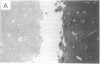
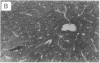
Abstract
To use the equilibrium established by creatine kinase (CK) to determine hepatic free ADP levels, the transcriptional control elements of the transthyretin gene were used to direct expression of the CK B isozyme to the livers of transgenic mice. Activities of CK ranging from 80-250 mumol per min per g (wet weight) were detected in liver extracts from five founder mice. The CK activity was stably transmitted to subsequent generations. Isozyme gels and immunoblots confirmed that the activity detected in extracts was due to the B isozyme of CK. Immunohistology indicated that the protein was expressed uniformly throughout the liver and was localized primarily to the cytoplasm. 31P NMR spectroscopy was used to detect the metabolic product of the CK reaction, phosphocreatine, demonstrating that the enzyme was active in vivo. The phosphocreatine level fell rapidly during anoxia (t1/2 = 1 min), indicating that the CK reaction was integrated into hepatic energy metabolism. The equilibrium established by CK was used to calculate a hepatic free ADP level of 0.059 +/- 0.004 mumol/g (wet weight). In vivo NMR studies of these mice will be valuable for studying the role of free ADP in regulating liver metabolism.
Full text
PDF

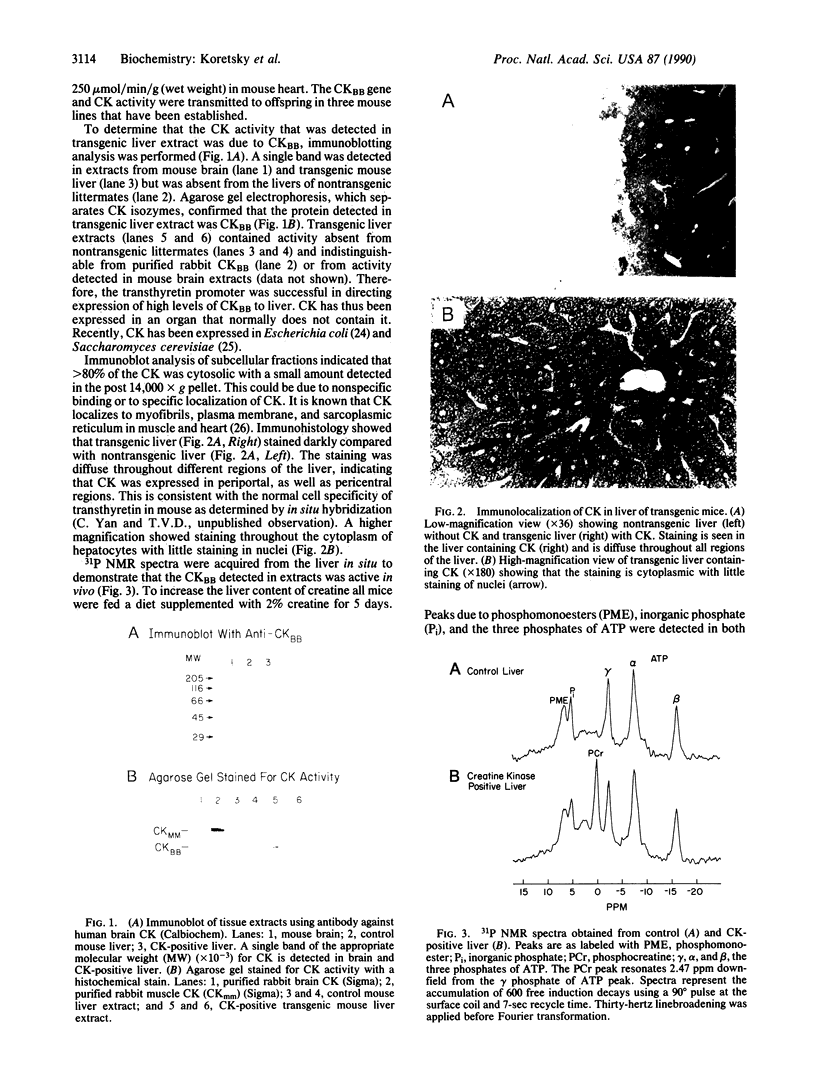
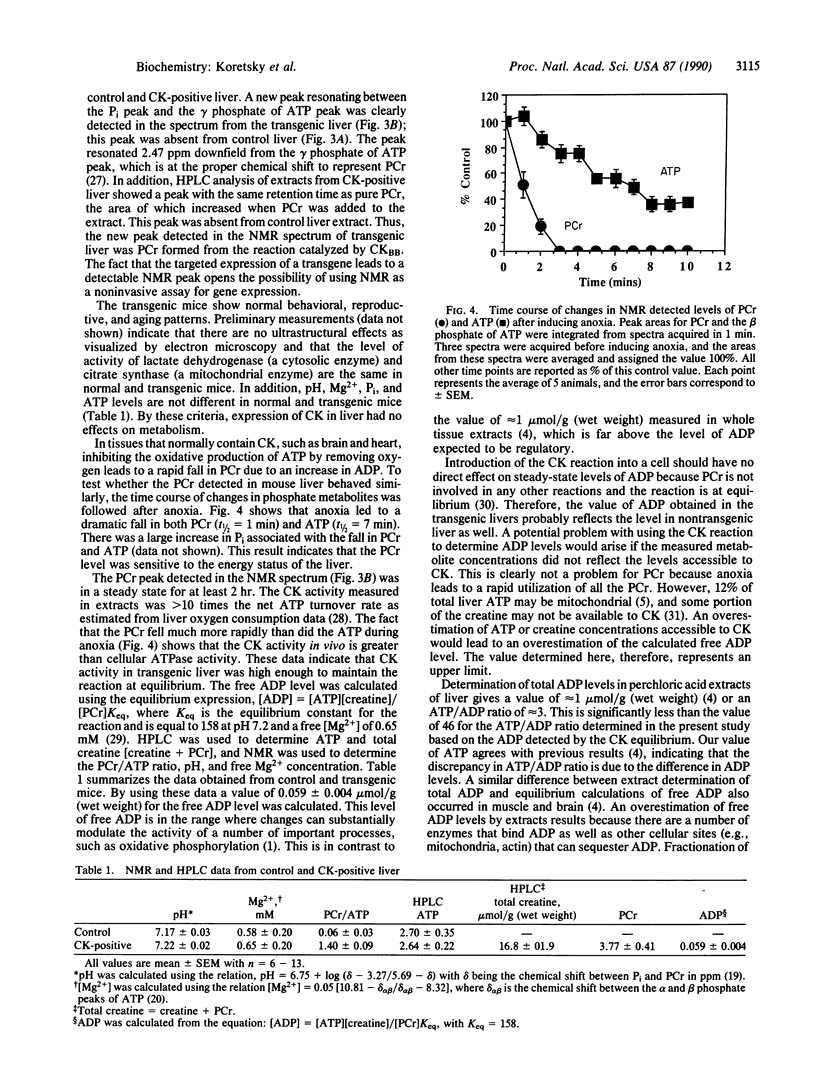

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balaban R. S., Kantor H. L., Katz L. A., Briggs R. W. Relation between work and phosphate metabolite in the in vivo paced mammalian heart. Science. 1986 May 30;232(4754):1121–1123. doi: 10.1126/science.3704638. [DOI] [PubMed] [Google Scholar]
- Benfield P. A., Graf D., Korolkoff P. N., Hobson G., Pearson M. L. Isolation of four rat creatine kinase genes and identification of multiple potential promoter sequences within the rat brain creatine kinase promoter region. Gene. 1988 Mar 31;63(2):227–243. doi: 10.1016/0378-1119(88)90527-6. [DOI] [PubMed] [Google Scholar]
- Bessman S. P., Carpenter C. L. The creatine-creatine phosphate energy shuttle. Annu Rev Biochem. 1985;54:831–862. doi: 10.1146/annurev.bi.54.070185.004151. [DOI] [PubMed] [Google Scholar]
- Brindle K. M., Rajagopalan B., Williams D. S., Detre J. A., Simplaceanu E., Ho C., Radda G. K. 31P NMR measurements of myocardial pH in vivo. Biochem Biophys Res Commun. 1988 Feb 29;151(1):70–77. doi: 10.1016/0006-291x(88)90560-8. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955 Nov;217(1):383–393. [PubMed] [Google Scholar]
- Chen J. D., Neilson K., Van Dyke T. Lymphotropic papovavirus early region is specifically regulated transgenic mice and efficiently induces neoplasia. J Virol. 1989 May;63(5):2204–2214. doi: 10.1128/jvi.63.5.2204-2214.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. M. Simultaneous 13C and 31P NMR studies of perfused rat liver. Effects of insulin and glucagon and a 13C NMR assay of free Mg2+. J Biol Chem. 1983 Dec 10;258(23):14294–14308. [PubMed] [Google Scholar]
- Costa R. H., Lai E., Darnell J. E., Jr Transcriptional control of the mouse prealbumin (transthyretin) gene: both promoter sequences and a distinct enhancer are cell specific. Mol Cell Biol. 1986 Dec;6(12):4697–4708. doi: 10.1128/mcb.6.12.4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C. C., Malloy C. R., Radda G. K. Effect of fasting and acute ethanol administration on the energy state of in vivo liver as measured by 31P-NMR spectroscopy. Biochim Biophys Acta. 1986 Jan 23;885(1):12–22. doi: 10.1016/0167-4889(86)90033-9. [DOI] [PubMed] [Google Scholar]
- GERBER G. B., GERBER G., KOSZALKA T. R., MILLER L. L. The rate of creatine synthesis in the isolated, perfused rat liver. J Biol Chem. 1962 Jul;237:2246–2250. [PubMed] [Google Scholar]
- Gupta R. K., Gupta P., Moore R. D. NMR studies of intracellular metal ions in intact cells and tissues. Annu Rev Biophys Bioeng. 1984;13:221–246. doi: 10.1146/annurev.bb.13.060184.001253. [DOI] [PubMed] [Google Scholar]
- Harmsen E., de Tombe P. P., de Jong J. W. Simultaneous determination of myocardial adenine nucleotides and creatine phosphate by high-performance liquid chromatography. J Chromatogr. 1982 Jun 11;230(1):131–136. doi: 10.1016/s0378-4347(00)81439-5. [DOI] [PubMed] [Google Scholar]
- Iles R. A., Stevens A. N., Griffiths J. R., Morris P. G. Phosphorylation status of liver by 31P-n.m.r. spectroscopy, and its implications for metabolic control. A comparison of 31P-n.m.r. spectroscopy (in vivo and in vitro) with chemical and enzymic determinations of ATP, ADP and Pi. Biochem J. 1985 Jul 1;229(1):141–151. doi: 10.1042/bj2290141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy B. G., Lunn G., Hoffman J. F. Effects of altering the ATP/ADP ratio on pump-mediated Na/K and Na/Na exchanges in resealed human red blood cell ghosts. J Gen Physiol. 1986 Jan;87(1):47–72. doi: 10.1085/jgp.87.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinugasa A., Thurman R. G. Differential effect of glucagon on gluconeogenesis in periportal and pericentral regions of the liver lobule. Biochem J. 1986 Jun 1;236(2):425–430. doi: 10.1042/bj2360425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koretsky A. P., Traxler B. A. The B isozyme of creatine kinase is active as a fusion protein in Escherichia coli: in vivo detection by 31P NMR. FEBS Lett. 1989 Jan 16;243(1):8–12. doi: 10.1016/0014-5793(89)81206-2. [DOI] [PubMed] [Google Scholar]
- Krebs H. A. The redox state of nicotinamide adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Adv Enzyme Regul. 1967;5:409–434. doi: 10.1016/0065-2571(67)90029-5. [DOI] [PubMed] [Google Scholar]
- LEE Y. C., VISSCHER M. B. On the state of creatine in heart muscle. Proc Natl Acad Sci U S A. 1961 Sep 15;47:1510–1515. doi: 10.1073/pnas.47.9.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson J. W., Veech R. L. Effects of pH and free Mg2+ on the Keq of the creatine kinase reaction and other phosphate hydrolyses and phosphate transfer reactions. J Biol Chem. 1979 Jul 25;254(14):6528–6537. [PubMed] [Google Scholar]
- Meyer R. A. A linear model of muscle respiration explains monoexponential phosphocreatine changes. Am J Physiol. 1988 Apr;254(4 Pt 1):C548–C553. doi: 10.1152/ajpcell.1988.254.4.C548. [DOI] [PubMed] [Google Scholar]
- Nageswara Rao B. D., Cohn M. 31P NMR of enzyme-bound substrates of rabbit muscle creatine kinase. Equilibrium constants, interconversion rates, and NMR parameters of enzyme-bound complexes. J Biol Chem. 1981 Feb 25;256(4):1716–1721. [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard J. W., Shulman R. G. NMR spectroscopy of brain metabolism in vivo. Annu Rev Neurosci. 1986;9:61–85. doi: 10.1146/annurev.ne.09.030186.000425. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schwenke W. D., Soboll S., Seitz H. J., Sies H. Mitochondrial and cytosolic ATP/ADP ratios in rat liver in vivo. Biochem J. 1981 Nov 15;200(2):405–408. doi: 10.1042/bj2000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thoma W. J., Uğurbil K. Effect of adenine on liver nucleotide after fructose loading by 31P-NMR. Am J Physiol. 1989 Jun;256(6 Pt 1):G949–G956. doi: 10.1152/ajpgi.1989.256.6.G949. [DOI] [PubMed] [Google Scholar]
- Tischler M. E., Friedrichs D., Coll K., Williamson J. R. Pyridine nucleotide distributions and enzyme mass action ratios in hepatocytes from fed and starved rats. Arch Biochem Biophys. 1977 Nov;184(1):222–236. doi: 10.1016/0003-9861(77)90346-0. [DOI] [PubMed] [Google Scholar]
- Veech R. L., Cook G. A., King M. T. Relationship of free cytoplasmic pyrophosphate to liver glucose content and total pyrophosphate to cytoplasmic phosphorylation potential. FEBS Lett. 1980 Aug 25;117 (Suppl):K65–K72. doi: 10.1016/0014-5793(80)80571-0. [DOI] [PubMed] [Google Scholar]
- Veech R. L., Lawson J. W., Cornell N. W., Krebs H. A. Cytosolic phosphorylation potential. J Biol Chem. 1979 Jul 25;254(14):6538–6547. [PubMed] [Google Scholar]