ABSTRACT
Acute graft versus host disease (aGVHD) remains a major problem after allogeneic hematopoietic stem cell transplantation. Standard frontline therapy for aGVHD involves corticosteroids. However, fewer than half of patients have a lasting complete response. The long-term mortality rate of steroid-refractory aGVHD (SR-aGVHD) remains around 70%. To date, no consensus has been reached regarding the optimal salvage treatment for SR-aGVHD. We performed the first prospective, multi-center clinical trial to assess the efficacy and safety of a novel approach to treat severe (grades III–IV) SR-aGVHD with the combination of basiliximab and etanercept. Sixty-five patients with severe SR-aGVHD from six centers were included. The median number of basiliximab infusions was 4 (range 2–11) and of etanercept was 9 (range 2–12). At day 28 after starting the combination treatment, overall response (complete and partial response: CR+PR) to second-line treatment was 90.8% with 75.4% being CR. The incidences of CR per organ were 100%, 73.8%, and 79.7% for skin, liver, and gut involvement, respectively. Patients >30-y old (p = 0.043, RR = 3.169), development of grades III–IV liver aGVHD (p = 0.007, RR = 5.034) and cytomegalovirus (CMV) reactivation (p = 0.035, RR = 4.02) were independent predictors for incomplete response. Combined treatment with basiliximab and etanercept resulted in improved CR to visceral aGVHD and significantly superior 2-y overall survival (54.7% vs. 14.8%, p <0.001) compared with classical salvage treatments. Our data suggest that the combination of basiliximab and etanercept may constitute a promising new treatment option for SR-aGVHD.
KEYWORDS: Basiliximab, etanercept, hematopoietic stem cell transplantation, steroid-refractory acute GVHD
Abbreviations
- aGVHD
acute graft versus host disease
- allo-HSCT
allogeneic hematopoietic stem cell transplantation
- ATG
anti-thymoglobulin
- CMV
cytomegalovirus
- CR
complete response
- EBV
Epstein–Barr virus
- FK506
tacrolimus
- IL-2R
IL-2 receptor
- MMF
mycophenolate mofetil
- NRM
non-relapse mortality
- ORR
overall response rate
- OS
overall survival
- PR
partial response
- SR-aGVHD
steroid-refractory aGVHD
- TRM
treatment-related mortality
Introduction
Acute graft versus host disease (aGVHD) remains a major problem after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Its incidence varies between 35% and 80% of transplants, depending on the type of transplantation.1 Standard frontline therapy for aGVHD involves corticosteroids. However, fewer than half of patients have a lasting complete response (CR).2,3 Steroid-refractory aGVHD (SR-aGVHD) is associated with increased mortality, and the long-term mortality rate remains around 70%.4
Second-line treatments included anti-thymoglobulin (ATG), mycophenolate mofetil, tacrolimus (FK506), IL-2 receptor (IL-2R) antibodies, alemtuzumab, etanercept, infliximab, sirolimus, and others. The overall CR rate from the 28 published retrospective studies evaluating agents for the second-line therapy of SR-aGVHD was 32%, the median survival was only about 6 mo, and no agent was clearly superior.5 To date, no consensus has been reached regarding the optimal salvage treatment for SR-aGVHD.
We have previously investigated the relationship between genetic variations in T-cell activation and effector pathways and important cytokine genes, such as TNF-α, TNF-β, and IL-10, and the risk of aGVHD, and confirmed the crucial roles of T cells and the inflammatory cascade in the induction of aGVHD.6-8 Mono-therapy targeting T-cell activation by IL-2R antibody (basiliximab)9-11 or single drug using TNF-α antagonisms (infliximab, etanercept)12, 13 has shown encouraging results in smaller, retrospective studies of patients with SR-aGVHD. We hypothesized that the simultaneous blockade of activated T cells and pivotal cytokines may further improve the outcomes of patients with SR-aGVHD. We developed a novel approach to treat severe (grades III–IV) SR-aGVHD by the combination of basiliximab and etanercept and first designed a multi-center prospective study to assess the efficacy and safety.
Results
Patients
Sixty-five patients with steroid-refractory grades III–IV aGVHD were included. All patients received the combination of basiliximab and etanercept as first alternative salvage therapy after failing to respond to corticosteroids. Patient characteristics are shown in Table 1. Median age of patients was 26 (range 9–55). The majority of patients were adult patients (n = 59) and only six pediatric patients (≤14-y old) were included. Fifty-five (84.6%) patients were diagnosed with acute leukemia, two patients (3.1%) with myelodysplastic syndrome (MDS), three patients (4.6%) with non-Hodgkin lymphoma, four patients (6.2%) with aplastic anemia, and one patient (1.5%) with chronic myeloid leukemia (CML). In 58 patients with a classifiable disease risk, 34 patients (58.6%) were in first CR (CR1), 14 patients (24.1%) in ≥ CR2, and 10 patients (17.3%) with advanced disease at the time of transplantation. All patients received a myeloablative-conditioning regimen. Thirteen patients (20%) received stem cells from an HLA-identical sibling, twelve (18.5%) received a transplant from a matched unrelated donor, and forty (61.5%) received a transplant from a haploidentical-related donor. Forty-four patients (67.7%) received peripheral blood stem cells (PBSCs) alone, and twenty one (32.3%) received the combination of PBSCs and bone marrow.
Table 1.
Patients' baseline characteristics.
| Combination cohort (n = 65) | Retrospective cohort (n = 27) | p value | |
|---|---|---|---|
| Median age, years (range) | 26 (9–55) | 27 (13–26) | 0.415 |
| Gender, n (%) | 0.819 | ||
| Male | 36 (55.4) | 16 (59.3) | |
| Female | 29 (44.6) | 11 (40.7) | |
| Disease, n (%) | 0.001 | ||
| AL | 55 (84.6) | 16 (59.4) | |
| CML | 1 (1.5) | 8 (29.6) | |
| MDS | 2 (3.1) | 2 (7.4) | |
| NHL | 3 (4.6) | 1 (3.7) | |
| SAA | 4 (6.2) | 0 (0) | |
| Disease status, n (%) | 0.184 | ||
| CR1 | 34 (58.6) | 12 (70.6) | |
| ≥CR2 | 14 (24.1) | 5 (29.4) | |
| Active | 10 (17.3) | 0 (0) | |
| Stem cell source, n (%) | <0.001 | ||
| PBSC | 44 (67.7) | 16 (59.3) | |
| BM | 0 (0) | 10 (37) | |
| PBSC+BM | 21 (32.3) | 1 (3.7) | |
| Combination cohort (n = 65) | Retrospective cohort (n = 27) | p value | |
| Conditioning regimen, n (%) | 0.293 | ||
| MAC | 65 (100) | 26 (96.3) | |
| RIC | 0 (0) | 1 (3.7) | |
| Donor type, n (%) | <0.001 | ||
| Identical sibling | 13 (20) | 3 (11.1) | |
| Unrelated | 12 (18.5) | 20 (74) | |
| Haploidentical | 40 (61.5) | 4 (14.8) | |
| Donor–patient gender, n (%) | 0.522 | ||
| Male–male | 23 (35.4) | 13 (48.1) | |
| Male–female | 18 (27.7) | 6 (22.2) | |
| Female–male | 14 (21.5) | 3 (11.1) | |
| Female–female | 10 (15.4) | 5 (18.5) | |
| ATG in conditioning regimen, n (%) | <0.001 | ||
| Yes | 47 (72.3) | 6 (22.2) | |
| No | 18 (27.7) | 21 (77.8) |
Abbreviations: AL: acute leukemia; CML: chronic myelogenous leukemia; MDS: myelodysplastic syndrome; NHL: non-Hodgkin's lymphoma; SAA: severe aplastic anemia; PBSC: peripheral blood stem cell; BM: bone marrow; MAC: myeloablative conditioning; RIC: reduced intensity conditioning.
The endpoint of the last follow-up for all of the surviving patients was December 31, 2015. The median follow-up for surviving patients after the initiation of the combination treatment was 18.5 (range 5.5–72.7) mo.
GVHD response
GVHD prophylaxis has been described previously14 All the patients received GVHD prophylaxis, consisting of cyclosporin A and methotrexate. ATG was given as GVHD prophylaxis during the conditioning regimen in 47 patients (72.3%). Patients engrafted to absolute neutrophil counts exceeding 0.5 × 109/L in a median time of 12 d (range 8–19 d). After myeloid recovery, all patients achieved sustained, full donor chimerism by day 30 post-transplantation. The median time of platelet engraftment was 13 d (range 8–22 d).
Median times to the onset of aGVHD were 20 d post-HSCT (range 5–85) for early aGVHD (n = 47), 182 d (range 108–262) for late-onset aGVHD (n = 4), and 24 d (range 7–49) after donor lymphocyte infusion (n = 14). In 21 patients (32.3%), the diagnosis was confirmed by colonoscopy biopsy. First-line treatment with 2 mg/kg/d steroids was initiated at GVHD onset. Total 39 patients (60%) were diagnosed with grades I–II aGVHD who eventually evolved into severe aGVHD during treatment with prednisolone, and 26 patients (40%) were diagnosed with severe aGVHD at onset. Median time from diagnosis of the onset of aGVHD to study enrollment was 8 d (range 3–49). Details concerning severe SR-aGVHD and response to second-line treatment are given in Table 2. At enrollment, 21 patients (32.3%) presented with overall grade III aGVHD and 44 (67.7%) with grade IV. Acute GVHD involved skin in 54 patients, gut in 59 patients, and liver in 42 patients. The median number of infusions of basiliximab was 4 (range 2–11), and the median number of etanercept was 9 (range 2–12). At day 28 after the initiation of treatment with the combination of basiliximab and etanercept, the overall response rate (ORR) (CR+PR) to second-line treatment was 90.8% (59/65) including 49 CRs (75.4%). The incidences of CR per organ were 100%, 73.8%, and 79.7% for skin, liver, and gut involvement, respectively. Of the 49 patients who achieved a CR, only one patient experienced a flare of aGVHD prior to day 90 post-transplantation. About 20 of 40 evaluable patients developed chronic GVHD (cGVHD), but only three patients developed moderate-to-severe cGVHD.
Table 2.
SR-aGVHD characteristics for the group.
| Combination cohort (n = 65) | Retrospective cohort (n = 27) | p value | |
|---|---|---|---|
| Overall grade, n (%) | 0.477 | ||
| III | 21 (32.3) | 11 (40.7) | |
| IV | 44 (67.7) | 16 (59.3) | |
| Skin grade, n (%) | 0.856 | ||
| 0 | 11 (16.9) | 5 (18.5) | |
| I | 4 (6.2) | 3 (11.1) | |
| II | 14 (21.5) | 5 (18.5) | |
| III–IV | 36 (55.4) | 14 (51.8) | |
| Gut grade, n (%) | 0.002 | ||
| 0 | 6 (9.2) | 2 (7.4) | |
| I | 2 (3.1) | 3 (11.1) | |
| II | 1 (1.5) | 6 (22.2) | |
| III–IV | 56 (86.1) | 16 (59.2) | |
| Liver grade, n (%) | 0.281 | ||
| 0 | 23 (35.4) | 15 (55.6) | |
| I | 8 (12.3) | 2 (7.4) | |
| II | 22 (33.8) | 5 (18.5) | |
| III–IV | 12 (18.5) | 5 (18.5) | |
| Combination cohort (n = 65) | Retrospective cohort (n = 27) | p value | |
| Response to treatment/patients evaluable, n (%) | |||
| ORR | 59/65 (90.8) | 12/27 (44.4) | <0.001 |
| CR | 49/65 (75.4) | 8/27 (29.6) | <0.001 |
| CR for skin aGVHD* | 54/54 (100) | 19/19 (100) | 1.0 |
| CR for gut aGVHD)* | 47/59 (79.7) | 8/22 (36.4) | <0.001 |
| CR for liver aGVHD* | 31/42 (73.8%) | 2/10 (20) | 0.003 |
Response per organ was assessed among evaluable patients with this organ involvement
The multivariate Cox regression analysis for the rate of CR in the total cohort of 65 patients including patient age, donor–patient sex mismatch, donor type, disease status at HSCT, conditioning regimen (ATG vs. non-ATG), stem cell source, grades of aGVHD occurred at onset, time of the onset of aGVHD post-HSCT, grades of SR-aGVHD at combined treatment enrollment, grades of liver aGVHD, grades of gut aGVHD, time from aGVHD onset to the combination treatment received, cytomegalovirus (CMV) reactivation (Table 3) confirmed that patient >30-y old (p = 0.043, RR = 3.169), development of grades III–IV liver aGVHD (p = 0.007, RR = 5.034), and CMV reactivation (p = 0.035, RR = 4.02) were the risk factors for incomplete response at day 28 after the initiation of the combined treatment in patients with severe SR-aGVHD.
Table 3.
Multivariate analysis for incomplete response to the combined therapy.
| Variable | RR (95%CI) | p | ||
|---|---|---|---|---|
| Patient age | > 30-y old | 3.169 (1.036–9.695) | 0.043 | |
| ≤ 30-y old | ||||
| Donor–patient gender | Female to male | 0.394 (0.055–2.84) | 0.356 | |
| Others | ||||
| Donor type | URD | 2.132 (0.093–49.081) | 0.636 | |
| HRD | 2.234 (0.078–63.841) | 0.638 | ||
| MSD | 1 | |||
| Disease status at HSCT | CR2 or advanced stage | 2.158 (0.452–10.297) | 0.335 | |
| CR1 | ||||
| ATG in conditioning regimen | No | 0.579 (0.034–9.78) | 0.705 | |
| Used in conditioning regimen | ||||
| Stem cell source | Bone marrow + PBSC | 1.198 (0.241–5.959) | 0.825 | |
| PBSC | ||||
| Grades of aGVHD occurred at onset | III–IV | 0.837 (0.177–3.965) | 0.822 | |
| II | ||||
| Time of the onset of aGVHD post-HSCT | ≤ 15 d | 1.239 (0.126–12.161) | 0.854 | |
| >15 d | ||||
| SR-aGVHD at combined treatment enrollment | IV | 1.745 (0.353–8.632) | 0.495 | |
| III | ||||
| Grades of liver aGVHD | III–IV | 5.034 (1.561–16.24) | 0.007 | |
| at treatment enrollment | I–II | |||
| Grades of gut aGVHD | IV | 0.312 (0.052–1.884) | 0.204 | |
| at treatment enrollment | I–III | |||
| Time from aGVHD onset to combination treatment | ≤14 d | 0.533 (0.054–5.305) | 0.592 | |
| >14 d | ||||
| CMV reactivation | Yes | 4.02 (1.1–14.689) | 0.035 | |
| No |
Toxicities and infectious complications
The most frequent extrahematologic toxicity was hemorrhagic cystitis, reported in 18 patients (27.7%). Cytopenias (anemia, leukopenia, or thrombocytopenia) are the most frequent hematologic toxicity and were observed in 49.2% of patients (32/65). Severe cytopenia (grades III and IV) was found in 32.3% (21/65) of patients. No anaphylactic reactions have been reported. No patients experienced grades III–V toxicities of the renal, neurologic, and cardiac systems.
Invasive fungal infections (IFIs) were defined using the standard criteria.15 All cases met the criteria for probable or definite IFIs. The cumulative incidence of an invasive pulmonary fungal infection at 12 mo post-transplantation was 36% (Fig. 1A). Although 37 patients (56.9%) experienced at least one CMV reactivation, all patients developed CMV viremia without CMV disease. Four patients (6.2%) experienced Epstein–Barr virus (EBV) reactivation, and one patient developed viral encephalitis by human herpesvirus 6 (HHV6). No post-transplantation lymphoproliferative disorder was observed.
Figure 1.
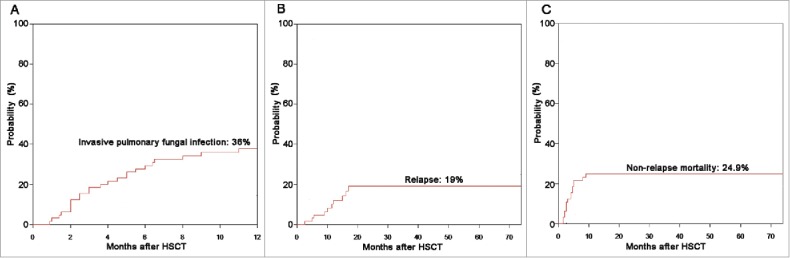
Probabilities of (A) pulmonary fungal infections, (B) relapse, and (C) NRM according to the novel combined second-line therapy.
Long-term follow-up and survival
The 2-y incidence of relapse was 19% (Fig. 1B). The 2-y incidence of NRM was 24.9% (Fig. 1C). A total of 26 patients died, and causes of death ordered by the number of patients were aGVHD (n = 9), pulmonary fungal infection (n = 8), relapse (n = 7), early complications of vascular origin (n = 1), and organ failure (n = 1). Overall survival (OS) probabilities at 3, 6, and 12 mo after initiation of the combination therapy were 78.5%, 70.6%, and 62.1%, respectively. Patients with severe SR-aGVHD receiving the salvage combination therapy achieved a 2-y OS of 54.7% (Fig. 2).
Figure 2.
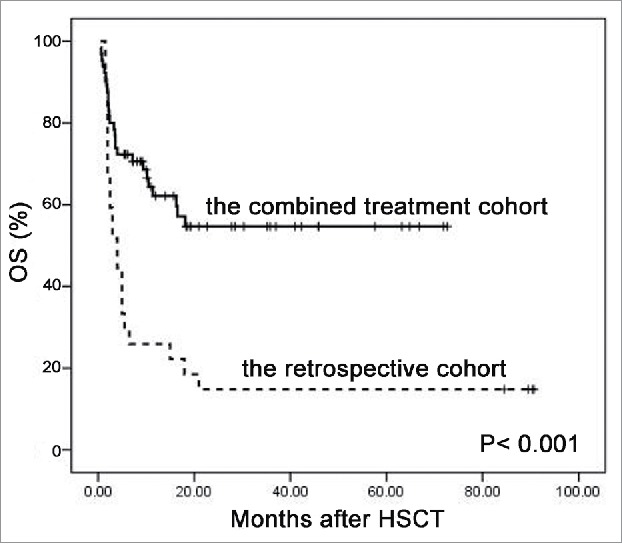
Overall survival by study arm (p < 0.001).
Comparison with a retrospective cohort
In order to compare response rates and survival outcomes, a retrospective cohort was constructed from database of the transplant center of the First Affiliated Hospital of Zhejiang University School of Medicine using all patients (n = 27) with severe SR-aGVHD before 2009, who received some other second-line salvage treatment including high-dose steroid (2–5 mg/kg) (n = 2), FK506 (n = 7), CD3 antibody (OKT3) (n = 5), CD25 antibody (daclizumab) alone (n = 9), or plasmapheresis (n = 6) at the discretion of physicians. Baseline clinical characteristics of the retrospective cohort can be found in Table 1. There were no significant differences in age, donor–patient gender relationship, or conditioning regimen between the combined treatment cohort and the retrospective cohort. The baseline characteristics that differed between the groups were more patients with CML, more cases used bone marrow as the stem cell source, and fewer cases used haploidentical donors, which contributed to fewer cases using ATG in the retrospective cohort than the combined treatment cohort, and these variables were not identified as significant in univariate or multivariate outcome analysis.
In the retrospective cohort, the ORR to second-line treatment was 44.4% with 29.6% of CR. The incidence of CR per organ was 100%, 20%, and 36.4% for skin, liver, and gut involvement, respectively. Although there were more cases experiencing grades III–IV gut aGVHD in our combination cohort, the combined treatment with basiliximab and etanercept showed higher ORR (90.8% vs. 44.4%, p <0.001), CR (75.4% vs. 29.6%, p < 0.001), response to liver aGVHD (73.8% vs. 20%, p = 0.003), and gut aGVHD (79.7% vs. 36.4%, p < 0.001), compared with the classical regimens (Table 2). Most important of all, our novel salvage therapy contributed to a significantly superior 2-y OS compared with controls (54.7% vs. 14.8%, p < 0.001) (Fig. 2). Acute GVHD was the most common cause of death in the retrospective cohort (68.2%).
Discussion
The number of unrelated donor HSCTs and haploidentical-related donor HSCTs continues to increase annually, and therapy for SR-aGVHD is still one of the most vexing and difficult problems faced by transplant physicians. The lack of progress and the absence of accepted standard treatments for SR-aGVHD contribute to a variety of immunosuppressive agents having been used in this setting but with a disappointing long-term outcome.5
Cytokines and cytokine receptors play key roles in the initiation and amplification of aGVHD, influencing T-cell differentiation and activation pathways and trafficking to GVHD target organs, and also mediating direct tissue injurious effects. Antagonists to the critical cytokines and cytokine receptors have been used in the treatment of SR-aGVHD. High-affinity receptors for interleukin-2 (IL-2), also known as CD25, are expressed on activated T cells. Binding of IL-2 to its receptor is a major requirement for T-cell clonal expansion.16 In previous studies, the chimeric IL-2R antibodies binding with high affinity to the α-chain of the IL-2R, daclizumab, or basiliximab were given as a single second-line agent to patients with SR-aGVHD. CR was achieved in 17%–37% of patients and 6-mo OS was poor, at only 28–55%,10, 17, 18 while 5-y OS was 20%.11 On the other hand, earlier animal models had suggested that TNF-α played a major role in aGVHD of gastrointestinal (GI) tract and skin.19 The use of monoclonal antibodies against TNF-α is one of the possible therapeutic approaches for SR-aGVHD, but the efficacy is also limited when given independently and the incidence of CR was less than 40% in pilot studies.12, 20
However, signaling through the IL-2R plays a pivotal role, not only in the proliferation of effector T cells, but also in that of regulatory T cells (Tregs).21 Anti-IL-2R monoclonal antibody therapy (basiliximab) has been reported to result in a reduction of circulating Treg percentage in the treatment of SR-aGVHD22 and its effect on Treg proliferation has been speculated to be one reason for the unsatisfactory response to inhibitors of IL-2 or IL-2R used alone in trials of aGVHD therapy.23 Although several reports have suggested that basiliximab selectively decreased the number of CD4+CD25+FOXP3+ Tregs but did not impair the suppressive function of Tregs by preserving or increasing the percentage of CD25−FOXP3+ Tregs.24-26 Taking into account the potential impacts of basiliximab on Treg function and the well-accepted fact that a complex network of cytokines, cellular receptors, and immune cell subsets resulting in the initiation and maintenance of aGVHD, we speculated that the blockade of multiple effector pathways may ultimately be necessary. To our knowledge, our study is the first prospective, multi-center clinical trial to develop a combined inflammatory cytokine inhibition therapy for SR-aGVHD by using basiliximab and etanercept. Our study enrolled 65 patients with grades III–IV SR-aGVHD of whom> 50% were grade IV, whose outcomes were historically dismal, with long-term mortality rates close to 90%. The patients with most severe SR-aGVHD achieved encouraging outcomes with 75.4% achieving CR and a 2-y OS of 54.7% after treatment with the combined therapy. Although improved supportive care after 2009 in our combined therapy cohort and heterogeneous salvage treatment in our retrospective cohort may enhance therapeutic benefits, the combination of basiliximab and etanercept to simultaneously block IL-2 and TNF-α activity as therapy for SR-GVHD could yield improved CR, response to visceral aGVHD and significantly superior 2-y OS (54.7% vs. 14.8%) compared with classical salvage treatment.
Our findings suggested that patients > 30-y old have an independent effect on incomplete response to the combined treatment among patients with SR-aGVHD, which is consistent with previous conclusions that older patients undergoing allo-HSCT may experience a high degree of morbidity from transplant-related complications, for significantly higher physical limitations and more impaired functional abilities. The role of aGVHD as a risk factor for CMV reactivation is well known. A retrospective study including 252 patients who were diagnosed with GI aGVHD indicated that 45% patients developed CMV viremia.27 In our cohort, 56.9% of patients with SR-aGVHD experienced CMV viremia, and CMV reactivation also contributed to incomplete response to the combined treatment. According to recent study, GVHD induced a profound dendritic cell defect that led to a failure in the generation of CMV-specific CD8+ T-cell responses and dramatically limited antiviral T-cell responses. On the other hand, CMV-infected endothelial cells have been shown to produce inflammatory cytokines such as IL-6, which plays a crucial role in the pathogenesis of aGVHD.28 Furthermore, in the presence of GVHD, CMV predisposes to induce a striking cytopathy resulting in universal mortality.29
Disappointing outcomes have also been reported in several small retrospective trials of therapy targeting both IL-2 and TNF in SR-aGVHD.30, 31 Rager et al. retrospectively reviewed their experience using combination anti-cytokine therapy of daclizumab and infliximab in a cohort that included 17 patients with SR-aGVHD. Twenty-four percent of patients had complete resolution of symptoms, but survival was limited and all the patients died at a median of 6.7 mo from transplant and 35 d from the initiation of daclizumab/infliximab.31 The reasons for the limited efficacy may be that many patients did not receive all planned doses of therapy, typically because of infection or death.31 Nadeau et al. retrospectively reviewed their experience with the combination of basiliximab and infliximab in 16 patients with steroid-refractory, grades III–IV GI aGVHD. The overall response rate was 76%, with 43% CR at a median time of 21 d after beginning the treatment. The survival at 1 y was 24%, with most deaths occurring due to the complications from GVHD.32 Recently, van Groningen et al. performed a prospective study on combination therapy with inolimomab (anti-IL-2Ra) and etanercept for 21 patients with SR-aGVHD. Their data indicated that overall response at day 28 was 48%, with the estimated rates of 6-mo and 2-y OS being dismal, 29% and 10%, respectively. They concluded that the combination of inolimomab and etanercept failed to improve the dismal prognosis of severe SR-aGVHD.33 Two factors may be related to our superior outcomes of the combination treatment by basiliximab and etanercept. First, as TNF inhibitors, etanercept, and infliximab have different structures and modes of action. Infliximab binds only to TNF-α, and etanercept binds to both TNF-α and TNF-β as a decoy receptor. TNF-β is a pro-inflammatory cytokine, considered to have almost the same effect as TNF-α.34, 35 Second, according to our results, patients >30-y old were independent predictor for incomplete response to the combined treatment. More than 50% of our patients were younger than 30-y old, whereas patients in above-mentioned three studies were older. The median age of patients was 26 (range 9–55) in our cohort, 47 (range 35–63) in Rager's study, 57 (20–71) in Nadeau's study, and 54 (range 24–66) in van Groningen's study.
Consistent with the fact that infections are the major complications in patients with SR-aGVHD,36 pulmonary fungal infection was one of the most common causes of death in our combined therapy cohort. Although active antifungal agents were administered to patients receiving secondary systemic therapy, the cumulative incidence of invasive pulmonary fungal infection at 12 mo post-transplantation was 36%. Data in adult patients treated with etanercept for SR-aGVHD indicated an incidence of complicating bacterial and fungal infections ranging from 14 to 80%.12, 37 When agents that cause profound depression of T-cell-function are administered, strategies for intensified surveillance and prophylaxis for opportunistic infections must be implemented, and, in particular, long-term prophylaxis against molds should be intensified.
Apart from the encouraging response to visceral SR-aGVHD and the superior OS, another compelling rationale for incorporating anti-cytokine therapy into GVHD management relies on the lack of attenuation of the graft versus leukemia (GVL) effect. The 2-y incidence of relapse in patients with SR-aGVHD was only 19%, which is no higher than that reported in contemporaneous patients receiving allo-HSCT in our center. We have reported that the 5-y incidence of relapse was 34% in patients receiving HLA-matched sibling HSCT, 21.2% in patients receiving unrelated donor HSCT, and 14.2% in patients receiving haploidentical HSCT.14
In conclusion, our data suggest that the combination of basiliximab and etanercept may constitute a promising new treatment option that is associated with long-term OS in 50% of patients suffering from severe SR-aGVHD, a group with otherwise high mortality, and does not impair GVL effects. Randomized prospective trials are necessary to address optimal dose, schedule, and management of infections.
Patients and methods
Patients
We conducted an open-label, non-randomized, phase II study involving patients recruited at transplant centers of the First Affiliated Hospital of Zhejiang University School of Medicine, the First Affiliated Hospital of Soochow University, Zhejiang Provincial People's Hospital, Guangzhou General Hospital of Guangzhou Military Command, Nanfang Hospital of Southern Medical University, and the First Affiliated Hospital of Wenzhou Medical University from January 2009 through June 2015. All patients who experienced severe (grades III–IV) SR-aGVHD were included. Severe SR-aGVHD was defined as fulfillment of one of the following criteria: (1) newly diagnosed with grades III–IV aGVHD or overlap syndrome and showed progression after 3 d of steroid therapy, or no improvement after at least 7 d of treatment with 2 mg/kg per day steroids, or inability to taper steroids.5, 38; or (2) de novo grades I–II aGVHD but eventually evolved into grades III–IV during treatment with 2 mg/kg per day prednisolone. Biopsy of the organs involved was not required for the diagnosis of aGVHD. This cohort was selected by including all patients who met the inclusion criteria by the different centers and no reported patient was excluded. All the patients gave their written informed consent. The protocol was approved by the ethics review committee of each institution and registered at the Chinese Clinical Trial Registry (www.chictr.org) (Identifier: ChiCTR-OCH- 12002890).
Treatment plan
Basiliximab was given intravenously at 20 mg per dose on day +1 and day +4. Injections were repeated weekly until GVHD was reduced to grade < II. Etanercept was given subcutaneously at 25 mg per dose twice a week for 4 weeks, and then subsequently at 25 mg once a week for another 4 weeks if necessary. During combined therapy, all patients received cyclosporine maintained at a therapeutic level. Prednisolone was tapered by 20% of the total dose weekly. All patients received prophylaxis for pneumocystis pneumonia with sulfamethoxazole, anti-fungal prophylaxis with micafungin, or itraconazole or voriconazole. Preemptive treatments were also used for CMV and EBV reactivation. Broad-spectrum intravenous antibacterial and as indicated antifungal antimicrobials were used when patients developed fever.
GVHD scoring and response determination
Acute GVHD was graded according to the Keystone Consensus Criteria39 We chose to report our clinical outcomes at day 28 after the initiation of combined treatment, which is referenced as a widely used endpoint for aGVHD treatment trials.40 The standards of GVHD response determination are also consistent with published studies.40, 41
CR was defined as complete resolution of all signs and symptoms of aGVHD in all evaluable organs without additional therapies. A partial response (PR) was defined as improvement of one stage in one or more GVHD-involved organs without progression in others. Progression was defined as worsening in one or more organs by one or more stage without improvement in any involved organ. No response was defined as stable disease or absence of improvement in any involved organ. A flare in aGVHD was defined as any increase in symptoms or therapy for aGVHD after an initial response (CR or PR).
All toxicities were reported regardless of whether or not they were thought to be related to the study treatment. Adverse events (AEs) were evaluated according to the NCI Common Terminology Criteria for AE (NCI- CTCAE), version 4.0. Cytopenias were defined according to NCI-CTCAE grades. Anemia: grade I: Hb < LLN to 10.0 g/dL, grade II: Hb <10.0 g/dL to 8.0 g/dL, grade III: Hb <8.0 g/dL; transfusion indicated, grade IV: life-threatening consequences; urgent intervention indicated. Neutropenia: grade I: Neutrophils <LLN to 1,500/mm3, grade II: Neutrophils <1,500 to 1,000/mm3, grade III: Neutrophils <1,000 to 500/mm3, grade IV: Neutrophils <500/mm3. Thrombocytopenia: grade I: Platelets <LLN to 75,000/mm3, grade II: <75,000 to 50,000/mm3, grade III: <50,000 to 25,000/mm3, grade IV: <25,000/mm3.
Statistical analysis
The sample size was calculated on the basis of expected CR rate to our combination treatment of 60% in patients with SR-aGVHD and 30% of the null hypothesis rate referenced by the 28 published retrospective studies evaluating agents for second-line therapy of SR-aGVHD.5 We estimated that a sample size of 66 patients would give the study at least 80% power to reject the null hypothesis with a type I error level of 5% (two-sided).
The primary end point was ORR to treatment. All patients who received at least one dose of the combination treatment were evaluated for efficacy and safety. The secondary end points included the incidences of relapse, nonrelapse-related death, and overall survival (OS) at 2 y. cGVHD, incidence of serious, life-threatening, or fatal infection, incidence of CMV or EBV reactivation, non-relapse mortality (NRM), and relapse were described using cumulative incidence, with relapse as the competing event for treatment-related mortality and death as the competing event for all other outcomes. OS was measured from the initiation of the combination treatment until death and estimated using the Kaplan–Meier method and compared between arms using the log-rank test. Multivariate Cox regression models using a forward stepwise procedure were used to analyze the effects of these characteristics and other known clinical and biological factors on HSCT outcomes. All variables in the univariate analysis with a p-value at or below 0.2 were included in the multivariate analysis.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thanks all transplant center physicians who participated in the study.
Funding
This work was funded in part by the Key Project of the National Natural Science Foundation of China (81230014), the National High Technology Research and Development Program of China (2012AA020905), the National Natural Science Foundation of China (81100387, 81170501, 81470309, 81471582, 81670169) and Medical Science and Technology Project of Zhejiang Province (2010KYA075).
Author contributions
Conception and design: Yamin Tan, Haowen Xiao, Yi Luo, and He Huang.
Collection, analysis, and interpretation of the data: Yamin Tan, Haowen Xiao, Yi Luo, Depei Wu, Jianping Lan, Qifa Liu, Kang Yu, Yishan Ye, and He Huang.
Drafting the article: Haowen Xiao and He Huang.
Provision of study materials or patients: Yamin Tan, Haowen Xiao, Depei Wu, Yi Luo, Jianping Lan, Qifa Liu, Kang Yu, Jimin Shi, Jingsong He, Weiyan Zheng, Xiaoyu Lai, Yuanyuan Zhu, Kaili Du, Yishan Ye, Yanmin Zhao, Gaofeng Zheng, Yongxian Hu, Xiaoyan Han, Yanlong Zheng, Guoqing Wei, Zhen Cai, and He Huang.
Obtaining funding: Yamin Tan, Haowen Xiao, Yi Luo, and He Huang.
References
- 1.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet 2009; 373(9674):1550-61; PMID:19282026; http://dx.doi.org/ 10.1016/S0140-6736(09)60237-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin PJ, Schoch G, Fisher L, Byers V, Anasetti C, Appelbaum FR et al.. A retrospective analysis of therapy for acute graft-versus-host disease: initial treatment. Blood 1990; 76(8):1464-72; PMID:2207321 [PubMed] [Google Scholar]
- 3.Roy J, McGlave PB, Filipovich AH, Miller WJ, Blazar BR, Ramsay NK et al.. Acute graft-versus-host disease following unrelated donor marrow transplantation: failure of conventional therapy. Bone Marrow Transpl 1992; 10(1):77-82; PMID:1515883 [PubMed] [Google Scholar]
- 4.Deeg HJ. How I treat refractory acute GVHD. Blood 2007; 109(10):4119-26; PMID:17234737; http://dx.doi.org/ 10.1182/blood-2006-12-041889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C et al.. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transpl 2012; 18(8):1150-63; PMID:22510384; http://dx.doi.org/ 10.1016/j.bbmt.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao HW, Lai XY, Luo Y, Shi JM, Tan YM, He JS et al.. Relationship between TNFA, TNFB and TNFRII gene polymorphisms and outcome after unrelated hematopoietic cell transplantation in a Chinese population. Bone Marrow Transpl 2011; 46(3):400-7; PMID:20548340; http://dx.doi.org/ 10.1038/bmt.2010.135 [DOI] [PubMed] [Google Scholar]
- 7.Xiao H, Cao W, Lai X, Luo Y, Shi J, Tan Y et al.. Immunosuppressive cytokine gene polymorphisms and outcome after related and unrelated hematopoietic cell transplantation in a Chinese population. Biol Blood Marrow Transpl 2011; 17(4):542-9; PMID:20457266; http://dx.doi.org/ 10.1016/j.bbmt.2010.04.013 [DOI] [PubMed] [Google Scholar]
- 8.Xiao H, Luo Y, Lai X, Fu S, Shi J, Tan Y et al.. Genetic variations in T-cell activation and effector pathways modulate alloimmune responses after allogeneic hematopoietic stem cell transplantation in patients with hematologic malignancies. Haematologica 2012; 97(12):1804-12; PMID:22733023; http://dx.doi.org/ 10.3324/haematol.2012.066159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massenkeil G, Rackwitz S, Genvresse I, Rosen O, Dorken B, Arnold R. Basiliximab is well tolerated and effective in the treatment of steroid-refractory acute graft-versus-host disease after allogeneic stem cell transplantation. Bone Marrow Transpl 2002; 30(12):899-903; PMID:12476283; http://dx.doi.org/ 10.1038/sj.bmt.1703737 [DOI] [PubMed] [Google Scholar]
- 10.Schmidt-Hieber M, Fietz T, Knauf W, Uharek L, Hopfenmuller W, Thiel E et al.. Efficacy of the interleukin-2 receptor antagonist basiliximab in steroid-refractory acute graft-versus-host disease. Br J Haematol 2005; 130(4):568-74; PMID:16098072; http://dx.doi.org/ 10.1111/j.1365-2141.2005.05631.x [DOI] [PubMed] [Google Scholar]
- 11.Funke VA, de Medeiros CR, Setubal DC, Ruiz J, Bitencourt MA, Bonfim CM et al.. Therapy for severe refractory acute graft-versus-host disease with basiliximab, a selective interleukin-2 receptor antagonist. Bone Marrow Transpl 2006; 37(10):961-5; PMID:16565744; http://dx.doi.org/ 10.1038/sj.bmt.1705306 [DOI] [PubMed] [Google Scholar]
- 12.Busca A, Locatelli F, Marmont F, Ceretto C, Falda M. Recombinant human soluble tumor necrosis factor receptor fusion protein as treatment for steroid refractory graft-versus-host disease following allogeneic hematopoietic stem cell transplantation. Am J Hematol 2007; 82(1):45-52; PMID:16937391; http://dx.doi.org/ 10.1002/ajh.20752 [DOI] [PubMed] [Google Scholar]
- 13.Yang J, Cheuk DK, Ha SY, Chiang AK, Lee TL, Ho MH et al.. Infliximab for steroid refractory or dependent gastrointestinal acute graft-versus-host disease in children after allogeneic hematopoietic stem cell transplantation. Pediatr Transpl 2012; 16(7):771-8; PMID:22905718; http://dx.doi.org/ 10.1111/j.1399-3046.2012.01756.x [DOI] [PubMed] [Google Scholar]
- 14.Luo Y, Xiao H, Lai X, Shi J, Tan Y, He J et al.. T-cell-replete haploidentical HSCT with low-dose anti-T-lymphocyte globulin compared with matched sibling HSCT and unrelated HSCT. Blood 2014; 124(17):2735-43; PMID:25214441; http://dx.doi.org/ 10.1182/blood-2014-04-571570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F, Denning DW, Donnelly JP, Edwards JE, Erjavec Z et al.. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis 2002; 34(1):7-14; PMID:11731939; http://dx.doi.org/ 10.1086/323335 [DOI] [PubMed] [Google Scholar]
- 16.Via CS, Finkelman FD. Critical role of interleukin-2 in the development of acute graft-versus-host disease. Int Immunol 1993; 5(6):565-72; PMID:8102248; http://dx.doi.org/ 10.1093/intimm/5.6.565 [DOI] [PubMed] [Google Scholar]
- 17.Przepiorka D, Kernan NA, Ippoliti C, Papadopoulos EB, Giralt S, Khouri I, Lu JG, Gajewski J, Durett A, Cleary K et al.. Daclizumab, a humanized anti-interleukin-2 receptor alpha chain antibody, for treatment of acute graft-versus-host disease. Blood 2000; 95(1):83-9; PMID:10607689 [PubMed] [Google Scholar]
- 18.Perales MA, Ishill N, Lomazow WA, Weinstock DM, Papadopoulos EB, Dastigir H, Chiu M, Boulad F, Castro-Malaspina HR, Heller G et al.. Long-term follow-up of patients treated with daclizumab for steroid-refractory acute graft-vs-host disease. Bone Marrow Transpl 2007; 40(5):481-6; PMID:17618322; http://dx.doi.org/ 10.1038/sj.bmt.1705762 [DOI] [PubMed] [Google Scholar]
- 19.Dignan FL, Clark A, Amrolia P, Cornish J, Jackson G, Mahendra P, Scarisbrick JJ, Taylor PC, Hadzic N, Shaw BE et al.. Diagnosis and management of acute graft-versus-host disease. Br J Haematol 2012; 158(1):30-45; PMID:22533831; http://dx.doi.org/ 10.1111/j.1365-2141.2012.09129.x [DOI] [PubMed] [Google Scholar]
- 20.Xhaard A, Rocha V, Bueno B, de Latour RP, Lenglet J, Petropoulou A, Rodriguez-Otero P, Ribaud P, Porcher R, Socié G et al.. Steroid-refractory acute GVHD: lack of long-term improved survival using new generation anticytokine treatment. Biol Blood Marrow Transpl 2012; 18(3):406-13; PMID:21736868; http://dx.doi.org/ 10.1016/j.bbmt.2011.06.012 [DOI] [PubMed] [Google Scholar]
- 21.Cheng G, Yu A, Malek TR. T-cell tolerance and the multi-functional role of IL-2R signaling in T-regulatory cells. Immunol Rev 2011; 241(1):63-76; PMID:21488890; http://dx.doi.org/ 10.1111/j.1600-065X.2011.01004.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakupurakal G, Garcia-Marquez MA, Shimabukuro-Vornhagen A, Theurich S, Holtick U, Hallek M, Scheid C, von Bergwelt-Baildon M. Immunological effects in patients with steroid-refractory graft-versus-host disease following treatment with basiliximab, a CD25 monoclonal antibody. Eur J Haematol 2016; 97(2):121-7; PMID:26492560; http://dx.doi.org/ 10.1111/ejh.12691 [DOI] [PubMed] [Google Scholar]
- 23.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol 2012; 12(6):443-58; PMID:22576252; http://dx.doi.org/ 10.1038/nri3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krystufkova E, Sekerkova A, Striz I, Brabcova I, Girmanova E, Viklicky O. Regulatory T cells in kidney transplant recipients: the effect of induction immunosuppression therapy. Nephrol Dial Transpl 2012; 27(6):2576-82; PMID:22167587; http://dx.doi.org/ 10.1093/ndt/gfr693 [DOI] [PubMed] [Google Scholar]
- 25.Bluestone JA, Liu W, Yabu JM, Laszik ZG, Putnam A, Belingheri M, Gross DM, Townsend RM, Vincenti F. The effect of costimulatory and interleukin 2 receptor blockade on regulatory T cells in renal transplantation. Am J Transpl 2008; 8(10):2086-96; PMID:18828769; http://dx.doi.org/ 10.1111/j.1600-6143.2008.02377.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okita R, Yamaguchi Y, Ohara M, Hironaka K, Okawaki M, Nagamine I, Ikeda T, Emi A, Hihara J, Okada M. Targeting of CD4+CD25high cells while preserving CD4+CD25 low cells with low-dose chimeric anti-CD25 antibody in adoptive immunotherapy of cancer. Int J Oncol 2009; 34(2):563-72; PMID:19148493; http://dx.doi.org/ 10.3892/ijo_00000182 [DOI] [PubMed] [Google Scholar]
- 27.Bhutani D, Dyson G, Manasa R, Deol A, Ratanatharathorn V, Ayash L, Abidi M, Lum LG, Al-Kadhimi Z, Uberti JP. Incidence, risk factors, and outcome of cytomegalovirus viremia and gastroenteritis in patients with gastrointestinal graft-versus-host disease. Biol Blood Marrow Transpl 2015; 21(1):159-64; PMID:25445637; http://dx.doi.org/ 10.1016/j.bbmt.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cantoni N, Hirsch HH, Khanna N, Gerull S, Buser A, Bucher C, Halter J, Heim D, Tichelli A, Gratwohl A et al.. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transpl 2010; 16(9):1309-14; PMID:20353832; http://dx.doi.org/ 10.1016/j.bbmt.2010.03.020 [DOI] [PubMed] [Google Scholar]
- 29.Wikstrom ME, Fleming P, Kuns RD, Schuster IS, Voigt V, Miller G, Clouston AD, Tey SK, Andoniou CE, Hill GR et al.. Acute GVHD results in a severe DC defect that prevents T-cell priming and leads to fulminant cytomegalovirus disease in mice. Blood 2015; 126(12):1503-14; PMID:26130706; http://dx.doi.org/ 10.1182/blood-2015-01-622837 [DOI] [PubMed] [Google Scholar]
- 30.Wolff D, Roessler V, Steiner B, Wilhelm S, Weirich V, Brenmoehl J, Leithaeuser M, Hofmeister N, Junghanss C, Casper J et al.. Treatment of steroid-resistant acute graft-versus-host disease with daclizumab and etanercept. Bone Marrow Transpl 2005; 35(10):1003-10; PMID:15806135; http://dx.doi.org/ 10.1038/sj.bmt.1704929 [DOI] [PubMed] [Google Scholar]
- 31.Rager A, Frey N, Goldstein SC, Reshef R, Hexner EO, Loren A, Luger SM, Perl A, Tsai D, Davis J et al.. Inflammatory cytokine inhibition with combination daclizumab and infliximab for steroid-refractory acute GVHD. Bone Marrow Transpl 2011; 46(3):430-5; PMID:20498647; http://dx.doi.org/ 10.1038/bmt.2010.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nadeau M, Perreault S, Seropian S, Foss F, Isufi I, Cooper DL. The use of basiliximab-infliximab combination for the treatment of severe gastrointestinal acute GvHD. Bone Marrow Transpl 2016; 51(2):273-6; PMID:26479982; http://dx.doi.org/ 10.1038/bmt.2015.247 [DOI] [PubMed] [Google Scholar]
- 33.van Groningen LF, Liefferink AM, de Haan AF, Schaap NP, Donnelly JP, Blijlevens NM, van der Velden WJ. Combination therapy with inolimomab and etanercept for severe steroid-refractory acute graft-versus-host disease. Biol Blood Marrow Transpl 2016; 22(1):179-82; PMID:26386320; http://dx.doi.org/ 10.1016/j.bbmt.2015.08.039 [DOI] [PubMed] [Google Scholar]
- 34.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 2001; 104(4):487-501; PMID:11239407; http://dx.doi.org/ 10.1016/S0092-8674(01)00237-9 [DOI] [PubMed] [Google Scholar]
- 35.Takeshita M, Suzuki K, Kikuchi J, Izumi K, Kurasawa T, Yoshimoto K, Amano K, Takeuchi T. Infliximab and etanercept have distinct actions but similar effects on cytokine profiles in rheumatoid arthritis. Cytokine 2015; 75(2):222-7; PMID:26095743; http://dx.doi.org/ 10.1016/j.cyto.2015.04.011 [DOI] [PubMed] [Google Scholar]
- 36.Messina C, Faraci M, de Fazio V, Dini G, Calo MP, Calore E. Prevention and treatment of acute GvHD. Bone Marrow Transpl 2008; 41(Suppl 2):S65-70; PMID:18545247; http://dx.doi.org/ 10.1038/bmt.2008.57 [DOI] [PubMed] [Google Scholar]
- 37.Alousi AM, Weisdorf DJ, Logan BR, Bolanos-Meade J, Carter S, Difronzo N, Pasquini M, Goldstein SC, Ho VT, Hayes-Lattin B et al.. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood 2009; 114(3):511-7; PMID:19443659; http://dx.doi.org/ 10.1182/blood-2009-03-212290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Te Boome LC, Mansilla C, van der Wagen LE, Lindemans CA, Petersen EJ, Spierings E, Thus KA, Westinga K, Plantinga M, Bierings M et al.. Biomarker profiling of steroid-resistant acute GVHD in patients after infusion of mesenchymal stromal cells. Leukemia 2015; 29(9):1839-46; PMID:25836589; http://dx.doi.org/ 10.1038/leu.2015.89 [DOI] [PubMed] [Google Scholar]
- 39.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED. 1994 consensus conference on acute GVHD grading. Bone Marrow Transpl 1995; 15(6):825-8; PMID:758107620388871 [PubMed] [Google Scholar]
- 40.MacMillan ML, DeFor TE, Weisdorf DJ. The best endpoint for acute GVHD treatment trials. Blood 2010; 115(26):5412-7; PMID:20388871; http://dx.doi.org/ 10.1182/blood-2009-12-258442 [DOI] [PubMed] [Google Scholar]
- 41.Bolanos-Meade J, Logan BR, Alousi AM, Antin JH, Barowski K, Carter SL, Goldstein SC, Hexner EO, Horowitz MM, Lee SJ et al.. Phase 3 clinical trial of steroids/mycophenolate mofetil vs steroids/placebo as therapy for acute GVHD: BMT CTN 0802. Blood 2014; 124(22):3221-7; quiz 335; PMID:25170121; http://dx.doi.org/ 10.1182/blood-2014-06-577023 [DOI] [PMC free article] [PubMed] [Google Scholar]


