMC Neurol. 2014;14:254. doi: 10.1186/s12883-014-0254-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dekker A.D., Coppus A.M., Vermeiren Y., Aerts T., van Duijn C.M., Kremer B.P. Serum MHPG strongly predicts conversion to Alzheimer's disease in behaviorally characterized subjects with Down syndrome. J Alzheimers Dis. 2015;43:871–891. doi: 10.3233/JAD-140783. [DOI] [PubMed] [Google Scholar]
- 57.Herbert M.K., Aerts M.B., Kuiperij H.B., Claassen J.A., Spies P.E., Esselink R.A. Addition of MHPG to Alzheimer's disease biomarkers improves differentiation of dementia with Lewy bodies from Alzheimer's disease but not other dementias. Alzheimers Dement. 2014;10:448–455. doi: 10.1016/j.jalz.2013.05.1775. e2. [DOI] [PubMed] [Google Scholar]
- 58.Wilcock D.M. Neuroinflammation in the aging Down syndrome brain; lessons from Alzheimer's disease. Curr Gerontol Geriatr Res. 2012;2012:170276. doi: 10.1155/2012/170276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iulita M.F., Ower A., Barone C., Pentz R., Gubert P., Romano C. An inflammatory and trophic disconnect biomarker profile revealed in Down syndrome plasma: relation to cognitive decline and longitudinal evaluation. Alzheimers Dement. 2016;12:1132–1148. doi: 10.1016/j.jalz.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 60.Naude P.J., Dekker A.D., Coppus A.M., Vermeiren Y., Eisel U.L., van Duijn C.M. Serum NGAL is associated with distinct plasma amyloid-beta peptides according to the clinical diagnosis of dementia in Down syndrome. J Alzheimers Dis. 2015;45:733–743. doi: 10.3233/JAD-142514. [DOI] [PubMed] [Google Scholar]
- 61.Dekker A.D., De Deyn P.P., Rots M.G. Epigenetics: the neglected key to minimize learning and memory deficits in Down syndrome. Neurosci Biobehav Rev. 2014;45C:72–84. doi: 10.1016/j.neubiorev.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 62.Janel N., Sarazin M., Corlier F., Corne H., de Souza L.C., Hamelin L. Plasma DYRK1A as a novel risk factor for Alzheimer's disease. Transl Psychiatry. 2014;4:e425. doi: 10.1038/tp.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martin S.B., Dowling A.L., Lianekhammy J., Lott I.T., Doran E., Murphy M.P. Synaptophysin and synaptojanin-1 in Down syndrome are differentially affected by Alzheimer's disease. J Alzheimers Dis. 2014;42:767–775. doi: 10.3233/JAD-140795. [DOI] [PMC free article] [PubMed] [Google Scholar]