Abstract
Reporting progress against targets for international biodiversity agreements is hindered by a shortage of suitable biodiversity data. We describe a cost-effective system involving Reef Life Survey citizen scientists in the systematic collection of quantitative data covering multiple phyla that can underpin numerous marine biodiversity indicators at high spatial and temporal resolution. We then summarize the findings of a continental- and decadal-scale State of the Environment assessment for rocky and coral reefs based on indicators of ecosystem state relating to fishing, ocean warming, and invasive species and describing the distribution of threatened species. Fishing impacts are widespread, whereas substantial warming-related change affected some regions between 2005 and 2015. Invasive species are concentrated near harbors in southeastern Australia, and the threatened-species index is highest for the Great Australian Bight and Tasman Sea. Our approach can be applied globally to improve reporting against biodiversity targets and enhance public and policymakers’ understanding of marine biodiversity trends.
Keywords: Convention on Biological Diversity, state of the environment, ecological indicator, Marine Trophic Index, community temperature index
A number of major international commitments and initiatives recognize the importance of biodiversity and healthy ecosystems, including the Convention on Biological Diversity (CBD; 1993), the UN Sustainable Development Goals (2016), and the EU Marine Strategy Framework Directive (MSFD). Targets associated with these, or policies implemented within countries in response to such commitments, require the assessment of and reporting on biodiversity status and trends across large scales (Scholes et al. 2008). For example, assessing progress toward a number of the Aichi targets for the CBD requires understanding biodiversity change in particular ecosystems and in relation to particular pressures or threats arising from human activities.
A proliferation of proposed indicator frameworks has evolved to assist with broadscale biodiversity assessments (Niemeijer and de Groot 2008, Pereira et al. 2013, Andersen et al. 2014), adding to a wealth of studies providing conceptual bases and the justification of indicators that summarize important characteristics or proxies of biodiversity (Loh et al. 2005, Butchart et al. 2007, Halpern et al. 2012). Such progress has arguably greatly outpaced the capacity to collect the data needed to underpin these indicators, as well as broader conservation efforts (Green et al. 2005). The majority of biodiversity assessments at a national scale or larger have necessarily required inferring status and trends through indirect means or using data that were collected for a different purpose (e.g., fisheries catch data). The Census of Marine Life provided a quantum advance in our understanding of the richness of life in the sea and provided a snapshot of abundance in some areas (Costello et al. 2010, Pauly and Froese 2010), but a shortfall persists in the development of suitable ongoing mechanisms for the collection of broadscale data—particularly those with sufficient detail to calculate responsive biodiversity indicators that can be clearly linked to pressures (Jones et al. 2011).
A consequence of the paucity of quantitative data for the assessment of biodiversity state and trends through time is that the majority of indicators applied (of the many proposed) have tended to be those associated with measuring pressures, drivers, or threats to biodiversity. Pressure indicators related to human activities (e.g., fishing effort or land use) are clearly required for reporting changing management practices, but more detailed biodiversity data across larger scales are needed to determine the realized impacts of changing pressures (Walpole et al. 2009, Butchart et al. 2010, Pereira et al. 2013). A report to the CBD concluded that “though a wide range of biodiversity information was available, it is unlikely that it would be possible to completely monitor progress towards the achievement of the Aichi Biodiversity Targets with current monitoring systems and indicators” (GEO BON 2011).
This is particularly true for the marine realm, where ecosystem condition is invisible to most of the human population. Declines in particular fish stocks may result in species replacements within markets that go unnoticed by the public (Miller and Mariani 2010), while changing community structure from ocean warming may result in numerous unrecognized broadscale changes in ecological functions for every local change that is readily observable. To date, data from commercial fishery catches have been extensively used for broadscale marine biodiversity assessments, including those designed to measure progress toward marine CBD targets (e.g., the Marine Trophic Index; Pauly et al. 1998, Pauly and Watson 2005). But there are some obvious limitations for measuring biodiversity state from fisheries catch data, including the limited subset of species considered and the confounding influences of fisher behavior (Branch et al. 2010, Shannon et al. 2014). It is ironic that one of the primary means of measuring the state of the world's marine biodiversity to date has been based on animals removed from the system.
Many biodiversity indicators could usefully inform national and international marine biodiversity assessments if suitable quantitative data were available. There has been growing impetus for a global marine biodiversity observation network to collect such data (Duffy et al. 2013). For example, the Smithsonian Institution has recently initiated the Marine Global Earth Observatory (MarineGEO), which includes coordinated field experiments and surveys to provide a functional perspective on biodiversity change from small to large scales. The Global Ocean Observing System (GOOS) has also recently developed a Biology and Ecosystems Panel with the aim of expanding its success in supporting monitoring of the physical ocean to its biota. In this article, we describe an application whereby scientific monitoring programs are greatly extended and integrated with a complementary cost-effective system, the Reef Life Survey program (RLS), to quantify ecological community structure in a standardized manner.
Reef Life Survey is a citizen-science program based on selective recruitment and intensive training of committed volunteer SCUBA divers, with the aim of greatly enhancing capacity in data generation without sacrificing detail and narrowing the potential range of applications. Since the establishment of the RLS in 2008, more than 230 divers have contributed 9300 surveys at more than 3000 sites in 49 countries, 7 continents, and 90 of the world's shallow marine ecoregions (as defined by Spalding et al. 2007). The program focuses on shallow rocky and coral reefs, which not only harbor the greatest concentrations of biodiversity in the sea (Roberts et al. 2002) but are also where major human pressures are often greatest. Fishing, climate change, pollution, and invasive species have consistently been recognized as the most serious and pervasive threats to marine biodiversity and can all be present and interacting on coastal reefs (Edgar et al. 2005, Crain et al. 2009).
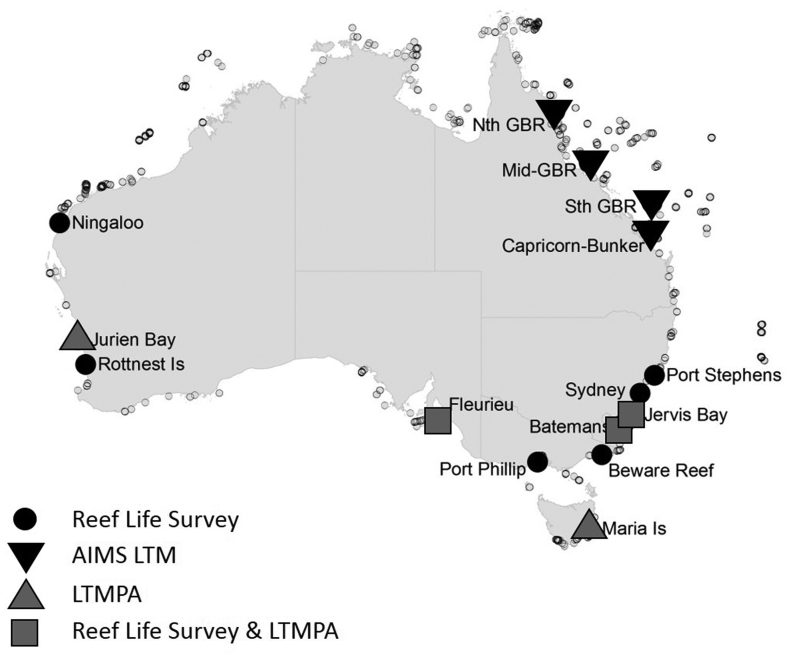
The RLS survey methods are globally standardized, based on underwater visual censuses along 50-meter transects. Each survey includes three separate methods: for fishes and larger mobile fauna, mobile invertebrates and cryptic fishes, and photoquadrats of substrate cover—together covering the majority of large biota on reefs that can be surveyed visually (i.e., more than 2.5 centimeters, cm, in size). Data collection occurs through three primary mechanisms: (1) the annual targeted monitoring of reef sites at dispersed locations in temperate and tropical waters (as is shown around Australia in figure 1), (2) targeted voyages of discovery to poorly surveyed locations, and (3) ad hoc data collection by divers in their local waters and when on vacation. The direction of the former two mechanisms by scientists in the field, as well as scientists and managers in the RLS advisory committee, minimizes sources of location bias, such as targeting the most accessible or attractive dive locations. Further efforts to minimize sources of bias (Bird et al. 2014) and assess the data quality of volunteer divers have been described in Edgar and Stuart-Smith (2009, 2014), including analyses that demonstrated that trained RLS volunteers produced data indistinguishable from those collected by professional scientists.
Figure 1.
A map of Reef Life Survey (RLS) sites surveyed from 2010 to 2015 and used in spatial analyses (small symbols, n = 1294), as well as long-term monitoring locations from RLS (n = 357), the Long-Term Temperate Marine Protected Area Monitoring (LTMPA; n = 182 sites) program, and the Australian Institute of Marine Science Long-Term Monitoring (AIMS LTM; n = 276 sites) program used for temporal trend assessment.
For terrestrial systems, amateur bird-watching initiatives have provided a wealth of species-level data that have allowed the incorporation of this taxonomic group in indicators for tracking progress toward relevant Aichi targets (Butchart et al. 2010, BirdLife International 2012). Here, we show how coordinated citizen science involving the systematic application of quantitative methods can contribute similarly to marine biodiversity assessments as a standalone data source and by complementing scientific programs. We synthesize results from the first Australian continental-scale reef biodiversity assessment using quantitative data, which relied on RLS data and fed into the national 5-yearly State of the Environment report (which forms the basis of much of Australia's international biodiversity reporting).
The three particular goals of this article are to (1) present an evaluation of a large suite of fishing indicators reported in the literature, including most indicators currently used for evaluating trends in fishing impacts globally, to confirm the most suitable indicator for assessing ecological state of shallow rocky and coral reefs in relation to this pressure; (2) describe the current status of reef biodiversity around Australia, including summarizing patterns in state indicators that relate to the specific pressures of fishing, climate change (ocean warming in this case), and invasive species, as well as an indicator that reports the contribution of threatened species to reef communities around the continent; and (3) integrate results from RLS monitoring with those from two other major long-term marine biodiversity monitoring programs that use compatible methods to describe temporal trends in fishing and climate-change indicators from 2005–2015. The University of Tasmania Long-Term Temperate MPA Monitoring program (LTMPA; Barrett et al. 2009) and the Australian Institute of Marine Sciences Great Barrier Reef Long-Term Monitoring program (AIMS LTM; Sweatman et al. 2011) have less spatial coverage than the RLS but have been operating from prior to the commencement of the RLS program and allow combined results to be shown from 2005.
Evaluation of fishing indicators
Numerous indicators have been developed that have a conceptual basis for assessing community-level responses to fishing pressure, and have typically applied for the assessment of impacts of large commercial fisheries. Application to the assessment of Australian reef biodiversity required determining which indicator would be most sensitive and specific to the types of fishing pressure that occur on shallow Australian reefs.
Taxonomic-based metrics such as indicator species are not comparable across tropical and temperate locations with completely different species compositions. This reduces the list of potential indicators to community-level or trait-based metrics. We compiled a list of candidate fishing indicators based on an extensive literature search and an initial screening for applicability to underwater visual census data. The vast majority of fishing indicators can be calculated from visual census data, many with less bias than when calculated using the trawl data most often applied. Few of the key studies comparing fishing indicators have used data as rich in detail as from visual censuses (Rochet and Trenkel 2003, Fulton et al. 2005). Our shortlist (table 1) includes those based on trophic level or group, biomass, exploited status, and size-based indicators (Blanchard et al. 2005). For the latter, the slope of the linear size (biomass) spectrum was specifically included because of its widespread use, relative specificity to fishing impacts, and broad applicability (Blanchard et al. 2005, Graham et al. 2005, Shin et al. 2005). We also included a new metric based on fitting a gamma distribution to the size spectrum of fishes to account for a consistent nonlinearity evident in visual census data.
Table 1.
Ranking and model results for evaluation of fishing indicators calculated from RLS surveys around Australia.
| A. Indicator | B. χ2 goodness of fit | C. Significant fishing effects | D. Significant SST effect | Rank |
|---|---|---|---|---|
| Vulnerability Indexa,b | 69.9*** | 3 (MPA, Pop, SF) | –0.87*** | |
| Lmaxb | 58.9*** | 2 (MPA, Pop) | –0.59*** | |
| Vulnerability Indexa,b (B) | 58.3*** | 3 (Pop, BR, SF) | –0.80*** | |
| B20c | 45.6*** | 3 (MPA, Pop, BR) | NS (0.07) | 1 |
| Total Biomassc | 42.8*** | 2 (Pop, BR) | 0.23* | |
| Lmaxb,d (B) | 41.3*** | 2 (MPA, Pop) | –0.33*** | |
| Gamma Scale | 41.2*** | 2 (Pop, SF) | NS (–0.07) | 2 |
| Trophic Levelb,e | 38.3*** | – | –0.30*** | |
| Mean Lengthd | 35.6*** | 2 (Pop, SF) | 0.62*** | |
| Max of Lmaxf | 34.7*** | 2 (MPA, Pop) | NS (0.12) | 3 |
| B303 | 34.6*** | 2 (MPA, Pop) | NS (0.09) | 4 |
| Mean biomass | 32.1*** | 2 (Pop, SF) | –0.49*** | |
| B Exploitedg | 32.0*** | 2 (MPA, Pop) | 0.18* | |
| Proportion pelagich,i | 28.9*** | 1 (MPA) | NS (0.05) | |
| Elasmobrach Bj | 23.7*** | 2 (MPA, Pop) | NS (0.05) | |
| Proportion piscivorousi | 23.3*** | 2 (MPA, Pop) | 0.57*** | |
| Trophic Levele (B) | 21.2*** | – | NS (–0.13) | |
| Proportion B Exploited | 16.5* | 1 (MPA) | NS (0.08) | |
| B spectrum slopef | 16.0* | 2 (Pop, BR) | –0.35*** | |
| Large Fish Index (20 cm)j | 15.9* | 2 (Pop, SF) | –0.52*** | |
| Richness spectra slope | 10.2* | 1 (MPA) | –0.42*** |
Note: Vulnerability, Lmax, and Trophic Level values are calculated as community-weighted means, with the mean index value of members of the community weighted by the log of their abundance (B indicates biomass weighting instead of abundance). The χ2 goodness of fit (column B) is from the likelihood ratio between models with all four fishing pressure variables versus models including environmental variables but no variables related to fishing pressure. The significant individual proxies of fishing pressure for which the trend was in the direction consistent with fishing are shown in column C (MPA, no take versus fished; Pop, human population index; BR, distance from nearest public boat ramp; SF, shore fishing index). Values in column D represent the standardized beta coefficient values for the effect of mean annual sea surface temperature. The final rank is shown for the top four indicators, following the rationale provided in the text. Full details of the modeling process are provided in the supplemental material. aCheung et al. (2005). bFishbase.org. cEdgar et al. (2014). dJennings et al. (1999).ePauly et al. (1998). fShin et al. (2005). gWillis et al. (2003). hRochet and Trenkel (2003). iMethratta and Link (2006). jCury and Christensen (2005). NS means that p > .05. p < .05. **p < .001.
The sensitivity and specificity of candidate fishing indicators to fishing pressure on a continental spatial scale were tested by calculating all indicators using the RLS data and modeling values in relation to spatial patterns in fishing pressure after accounting for the large variation in environmental factors around the country. Effective marine protected areas (MPAs), human population density, and two metrics of geographic isolation (distance from boat ramps and a shore fishing accessibility index; see supplemental material) were used as proxies for fishing pressure. Proxies for fishing pressure were necessary because no reliable catch data were available to quantify the intense recreational fishing pressure on many of the shallow reef systems around Australia, nor were they available at an appropriate resolution or scale from most commercial fisheries operating in this environment. Isolation (by distance) from recreational fisher access has been shown to be a useful predictor of fishing impacts in shallow rocky reef communities in Tasmania (Stuart-Smith et al. 2008), and MPAs that have been proven effective (Edgar et al. 2009, Edgar et al. 2014) include an experimental removal of fishing pressure. Full details of the variables and the modeling process used to assess relationships among indicators, fishing pressure, and environmental factors are provided in the supplemental material.
The most appropriate fishing indicator for our purposes was selected using the following procedure: (a) the candidate indicators were ranked on the basis of their ability to describe the overall spatial variation in fishing pressure, (b) those indicators for which none of the fishing pressure proxies showed significant relationships in the direction consistent with fishing pressure (when proxies were considered individually) were excluded, and (c) those indicators that were strongly and significantly related to the mean annual sea surface temperature (SST) were also excluded. The latter was needed because close relationships with SST will reduce the interpretability of spatial and temporal trends in indicator values (Blanchard et al. 2005) and may confound warming and fishing impacts in the longer term.
Table 1 shows the outcome of the ranking process, with all fishing indicators tested displaying a significant relationship with fishing pressure, albeit not all in the expected direction. The community-weighted mean (CWM) of the vulnerability index (Cheung et al. 2005) showed the best fit to modeled fishing pressure when weighted by species abundance and the third-best fit when weighted by biomass of species. This index is based on a range of life-history parameters, including age at maturity, fecundity, longevity, and range size (Cheung et al. 2005), and was developed for the purpose of identifying species that are vulnerable to fishing. However, for data-poor species (i.e., most species, including virtually all those not commercially exploited), the vulnerability index reduces to an index of maximum size (Lmax), which is the only life-history parameter available for all species. When assessed separately, the Lmax CWM was second in the rankings on the basis of model fit. Therefore, the top three fishing indicators effectively describe the same effect of fishing in changing the composition of fishes observed on RLS surveys around Australia based on the maximum size species attain.
The vulnerability index and Lmax were also significantly related to mean annual SST, with strong natural gradients toward lower values in northern Australia (smaller, short-lived fishes dominate by abundance in warm areas). Although it might be possible to standardize CWM values of the vulnerability index by local SST to provide a metric that is comparable among regions, the tight relationship with temperature indicates that future changes in its values may be influenced by ocean warming. In addition to this, a recent study showed that the CWM of the vulnerability index based on RLS data (as we used here) was very closely associated with a pollution gradient, with a trend in the opposite direction to that expected from fishing pressure (i.e., increasing prevalence of vulnerable species with greater fishing pressure; Stuart-Smith et al. 2015b). This and the strong relationship with SST imply that the vulnerability index has poor specificity for fishing impacts (Shin et al. 2010), and although it describes important variation related to overall human impacts, its interpretation as a fishing indicator could be confounded by changes arising from pollution or warming when these pressures geographically overlap.
The biomass of all fishes in size classes 20 cm and above, hereafter referred to as B20, was the most sensitive indicator that was not significantly related to mean SST at the continental scale (table 1) and was followed closely in rankings by the scale parameter from a gamma model of the size spectrum of fishes. The latter also described the trend for a reduced density of larger fishes in the size spectrum (regardless of species identity) with increasing fishing pressure and is theoretically specific to fishing impacts, although complete specificity is unlikely to be realistic for any fishing indicator.
The current status of reef biodiversity around Australia
Spatial patterns in indicators related to specific pressures and values were assessed using RLS data collected from 1,294 reef sites dispersed around Australia from 2010 to 2015.
Fishing pressure
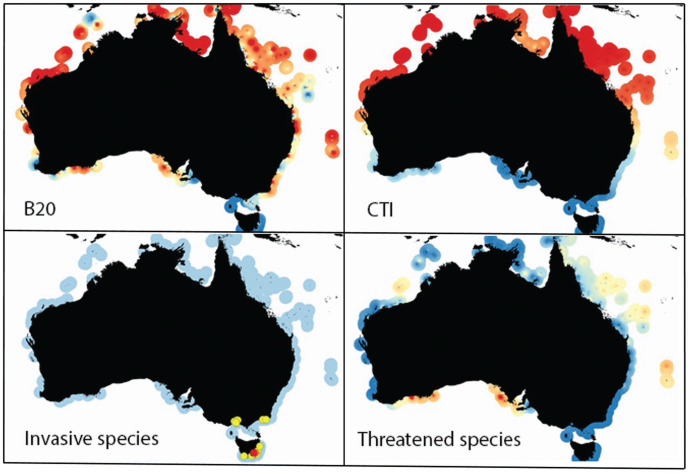
Guided by the ranking of fishing indicators (above), the B20 from surveys of Australian rocky and coral reefs by the RLS was mapped nationally (figure 2). The spatial distribution of B20 values suggests some relationship with mean SST, even though B20 was one of the fishing indicators least related to SST of all indicators compared (the effect of SST was not statistically significant for B20; table 1). Reef fish communities in temperate southern Australia are characterized by lower biomass of large fishes than those in the tropical reefs around the north. Clear local deviations can be seen from natural gradients, however, with numerous localized areas of depressed B20 relative to surrounding areas observable at population centers along the east coast, in the southwest, at Ningaloo Reef, and at Ashmore and Hibernia Reefs in the northwest.
Figure 2.
The distribution of values of indicators of reef biodiversity in relation to fishing pressure, ocean warming, invasive species, and threatened species, based on quantitative surveys of coral and rocky reefs by the Reef Life Survey program (n = 1294 sites). B20 is the total biomass of fishes 20 centimeters or larger, and CTI is the community temperature index. The CTI is calculated as a community-weighted mean of the midpoint of the realized thermal range of each species, weighted by the log of their abundance. It represents the current mean thermal affinity of reef fish communities rather than implying any warming-related change (shown in figure 3). Invasive species were only plotted for sites at which they were recorded, with yellow indicating up to 30% of individuals belonging to invasive species and red indicating values from 30% to 95% of individuals. Otherwise, the color scales are interpreted as red being the highest values in the data set and blue as the lowest (zero for invasive and threatened species). The values have been interpolated and extended to a maximum of 100 kilometers from the survey sites to enable visualization of a broader strip of color around the coastline (see supplemental material). The values only apply to shallow reef habitats within the colored areas of the maps.
Ocean warming
Few effective indicators of ecological state in relation to ocean warming have been developed or proposed (Gregory et al. 2009). Recent research on birds and butterflies (Devictor et al. 2008, Tayleur et al. 2016) has shown the community temperature index (CTI) to capture biodiversity responses to long-term warming, and previous studies on fishes and invertebrates using the RLS and LTMPA data have suggested the CTI to be a sensitive and specific indicator of reef biodiversity responses to ocean warming (Bates et al. 2014, Stuart-Smith et al. 2015a). Preliminary analyses have shown the CTI for fishes to be more responsive to temperature change than the CTI calculated for invertebrates.
Current spatial patterns in reef fish CTI, as are shown in figure 2, provide little indication of the distribution of warming impacts per se (see temporal trends below for warming impacts) but provide a baseline of reef communities for future assessment. Importantly, the map of CTI (figure 2) reveals a lack of obvious north–south gradients along the Great Barrier Reef and the northwest coastline despite regional gradients in SST along these coasts. These are related to high similarity in the thermal composition of reef communities over large temperature gradients, which results in regional differences in community thermal bias and predicted sensitivity to warming (reported in Stuart-Smith et al. 2015a). Such patterns emphasize the importance of tracking a community metric such as the CTI instead of inferring ecological change from changes in SST without a baseline such as this.
Invasive species
The ecological impacts of invasive species can be difficult to tease apart from those due to numerous other pressures. The threat posed by invasive species can also be considered part of the ecological state at a given location and so is not clearly placed among the categories of indicators in standard frameworks (i.e., it could be considered a pressure or state in the drivers–pressure–state–impact–response, or DPSIR, framework; Smeets and Weterings 1999). We consider the abundance of invasive species here as a direct metric of ecological state relating to this pressure and summarize national patterns in their proportional abundance among mobile invertebrates and small bottom-dwelling fishes recorded from RLS surveys (as was previously applied to the RLS data in Stuart-Smith et al. 2015b).
Invasive species were absent from reef fish and mobile invertebrate survey data across most of the continent (figure 2), but localized high densities were found in the southeast, ranging up to 100% of individuals surveyed. These comprised nine non-native species from four phyla: Arthropoda, Chordata, Echinodermata, and Mollusca.
Species vulnerability
Marine species are poorly covered by the Australian threatened species listing system (the 1999 EPBC Act), so the distribution of threatened species around Australia was assessed on the basis of International Union for Conservation of Nature (IUCN) threat status. Thirty-four species listed as vulnerable, endangered, or critically endangered on the IUCN Red List were recorded on RLS surveys around Australia over the assessment time period (2010–2015). These included bony fishes, elasmobranchs, marine mammals, reptiles, echinoderms, and molluscs. The proportion of these species on RLS surveys constituted an indicator relating to species vulnerability, which also revealed localized areas of high values (figure 2). Western blue groper (Achoerodus gouldii; VU) and Australian sea lions (Neophoca cinerea; EN) were recorded on many surveys in the Great Australian Bight, leading to high index values in this region. This was also amplified by relatively low local species richness, which meant that each threatened species formed a greater proportion of the community compared with in tropical locations. The maximum number of threatened species recorded on any RLS survey was four, at three sites in the Coral Sea and two offshore sites in the northwest, both areas with relatively high species richness, leading to moderate index values for these sites (4% to 7.5%).
Temporal trends in biodiversity indicators
Only fishing and warming indicators were assessed for temporal trends, because few invasive and threatened species were present at any location with adequate time series. Overall, the integration and reporting of data from the three programs for 15 locations across the continent suggest that a number of reef communities have changed over the past decade as a result of fishing pressure and warm-water events.
Fishing pressure
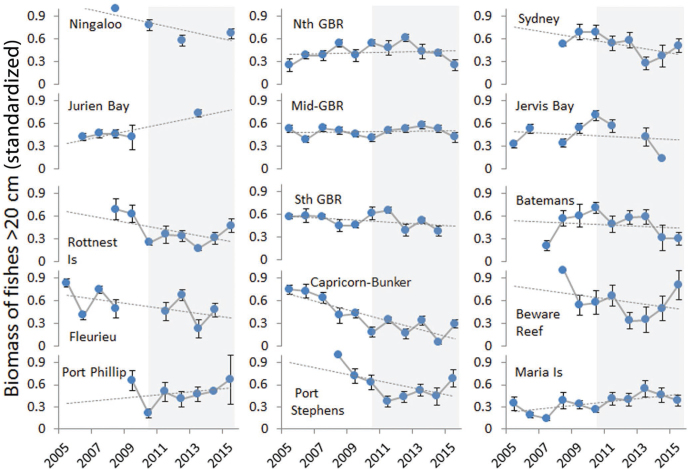
Despite some variation, only 4 of the 15 monitored locations showed an increasing trend in B20 (figure 3), whereas decreases were apparent in at least 8. Some of the declines were very steep, with B20 values dropping by more than 60% at the Capricorn Bunker Group (Queensland), Fleurieu Peninsula (South Australia), Beware Reef (Victoria), and Port Stephens (New South Wales) at some point during the monitored time series, although values appeared to be increasing in the last 3 years for the latter two locations (and at Sydney, Port Phillip, and Rottnest Island).
Figure 3.
Trends in biomass of large reef fishes (20 centimeters or greater) at monitoring locations from 2005 to 2015. The values for each site have been standardized by the maximum for that site over the time series, and the means of the standardized values among a number of sites at each location are shown (overall mean of 23 sites per location per year). The error bars represent the standard error. Long-term trends shown by the dotted gray line are linear smoothers, and background shading provides a visual reference to the middle of the period covered (5 years). The locations at which marine protected areas (MPAs) were monitored include sites within and outside MPA boundaries.
Ocean warming
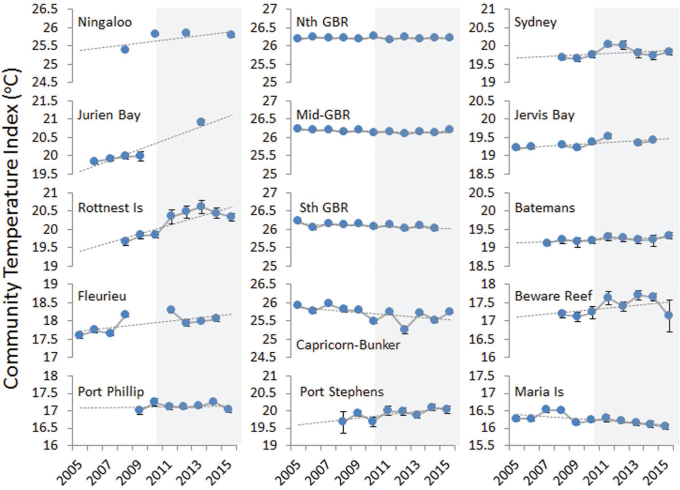
Trends in the CTI (figure 4) closely match expectations based on interannual trends in SST over the same period (Foster et al. 2014). On one hand, stability in the CTI in most tropical locations, particularly the northeast, reflected low interannual SST variability and no positive linear trend in SST in these regions over these years. On the other hand, distinct impacts of a marine heatwave were observed in a spike in the CTI in the temperate Western Australian locations of Rottnest Island and Jurien Bay in 2011 and in a subsequent warm year in 2012. The change in the CTI at Rottnest Island over the course of the heatwave was very large, equivalent to the difference in fish communities observed between Rottnest Island and locations more than 250 kilometers farther north.
Figure 4.
Trends in the community temperature index (CTI) for reef fishes at monitoring locations from 2005 to 2015. Each point represents the mean (±SE) of CTI values among sites surveyed at each location in that year (overall mean of 23 sites per location per year). The long-term trends shown by the dotted gray line are linear smoothers, and background shading provides a visual reference to the middle of the period covered (5 years). The locations at which marine protected areas (MPAs) were monitored include sites within and outside MPA boundaries.
National biodiversity assessment
Assessment of the current state of Australian rocky and coral reef biodiversity and temporal trends over the last decade suggest that the impacts of fishing are the most substantial and spatially widespread among the pressures examined here, although we also note locations where significant impacts were observed as a result of extreme warming events.
Aichi target 6 for the Convention on Biological Diversity (CBD), which aims to increase the sustainability of fishing and avoid overfishing, has so far used the mean trophic level, or Marine Trophic Index (MTI; Pauly et al. 1998, Pauly and Watson 2005), based on fisheries catch data as a headline indicator. MTI based on catch data poorly covers impacts on noncommercial species and inshore marine systems where recreational and unreported subsistence fisheries typically operate and where fishing is generally much less regulated than for large commercial fisheries. In our comparison of fishing indicators, we found the direction of spatial patterns in this index the opposite to expectations from fishing pressure, including lower values evident inside effective MPAs. Including only fishes with a trophic level exceeding 3.25, as was later suggested by Pauly and Watson (2005), also made no difference to results.
It is unclear why mean trophic level was lower in effective MPAs, but it may be that the geographic distribution of effective Australian MPAs is biased in relation to stronger drivers of trophic structure that are broadly related to environment and/or habitat. Regardless, a recent global study revealed the recovery of shallow reef fishes in the global MPA network to be more clearly based on size rather than the trophic group (Soler et al. 2015), and we found little empirical support here for using mean trophic level to track changes in shallow reef fish communities due to fishing pressure (in line with the findings of Branch et al. 2010). Conversely, the biomass of fishes over 20 cm (and to a lesser extent over 30 cm) and the gamma scale parameter for the size spectrum appear to be useful indicators for this purpose and can easily be calculated from a range of available data sets. The vulnerability index also offers an informative means to measure fishing impacts over large scales, but interactions with pollution—and potentially with warming—should be considered when interpreting trends.
The variable nature of trends in the fishing indicator (B20) at monitored locations suggests that either fishing pressure is highly dynamic or, more likely, that longer time series are needed to separate true fishing impacts from other sources of natural variation that affect fish production. Our spatial assessment showed clear fishing impacts, probably in part because it integrated observations over multiple years in many locations (2010–2015), but it also likely represents spatial patterns in the accumulated impacts from fishing over a much longer timescale. The length of time series needed to statistically disentangle fishing impacts from natural variation in fish communities is an important consideration for most fishing indicators (Piet and Jennings 2005). Our findings suggest that caution is required in interpreting year-to-year changes in B20, with longer-term trends of over 5 years offering more robust insights. We highlight that the purpose of assessing B20 here is not to guide an immediate fisheries management response but rather to identify locations where fishing pressure is having the greatest impact and to provide a quantitative comparison of its magnitude in relation to other pressures. The longer-term trends in B20 suggest that few improvements have occurred in ecological condition around Australia over the last decade, with the exception of some areas where MPAs form part of the seascape and where B20 was initially low by national standards (including Jurien Bay and Maria Island). Local changes in B20 have been more prevalent across temperate and tropical regions than warming-driven changes, as have been measured by the CTI (figure 4). Figure 2 suggests fishing impacts appear to be greatest at locations close to large population centers on the east coast, in the southwest, and also at the remote Ashmore and Hibernia Reefs in the northwest (the “MOU Box”), where traditional fishing by fishers from nearby Indonesian islands is permitted.
Long-term ocean warming is beyond the 10-year scope of this assessment, but the impacts of short-term warming events were marked. In particular, the CTI changes at Rottnest Island and Jurien Bay in Western Australia following a marine heatwave were substantial; the mean thermal affinities of fish communities at these locations have changed the most of all the monitored locations presented in figure 4. A number of impacts of this marine heatwave have been documented (Wernberg et al. 2013, 2016), but more investigation is needed to determine the extent and longevity of meaningful ecological change, including loss of species and functions.
The CTI has been relatively stable at most tropical locations, which is consistent with the SST trends at these locations over the monitoring periods (which were stable or slightly cooling). The long-term warming trend in the fish community on the east coast of Tasmania that was previously observed based on species identities (1992–2012; Bates et al. 2014) appears to have stalled, with a slight cooling trend evident in our results when only the last 10 years are considered and the CTI is abundance weighted (thereby capturing the important contributions of abundance changes to overall community change). Longer time series will likely show this to represent a temporary downward portion of the decadal cycle that overlays a longer-term warming trend (Stuart-Smith et al. 2010, Bates et al. 2014).
The baseline of the CTI provided in figure 2 will be important through the future, providing insights into where the most temperature-sensitive communities are found (Tayleur et al. 2016). Reporting on changes in CTI, as well as other indicators assessed here, is important for improving public and policymakers’ awareness of biodiversity change, guiding where long-term changes in human behavior and management practices are required, and assessing the success of current policies. Assessing progress toward Aichi target 10 for the CBD also requires understanding the impacts on biodiversity of coral reefs and other vulnerable ecosystems that are related to climate change. To our knowledge, this represents the first nationwide assessment of marine biodiversity related to this pressure. Our study supports recent calls for the CTI to be included in the CBD indicator suite (Devictor et al. 2012) and demonstrates a cost-effective mechanism for ongoing reporting for marine communities.
The identification of trends in invasive species provides a basis to evaluate Aichi target 9, but invasive species are not monitored by any national system in Australia—despite having substantial impacts (Bax et al. 2003). Although we were unable to assess temporal trends in invasive reef species in this study, this was largely due to their rarity in reef surveys at long-term monitoring locations. The paucity of invasive species in the reef data suggests that this threat is not currently as pervasive as fishing or warming. Nevertheless, invasive species assessed here only include mobile species recorded on hard substrates, and our map overlooks aggregations of invasive species amongst anthropogenic structures and soft sediments in Sydney and Melbourne (Hewitt et al. 1999, Glasby et al. 2006). The threat posed by invasive species should not be regarded as negligible outside locations identified in figure 2; however, highlighted locations appear to be most at risk. A recent global study also identified southeastern Australia as a hotspot for invasive species impacts (Molnar et al. 2008).
The distribution of threatened species in figure 2 indicates Australian locations with relatively high global conservation value. Locations where threatened species constitute a greater proportion of species present on reefs are clearly important for conservation, but appropriate management still relies on considering the most significant pressures on the particular threatened species in that area. In the case of the Great Australian Bight and Tasman Sea, some of the key threatened species (e.g., western blue groper and doubleheader wrasse) are threatened primarily by exploitation; therefore, MPAs and carefully targeted fisheries regulations or closures are likely effective conservation strategies in these areas. The Tasman Sea reefs (Lord Howe Island, Elizabeth Reef and Middleton Reef) already have some no-take MPAs that appear to be well placed in this regard.
The values of the threatened species indicator were low, however, with the highest value at any single site being 9% of the species recorded (from the six animal classes included in the calculation of the index). Generally, low values could be seen as promising in terms of suggesting that only a small proportion of the mobile reef species making up ecological communities around Australia are globally threatened. But low values may also relate to the fact that historically-limited population trend data have prohibited effective threat assessment for the majority of unexploited or less charismatic marine species. Low indicator values and the natural rarity of threatened species also made it difficult to assess trends in this aspect of marine biodiversity at a national scale, even with the RLS data set, which includes site- and species-level abundance data and covers more than 2500 Australian species. An additional important consideration for tracking changes in this indicator through the future is that improvements in data availability and knowledge may result in more species being listed as threatened, which could result in increases in the indicator value, even if some species are lost to extinction. Therefore, this indicator will not provide a substitute for tracking population trends in individual threatened species, which is also required for reporting against Aichi target 12 (the prevention of extinction of known threatened species).
An important limitation of this pressure-specific assessment of the state of Australian reefs is that we have not directly considered habitats. Habitat integrity is an important component of ecological condition, and degradation may also lead to changes in values of any of the indicators reported here. In particular, it is likely that the observed decline in B20 in the Capricorn-Bunker Group may be at least in part a result of the coral loss associated with recent cyclones rather than purely due to increasing fishing pressure. This reinforces a process whereby indicator trends should trigger detailed local investigation before applying specific local management actions. Complimentary investigation of trends in small fishes could help separate influences of habitat loss versus fishing impacts in the particular case of the Capricorn-Bunker Group B20 trend.
Scientific monitoring programs such as the LTMPA and AIMS LTM cover different elements of habitat integrity, but as yet, no nationally coordinated system to collate and report on habitat trends exists in Australia. Quantifying habitat degradation through coral bleaching and storms in the tropics, as well as ecological interaction and climate-driven loss of kelp in temperate locations, are both needed to report against Aichi target 5. The recent mass bleaching observed on the Great Barrier Reef and off northwestern Australia resulted in a thorough assessment of coral loss in 2016.
Pollution is also a major threat to marine biodiversity (Aichi target 8) but was not considered here. The impacts of pollution from metropolitan point sources may be locally severe but often dissipate relatively quickly with distance from the source (Islam and Tanaka 2004, Oh et al. 2015). Sedimentation and runoff from intensively managed landscapes may have more widespread impacts but are still arguably less widespread than those of fishing and warming. Pollution covers a broad suite of pressures that are typically referred to in aggregate as a single pressure, with targeted research required to identify the most appropriate broadscale indicators for the impacts of different types of pollution on reef biodiversity. Indicators trialed to date have been closely linked to other pressures and ecological interactions that are difficult to disentangle (Stuart-Smith et al. 2015b).
Global biodiversity observation
A recent report to the CBD on “Essential Biodiversity Variables” (Walters et al. 2013) highlighted the need for “an observation system that collects data on species abundance for several taxa at multiple locations on our planet, can support the derivation of the Living Planet Index…, the Community Temperature Index, measures of species range shifts, and a number of other high-level indicators on the CBD's indicative list of indicators….” Here, we have presented such a system for the shallow marine environment and demonstrated its utility for calculating a small suite of indicators suitable for the assessment of the state of reef biodiversity in relation to particular pressures.
The RLS survey data have already been used to quantify species range shifts (Bates et al. 2015, Sunday et al. 2015) and contain the detail necessary to calculate numerous other indicators retrospectively should future research determine that alternative indicators are more informative. The Living Planet Index (Loh et al. 2005) could also be calculated for marine species on the basis of species’ abundance data collected by RLS divers using standardized methods for animals in 11 classes—all from a single data source. This has not been previously possible for any group of animals; even the vast quantities of data from bird-watching organizations encompass a single animal class. We did not calculate the Living Planet Index for this assessment, in part because of the need for further analyses to support interpretation, because negative trends in abundance do not always indicate unhealthy ecosystems. This can be particularly marked in MPAs, where the abundance of many prey species may decrease as a result in recovery of a few predator species (Babcock et al. 2010).
Although time series are presently only available for locations in Australia, the current RLS data set provides global context and opportunities for empirically assessing the sensitivity of particular indicators. Further monitoring locations around the globe will allow more rigorous tests of temporal responsiveness of the indicators presented here and inform ecologically relevant reference levels and targets for indicators. We have not attempted to provide these here, but research on ecological thresholds and tipping points in relation to indicator values represents an important next step, along with modeling responses to alternative management scenarios (Collen and Nicholson 2014).
Pragmatic solutions are clearly required to gather the necessary data for assessing and understanding global biodiversity trends. Given the limited funding available and the comparatively small size of the scientific community, the future of biodiversity reporting over large scales likely relies on the well-designed application of citizen science in addition to technological advances (Pimm et al. 2015, Edgar et al. 2016). The RLS model, in which species-level abundance data are collected for multiple phyla, shows that engaging citizens in the collection of marine biodiversity data does not necessarily require simplifying data-collection methods and consequently reducing the capacity to calculate a large range of biodiversity indicators. Extension of this model to generate high-resolution time-series data on a global scale will likely best be achieved through integration and coordination with existing international initiatives such as GEO BON, GOOS, and MarineGEO. The mobilization of skilled recreational divers to contribute big data should herald the start of a new era in which the public and policymakers will have access to a vastly greater understanding of the true state and trends in marine biodiversity.
Supplementary Material
Supplementary data are available at BIOSCI online.
Acknowledgments
We thank the many Reef Life Survey (RLS) divers who participated in data collection and provide ongoing expertise and commitment to the program, as well as University of Tasmania staff including Marlene Davey, Justin Hulls, Elizabeth Oh, and Jemina Stuart-Smith. Development of RLS was supported by the former Commonwealth Environment Research Facilities Program, whereas analyses were supported by the Australian Research Council (ARC), the Institute for Marine and Antarctic Studies, and the Marine Biodiversity Hub, a collaborative partnership supported through the Australian Government's National Environmental Science Programme (NESP). Additional funding and support for field surveys were provided by grants from the Ian Potter Foundation, Parks Australia, CoastWest, the WA State NRM program, and the Royalties for Regions program. The LTMPA surveys were supported by a wide range of state agencies in temperate Australia, including the Department of Environment, Water, and Natural Resources (South Australia); the Parks and Wildlife Service (Tasmania); the Marine Parks Authority (New South Wales); the Department of Parks and Wildlife (Western Australia); the ARC; a the Fisheries Research and Development Corporation; Department of Primary Industries, Parks, Water, and Environment (Tasmania); and the Institute for Marine and Antarctic Studies. The AIMS LTMP was supported by the Australian Institute of Marine Science and NESP.
Supplemental material
Supplementary data are available at BIOSCI online.
References cited
- Andersen JH, Dahl K, Göke C, Hartvig M, Murray C, Rindorf A, Skov H, Vinther M, Korpinen S, 2014, Integrated assessment of marine biodiversity status using a prototype indicator-based assessment tool, Frontiers in Marine Science, 1art 55doi:10.3389/fmars.2014.00055 [Google Scholar]
- Babcock RC, Shears NT, Alcala A, Barrett NS, Edgar GJ, Lafferty KD, McClanahan TR, Russ GR, 2010, Decadal trends in marine reserves reveal differential rates of change in direct and indirect effects, Proceedings of the National Academy of Sciences, 107, 18251, 18255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett NS, Buxton CD, Edgar GJ, 2009, Changes in invertebrate and macroalgal populations in Tasmanian marine reserves in the decade following protection, Journal of Experimental Marine Biology and Ecology, 370, 104, 119 [Google Scholar]
- Bates AE, Barrett NS, Stuart-Smith RD, Holbrook NJ, Thompson PA, Edgar GJ, 2014, Resilience and signatures of tropicalization in protected reef fish communities, Nature Climate Change, 4, 62, 67 [Google Scholar]
- Bates AE, et al. , 2015, Distinguishing geographical range shifts from artefacts of detectability and sampling effort, Diversity and Distributions, 21, 13, 22 [Google Scholar]
- Bax N, Williamson A, Aguero M, Gonzalez E, Geeves W, 2003, Marine invasive alien species: A threat to global biodiversity, Marine Policy, 27, 313, 323 [Google Scholar]
- Bird TJ, et al. , 2014, Statistical solutions for error and bias in global citizen science datasets, Biological Conservation, 173, 144, 154 [Google Scholar]
- BirdLife International , 2012, Developing and Implementing National Biodiversity Strategies and Action Plans: How to Set, Meet and Track the Aichi Biodiversity Targets, BirdLife International [Google Scholar]
- Blanchard JL, Dulvy NK, Jennings S, Ellis JR, Pinnegar JK, Tidd A, Kell LT, 2005, Do climate and fishing influence size-based indicators of Celtic Sea fish community structure?, ICES Journal of Marine Science, 62, 405, 411 [Google Scholar]
- Branch TA, Watson R, Fulton EA, Jennings S, McGilliard CR, Pablico GT, Ricard D, Tracey SR, 2010, The trophic fingerprint of marine fisheries, Nature, 468, 431, 435 [DOI] [PubMed] [Google Scholar]
- Butchart SHM, Resit Akçakaya H, Chanson J, Baillie JEM, Collen B, Quader S, Turner WR, Amin R, Stuart SN, Hilton-Taylor C, 2007, Improvements to the Red List Index, PLOS ONE, 2art. e140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butchart SHM, et al. , 2010, Global biodiversity: Indicators of recent declines, Science, 328, 1164, 1168 [DOI] [PubMed] [Google Scholar]
- Cheung WWL, Pitcher TJ, Pauly D, 2005, A fuzzy logic expert system to estimate intrinsic extinction vulnerabilities of marine fishes to fishing, Biological Conservation, 124, 97, 111 [Google Scholar]
- Collen B, Nicholson E, 2014, Taking the measure of change, Science, 346, 166, 167 [DOI] [PubMed] [Google Scholar]
- Costello MJ, Coll M, Danovaro R, Halpin P, Ojaveer H, Miloslavich P, 2010, A census of marine biodiversity knowledge, resources, and future challenges, PLOS ONE 5 (art. e12110). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain CM, Halpern BS, Beck MW, Kappel CV, 2009, Understanding and managing human threats to the coastal marine environment, Annals of the New York Academy of Sciences, 1162, 39, 62 [DOI] [PubMed] [Google Scholar]
- Cury PM, Christensen V, 2005, Quantitative ecosystem indicators for fisheries management, ICES Journal of Marine Science, 62, 307, 310 [Google Scholar]
- Devictor V, Julliard R, Couvet D, Jiguet F, 2008, Birds are tracking climate warming, but not fast enough, Proceedings of the Royal Society B, 275, 2743, 2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devictor V, et al. , 2012, Differences in the climatic debts of birds and butterflies at a continental scale, Nature Climate Change, 2, 121, 124 [Google Scholar]
- Duffy JE, Amaral-Zettler LA, Fautin DG, Paulay G, Rynearson TA, Sosik HM, Stachowicz JJ, 2013, Envisioning a marine biodiversity observation network, BioScience, 63, 350, 361 [Google Scholar]
- Edgar GJ, Stuart-Smith RD, 2009, Ecological effects of marine protected areas on rocky reef communities: A continental-scale analysis, Marine Ecology Progress Series, 388, 51, 62 [Google Scholar]
- Edgar GJ, Stuart-Smith RD, 2014, Systematic global assessment of reef fish communities by the Reef Life Survey program, Scientific Data 1 (art. 140007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar GJ, Samson CR, Barrett NS, 2005, Species extinction in the marine environment: Tasmania as a regional example of overlooked losses in biodiversity, Conservation Biology, 19, 1294, 1300 [Google Scholar]
- Edgar GJ, Barrett NS, Stuart-Smith RD, 2009, Exploited reefs protected from fishing transform over decades into conservation features otherwise absent from seascapes, Ecological Applications, 19, 1967, 1974 [DOI] [PubMed] [Google Scholar]
- Edgar GJ, et al. , 2014, Global conservation outcomes depend on marine protected areas with five key features, Nature, 506, 216, 220 [DOI] [PubMed] [Google Scholar]
- Edgar GJ, Bates AE, Bird TJ, Jones AH, Kininmonth S, Stuart-Smith RD, Webb TJ, 2016, New approaches to marine conservation through the scaling up of ecological data, Annual Review of Marine Science, 8, 435, 461 [DOI] [PubMed] [Google Scholar]
- Foster SD, Griffin DA, Dunstan PK, 2014, Twenty years of high-resolution sea surface temperature imagery around Australia: Inter-annual and annual variability, PLOS ONE, 9art. e100762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton E, Smith ADM, Punt AE, 2005, Which ecological indicators can robustly detect effects of fishing?, Journal of Marine Science, 62, 540, 551 [Google Scholar]
- [GEO BON] Group on Earth Observations Biodiversity Observation Network , 2011, Adequacy of Biodiversity Observation Systems to support the CBD 2020 Targets. A Report Prepared for the Convention on Biological Diversity, GEO BON, (13 December 2016; www.earthobservations.org/documents/cop/bi_geobon/2011_cbd_adequacy_report.pdf) [Google Scholar]
- Glasby TM, Connell SD, Holloway MG, Hewitt CL, 2006, Nonindigenous biota on artificial structures: Could habitat creation facilitate biological invasions?, Marine Biology, 151, 887, 895 [Google Scholar]
- Graham NAJ, Dulvy NK, Jennings S, Polunin NVC, 2005, Size-spectra as indicators of the effects of fishing on coral reef fish assemblages, Coral Reefs, 24, 118, 124 [Google Scholar]
- Green RE, Balmford A, Crane PR, Mace GM, Reynolds JD, Turner RK, 2005, A framework for improved monitoring of biodiversity: Responses to the World Summit on Sustainable Development, Conservation Biology, 19, 56, 65 [Google Scholar]
- Gregory RD, Willis SG, Jiguet F, Voříšek P, Klvaňová A, van Strien A, Huntley B, Collingham YC, Couvet D, Green RE, 2009, An indicator of the impact of climatic change on European bird populations, PLOS ONE, 4art. e4678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern BS, et al. , 2012, An index to assess the health and benefits of the global ocean, Nature, 488, 615, 620 [DOI] [PubMed] [Google Scholar]
- Hewitt CL, Campbell ML, Thresher RE, Martin RB, 1999, Marine Biological Invasions of Port Phillip Bay, Victoria, Centre for Research on Introduced Marine Pests Technical Report no. 20 [Google Scholar]
- Islam S, Tanaka M, 2004, Impacts of pollution on coastal and marine ecosystems including coastal and marine fisheries and approach for management: A review and synthesis, Marine Pollution Bulletin, 48, 624, 649 [DOI] [PubMed] [Google Scholar]
- Jennings S, Simon PRG, Reynolds JD, 1999, Structural change in an exploited fish community: A consequence of differential fishing effects on species with contrasting life histories, Journal of Animal Ecology, 68, 617, 627 [Google Scholar]
- Jones JPG, et al. , 2011, The why, what, and how of global biodiversity indicators beyond the 2010 target, Conservation Biology, 25, 450, 457 [DOI] [PubMed] [Google Scholar]
- Loh J, Green RE, Ricketts T, Lamoreux J, Jenkins M, Kapos V, Randers J, 2005, The Living Planet Index: Using species population time series to track trends in biodiversity, Philosophical Transactions of the Royal Society of London B, 360, 289, 295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Methratta ET, Link JS, 2006, Evaluation of quantitative indicators for marine fish communities, Ecological Indicators, 6, 575, 588 [Google Scholar]
- Miller DD, Mariani S, 2010, Smoke, mirrors, and mislabeled cod: Poor transparency in the European seafood industry, Frontiers in Ecology and the Environment, 8, 517, 521 [Google Scholar]
- Molnar JL, Gamboa RL, Revenga C, Spalding MD, 2008, Assessing the global threat of invasive species to marine biodiversity, Frontiers in Ecology and the Environment, 6, 485, 492 [Google Scholar]
- Niemeijer D, de Groot RS, 2008, A conceptual framework for selecting environmental indicator sets, Ecological Indicators, 8, 14, 25 [Google Scholar]
- Oh ES, Edgar GJ, Kirkpatrick JB, Stuart-Smith RD, Barrett NS, 2015, Broad-scale impacts of salmon farms on temperate macroalgal assemblages on rocky reefs, Marine Pollution Bulletin, 98, 201, 209 [DOI] [PubMed] [Google Scholar]
- Pauly D, Froese R, 2010, A count in the dark, Nature Geoscience, 3, 662, 663 [Google Scholar]
- Pauly D, Watson R, 2005, Background and interpretation of the “Marine Trophic Index” as a measure of biodiversity, Philosophical Transactions of the Royal Society B, 360, 415, 423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly D, Christensen V, Dalsgaard J, Froese R, Torres F Jr, 1998, Fishing down marine food webs, Science, 279, 860, 863 [DOI] [PubMed] [Google Scholar]
- Pereira HM, et al. , 2013, Essential biodiversity variables, Science, 339, 277, 278 [DOI] [PubMed] [Google Scholar]
- Piet GJ, Jennings S, 2005, Response of potential fish community indicators to fishing, ICES Journal of Marine Science, 62, 214, 225 [Google Scholar]
- Pimm SL, Alibhai S, Bergl R, Dehgan A, Giri C, Jewell Z, Joppa L, Kays R, Loarie S, 2015, Emerging Technologies to Conserve Biodiversity, Trends in Ecology and Evolution, 30, 685, 696 [DOI] [PubMed] [Google Scholar]
- Roberts CM, et al. , 2002, Marine biodiversity hotspots and conservation priorities for tropical reefs, Science, 295, 1280, 1284 [DOI] [PubMed] [Google Scholar]
- Rochet MJ, Trenkel VM, 2003, Which community indicators can measure the impact of fishing? A review and proposals, Canadian Journal of Fisheries and Aquatic Sciences, 60, 86, 99 [Google Scholar]
- Scholes RJ, Mace GM, Turner W, Geller GN, Jürgens N, Larigauderie A, Muchoney D, Walther BA, Mooney HA, 2008, Toward a global biodiversity observing system, Science, 321, 1044, 1045 [DOI] [PubMed] [Google Scholar]
- Shannon L, et al. , 2014, Trophic level–based indicators to track fishing impacts across marine ecosystems, Marine Ecology Progress Series, 512, 115, 140 [Google Scholar]
- Shin Y-J, Rochet M-J, Jennings S, Field JG, Gislason H, 2005, Using size-based indicators to evaluate the ecosystem effects of fishing, ICES Journal of Marine Science, 62, 384, 396 [Google Scholar]
- Shin Y-J, et al. , 2010, Using indicators for evaluating, comparing, and communicating the ecological status of exploited marine ecosystems 2. Setting the sceneICES Journal of Marine Science, 67, 692, 716 [Google Scholar]
- Smeets E, Weterings R, 1999, Environmental Indicators: Typology and Overview, European Environment Agency, Report no. 25/1999 [Google Scholar]
- Soler GA, et al. , 2015, Reef fishes at all trophic levels respond positively to effective marine protected areas, PLOS ONE, 10art. e0140270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding MD, et al. , 2007, Marine ecoregions of the world: A bioregionalization of coastal and shelf areas, BioScience, 57, 573, 583 [Google Scholar]
- Stuart-Smith RD, Barrett NS, Crawford CM, Frusher SD, Stevenson DG, Edgar GJ, 2008, Spatial patterns in impacts of fishing on temperate rocky reefs: Are fish abundance and mean size related to proximity to fisher access points?, Journal of Experimental Marine Biology and Ecology, 365, 116, 125 [Google Scholar]
- Stuart-Smith RD, Barrett NS, Stevenson DG, Edgar GJ, 2010, Stability in temperate reef communities over a decadal time scale despite concurrent ocean warming, Global Change Biology, 16, 122, 134 [Google Scholar]
- Stuart-Smith RD, Edgar GJ, Barrett NS, Kininmonth SJ, Bates AE, 2015a, Thermal biases and vulnerability to warming in the world's marine fauna, Nature, 528, 88, 92 [DOI] [PubMed] [Google Scholar]
- Stuart-Smith RD, et al. , 2015b, Loss of native rocky reef biodiversity in Australian metropolitan embayments, Marine Pollution Bulletin, 95, 324, 332 [DOI] [PubMed] [Google Scholar]
- Sunday JM, et al. , 2015, Species traits and climate velocity explain geographic range shifts in an ocean-warming hotspot, Ecology Letters, 18, 944, 953 [DOI] [PubMed] [Google Scholar]
- Sweatman H, Delean S, Syms C, 2011, Assessing loss of coral cover on Australia's Great Barrier Reef over two decades, with implications for longer-term trends, Coral Reefs, 30, 521, 531 [Google Scholar]
- Tayleur CM, Devictor V, Gaüzère P, Jonzén N, Smith HG, Lindström Å, 2016, Regional variation in climate change winners and losers highlights the rapid loss of cold-dwelling species, Diversity and Distributions, 22, 468, 480 [Google Scholar]
- Walpole M, et al. , 2009, Tracking progress toward the 2010 biodiversity target and beyond, Science, 325, 1503, 1504 [DOI] [PubMed] [Google Scholar]
- Walters M, Pereira HM, Ferrier S, Geller GN, Jongman R, Scholes RJ, Bruford M, Reyers B, 2013, Essential Biodiversity Variables, Convention on Biological Diversity, Subsidiary Body on Scientific, Technical, and Technological Advice [Google Scholar]
- Wernberg T, Smale DA, Tuya F, Thomsen MS, Langlois TJ, de Bettignies T, Bennett S, Rousseaux CS, 2013, An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot, Nature Climate Change, 3, 78, 82 [Google Scholar]
- Wernberg T, et al. , 2016, Climate-driven regime shift of a temperate marine ecosystem, Science, 353, 169, 172 [DOI] [PubMed] [Google Scholar]
- Willis TJ, Millar RB, Babcock RC, 2003, Protection of exploited fishes in temperate regions: High density and biomass of snapper Pagrusauratus (Sparidae) in northern New Zealand marine reserves, Journal of Applied Ecology, 40, 214, 227 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data are available at BIOSCI online.