Abstract
Current understanding on the mechanisms of brain injury and neurodegeneration highlights an appreciation of multicellular interactions within the neurovascular unit (NVU), which include the evolution of blood-brain barrier (BBB) damage, neuronal cell death or degeneration, glial reaction, and immune cell infiltration. Aging is an important factor that influences the integrity of the NVU. The age-related physiological or pathological changes in the cellular components of the NVU have been shown to increase the vulnerability of the NVU to ischemia/reperfusion injury or neurodegeneration, and to result in deteriorated brain damage. This review describes the impacts of aging on each NVU component and discusses the mechanisms by which aging increases NVU sensitivity to stroke and neurodegenerative diseases. Prophylactic or therapeutic perspectives that may delay or diminish aging and thus prevent the incidence of these neurological disorders will also be reviewed.
Keywords: Aging, Neurovascular unit, Vulnerability, Ischemic stroke, neurodegeneration
1. Introduction
Aging inevitably starts as early as a new life begins. The factors that influence biological aging fall into two categories, the programmed factors and the damage-related factors. The programmed factors of aging refer to the innate functions that decline or change over time, such as shortened telomeres, reduced production of growth hormone, dysregulated reproductive hormones and dampened immune responses. The damage-related factors occur as results of routine damage at the cellular level and slowly build up to cause aging. These factors usually lead to cellular injuries when they outrange the body’s repair capacity. The best examples of damage-related factors include improperly metabolized cell wastes, insufficiently repaired DNA damage and free radicals derived from normal metabolism or environmental toxins. Both the programmed factors and the damage-related factors of aging may impair cell functions and increase the vulnerability of the brain to injuries or other noxious stimuli. Indeed, aging is an important risk factor for a variety of neurological disorders.
The current understanding of the mechanisms of ischemic brain injury includes an appreciation of multicellular interactions within the neurovascular unit (NVU), which may determine the evolution of blood-brain barrier (BBB) damage, neuronal cell death, glial reaction, and immune cell infiltration (Sohrabji et al., 2013). Evidence from recent studies indicates that aging may aggravate the damage and dysfunction of different components of the NVU and thus accelerate the progress of brain injuries. In this article, we will discuss how aging influences the integrity of the NVU and its subsequent impact on the pathology and outcomes of ischemic stroke. Prophylactic or therapeutic perspectives that may delay or diminish the aging effects will also be reviewed.
2. Basics of the Neurovascular Unit (NVU)
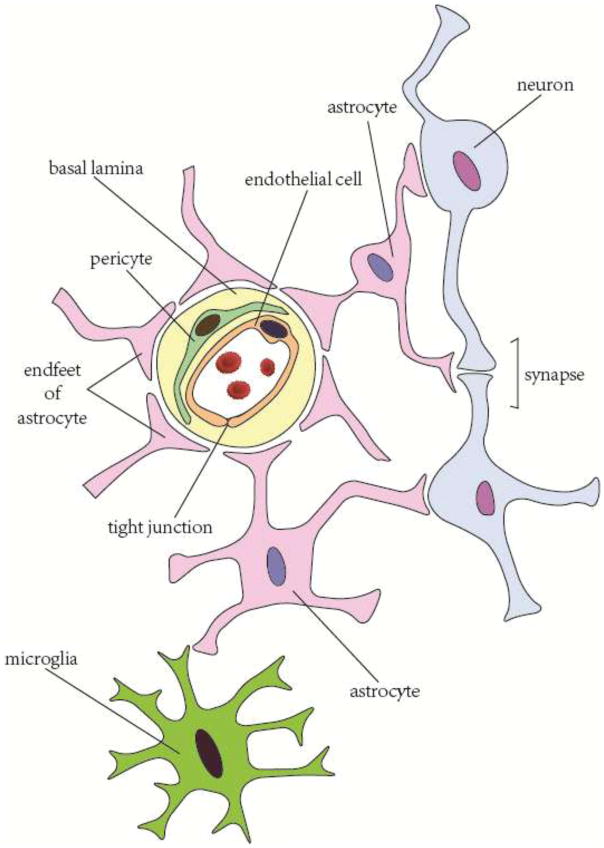
In normal brain, neurons are connected to each other through dendrites and axons, forming a network for signal transmission and communication. For many decades, neuronal injury was considered to be the main reason for functional deficits after brain injuries or diseases. Accordingly, almost all therapeutic strategies were targeted at rescuing neurons and repairing neuronal damage. This neurocentric view of brain diseases, however, has been revised as it gradually became clear that the normal functions of brain depend not only on neuron-to-neuron connections but also on functional interactions among the different components in the NVU, including neurons, glial cells (oligodendrocytes, microglia and astrocytes), vascular cells (endothelial cells, pericytes and smooth muscle cells (SMC)) as well as the basal lamina matrix within brain vasculature (Lo and Rosenberg, 2009) (Figure 1).
Figure 1.
Schematic of neurovascular unit components.
2.1 Glial cells in the NVU
All the structures in NVU exert specific functions to maintain the central nervous system (CNS) homeostasis. Within the NVU, neurons are surrounded by glial cells, which keep them from direct contact with vascular cells and buffer the blows of blood-borne substances. In particular, astrocytes serve as a bridge that allows neuron-glial crosstalk and links the neuroglial part with the vascular part in the NVU. They maintain the metabolic and ion homeostasis of neuronal cells, regulate synaptic glutamate balance and retard glutamate-induced excitatory signals via Ca2+ oscillation (Salminen et al., 2011). In addition, astrocytes regulate cerebral blood flow (CBF) and capillary permeability by stretching out their endfeet to the microvessel and forming a proximal connection with the capillary (Abbott et al., 2006). Oligodendrocytes produce lipid-enriched myelin to wrap axons and accelerate impulse conduction. Endowed with pathogen recognition and phagocytotic functions, microglia serve as the first defense in the CNS and continuously monitor their territory with high mobility (Hu et al., 2014).
2.2 Microvascular components in the NVU
The CNS is in huge demand of energy while its energy storage capacity is rather limited. Researchers have reached a consensus regarding the coupling of neuronal activity with cerebral blood flow (CBF). Nearly every neuron has its own capillaries to provide sufficient energy and nutrition (Zlokovic, 2005). Astrocytes are known to have the ability to monitor neuronal activity and contribute to neurovascular coupling. On the one hand, astrocytes sense and respond to the metabolic changes of neurons via unknown mechanisms, possibly through glutamate signaling (Filous and Silver, 2016). On the other hand, the endfeet of astrocytes reach to pericytes and SMCs. By releasing ions or secreting various vasoactive substances, astrocytes adjust the constriction or relaxation tone of pericytes/SMCs. In this way, astrocytes instantaneously regulate CBF according to neuronal activity (Zlokovic, 2008).
In addition to the precise regulation of CBF and energy supply, the vascular part of the NVU also provides permeability regulation to maintain CNS homeostasis. Endothelial cells, pericytes and astrocytes interact with each other to form inter-endothelial tight junctions (TJ) (Bonkowski et al., 2011) (Jo et al., 2013). TJ are expressed on all barrier structures (Förster, 2008). The abundance of endothelial TJ in CNS makes them one of the pivotal gatekeepers (Luissint et al., 2012). Both endothelial cells and pericytes produce extracellular matrix, which forms the basal lamina of CNS capillary (Bergers and Song, 2005). TJ-sealed endothelial cells, pericytes, astrocyte endfeet, and the basal lamina form a functional BBB and preserve the metabolic homeostasis of CNS.
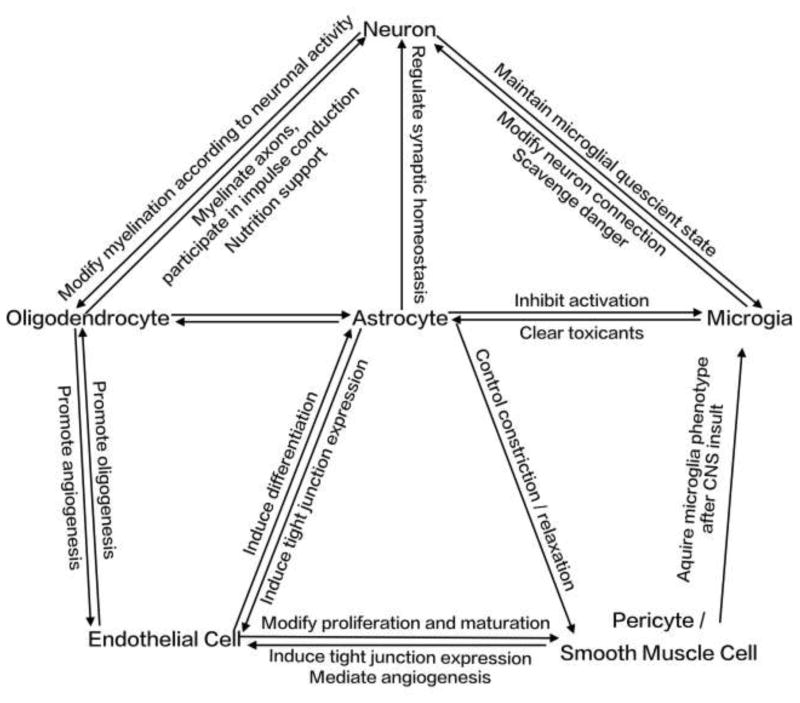
Taken together, all the components in the NVU interact with each other and work in concert to maintain the normal physiological functions of the brain (Figure 2).
Figure 2.
Brief summary of interactions among neurovascular unit components.
3. Impact of aging on the components of the NVU
Every living organism is subject to the aging process. The normal functions of an organism rely on the energetic metabolism within mitochondria or cytoplasm. The process of energy metabolization induces damage-related factors of aging, such as oxidative stress, which may cause injuries to cells. Some of the injuries are reversible, but some are not. Those irreversible injuries accumulate over time and eventually impair normal cellular functions. Neurons, with their high metabolic rate, turn out to be the most susceptible cell type to the damage-related factors of aging.
3.1 Impact of aging on neurons
The disruption to nucleic acids frequently occurs in both the nucleus and mitochondria of neurons. Among all the nuclear DNA lesions, the ones easily accumulated in the aging process are those that encode proteins involved in insulin signaling, DNA protein methylation and acetylation, DNA repair, and lipid metabolism (Mattson and Magnus, 2006). Meanwhile, the endogenous DNA repair function decreases over time with aging (Rutten et al., 2007), resulting in the further expansion of DNA lesions. Mitochondrial DNA is especially vulnerable to age-related injuries due to the following reasons (Salminen et al., 2015). First, mitochondrial DNA location is in proximity to the electron transport chain, which contributes to 90% of cellular reactive oxygen species (ROS) production. Second, the lack of protective histones in mitochondria facilitates the accumulation of injuries. Third, mitochondrial DNA has a high replication rate. As the injured clonal expand, the errors of mitochondrial DNA propagate. Finally, parts of the mitochondrial proteins are encoded by nuclear DNA. Injuries of these nuclear DNA can also cause mitochondrial dysfunction (Rutten et al., 2007).
Other than nucleic acids, proteins and lipids are also progressively modified by metabolic and oxidative stress with aging. Age-related modifications to proteins include carbonylation, nitration and covalent binding of the lipid peroxidation products 4-hydroxynonenal (Lardenoije et al., 2015). These modifications impair normal activity of both structural and functional proteins, and thus lead to toxic protein aggregation. Abnormal protein activity also brings in oxidative stress, which further exacerbates the aging process. Malfunction of both cytoplasm- and mitochondrion-derived proteins, such as enzymes in the respiratory chain, impairs the functional activity of mitochondria. The accumulation of intracellular toxic proteins, such as Aβ, further compromises mitochondrial function (Mattson and Magnus, 2006).
Mitochondrion and endoplasmic reticulum (ER) dysfunctions are key factors that contribute to cellular functional impairments (Ham and Raju, 2016). Intracellular concentration of Ca2+ is tightly regulated by ion channels and transporters because of its broad involvement in cellular processes ranging from enzyme activity to programmed cell death (Cai and Patel, 2010; Curcio et al., 2016). Mitochondria and ER are both capable of sequestrating Ca2+. With aging, the calcium processing capability of these organelles declines due to their functional impairment (Mattson and Magnus, 2006). Sustained elevation of cellular Ca2+ concentration activates proteases, induces ROS production, and results in neuronal apoptosis (He et al., 1997). The capacity for ROS clearance reduces with aging, rending the aged neurons more vulnerable to oxidative stress (Mattson and Magnus, 2006).
ER, the cellular machinery responsible for protein production, delivery and degradation, is another organelle that shows apparent impairment during aging (Chadwick et al., 2012; Ghavami et al., 2014). Retardations in protein production and delivery greatly compromise protein renewal. Protein degradation deficiency is related to toxic protein aggregation. Mounting evidence has shown that with advancing age, ER-related stress gradually elevates in brain (Chen et al., 2013; Naidoo et al., 2011). Moreover, it was recently shown that the ER chaperones, such as glucose-regulated protein 78 (GRP78), decline significantly with aging (Naidoo et al., 2008; Salganik et al., 2015). The decline of GRP78 could be potentially related to the development of Parkinson’s disease. Moreover, the degree of chaperone decline corresponds with the severity of neurodegeneration (Salganik et al., 2015).
Although aging affects all types of cells in the CNS, neuron seems to be one of the most vulnerable populations due to its intrinsic properties. Firstly, although neurogenesis is detected in some specific regions in brain such as the subventricular zone (SVZ) and subgranular zone (SGZ) of the dentate gyrus (Gemma et al., 2010; Irwin and Brinton, 2014), most neurons throughout the CNS are unrenewable. Furthermore, the regenerative capacities of SVZ and SGZ neurons decline in an age-dependent manner (Smith et al., 2015). Beta 2-microglobulin (B2M), a component of major histocompatibility complex class 1 (MHC I) molecules, was recently identified as a circulating factor that negatively regulates neurogenesis in the aged hippocampus (Smith et al., 2015). Insulin-like growth factor-1 (IGF-1) is another negative regulator of neurogenesis in aging brain based on evidence showing that the knocking down of IGF-1 enhances neurogenesis during aging (Chaker et al., 2015). Second, the highly active metabolism of neuron determines its extreme dependency on mitochondrial stability (Nicholls and Budd, 2000). However, neuronal mitochondria are especially susceptible to oxidative stress, as we discussed above. Third, large amounts of neurons receive synaptic inputs from glutamate, the main excitatory neurotransmitter in the CNS (van den Pol et al., 1990). Therefore, the glutamate receptors such as α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA) receptors are highly expressed in neurons (Ben-Ari et al., 1997). During aging, negative regulation from inhibitory neurons decreases (Caspary et al., 2008), which results in the excessive activation of glutamate receptors and subsequent excitotoxicity. Meanwhile, calcium dysregulation and low levels of protective calcium binding proteins further contribute to neuronal vulnerability to excitotoxicity (Mattson and Magnus, 2006). Fourth, neurons elongate their axons to form a central neuronal network and to connect the periphery for signal transmission. The long projection of axons requires stable neurofilament (Root et al., 2015). However, the long travel distance from soma reduces the toxin elimination capacity within axons, which makes the axonal cytoskeleton prone to dysfunction (Mukaetova-Ladinska et al., 1996). Moreover, the board seating site of axons increases their exposure to toxins, further pushing up the odds of injury (Mattson and Magnus, 2006).
3.2 Impact of aging on glial cells in NVU
3.2.1 Microglia
Age-related impairments affect not only neurons but also the three kinds of glial cells. A growing body of evidence has shown that although the number of microglia increases in the aged brain, their functions decline. Aged microglia display retracted processes, semi-ameboid shapes and enlarged cell bodies in which accumulated lipofuscin granules are stored (Lai et al., 2013; Lee et al., 2015). They become more rigid and less flexible in mobility, making them less efficient in the daily CNS patrol (Lee et al., 2015). Their capacity to eliminate either exogenous invasion (e.g. E. Coli) (Schutze et al., 2014) or endogenous metabolites (e.g. amyloid-beta peptides) (Babcock et al., 2015) also decreases with aging. On the other hand, aged microglia tend to polarize in to M1 phenotype. They have elevated expression of pro-inflammatory molecules, such as MHC-II, CD16/32 and CD86. Their secretion of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), inducible nitric oxide synthase (iNOS), interleukin-6 (IL-6), and interleukin-1β (IL-1 β) also dramatically increases in response to noxious stimuli. In contrast, aged microglia are less responsive to regulatory signals such as tumor necrosis factor-β (TGF-β) and colony-stimulating factor-1 (CSF-1) (Lourbopoulos et al., 2015). Indeed, RNA CHIP-Seq data show a decrease in expression of the anti-inflammatory M2a markers and relatively stable expression of the pro-inflammatory M1 markers in aged microglia (Leovsky et al., 2015). Such phenotypic shift in aged microglia could be attributed to multiple mechanisms that still remain elusive. So far, it is known that microglia phenotype could be regulated by its interactions with neurons and astrocytes in the NVU. Healthy neurons maintain microglia in their resting state via CD200 (ligand of CD200R), CX3CL1 (fractalkine, ligand of CX3CR1 on microglia), neurotransmitters (e.g. GABA) and neurotrophins (Hu et al., 2014). Astrocytes inhibit microglia activation through secreting soluble factors such as nuclear factor erythroid 2-related factor 2 (Nrf2), TGF-β, macrophage colony-stimulating factor (MCSF), and granulocyte/macrophage colony-stimulating factor (G/MCSF). Reduction in these regulatory factors during aging, together with excessive glutamate and various pro-inflammatory mediators and toxins, may challenge microglia and trigger their phenotypic shift toward M1 (Barakat and Redzic, 2016).
3.2.2 Oligodendrocytes
Oligodendrocytes produce myelin, which acts as an insulator of neuronal axons to allow high nerve conduction velocity (Plemel et al., 2014). Some oligodendrocytes are not associated with axons, but exist in the brain either as oligodendrocyte precursor cells (OPCs) or as perineuronal (satellite) oligodendrocytes with ramified processes around neuronal soma (Mancall and Brock, 2011). Senile oligodendrocytes have swelling morphology, as is shown in a primate study (Peters, 2009) in which the origin of swelling turned out to be inclusions of degenerated myelin sheaths in the cytoplasm. The degeneration of myelin sheaths leaves axons unwrapped, which in turn stimulates the proliferation of OPCs and remyelination. When the injury to myelin and the oligodendrocytes is beyond their repair and renewal capacity, broad and irreversible demyelination occurs, which is one of the most important steps of various age-related CNS insults. The efficiency of remyelination in senescence is much lower compared with that in the young. It was demonstrated that the recruitment and differentiation of OPCs are impaired during aging, and this could result in decreased CNS remyelination efficiency (Sim et al., 2002). Moreover, senile oligodendrocytes form myelin of short inter-nodal lengths with thin sheath. As a result, the axonal conduction velocity is much lower in the aged than the young (Peters, 2009; Peters and Sethares, 2004).
3.2.3 Astrocytes
It is believed that astrocyte is the least affected glial cell during aging. The numbers of astrocytes do not change dramatically in the old compared to the young, at least in human (Rodriguez-Arellano et al., 2016; Vartak-Sharma et al., 2016). Yet, age-related alterations can still be detected in senile astrocytes. For example, the expression of glial fibrillary acidic protein (GFAP) increases in aged astrocytes (Diniz et al., 2010), which was shown to be associated with a flat, senescent morphology of astrocytes and could repress their supportive capacity (Salminen et al., 2011). On the other hand, the density of glutamate transporters (e.g. glutamate-transporter-1 (GLT-1) and glutamate-aspartate transporter (GLAST)) and purinergic receptors in astrocytes decreases with aging (Barreto et al., 2011), limiting their regulation on inter-synaptic glutamate concentration. Since excessive glutamate input is the key mechanism of neuronal excitotoxicity, the loss of glutamate regulation in astrocytes may exacerbate neuronal injury during aging. The function of aquaporin 4 in aged astrocytes is down-regulated as well (Moftakhar et al., 2010), which leads to diminished clearance of CNS metabolites. Neurotransmitter-induced Ca2+ signaling reduces in aged astrocytes, which affects their release of neuro-active substances that support neuronal functions (Salminen et al., 2011).
Abnormal protein accumulation also happens to astrocytes during aging. Lipofuscin and intermediate filament bundle aggregation are observed (Salminen et al., 2011). In parallel, oxidative metabolism in aged astrocytes increases in the elderly, which affects their capacity to offer metabolic support to neurons (Simpson et al., 2010).
Reduction of inter-astrocytic gap junction is another characteristic in senile astrocytes (Cotrina et al., 2001). Gap junction is of importance for astrocytic cross-talk and mediates long-distance synaptic homeostasis. Astrocytic gap junction impairment further disturbs synaptic function during aging.
In respect to their innate immune property, astrocytes express several pattern recognition receptors (PARs) and can secrete a large array of soluble mediators such as interleukins (ILs), chemokines and ROS (Farina et al., 2007). When exposed to chronic stress during aging, the secretory profile of astrocytes turns into a pro-inflammatory phenotype. Further studies showed that oxidative stress and aggregated toxic proteins could induce astrocytes to secrete pro-inflammatory factors, such as IL-6, monocyte chemoattractant protein (MCP)-1 and metalloproteinase (MMP)-9, which could induce BBB disruption and contribute to inflammation in the senile brain (Salminen et al., 2011).
As discussed above, the supportive functions of glial cells decrease during aging. The secretion of neurotrophic factors is also impaired. In contrast, the detrimental factors are increasingly released from senile glial cells.
3.3 Impact of aging on microvascular components in the NVU
In the vascular part of the NVU, which directly confronts all kinds of dangerous signals from the blood flow, the effect of senility is even more overwhelming. Decrease of microcirculation blood flow, impaired angiogenesis and development of atherosclerosis are all common in senescence.
3.3.1 Endothelial cells
Endothelial cells are physiologically versatile. They help to maintain vascular homeostasis, regulate vaso-constriction and dilation, participate in angiogenesis and secrete anti-coagulation factors (Shaik et al., 2013). Cerebral endothelial cells are the main component of the BBB. They play a critical role in controlling material exchange between the CNS and periphery, and also regulate the transportation of ions and nutrient. Cerebral endothelial cells are enriched with mitochondria to maintain normal function of ATP-dependent active transporters, such as the ATP-binding cassette (ABC) and sodium potassium ATPase. Therefore, mitochondrial senescence has catastrophic effect on cerebral endothelial cells (Zlokovic, 2011).
Age-related mitochondrial malfunction affects not only material exchange function but also the CBF regulation ability of endothelial cells (Seals et al., 2011). Vasodilation capacity is apparently impaired in senile endothelial cells, and it is associated with the decline of the three major endothelium-derived vasodilators--nitric oxide (NO) (Prisby et al., 2007), prostacyclin (Nicholson et al., 2009) and endothelium-derived hyperpolarizing factor (EDHF) (Long et al., 2005). Among these vasodilators, NO is the most important and intensively studied one. Reduction of NO production with age involves multiple mechanisms. First, as a result of impairment of mitochondrion function and the anti-oxidative defense system, cellular oxidative stress increases in aged endothelial cells. Increased O2-anions react with NO and form peroxynitrite (Van Der Loo et al., 2000), a potent free radical, which results in the deprivation of NO in aged endothelial cells. In addition, the activity of endothelial NO synthase (eNOS), which catalyzes the production of NO, declines with aging (Puca et al., 2012).
Generally, whenever the nutrient and energy supply cannot meet the demand, angiogenesis is triggered as an endogenous compensatory mechanism. However, in the elderly, endothelial-dependent angiogenesis is impaired (Lähteenvuo and Rosenzweig, 2012) as a result of growth arrest. The reduced capacity of regeneration in senile endothelial cells could be attributed, at least partially, to the impaired secretion of growth factors such as VEGF (Lähteenvuo and Rosenzweig, 2012). Meanwhile, the number of circulating endothelial progenitor cells (EPC) decreases with age, probably as a consequence of reduced mobility (Marín et al., 2013). MMPs play important roles in angiogenesis. However, aged endothelial cells express high levels of tissue inhibitor of metalloproteinase-2 (TIMP-2) (Koike et al., 2003), which is a natural inhibitor of MMP-9. Moreover, the newly generated endothelial cells in the aged population display altered phenotype, with upregulated low-density lipoprotein (LDL) and down-regulated NO production (Gradinaru et al., 2015). Expression of endothelin also increases with age (Brandes et al., 2005), which is associated with atherosclerosis development. Therefore, aged endothelial cells exhibit decreased capacity of regeneration, and newly generated endothelial cells in aged organisms possess impaired functions.
3.3.2 Impact of aging on SMCs and pericytes
Oxidative stress is one of the major contributors to the impairment of endothelial functions. The insult comes not only from circulation but also from SMCs and pericytes. Increased expression of iNOS in senile SMCs leads to excessive generation of NO. SMC-derived NO reacts with O2-anions, which results in the production of peroxynitrite and subsequent severe oxidative stress. It is also reported that during the aging process, SMCs migrate from tunica media to tunica intima, where they accumulate and lose their vasoconstrictory and vasodilatory functions (Brandes et al., 2005).
In pericytes, increased alpha smooth muscle actin (α-SMA) expression, as well as an altered length and orientation of desmin, are found in senescence (Hughes et al., 2006). In addition, pericytes could secrete various neural trophic factors, which are impaired in aged brains (Bell et al., 2010).
In summary, aging has great impact on different components of the NVU and might therefore influence the incidence and progress of age-related diseases including stroke and neurodegeneration. Interestingly, it is noted that there might be sex differences in aging-mediated NVU changes. Specifically, female sex steroids have been found to be beneficial to NVU components (Sohrabji et al., 2013). Female astrocytes produce larger amounts of growth factors than those of male astrocytes (Sohrabji et al., 2013). Additionally, female sex steroids could promote differentiation and myelination of OPCs (Marin-Husstege et al., 2004). In consequence, female patients have better prognosis than males after some age-related diseases (Engler-Chiurazzi et al., 2016). However, as aging processes and estrogen levels drop after menopause, aged females display significantly higher mortality and poorer stroke outcomes than aged males. The sexual differences in the aged NVU warrant further exploration and are important factors to be considered for disease treatments.
4. Vulnerability of aged NVU to ischemic stroke
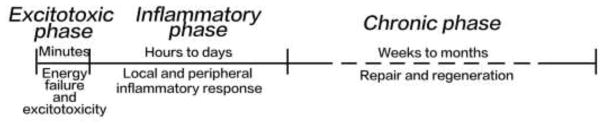
Structural and functional impairments of a variety of NVU components result in the vulnerability of aged NVU to brain injuries, including ischemic stroke. Ischemic stroke is one of the leading causes of death worldwide, especially among the elderly. Aging is not only a main risk factor of ischemic stroke, but also an indicator of poor outcome (Denti et al., 2010). The pathophysiological process of ischemic stroke can be divided into three phases (Figure 3): (i) the excitotoxic phase, which happens within minutes after the ischemic onset; (ii) the inflammatory phase, which begins hours after ischemic stroke and lasts for days; and (iii) the chronic phase, mainly at weeks to months after ischemia (Jin et al., 2010). Our recent study found that compared with the juvenile mice, aged mice developed larger infarct volume and more severe neuronal damage (Suenaga et al., 2015). However, the underlying mechanisms still remain obscure and further studies are warranted.
Figure 3.
General pathophysiological phases of ischemic stroke. There are three pathophysiological phases after the onset of ischemic stroke: the excitotoxicity phase, the inflammatory phase, and the chronic phase.
4.1 Excitotoxic phase
In the excitotoxic phase, cellular energy failure and subsequent excitotoxicity are the main detrimental events (Jin et al., 2010). Oxygen and glucose deprivation causes cell necrosis in the ischemic core, and apoptosis in the penumbra (Broughton et al., 2009). When the ischemic cells are reperfused, the consequent oxidative stress is catastrophic to the NVU (Chen et al., 2011). Due to energy depletion and oxidative stress, cellular ion channels fail to exert normal function (e.g. sodium potassium ATPase and sodium calcium pump). As a result, abnormalities in trans-membrane ion gradient arise both at the cellular and at the organelle levels, leading to excessive neuronal depolarization as well as excitatory neurotransmitters, such as glutamate (Weilinger et al., 2013). Excessive glutamate depolarizes neurons and increases the toxic calcium level. This process is defined as glutamate-mediated excitotoxicity (Weilinger et al., 2013).
Many cellular components of NVU demonstrate age-related abnormalities in their responses to ischemic injury. Senile neurons have already been suffering from accumulated metabolic and oxidative stress as well as mitochondrial malfunction. They have a relatively low level of energy supply, so it could be predicted that they will succumb to ischemia and the subsequent necrosis and apoptosis more easily. Astrocytes are responsible for the inter-synaptic glutamate level with their glutamate transporters such as GLT-1 and GLAST (Santos-Carvalho et al., 2014). However, the density of these transporters decreases with aging (Bradford et al., 2009). Moreover, the regulation capacity of astrocytes during stroke is impaired since their glutamate transporter level drops acutely soon after ischemia (Yeh et al., 2005), which further exacerbates glutamate-mediated excitotoxicity in aged cerebral ischemia. In the elderly, cerebral blood flow is not at the same level as that in the young. And we found that aged animals exhibit an even greater reduction of cerebral reperfusion after ischemic stroke (Suenaga et al., 2015).
4.2 Inflammatory phase
Neural inflammation after stroke is complicated due to the infiltration of various kinds of immune cells into the brain. Soon after the ischemic onset, resident microglia and astrocytes are activated. They secrete cytokines, chemokines and adhesion molecules. These inflammatory factors increase BBB permeability, further elevate endothelial adhesion molecule expression and recruit the peripheral immune cells into the injury site (Hu et al., 2014; Ma et al., 2016). Inflammation-induced BBB dysfunction is one of the key mechanisms underlying acute brain damage after ischemic stroke. Of note, BBB permeability increases with age (Wardlaw et al., 2013) and is associated with severe cognitive decline and poor functional outcome in elderly stroke patients (Di Napoli and Shah, 2011). The malfunctions of several components in the senile NVU contribute to the compromised BBB integrity in aged brain. In this context, more leukocytes migrate to the aged ischemic brain through the impaired BBB, including T cells, neutrophils, macrophage and dendritic cells (Manwani et al., 2013).
In a previous study, we found that M2 microglia phenotype was dominant in the ischemic brain at the early phase of injury, but gradually shifted toward M1 phenotype at a later phase in young adult mice (Hu et al., 2012; Xiong et al., 2016). Interestingly, microglial M2 polarization in the early stage was impaired in aged mice after ischemia (Suenaga et al., 2015). Similarly, astrocytes also display a pro-inflammatory phenotype after stroke in the aged population (Dinapoli et al., 2010).
Aging affects stroke outcome not only by increasing pro-inflammatory tone, but also by weakening the anti-inflammatory system. In the elderly, the expression of the forkhead box P3 (Foxp3) level and the immune suppression function of regulatory T cells (Treg) (Zhao et al., 2007), which effectively protect CNS from ischemic insult, are decreased.
After ischemic insult, phagocytosis is required for debris clearance (Chiu et al., 2011). In the aged animal, the phagocytosis capacity of immune cells declines, leading to an accumulation of neural debris. An important example is Tau, the cytoskeleton protein which becomes toxic after structural damage. In old age, the efficiency of impaired tau elimination is down-regulated, thus causing neural functional impairment and neuronal cell death, which further increases the risk of developing neurodegeneration in aged stroke animals (Kurata et al., 2014).
4.3 Chronic phase
In the chronic phase after stroke, repair and regeneration ensue to restore NVU functions. The limited capacity of neurogenesis results in insufficient neuronal replacement in aged mice subjected to ischemic stroke. Angiogenesis, which is activated to ensure sufficient energy supply for the repair process, also dramatically reduces in the aged population. The impaired angiogenesis during aging is a result of multiple mechanisms, including endothelial growth arrest, lack of growth factors, reduced circulating EPC and increased TIMP. In aged brain, reduced reperfusion and cerebral blood flow after stroke further attenuates angiogenesis and consequently diminishes energy supply to the injured NVU.
Oligodendrocytes and their precursor cells initiate the process of remyelination to promote white matter repair (Sim et al., 2002). It has recently been demonstrated that decreased remyelination in response to cerebral ischemic insults in the elderly leaves the white matter injury incompletely repaired (Sim et al., 2002; Suenaga et al., 2015).
The polarization of M2 microglia, which contributes to neuro-regeneration by increasing phagocytosis-secreting growth factors, and resolving cerebral inflammation, is also weakened in the elderly (Hu et al., 2015). In summary, the repair of the NVU during the chronic phase of ischemic stroke is down-regulated overall.
Taken together, the increased neuronal necrosis and apoptosis in the excitotoxic phase, the amplified detrimental immune response in the inflammatory phase and the impaired regeneration in the chronic phase coordinately contribute to the poor outcome of ischemic stroke in the elderly. Understanding the underlying mechanism of these aged-related alterations may identify novel therapeutic targets for stroke in the aged population.
5. NVU impairment is a cause rather than a consequence in aging-related neurodegenerative diseases
Neurodegenerative diseases could be considered as accelerated aging because their pathophysiological mechanisms share commonalities with the normal aging process (Butterfield et al., 2001). Unlike ischemic stroke, which is explicitly a vascular disease, the relationship between neurodegenerative diseases and age-related NVU impairment seems relatively obscure. However, more and more evidence supports the notion that the impairments in both the microvascular and the neuroglial components of the NVU facilitate the pathophysiological process of Alzheimer’s disease (AD) and Parkinson’s disease (PD).
5.1 The aged NVU and AD
The pathologic role of aging-related vascular injury in the progress of AD was not reported until recently. It is known that microvascular deficits and CBF reduction can be detected prior to the symptoms of cognitive dysfunction in AD patients (Wierenga et al., 2014). Indeed, BBB breakdown serves as a key pathway that leads to the onset of AD (Sharma et al., 2012).
As aging proceeds, cellular damage accumulates and functions of the BBB are progressively compromised. Since the barrier of the CNS is weakened, red blood cells (RBC) and serum proteins, including albumin, prothrombin, plasminogen and fibrinogen can leak from the circulation into interstitial fluid. RBC invasion leads to hemoglobin and ion accumulation. Excessive ions increase ROS damage to the NVU (Zhong et al., 2008; Zhong et al., 2009). Thrombin not only directly damages neurons, but also activates microglia and astrocytes and provokes inflammatory neurotoxicity (Grammas, 2011). Plasmin catalyzes the degradation of laminin, which is a key component of BBB basal lamina. In addition, leakage of albumin and immunoglobulins results in vasogenic edema, which suppresses vascular diameter and results in the hypoperfusion within the NVU (Bell et al., 2010). Hypoxia in turn increases ROS production in mitochondria and causes injury to NVU components, especially in endothelial cells and high metabolic neurons. All these pathological changes will culminate in deteriorated NVU integrity and predispose the aged brain to neurodegenerative diseases.
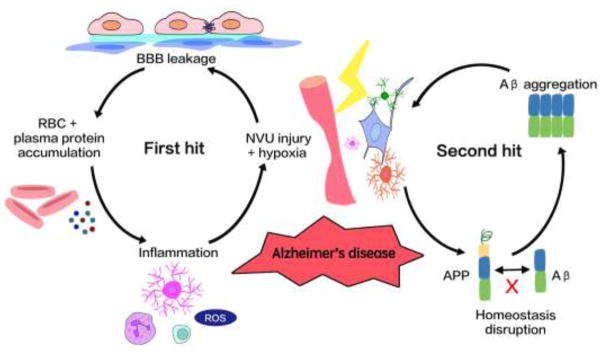
Zlokovic first proposed the two-hit hypothesis for AD (Figure 4) (Zlokovic, 2011). In his theory, NVU dysfunction is the first step in the pathogenesis of AD, while the disruption of CNS amyloid-β (Aβ) homeostasis serves as the second hit. Aβ is produced from Aβ precursor protein (APP) in both CNS and periphery. In normal conditions, the peripheral Aβ is prevented from entering the brain by the intact BBB, and the CNS Aβ is cleared in a timely fashion (O’Brien and Wong, 2011). Aβ in the interstitial fluid is bound by low-density lipoprotein receptor-related protein 1 (LRP1) on the BBB and released to the circulation (O’Brien and Wong, 2011). The above-mentioned NVU dysfunction in aged brain will not only increase vascular-mediated neuronal injury but also impair Aβ clearance. The disruption of Aβ homeostasis may cause its accumulation within the CNS and the formation of Aβ plaques. The Aβ plaques could further damage the NVU and lead to neurodegeneration (Mattson and Magnus, 2006; Zlokovic, 2011). According to this two-hit hypothesis, NVU injury, which progresses with aging, is the primary insult that initiates a cascade of pathological events in AD.
Figure 4.
Two-hit hypothesis of Alzheimer’s disease. The two-hit hypothesis of Alzheimer’s disease proposed a view that vascular risk factors and cell cycle-related abnormalities could lead to AD synergistically. As the first hit, the aged-related vascular risk factors impair the NVU function and result in hypoperfusion and hypoxia infraction. The second hit comes from the abnormal accumulation of amyloid-β (Aβ), which is caused by increased production and reduced clearance of Aβ. The NVU damage and Aβ accumulation together accelerate neurodegeneration and dementia in Alzheimer’s disease.
5.2 The aged NVU and PD
Compared with AD, BBB disruption seems to be less important in PD, and the effect of neuron and glial cell injury is more crucial. The loss of dopaminergic neurons is the hallmark of PD. As high-metabolic cells, dopaminergic neurons are equipped with abundant amounts of mitochondria, and are therefore easily exposed to high levels of ROS (Liang et al., 2007). Moreover, dopamine is metabolized by monoamine oxidase (Yamato et al., 2010), dopamine auto-oxidation and the Fenton reaction (Youdim et al., 2004), all of which produce large amounts of ROS. Mitochondrial DNA is easily damaged by ROS, which results in mitochondrial malfunction. In aged cells, mitophagy is disturbed (Wenz and Passos, 2012). Meanwhile, low energy support results in reduced mitochondria repair (Gredilla et al., 2010). All these changes will contribute to dopaminergic neuronal loss.
In physiological conditions, dopaminergic neurons are well equipped to deal with oxidative stress. Superoxide dismutase (SOD) and glutathione peroxidase (GPX) nonspecifically decompose ROS / NOS. Meanwhile, dopamine is specifically transported by dopamine active transporter (DAT) and vesicular monoamine transporter 2 (VMAT2) from the extracellular space to synaptic vesicles, where it undergoes self-oxidation (Bisaglia et al., 2013). However, when these protective mechanisms are compromised during aging, large amounts of ROS will be produced. The subsequent oxidative stress will then facilitate the aggregation of α-synuclein (Takahashi et al., 2007). Aggregation of α-synuclein forms Lewy bodies and Lowy neurites, both of which are hallmarks of PD (Rodriguez et al., 2016; Rodriguez et al., 2015).
In addition to neuronal injury, glial cells play a pivotal part in PD development. Dopaminergic neurons are equipped with unmyelinated axons, and thus astrocytes make the most intimate contact with them (Rodriguez et al., 2016). Intact astrocytes provide protection and support to dopaminergic neurons through producing antioxidants such as glutathione, and through removing toxic molecules including glutamate and α-synuclein (Rappold and Tieu, 2010). Meanwhile, astrocytic neurotrophic factors protect neurons from damage (Drinkut et al., 2012). However, chronic inflammation in the aged CNS changes the phenotype of astrocytes. The senile astrocytes fail to provide protection and support. Instead, they produce cytokines, chemokines and ROS, which harm the integrity of the NVU (Rodriguez et al., 2015). Microglia are also activated in the inflammatory milieu of aged CNS. Dying neurons release α-synuclein aggregates, MMP-3 and neuromelanin, all of which could prime microglia to a pro-inflammatory status. Activated microglia produce even more ROS and pro-inflammatory factors, and propel disease progression (Collins et al., 2012).
Although the detailed mechanisms of various neurodegenerative diseases remain veiled, it is clear that aging is a vital risk factor in neurodegeneration. Age-related impairments in the NVU, together with alterations in genes and environmental factors, initiate and maintain a self-perpetuating cycle of neurodegeneration. Therefore, targeting aging-related alterations in the NVU is a promising therapeutic strategy for neurodegenerative diseases.
6. Prevention perspectives
Population aging is becoming a serious societal issue worldwide. Age-related changes to the NVU have been increasingly accepted as important factors that promote the vulnerability to ischemic injury and neurodegeneration. Despite the monumental progress in the research on aging, it is so far still impossible to reverse the process of aging in the senile NVU. Nevertheless, alternative approaches have been developed to ameliorate the impact of aging on NVU and protect the aged brain against ischemia/reperfusion attack or neurodegeneration.
One of the effective approaches is to counteract the aging-related detrimental factors in the NVU. As discussed in pervious sections, excessive oxidative stress is one of the most important catastrophic factors in the aged NVU. Antioxidants therefore represent an effective strategy to slow down the aging process in the senile brain. Various antioxidants, such as statins (Stoll et al., 2004) and polyunsaturated fatty acids (PUFAs) (Richard et al., 2008), have been proved to lessen ROS damage and to protect aged brain from stroke (Di Napoli and Papa, 2001; Zhang et al., 2010).
In addition to reducing detrimental factors, boosting the defense and repair system is equally important in the battle with aging. Enhancing the DNA repair system, upregulating neurogenesis and angiogenesis and promoting white matter repair are all promising strategies that reduce aging-related damage (Liu et al., 2014; Ruan et al., 2014).
Finally, recent research has highlighted the success in the effort to slow down the aging process in the NVU. The pace of aging in each individual greatly depends on living environment and daily lifestyle, including diet and sleep. Different styles of diet have been shown to contribute to the incidence of stroke as well as other CNS disorders (Campbell and Junshi, 1994; Liang et al., 2011; Sacco et al., 2006). Proper diet could help to protect against CNS injury and neurodegeneration by lessening ROS production and boosting neurotrophic factors (Heilbronn and Ravussin, 2003). For example, the Mediterranean diet has been shown to delay the process of aging via multiple mechanisms, including ameliorating oxidative stress (Marín et al., 2013). Recently, a diet called Fortasyn has been recommended to AD patients. The Fortasyn diet is comprised of the n3-LCPUFAs docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), and some other precursors and cofactors for membrane synthesis, such as uridine, choline, phospholipids, folic acid, vitamins B12, B6, C, and E, and selenium (Kamphuis and Scheltens, 2010). It is reported that the Fortasyn diet an increase CBF and restore CNS integrity (Wiesmann et al., 2016). The effect of the Fortasyn diet in stroke outcomes need to be further investigated. Interestingly, there is an emerging literature about the importance of microbiota in CNS homeostasis and in the outcomes of CNS diseases (Benakis et al., 2016; Shoemark and Allen, 2015). Food consumed has been reported to change the gut microbiota, which in turn modulate CNS functions through regulating energy metabolism as well as peripheral immune profile (Saraswati and Sitaraman, 2014). It is therefore possible that the impact of diet on the “microbiota-gut-brain” axis could influence the integrity of aging NVU in both physiological and pathological conditions.
In addition to proper diet, other elements of a healthy lifestyle, such as proper medication, high-quality sleep (Scullin and Bliwise, 2015) and appropriate physical activity (Reimers et al., 2012) help to slow down the aging process. For example, most aged people have to take a variety of medicines to treat comorbid conditions, such as hypertension, diabetes and chronic pain. These medicines could reduce the detrimental impact of comorbidities to the aged NVU; however, these drugs themselves may impair BBB integrity and NVU functions. The interaction between analgesic drugs and the NVU has been previously reviewed (Radu et al., 2013). Therefore, medication regimens should be carefully adjusted to achieve the best protection for aged NVU. In addition, physical exercises, such as running and swimming, have been demonstrated to stimulate neurogenesis (Chae et al., 2014; van Praag et al., 1999). Indeed, lifestyle shift could be the most efficient and economical strategy to prevent the incidence of stroke and to ameliorate ischemic brain injury in the geriatric population.
7. Conclusion
Aging is an unescapable and ever-progressing process that affects every living organism from birth. It causes molecular damage, organelle dysfunction and cellular injury in the components of the NVU and leads to structural and functional impairments. As a result, the vulnerability of the NVU to ischemic stroke and neurodegeneration increases with aging. Prophylactic or therapeutic strategies that target at the aging process will bring new hope for management of CNS injuries and diseases.
Highlights.
The aging process causes structural and functional impairments to the neurovascular unit (NVU).
Age-related vulnerability of the NVU increases the risk and exacerbates the severity of ischemic stroke.
NVU impairment is a cause rather than consequence in aging-related neurodegenerative diseases.
Alterations of diet and lifestyle could slow down the aging process and ameliorate the risk and outcome of neurological disorders.
Acknowledgments
Sources of support:
This work was supported by VA merit grants (I01BX002495 and I01RX000420 to Jun Chen), NIH grants (NS095671, NS089534 and NS45048 to Jun Chen); and the U.S. Department of Veterans Affairs Senior Research Career Scientist Award (to Jun Chen).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nature reviews Neuroscience. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Babcock AA, Ilkjaer L, Clausen BH, Villadsen B, Dissing-Olesen L, Bendixen AT, Lyck L, Lambertsen KL, Finsen B. Cytokine-producing microglia have an altered beta-amyloid load in aged APP/PS1 Tg mice. Brain, behavior, and immunity. 2015;48:86–101. doi: 10.1016/j.bbi.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Barakat R, Redzic Z. The Role of Activated Microglia and Resident Macrophages in the Neurovascular Unit during Cerebral Ischemia: Is the Jury Still Out? Medical principles and practice : international journal of the Kuwait University, Health Science Centre. 2016;25(Suppl 1):3–14. doi: 10.1159/000435858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto GE, Capani F, Gonzalez J, Morales L. Role of astrocytes in neurodegenerative diseases. INTECH Open Access Publisher; 2011. [Google Scholar]
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL. GABAA, NMDA and AMPA receptors: a developmentally regulated ‘menage a trois’. Trends Neurosci. 1997;20:523–529. doi: 10.1016/s0166-2236(97)01147-8. [DOI] [PubMed] [Google Scholar]
- Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, Sita G, Racchumi G, Ling L, Pamer EG, Iadecola C, Anrather J. Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat Med. 2016;22:516–523. doi: 10.1038/nm.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro-oncology. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaglia M, Greggio E, Beltramini M, Bubacco L. Dysfunction of dopamine homeostasis: clues in the hunt for novel Parkinson’s disease therapies. The FASEB Journal. 2013;27:2101–2110. doi: 10.1096/fj.12-226852. [DOI] [PubMed] [Google Scholar]
- Bonkowski D, Katyshev V, Balabanov RD, Borisov A, Dore-Duffy P. The CNS microvascular pericyte: pericyte-astrocyte crosstalk in the regulation of tissue survival. Fluids and barriers of the CNS. 2011;8:8. doi: 10.1186/2045-8118-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford J, Shin JY, Roberts M, Wang CE, Li XJ, Li S. Expression of mutant huntingtin in mouse brain astrocytes causes age-dependent neurological symptoms. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:22480–22485. doi: 10.1073/pnas.0911503106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovascular research. 2005;66:286–294. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331–e339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Howard BJ, LaFontaine MA. Brain oxidative stress in animal models of accelerated aging and the age-related neurodegenerative disorders, Alzheimer’s disease and Huntington’s disease. Current medicinal chemistry. 2001;8:815–828. doi: 10.2174/0929867013373048. [DOI] [PubMed] [Google Scholar]
- Cai X, Patel S. Degeneration of an intracellular ion channel in the primate lineage by relaxation of selective constraints. Molecular biology and evolution. 2010;27:2352–2359. doi: 10.1093/molbev/msq122. [DOI] [PubMed] [Google Scholar]
- Campbell TC, Junshi C. Diet and chronic degenerative diseases: perspectives from China. The American journal of clinical nutrition. 1994;59:1153S–1161S. doi: 10.1093/ajcn/59.5.1153S. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. The Journal of experimental biology. 2008;211:1781–1791. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick W, Mitchell N, Martin B, Maudsley S. Therapeutic Targeting of the Endoplasmic Reticulum in Alzheimer’s Disease. Current Alzheimer research. 2012;9:110–119. doi: 10.2174/156720512799015055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae CH, Jung SL, An SH, Park BY, Kim TW, Wang SW, Kim JH, Lee HC, Kim HT. Swimming exercise stimulates neuro-genesis in the subventricular zone via increase in synapsin I and nerve growth factor levels. Biology of sport. 2014;31:309–314. doi: 10.5604/20831862.1132130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaker Z, Aid S, Berry H, Holzenberger M. Suppression of IGF-I signals in neural stem cells enhances neurogenesis and olfactory function during aging. Aging cell. 2015;14:847–856. doi: 10.1111/acel.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Gong M, Yan M, Zhang X. Sevoflurane induces endoplasmic reticulum stress mediated apoptosis in hippocampal neurons of aging rats. PloS one. 2013;8:e57870. doi: 10.1371/journal.pone.0057870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, Maier CM, Narasimhan P, Goeders CE, Chan PH. Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxidants & redox signaling. 2011;14:1505–1517. doi: 10.1089/ars.2010.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CH, Yeh KM, Siu LK, Fung CP, Lin JC, Chang FY. Impact of age on neutrophil phagocytic reaction with different capsular serotypes of Klebsiella pneumoniae. Journal of Microbiology, Immunology and Infection. 2011;44:333–337. doi: 10.1016/j.jmii.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Collins LM, Toulouse A, Connor TJ, Nolan YM. Contributions of central and systemic inflammation to the pathophysiology of Parkinson’s disease. Neuropharmacology. 2012;62:2154–2168. doi: 10.1016/j.neuropharm.2012.01.028. [DOI] [PubMed] [Google Scholar]
- Cotrina ML, Gao Q, Lin JHC, Nedergaard M. Expression and function of astrocytic gap junctions in aging. Brain research. 2001;901:55–61. doi: 10.1016/s0006-8993(01)02258-2. [DOI] [PubMed] [Google Scholar]
- Curcio M, Salazar IL, Mele M, Canzoniero LM, Duarte CB. Calpains and neuronal damage in the ischemic brain: The swiss knife in synaptic injury. Progress in neurobiology. 2016 doi: 10.1016/j.pneurobio.2016.06.001. [DOI] [PubMed] [Google Scholar]
- Denti L, Scoditti U, Tonelli C, Saccavini M, Caminiti C, Valcavi R, Benatti M, Ceda GP. The poor outcome of ischemic stroke in very old people: a cohort study of its determinants. Journal of the American Geriatrics Society. 2010;58:12–17. doi: 10.1111/j.1532-5415.2009.02616.x. [DOI] [PubMed] [Google Scholar]
- Di Napoli M, Papa F. Inflammation, statins, and outcome after ischemic stroke. Stroke. 2001;32:2446–2447. [PubMed] [Google Scholar]
- Di Napoli M, Shah IM. Neuroinflammation and cerebrovascular disease in old age: a translational medicine perspective. Journal of aging research. 2011;2011 doi: 10.4061/2011/857484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinapoli VA, Benkovic SA, Li X, Kelly KA, Miller DB, Rosen CL, Huber JD, O’Callaghan JP. Age exaggerates proinflammatory cytokine signaling and truncates signal transducers and activators of transcription 3 signaling following ischemic stroke in the rat. Neuroscience. 2010;170:633–644. doi: 10.1016/j.neuroscience.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz DG, Foro CA, Rego CM, Gloria DA, de Oliveira FR, Paes JM, de Sousa AA, Tokuhashi TP, Trindade LS, Turiel MC, Vasconcelos EG, Torres JB, Cunnigham C, Perry VH, Vasconcelos PF, Diniz CW. Environmental impoverishment and aging alter object recognition, spatial learning, and dentate gyrus astrocytes. The European journal of neuroscience. 2010;32:509–519. doi: 10.1111/j.1460-9568.2010.07296.x. [DOI] [PubMed] [Google Scholar]
- Drinkut A, Tereshchenko Y, Schulz JB, Bähr M, Kügler S. Efficient gene therapy for Parkinson’s disease using astrocytes as hosts for localized neurotrophic factor delivery. Molecular Therapy. 2012;20:534–543. doi: 10.1038/mt.2011.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler-Chiurazzi EB, Brown CM, Povroznik JM, Simpkins JW. Estrogens as neuroprotectants: Estrogenic actions in the context of cognitive aging and brain injury. Progress in neurobiology. 2016 doi: 10.1016/j.pneurobio.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends in immunology. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Filous AR, Silver J. Targeting astrocytes in CNS injury and disease: A translational research approach. Progress in neurobiology. 2016 doi: 10.1016/j.pneurobio.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster C. Tight junctions and the modulation of barrier function in disease. Histochemistry and Cell Biology. 2008;130:55–70. doi: 10.1007/s00418-008-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemma C, Bachstetter AD, Bickford PC. Neuron-Microglia Dialogue and Hippocampal Neurogenesis in the Aged Brain. Aging and disease. 2010;1:232–244. [PMC free article] [PubMed] [Google Scholar]
- Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M, Christoffersson J, Chaabane W, Moghadam AR, Kashani HH, Hashemi M, Owji AA, Los MJ. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Progress in neurobiology. 2014;112:24–49. doi: 10.1016/j.pneurobio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Gradinaru D, Borsa C, Ionescu C, Prada GI. Oxidized LDL and NO synthesis—biomarkers of endothelial dysfunction and ageing. Mechanisms of ageing and development. 2015;151:101–113. doi: 10.1016/j.mad.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Grammas P. Neurovascular dysfunction, inflammation and endothelial activation: implications for the pathogenesis of Alzheimer’s disease. J Neuroinflammation. 2011;8:2094–2098. doi: 10.1186/1742-2094-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gredilla R, Bohr VA, Stevnsner T. Mitochondrial DNA repair and association with aging–an update. Experimental gerontology. 2010;45:478–488. doi: 10.1016/j.exger.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham PB, 3rd, Raju R. Mitochondrial function in hypoxic ischemic injury and influence of aging. Progress in neurobiology. 2016 doi: 10.1016/j.pneurobio.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Lam M, McCormick TS, Distelhorst CW. Maintenance of calcium homeostasis in the endoplasmic reticulum by Bcl-2. The Journal of cell biology. 1997;138:1219–1228. doi: 10.1083/jcb.138.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. The American journal of clinical nutrition. 2003;78:361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, Chen J. Microglial and macrophage polarization [mdash] new prospects for brain repair. Nature reviews Neurology. 2015;11:56–64. doi: 10.1038/nrneurol.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, Gao Y, Chen J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- Hu X, Liou AK, Leak RK, Xu M, An C, Suenaga J, Shi Y, Gao Y, Zheng P, Chen J. Neurobiology of microglial action in CNS injuries: receptor-mediated signaling mechanisms and functional roles. Progress in neurobiology. 2014;119–120:60–84. doi: 10.1016/j.pneurobio.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S, Gardiner T, Hu P, Baxter L, Rosinova E, Chan-Ling T. Altered pericyte-endothelial relations in the rat retina during aging: implications for vessel stability. Neurobiol Aging. 2006;27:1838–1847. doi: 10.1016/j.neurobiolaging.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Irwin RW, Brinton RD. Allopregnanolone as regenerative therapeutic for Alzheimer’s disease: translational development and clinical promise. Progress in neurobiology. 2014;113:40–55. doi: 10.1016/j.pneurobio.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. Journal of leukocyte biology. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo DH, Kim JH, Heo JI, Kim JH, Cho CH. Interaction between pericytes and endothelial cells leads to formation of tight junction in hyaloid vessels. Molecules and cells. 2013;36:465–471. doi: 10.1007/s10059-013-0228-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphuis PJ, Scheltens P. Can nutrients prevent or delay onset of Alzheimer’s disease? Journal of Alzheimer’s Disease. 2010;20:765–775. doi: 10.3233/JAD-2010-091558. [DOI] [PubMed] [Google Scholar]
- Koike T, Vernon RB, Gooden MD, Sadoun E, Reed MJ. Inhibited angiogenesis in aging: a role for TIMP-2. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2003;58:B798–B805. doi: 10.1093/gerona/58.9.b798. [DOI] [PubMed] [Google Scholar]
- Kurata T, Lukic V, Kozuki M, Wada D, Miyazaki K, Morimoto N, Ohta Y, Deguchi K, Ikeda Y, Kamiya T, Abe K. Telmisartan reduces progressive accumulation of cellular amyloid beta and phosphorylated tau with inflammatory responses in aged spontaneously hypertensive stroke resistant rat. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2014;23:2580–2590. doi: 10.1016/j.jstrokecerebrovasdis.2014.05.023. [DOI] [PubMed] [Google Scholar]
- Lähteenvuo J, Rosenzweig A. Effects of aging on angiogenesis. Circulation research. 2012;110:1252–1264. doi: 10.1161/CIRCRESAHA.111.246116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai AY, Dibal CD, Armitage GA, Winship IR, Todd KG. Distinct activation profiles in microglia of different ages: a systematic study in isolated embryonic to aged microglial cultures. Neuroscience. 2013;254:185–195. doi: 10.1016/j.neuroscience.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Lardenoije R, Iatrou A, Kenis G, Kompotis K, Steinbusch HW, Mastroeni D, Coleman P, Lemere CA, Hof PR, van den Hove DL, Rutten BP. The epigenetics of aging and neurodegeneration. Progress in neurobiology. 2015;131:21–64. doi: 10.1016/j.pneurobio.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Wu Y, Shi XQ, Zhang J. Characteristics of spinal microglia in aged and obese mice: potential contributions to impaired sensory behavior. Immunity & ageing : I & A. 2015;12:22. doi: 10.1186/s12979-015-0049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leovsky C, Fabian C, Naaldijk Y, Jager C, Jang HJ, Bohme J, Rudolph L, Stolzing A. Biodistribution of in vitro-derived microglia applied intranasally and intravenously to mice: effects of aging. Cytotherapy. 2015;17:1617–1626. doi: 10.1016/j.jcyt.2015.07.019. [DOI] [PubMed] [Google Scholar]
- Liang CL, Wang TT, Luby-Phelps K, German DC. Mitochondria mass is low in mouse substantia nigra dopamine neurons: implications for Parkinson’s disease. Experimental neurology. 2007;203:370–380. doi: 10.1016/j.expneurol.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Liang W, Lee AH, Binns CW. Dietary intake of minerals and the risk of ischemic stroke in Guangdong Province, China, 2007–2008. Preventing chronic disease. 2011;8:A38. [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang Y, Akamatsu Y, Lee CC, Stetler RA, Lawton MT, Yang GY. Vascular remodeling after ischemic stroke: mechanisms and therapeutic potentials. Progress in neurobiology. 2014;115:138–156. doi: 10.1016/j.pneurobio.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo EH, Rosenberg GA. The Neurovascular Unit in Health and Disease Introduction. Stroke. 2009;40:S2–S3. doi: 10.1161/STROKEAHA.108.534404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long DA, Newaz MA, Prabhakar SS, Price KL, D TRUONG L, Feng L, Mu W, Oyekan AO, Johnson RJ. Loss of nitric oxide and endothelial-derived hyperpolarizing factor–mediated responses in aging. Kidney international. 2005;68:2154–2163. doi: 10.1111/j.1523-1755.2005.00671.x. [DOI] [PubMed] [Google Scholar]
- Lourbopoulos A, Erturk A, Hellal F. Microglia in action: how aging and injury can change the brain’s guardians. Frontiers in cellular neuroscience. 2015;9:54. doi: 10.3389/fncel.2015.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luissint AC, Artus C, Glacial F, Ganeshamoorthy K, Couraud PO. Tight junctions at the blood brain barrier: physiological architecture and disease-associated dysregulation. Fluids and barriers of the CNS. 2012;9:23. doi: 10.1186/2045-8118-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Wang J, Wang Y, Yang GY. The biphasic function of microglia in ischemic stroke. Progress in neurobiology. 2016 doi: 10.1016/j.pneurobio.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Mancall EL, Brock DG. Gray’s clinical neuroanatomy. Elsevier Health Sciences; 2011. [Google Scholar]
- Manwani B, Liu F, Scranton V, Hammond MD, Sansing LH, McCullough LD. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Experimental neurology. 2013;249:120–131. doi: 10.1016/j.expneurol.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Husstege M, Muggironi M, Raban D, Skoff RP, Casaccia-Bonnefil P. Oligodendrocyte progenitor proliferation and maturation is differentially regulated by male and female sex steroid hormones. Developmental neuroscience. 2004;26:245–254. doi: 10.1159/000082141. [DOI] [PubMed] [Google Scholar]
- Marín C, Yubero-Serrano EM, López-Miranda J, Pérez-Jiménez F. Endothelial aging associated with oxidative stress can be modulated by a healthy mediterranean diet. International journal of molecular sciences. 2013;14:8869–8889. doi: 10.3390/ijms14058869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nature reviews Neuroscience. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moftakhar P, Lynch MD, Pomakian JL, Vinters HV. Aquaporin expression in the brains of patients with or without cerebral amyloid angiopathy. Journal of Neuropathology & Experimental Neurology. 2010;69:1201–1209. doi: 10.1097/NEN.0b013e3181fd252c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaetova-Ladinska EB, Harrington CR, Roth M, Wischik CM. Alterations in tau protein metabolism during normal aging. Dementia (Basel, Switzerland) 1996;7:95–103. doi: 10.1159/000106861. [DOI] [PubMed] [Google Scholar]
- Naidoo N, Ferber M, Master M, Zhu Y, Pack AI. Aging impairs the unfolded protein response to sleep deprivation and leads to proapoptotic signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:6539–6548. doi: 10.1523/JNEUROSCI.5685-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo N, Zhu J, Zhu Y, Fenik P, Lian J, Galante R, Veasey S. Endoplasmic reticulum stress in wake-active neurons progresses with aging. Aging cell. 2011;10:640–649. doi: 10.1111/j.1474-9726.2011.00699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG, Budd SL. Mitochondria and Neuronal Survival. Physiological Reviews. 2000;80:315–360. doi: 10.1152/physrev.2000.80.1.315. [DOI] [PubMed] [Google Scholar]
- Nicholson WT, Vaa B, Hesse C, Eisenach JH, Joyner MJ. Aging is associated with reduced prostacyclin-mediated dilation in the human forearm. Hypertension. 2009;53:973–978. doi: 10.1161/HYPERTENSIONAHA.108.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer’s disease. Annual review of neuroscience. 2011;34:185. doi: 10.1146/annurev-neuro-061010-113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A. The effects of normal aging on myelinated nerve fibers in monkey central nervous system. Frontiers in neuroanatomy. 2009;3:11. doi: 10.3389/neuro.05.011.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Sethares C. Oligodendrocytes, their progenitors and other neuroglial cells in the aging primate cerebral cortex. Cerebral cortex (New York, NY : 1991) 2004;14:995–1007. doi: 10.1093/cercor/bhh060. [DOI] [PubMed] [Google Scholar]
- Plemel JR, Keough MB, Duncan GJ, Sparling JS, Yong VW, Stys PK, Tetzlaff W. Remyelination after spinal cord injury: is it a target for repair? Progress in neurobiology. 2014;117:54–72. doi: 10.1016/j.pneurobio.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Prisby RD, Ramsey MW, Behnke BJ, Dominguez JM, Donato AJ, Allen MR, Delp MD. Aging Reduces Skeletal Blood Flow, Endothelium-Dependent Vasodilation, and NO Bioavailability in Rats. Journal of Bone and Mineral Research. 2007;22:1280–1288. doi: 10.1359/jbmr.070415. [DOI] [PubMed] [Google Scholar]
- Puca AA, Carrizzo A, Ferrario A, Villa F, Vecchione C. Endothelial nitric oxide synthase, vascular integrity and human exceptional longevity. Immunity & Ageing. 2012;9:1. doi: 10.1186/1742-4933-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radu BM, Bramanti P, Osculati F, Flonta ML, Radu M, Bertini G, Fabene PF. Neurovascular unit in chronic pain. Mediators of inflammation. 2013;2013:648268. doi: 10.1155/2013/648268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappold PM, Tieu K. Astrocytes and therapeutics for Parkinson’s disease. Neurotherapeutics. 2010;7:413–423. doi: 10.1016/j.nurt.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers C, Knapp G, Reimers A. Does physical activity increase life expectancy? A review of the literature. Journal of aging research. 2012;2012 doi: 10.1155/2012/243958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard D, Kefi K, Barbe U, Bausero P, Visioli F. Polyunsaturated fatty acids as antioxidants. Pharmacological Research. 2008;57:451–455. doi: 10.1016/j.phrs.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Arellano JJ, Parpura V, Zorec R, Verkhratsky A. Astrocytes in physiological aging and Alzheimer’s disease. Neuroscience. 2016;323:170–182. doi: 10.1016/j.neuroscience.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Morales I, Rodriguez-Sabate C, Sanchez A, Castro R, Brito JM, Sabate M. The degeneration and replacement of dopamine cells in Parkinson’s disease: the role of aging. Parkinson’s Disease: Cell Vulnerability and Disease Progression. 2016:60. doi: 10.3389/fnana.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Rodriguez-Sabate C, Morales I, Sanchez A, Sabate M. Parkinson’s disease as a result of aging. Aging cell. 2015;14:293–308. doi: 10.1111/acel.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Melendez RI, Zaborszky L, Napier TC. The ventral pallidum: Subregion-specific functional anatomy and roles in motivated behaviors. Progress in neurobiology. 2015;130:29–70. doi: 10.1016/j.pneurobio.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan L, Lau BW, Wang J, Huang L, Zhuge Q, Wang B, Jin K, So KF. Neurogenesis in neurological and psychiatric diseases and brain injury: from bench to bedside. Progress in neurobiology. 2014;115:116–137. doi: 10.1016/j.pneurobio.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Rutten BPF, Schmitz C, Gerlach OHH, Oyen HM, de Mesquita EB, Steinbusch HWM, Korr H. The aging brain: Accumulation of DNA damage or neuron loss? Neurobiology of Aging. 2007;28:91–98. doi: 10.1016/j.neurobiolaging.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB, Gorelick P, Halperin J, Harbaugh R. Guidelines for Prevention of Stroke in Patients With Ischemic Stroke or Transient Ischemic Attack A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association Council on Stroke: Co-Sponsored by the Council on Cardiovascular Radiology and Intervention: The American Academy of Neurology affirms the value of this guideline. Circulation. 2006;113:e409–e449. [PubMed] [Google Scholar]
- Salganik M, Sergeyev VG, Shinde V, Meyers CA, Gorbatyuk MS, Lin JH, Zolotukhin S, Gorbatyuk OS. The loss of glucose-regulated protein 78 (GRP78) during normal aging or from siRNA knockdown augments human alpha-synuclein (alpha-syn) toxicity to rat nigral neurons. Neurobiol Aging. 2015;36:2213–2223. doi: 10.1016/j.neurobiolaging.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Haapasalo A, Kauppinen A, Kaarniranta K, Soininen H, Hiltunen M. Impaired mitochondrial energy metabolism in Alzheimer’s disease: Impact on pathogenesis via disturbed epigenetic regulation of chromatin landscape. Progress in neurobiology. 2015;131:1–20. doi: 10.1016/j.pneurobio.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Salminen A, Ojala J, Kaarniranta K, Haapasalo A, Hiltunen M, Soininen H. Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. The European journal of neuroscience. 2011;34:3–11. doi: 10.1111/j.1460-9568.2011.07738.x. [DOI] [PubMed] [Google Scholar]
- Santos-Carvalho A, Alvaro AR, Martins J, Ambrosio AF, Cavadas C. Emerging novel roles of neuropeptide Y in the retina: from neuromodulation to neuroprotection. Progress in neurobiology. 2014;112:70–79. doi: 10.1016/j.pneurobio.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Saraswati S, Sitaraman R. Aging and the human gut microbiota-from correlation to causality. Frontiers in microbiology. 2014;5:764. doi: 10.3389/fmicb.2014.00764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutze S, Ribes S, Kaufmann A, Manig A, Scheffel J, Redlich S, Bunkowski S, Hanisch UK, Bruck W, Nau R. Higher mortality and impaired elimination of bacteria in aged mice after intracerebral infection with E. coli are associated with an age-related decline of microglia and macrophage functions. Oncotarget. 2014;5:12573–12592. doi: 10.18632/oncotarget.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scullin MK, Bliwise DL. Sleep, Cognition, and Normal Aging Integrating a Half Century of Multidisciplinary Research. Perspectives on Psychological Science. 2015;10:97–137. doi: 10.1177/1745691614556680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clinical science. 2011;120:357–375. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaik S, Inuzuka H, Liu P, Wei W, Wang Z. Endothelium aging and vascular diseases. INTECH Open Access Publisher; 2013. [Google Scholar]
- Sharma HS, Castellani RJ, Smith MA, Sharma A. The blood-brain barrier in Alzheimer’s disease: novel therapeutic targets and nanodrug delivery. Int Rev Neurobiol. 2012;102:47–90. doi: 10.1016/B978-0-12-386986-9.00003-X. [DOI] [PubMed] [Google Scholar]
- Shoemark DK, Allen SJ. The microbiome and disease: reviewing the links between the oral microbiome, aging, and Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2015;43:725–738. doi: 10.3233/JAD-141170. [DOI] [PubMed] [Google Scholar]
- Sim FJ, Zhao C, Penderis J, Franklin RJ. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:2451–2459. doi: 10.1523/JNEUROSCI.22-07-02451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JE, Ince PG, Haynes LJ, Theaker R, Gelsthorpe C, Baxter L, Forster G, Lace GL, Shaw PJ, Matthews FE, Savva GM, Brayne C, Wharton SB. Population variation in oxidative stress and astrocyte DNA damage in relation to Alzheimer-type pathology in the ageing brain. Neuropathology and applied neurobiology. 2010;36:25–40. doi: 10.1111/j.1365-2990.2009.01030.x. [DOI] [PubMed] [Google Scholar]
- Smith LK, He Y, Park JS, Bieri G, Snethlage CE, Lin K, Gontier G, Wabl R, Plambeck KE, Udeochu J, Wheatley EG, Bouchard J, Eggel A, Narasimha R, Grant JL, Luo J, Wyss-Coray T, Villeda SA. beta2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nature medicine. 2015;21:932–937. doi: 10.1038/nm.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Bake S, Lewis DK. Age-related changes in brain support cells: Implications for stroke severity. Neurochemistry international. 2013;63:291–301. doi: 10.1016/j.neuint.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll LL, McCormick ML, Denning GM, Weintraub NL. Antioxidant effects of statins. Drugs of Today. 2004;40:975–990. doi: 10.1358/dot.2004.40.12.872573. [DOI] [PubMed] [Google Scholar]
- Suenaga J, Hu X, Pu H, Shi Y, Hassan SH, Xu M, Leak RK, Stetler RA, Gao Y, Chen J. White matter injury and microglia/macrophage polarization are strongly linked with age-related long-term deficits in neurological function after stroke. Exp Neurol. 2015;272:109–119. doi: 10.1016/j.expneurol.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Ko Lw, Kulathingal J, Jiang P, Sevlever D, Yen SHC. Oxidative stress-induced phosphorylation, degradation and aggregation of α-synuclein are linked to upregulated CK2 and cathepsin D. European Journal of Neuroscience. 2007;26:863–874. doi: 10.1111/j.1460-9568.2007.05736.x. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Wuarin JP, Dudek FE. Glutamate, the dominant excitatory transmitter in neuroendocrine regulation. Science. 1990;250:1276–1278. doi: 10.1126/science.1978759. [DOI] [PubMed] [Google Scholar]
- Van Der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T. Enhanced peroxynitrite formation is associated with vascular aging. The Journal of experimental medicine. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature neuroscience. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Vartak-Sharma N, Nooka S, Ghorpade A. Astrocyte elevated gene-1 (AEG-1) and the A(E)Ging HIV/AIDS-HAND. Progress in neurobiology. 2016 doi: 10.1016/j.pneurobio.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw JM, Doubal FN, Valdes-Hernandez M, Wang X, Chappell FM, Shuler K, Armitage PA, Carpenter TC, Dennis MS. Blood–brain barrier permeability and long-term clinical and imaging outcomes in cerebral small vessel disease. Stroke. 2013;44:525–527. doi: 10.1161/STROKEAHA.112.669994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weilinger NL, Maslieieva V, Bialecki J, Sridharan SS, Tang PL, Thompson RJ. Ionotropic receptors and ion channels in ischemic neuronal death and dysfunction. Acta Pharmacologica Sinica. 2013;34:39–48. doi: 10.1038/aps.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenz T, Passos JF. Mitophagy in neurodegeneration and aging 2012 [Google Scholar]
- Wierenga CE, Hays CC, Zlatar ZZ. Cerebral blood flow measured by arterial spin labeling MRI as a preclinical marker of Alzheimer’s disease. Journal of Alzheimer’s Disease. 2014:42. doi: 10.3233/JAD-141467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesmann M, Zerbi V, Jansen D, Haast R, Lütjohann D, Broersen LM, Heerschap A, Kiliaan AJ. A Dietary Treatment Improves Cerebral Blood Flow and Brain Connectivity in Aging apoE4 Mice. Neural Plasticity. 2016;2016 doi: 10.1155/2016/6846721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong XY, Liu L, Yang QW. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Progress in neurobiology. 2016;142:23–44. doi: 10.1016/j.pneurobio.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Yamato M, Kudo W, Shiba T, Yamada KI, Watanabe T, Utsumi H. Determination of reactive oxygen species associated with the degeneration of dopaminergic neurons during dopamine metabolism. Free radical research. 2010;44:249–257. doi: 10.3109/10715760903456084. [DOI] [PubMed] [Google Scholar]
- Yeh TH, Hwang HM, Chen JJ, Wu T, Li AH, Wang HL. Glutamate transporter function of rat hippocampal astrocytes is impaired following the global ischemia. Neurobiology of disease. 2005;18:476–483. doi: 10.1016/j.nbd.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Youdim MB, Stephenson G, Ben Shachar D. Ironing iron out in Parkinson’s disease and other neurodegenerative diseases with iron chelators: a lesson from 6-hydroxydopamine and iron chelators, desferal and VK-28. Annals of the New York Academy of Sciences. 2004;1012:306–325. doi: 10.1196/annals.1306.025. [DOI] [PubMed] [Google Scholar]
- Zhang W, Hu X, Yang W, Gao Y, Chen J. Omega-3 polyunsaturated fatty acid supplementation confers long-term neuroprotection against neonatal hypoxic-ischemic brain injury through anti-inflammatory actions. Stroke. 2010;41:2341–2347. doi: 10.1161/STROKEAHA.110.586081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Sun L, Wang H, Ma H, Liu G, Zhao Y. Changes of CD4+CD25+Foxp3+ regulatory T cells in aged Balb/c mice. J Leukoc Biol. 2007;81:1386–1394. doi: 10.1189/jlb.0506364. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Deane R, Ali Z, Parisi M, Shapovalov Y, O’Banion MK, Stojanovic K, Sagare A, Boillee S, Cleveland DW. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nature neuroscience. 2008;11:420–422. doi: 10.1038/nn2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Ilieva H, Hallagan L, Bell R, Singh I, Paquette N, Thiyagarajan M, Deane R, Fernandez JA, Lane S, Zlokovic AB, Liu T, Griffin JH, Chow N, Castellino FJ, Stojanovic K, Cleveland DW, Zlokovic BV. Activated protein C therapy slows ALS-like disease in mice by transcriptionally inhibiting SOD1 in motor neurons and microglia cells. The Journal of clinical investigation. 2009;119:3437–3449. doi: 10.1172/JCI38476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends in Neurosciences. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nature reviews Neuroscience. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]