Highlights
-
•
We report a case of intramuscular hemangioma mimicking osteoid osteoma.
-
•
Magnetic resonance image (MRI) is the most precise diagnostic tool for the identification of soft-tissue mass adjacent to the bone.
-
•
Precise preoperative diagnosis is essential to avoid excessive surgery.
Keywords: Intramuscular hemangioma, Periosteal reaction, Osteoid osteoma
Abstract
Introduction
Intramuscular hemangioma in the periosteal region is rare. Although comprising less than 1% of all hemangiomas, they represent the most common type of intramuscular tumors. When located adjacent to bone, a periosteal reaction can occur. The deep localization of the hemangioma poses the diagnosis difficult. Only 8% to 19% of cases were diagnosed before surgery according to the literature review.
Presentation of case
We present a case of forty-one-year-old female diagnosed with intramuscular hemangioma, mimicking osteoid osteoma, adjacent to the periosteal region of tibia diaphysis treated by surgical excision.
Discussion
When intramuscular hemangioma occurs nearby a bone structure, it can cause cortical, medullary and periosteal bone changes that are frequently misdiagnosed by plain radiography. Due to their infrequency, deep location, and atypical presentation, these lesions are seldom diagnosed at presentation. The hemangioma of the periosteal region can be locally destructive due to compression exerted on neighboring structures. It does not regress spontaneously, and surgical excision is frequently needed.
Conclusion
Intramuscular hemangioma of periosteal region occurs most commonly adjacent to long bones of the lower limb. They can cause hypertrophic periosteal reactions mimicking a periosteal or parosteal tumor. Although osteoid osteoma was considered in the differential diagnosis, MRI with enhancement should be performed to exclude intramuscular hemangioma. This may avoid unnecessary aggressive en-bloc tumor excisions resulting in bone weakness and prolonged rehabilitation.
This case report has been written in line with the SCARE criteria (Agha et al., 2016 [1]).
1. Introduction
Intramuscular hemangiomas are benign neoplastic proliferations of blood vessels that arise from skeletal muscle. Although comprising less than 1% of all hemangiomas, they represent the most common type of intramuscular tumors [2], [3] with a preoperative diagnostic rate of only 8%–19% [4]. When intramuscular hemangioma occurs nearby a bone structure, it can cause cortical, medullary and periosteal bone changes that are frequently misdiagnosed by plain radiography. Due to their infrequency, deep location, and atypical presentation, these lesions are seldom diagnosed at presentation. The hemangioma of the periosteal region can be locally destructive due to compression exerted on neighboring structures. It does not regress spontaneously, and surgical excision is frequently needed [5]. By recognizing that skeletal muscle is the most common site of deeply located hemangiomas, should orthopedic surgeons avoid misdiagnosis of this pathology and evolve a better understanding of its clinical and radiographic presentation. We reported a case of intramuscular hemangioma diagnosed by histology and localized adjacent to the bone in the lower limb with periosteal reaction mimicking an osteoid osteoma.
2. Presentation of case
A 41-year-old female presented with a 6-year history of calf pain in her right lower leg. Initial conservative therapy at a local medical doctor that consisted of aspirin or no steroid anti-inflammatory drugs failed to alleviate the patient’s symptoms. The pain progressed to the point of limiting her normal daily activities in the last three months. Although typical night pain was not observed, minimal pressure on the affected area resulted in severe pain that awakens her from sleep. There was no history of prior trauma or infection. The remaining medical history was unremarkable. On physical examination, there was a significant amount of pain elicited with palpation on mid-calf. No local palpable mass, warmth or bruit was noted. There was no obvious skin change overlying the painful area. The range of movements of knee and ankle were full and free. All laboratory studies were normal. Plain radiographs showed the irregular sclerotic periosteal reaction of the posterior mid-diaphyseal cortex of the right tibia. Although the mass lesion was not well visualized on images in the anteroposterior plane, it was well pictured on the lateral projection. There was no associated osseous destruction or fracture, nor were there calcifications adjacent to the mass. Soft tissue abnormalities were not identified within the tibia, and the visualized knee and ankle joints appeared normal (Fig. 1). Axial computed tomography (CT) scans with contrast revealed a cortically based sclerotic lesion that emanated from the posterior aspect of the tibial diaphysis (2.01 cm × 4.79 cm × 0.67 cm in the sagittal, coronal, and axial planes, respectively). There was no identifiable cortical breakthrough (Fig. 2).
Fig 1.
Plain radiograph showing periosteal reaction with cortical hypertrophy of the posterior mid-diaphyseal segment of the right tibia (white arrow).
Fig. 2.
Axial CT scan revealed a cortically based sclerotic lesion emanating from the posterior aspect of the tibial diaphysis (white arrow). A poorly defined high density heterogeneous mass was seen immediately posterior to the cortically based lesion, within the adjacent musculature, likely representing a phlebolith (red circle).
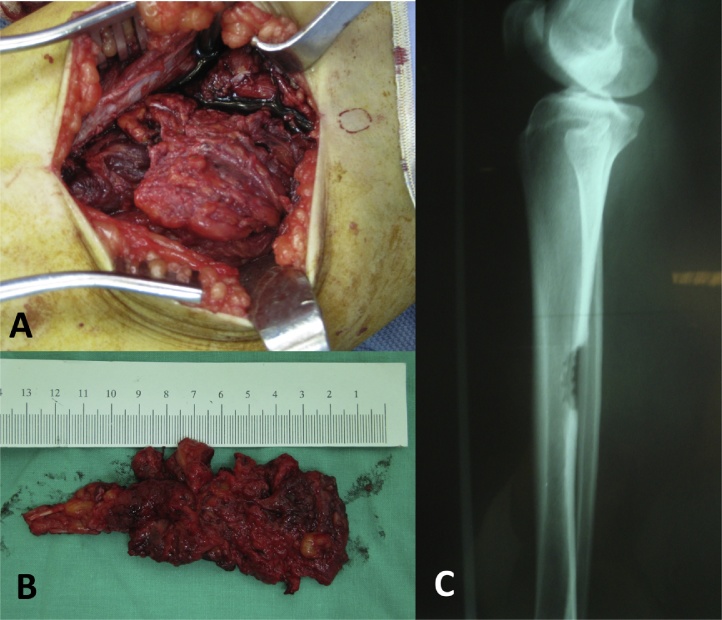
Although the clinical symptoms and the radiological assessment were not typical, osteoid osteoma was still a concern, and other pathological osteo skeletal processes such as periostitis, eosinophilic granuloma, hemangioma of bone, non-ossifying fibroma, Paget's disease and avascular necrosis of the bone also should be considered. An open biopsy was performed. The operative strategy was pre-planned based on the preoperative imaging findings, dictating the position of the patient on the operating table. The palpable tender point over the posterior aspect of the right lower leg was marked using dermographic pencil. The lesion was localized and needle trajectory planned using a fluoroscopic guiding. The patient was set in prone position and under spinal anesthesia. A tourniquet was applied to the affected extremity prepped aseptically in the usual way. An incision was made longitudinally over middle calf according to the landmark prepared previously. The skin and soft tissue were dissected deep to periosteal area. The periosteum was dissected with elevator and cortical margins just underneath the posterior border of the shinbone. A large friable soft, lobulated mass, measuring 10 cm × 4 cm × 2 cm in length, wide and thickness respectively, was located on the surface of the tibial bone, interpolated within the soleus muscle fibers, and eroding the tibial cortex without evidence of intramedullary involvement (Fig. 3A). The thickened cortex was excised en-bloc with the adjacent soft tumorous mass (Fig. 3B and C). The resection was made with a little saw and osteotome.
Fig. 3.
A large friable soft lobulated dark-red mass was located on the surface of the hypertrophic tibia bone and interpolated within the soleus muscle fibers. Photographs showed the tumorous mass before (A) and after (B) surgical excision. (C) Plain radiograph showing en-bloc excision of the hypertrophic cortex of the posterior mid-diaphyseal segment of the right tibia.
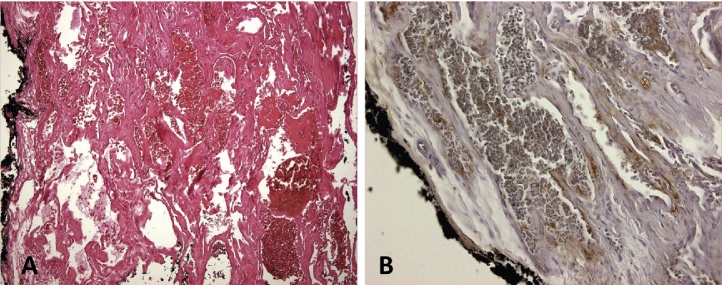
Histology revealed poorly circumscribed dilated vascular channels in a loose fibrous stroma interspersed between striated muscle bundles (Fig. 4A). The presence of cells that stained positively for factor VIII confirmed the diagnosis of intramuscular cavernous hemangioma (Fig. 4B). The cortical bone was normal histologically, and a reactive sclerosis was identified. No nidus was found.
Fig. 4.
Microscopically (hematoxylin-eosin, magnification 100×), red blood cells are visible within multiple dilated vascular channels (A) with interpositions of fibrous stroma and surrounded by skeletal muscle. The lining endothelial cells are stained for factor VIII (B). No nidus was found.
The patient was involved in light exercises and partial weight bearing walk for three weeks and then allowed to increase activity level as tolerated. Physical therapy was focused on muscle strengthening, proprioception, and range of motion exercises. Within one month, the patient reported much pain relief and discontinued all pain medications. Full weight bearing walk with no external support was permitted at the sixth-month follow-up. The symptoms resolved after the surgery and at eight months’ follow-up, she was pain-free without evidence of tumor recurrence.
3. Discussion
Intramuscular hemangioma in the periosteal region is rare [3], [6], [7] and account for 0.8% of all benign vascular tumors [8]. The diagnosis is usually difficult due to the deep localization of the hemangioma. Approximately 45% of described cases were located in the lower extremities, occurring predominantly in the 3rd decades of life in 90% of cases [4], [9], [10]. The highest prevalence was noted in young women [9]. The typical presentation is a painful mass, usually not associated with cutaneous changes [2], [11]. Because of its location in the deep tissue, painful tendon contracture and functional impairment of the extremity can be complications of this slow growing tumor [4].
The hemangioma of the periosteal region can be locally destructive due to compression exerted on neighboring structures. It most commonly affects the diaphysis of fibula, tibia or ulna [2]. The mechanism by which it causes bone changes is unknown. Plain radiographic findings have been described as cortical thickening or depression, periosteal reaction, soft tissue mass and osteopenia [6], [7], [12], [13], [14], [15], [16], [17], [18], [19], [20]. Unusual calcification with phleboliths were also reported. Histological and radiographic correlations are essential for its differential diagnosis from other pathological processes [21].
Intramuscular hemangioma does not undergo spontaneous regression. Definitive treatment is surgical excision. Lesions which do not enlarge or is associated with only minor local discomfort can be treated with a more conservative approach [13]. No barely, persistent pain and cortical thickening of the tibia shaft can mimic clinical symptoms of osteoid osteoma [22]. In such cases, affected individuals are usually male patients in their second and third decades of life, complaining of severe night pain that extends from weeks to years, only relieved by the use of nonsteroidal anti-inflammatory drugs (NSAIDs). However, these classical symptoms are only present in no more than one-third of cases of patients with osteoid osteoma [23].
In our reported case, the clinical presentation simulated an osteoid osteoma though the nidus could not be found at CT scan, surgical treatment was performed with open biopsy and removal of the soft tissue mass and involved adjacent tibial cortex (Fig. 3B). It made the need for weight-bearing protection with crutches for more than half year. According to Kudawara et al., magnetic resonance image (MRI) gives the most precise diagnostic information for the identification of soft tissue mass adjacent to bone and distinction of intramuscular hemangiomata from other soft tissue tumors, both benign and malignant [13]. Unfortunately, our case was misdiagnosed initially as osteoid osteoma, and MRI was not performed.
4. Conclusion
In summary, intramuscular hemangioma of periosteal region occurs most commonly adjacent to long bones of the lower limb. They can cause hypertrophic periosteal reactions mimicking a periosteal or parosteal tumor. Although osteoid osteoma should be considered in the differential diagnosis, MRI with enhancement should be performed to exclude intramuscular hemangioma. This applies in particular when the clinical symptom is atypical and the absence of nidus of osteoid osteoma on the CT scans. In our presented case, the initial failure to obtain MRI has resulted in unnecessarily aggressive en-bloc excision of the tumor. This may create more postoperative pain and blood loss as well. The extensive bone defect and subsequent structural bone weakness enable the delayed weight bearing of the affected lower limb, and prolonged rehabilitative process is mandatory.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Conflicts of interest
The authors have no conflict of interest to declare.
Funding
There is no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Approval to publish case report is waived by the institution.
Author contributions
Ya-Lin Yeh, MD: make substantial contributions to conception and design, acquisition of data, analysis, interpretation of data and manuscript writing.
Shu-I Yeh, MD: participate in revising the manuscript critically for important intellectual content.
Chih-Ting Cheng, MD: participate in drafting the article and revising it critically for important intellectual content.
Guarantor
This is not a research study. All authors read and approved the final manuscript. Therefore, all authors are responsible for this article.
Ya-Lin Yeh, M.D.
Chih-Ting Cheng, M.D.
Acknowledgement
Not applicable.
Footnotes
No proprietary interest by any of the authors was given to any product or manufacturer mentioned.
References
- 1.Agha R.A. The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Memis A. Magnetic resonance imaging of intramuscular haemangiomas with emphasis on contrast enhancement patterns. Clin. Radiol. 1996;51(3):198–204. doi: 10.1016/s0009-9260(96)80323-0. [DOI] [PubMed] [Google Scholar]
- 3.Welsh D., Hengerer A.S. The diagnosis and treatment of intramuscular hemangiomas of the masseter muscle. Am. J. Otolaryngol. 1980;1(2):186–190. doi: 10.1016/s0196-0709(80)80014-7. [DOI] [PubMed] [Google Scholar]
- 4.Shallow T.A., Eger S.A., Wagner F.B. Primary hemangiomatous tumors of skeletal muscle. Ann. Surg. 1944;119(5):700–740. doi: 10.1097/00000658-194405000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goto T. Soft-tissue haemangioma and periosteal new bone formation on the neighbouring bone. Arch. Orthop. Trauma Surg. 2001;121(10):549–553. doi: 10.1007/s004020100302. [DOI] [PubMed] [Google Scholar]
- 6.Loxley S.S., Thiemeyer J.S., Jr., Ellsasser J.C. Periosteal hemangioma. A report of two cases. Clin. Orthop. Relat. Res. 1972;85:151–154. [PubMed] [Google Scholar]
- 7.Sugiura I. Tibial periosteal hemangioma. Clin. Orthop. Relat. Res. 1975;106:242–244. doi: 10.1097/00003086-197501000-00035. [DOI] [PubMed] [Google Scholar]
- 8.Jin W. Intramuscular hemangioma with ossification: emphasis on sonographic findings. J. Ultrasound Med. 2008;27(2):281–285. doi: 10.7863/jum.2008.27.2.281. [DOI] [PubMed] [Google Scholar]
- 9.Allen P.W., Enzinger F.M. Hemangioma of skeletal muscle. An analysis of 89 cases. Cancer. 1972;29(1):8–22. doi: 10.1002/1097-0142(197201)29:1<8::aid-cncr2820290103>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 10.Scott J.E. Haemangiomata in skeletal muscle. Br. J. Surg. 1957;44(187):496–501. doi: 10.1002/bjs.18004418713. [DOI] [PubMed] [Google Scholar]
- 11.Melman L., Johnson F.E. Intramuscular cavernous hemangioma. Am. J. Surg. 2008;195(6):816–817. doi: 10.1016/j.amjsurg.2007.08.064. [DOI] [PubMed] [Google Scholar]
- 12.Pena J.M. Periosteal hemangioma of left fibula. Case report 324. Skeletal Radiol. 1985;14(2):133–135. doi: 10.1007/BF00349749. [DOI] [PubMed] [Google Scholar]
- 13.Kudawara I. Intramuscular haemangioma adjacent to the bone surface with periosteal reaction: report of three cases and review of the literature. J. Bone Joint Surg. Br. 2001;83(5):659–662. doi: 10.1302/0301-620x.83b5.11697. [DOI] [PubMed] [Google Scholar]
- 14.Hall F.M. Case report 131: periosteal hemangioma of the fibula. Skeletal Radiol. 1980;5(4):275–278. doi: 10.1007/BF00580606. [DOI] [PubMed] [Google Scholar]
- 15.Kenan S. Subperiosteal hemangioma. A case report and review of the literature. Clin. Orthop. Relat. Res. 1988;232:279–283. [PubMed] [Google Scholar]
- 16.Devaney K., Vinh T.N., Sweet D.E. Surface-based hemangiomas of bone. A review of 11 cases. Clin. Orthop. Relat. Res. 1994;300:233–240. [PubMed] [Google Scholar]
- 17.DeFilippo J.L. Soft tissue hemangioma with adjacent periosteal reaction simulating a primary bone tumor. Skeletal Radiol. 1996;25(2):174–177. doi: 10.1007/s002560050057. [DOI] [PubMed] [Google Scholar]
- 18.Johnson E.W., Jr., Ghormley R.K., Dockerty M.B. Hemangiomas of the extremities. Surg. Gynecol. Obstet. 1956;102(5):531–538. [PubMed] [Google Scholar]
- 19.Greenspan A. Imaging strategies in the evaluation of soft-tissue hemangiomas of the extremities: correlation of the findings of plain radiography, angiography, CT, MRI, and ultrasonography in 12 histologically proven cases. Skeletal Radiol. 1992;21(1):11–18. doi: 10.1007/BF00243086. [DOI] [PubMed] [Google Scholar]
- 20.Sung M.S., Kang H.S., Lee H.G. Regional bone changes in deep soft tissue hemangiomas: radiographic and MR features. Skeletal Radiol. 1998;27(4):205–210. doi: 10.1007/s002560050366. [DOI] [PubMed] [Google Scholar]
- 21.Jackson R.P., Reckling F.W. Intracortical and subperiosteal lesion of unknown etiology. Clin. Orthop. Relat. Res. 2016;1978(130):260–262. [PubMed] [Google Scholar]
- 22.Kransdorf M.J. Osteoid osteoma. Radiographics. 1991;11(4):671–696. doi: 10.1148/radiographics.11.4.1887121. [DOI] [PubMed] [Google Scholar]
- 23.Lee E.H., Shafi M., Hui J.H. Osteoid osteoma: a current review. J. Pediatr. Orthop. 2006;26(5):695–700. doi: 10.1097/01.bpo.0000233807.80046.7c. [DOI] [PubMed] [Google Scholar]