Abstract
A narrative is a brief summary of specific events experienced by patients, during the course of a clinical trial. Narrative writing involves multiple activities such as generation of patient profiles, review of data sources, and identification of events for which narratives are required. A sponsor outsources narrative writing activities to leverage the expertise of service providers which in turn requires effective management of resources, cost, time, quality, and overall project management. Narratives are included as an appendix to the clinical study report and are submitted to the regulatory authorities as a part of dossier. Narratives aid in the evaluation of the safety profile of the investigational drug under study. To deliver high-quality narratives within the specified timeframe to the sponsor can be achieved by standardizing processes, increasing efficiency, optimizing working capacity, implementing automation, and reducing cost. This paper focuses on effective ways to design narrative writing process and suggested best practices, which enable timely delivery of high-quality narratives to fulfill the regulatory requirement.
Keywords: Clinical study report, narrative writing process, patient narrative, project management, safety narrative
INTRODUCTION
Medical writing is a specialized field in clinical research domain, where scientific documents are prepared for regulatory submissions. These documents require adherence to regulatory guidelines in terms of structure, content, and format. Narrative writing is an integral part of medical writing services. The purpose of writing patient narratives is to provide a concise summary of identified/specific adverse events (AEs) occurring in a patient to conclude causal relationship between the investigational drug and AE. These narratives are submitted along with the clinical study report (CSR). The approximate length of narrative may vary from one to four pages depending on the number of AEs, and data availability for a particular patient/event. In a CSR, the number of narratives could be as huge as 1000, depending on the phase of the trial. Thus, the sponsor may choose to outsource narrative writing activity to effectively manage cost, time, quality, and in turn overall project management. Considering current trends, this article focuses on providing guidance to a medical writer for better understanding of the process, how to drive the narrative writing process (NWP) effectively, regulatory norms, challenges, mitigation strategies, and trends toward automation of narratives.
Patient narrative is a summary of AEs occurring in a clinical trial patient/subject. It is generally written for the following criteria: Death, serious AE (SAE), event(s) of special interest, AE leading to study drug/trial discontinuation, and adjudication event(s). It should be written in a structured way with clear, concise, and logical flow of information. It should follow succinct style and presentation and should provide a medical and scientific context in terms of the event(s) for which it is being written. The logical flow to describe event(s) in a patient narrative is as described below:
Clinical course of the events, with an indication of timing of event corresponding to study drug administration
Nature, intensity/severity, and outcome of the event
Relevant laboratory findings
Treatment administered for the event
Action taken with respect to the study drug
Postmortem findings (if applicable)
Investigator's and sponsor's assessment (if required) on causality.[1]
In addition, it should also include patient identifier, age, sex, race (height and weight, if relevant), disease being treated (if relevant), prior and concomitant medications, medical history, and active/ongoing medical conditions.
TYPES OF NARRATIVES
The type of narratives depends on whether the trial is ongoing (interim analysis) or completed. For completed studies, if the number of narratives is large (approximately >150–200), narrative writing activity usually starts along with cleaning of database. These are usually referred to as Predatabase lock (DBL) narratives. If the number of narratives is less (<150), narrative writing activity may start along with CSR preparation. If the narratives are written based on pre-DBL data, these narratives will be updated/modified based on post-DBL data which is considered clean and final. Final narratives are submitted with the CSR as an appendix. For studies under interim analysis (ongoing studies), it is advisable to initiate narrative writing activities well before the data cutoff date and per protocol recommendations. In such cases, only the events which occurred before the cutoff date will have the narratives written for. Narratives may be written in text or tabular format depending on the requirement.
Patient and safety (pharmacovigilance) narratives
A patient narrative is written for each patient who experiences at least one of the qualifying events as per the criteria defined and agreed upon with the regulatory authority. Thus, a patient narrative may have more than one qualifying events and is submitted as an appendix to the CSR. However, for spontaneous reporting, a safety narrative is written for each event or a couple of events that occurred together.
DATA SOURCES
Data sources required for drafting patient narratives usually comprise (a) primary data source which includes clinical database (patient profiles in the form of pdf/excel/rtf, or patient listings in the form of pdf/excel/rtf/doc, or case report form) and (b) supplementary data sources such as Argus/the Council for International Organization of Medical Sciences/MedWatch forms/data clarification forms that aid in providing details about the event(s) and its course in the narrative.
THERAPEUTIC AREAS
Patient narratives are written across all therapeutic areas and for all phases of clinical trials (Phase I–IV) depending on the drug under evaluation (molecule and its mechanism of action). Framing the template for the narratives is a critical step that requires extensive knowledge of the relevant therapeutic area.
It is important for a medical writer to have a thorough understanding of the therapeutic area for working on a narrative project, as it helps in having a better understanding of the safety profile of the drug class and its derivatives.
REGULATORY REQUIREMENTS
Patient narratives are submitted to regulatory authorities along with the CSR. They are included either as a text in Section 12.3.2 of the CSR, if the number of narratives is less (6–10), or provided in Section 14.3.3 as an appendix. The narratives can also be appended in the CSR addendum (usually required for legacy studies, if the narratives are not submitted earlier). They may also be submitted as Food and Drug Administration/other health authority response document, if the regulatory authority identifies and requests narratives for any event/s of interest due to safety concerns. Patient narratives are also submitted for Data Safety Monitoring Board review, if there are any safety concerns for the study drug which need evaluation by an independent committee on an ongoing basis.
REGULATORY REVIEW PERSPECTIVE
A medical writer has to keep several factors in mind while drafting a narrative. As the narratives get reviewed by regulatory authorities, it is important for a medical writer to understand their role and expectations while drafting a narrative. Usually, regulatory authorities check the narratives with the following objectives during their review:
To identify and closely examine AEs/SAEs that require further monitoring[2]
To identify and monitor frequency, severity, and seriousness of unexpected AEs
To identify the factors which affect and predict for the occurrence of adverse drug reactions including patient-related factors such as age, gender, ethnicity, race, target illness, abnormalities of renal or hepatic function, comorbid diseases, genetic characteristics such as metabolic status, environment, and drug-related factors such as dose, plasma concentration, duration of exposure, and concomitant medication[2]
To evaluate whether the data are suitable for support of the safety analysis[2]
To identify the limitations of the drug on the basis of data[2]
To evaluate and analyze drug–drug interaction, if applicable.
NARRATIVE WRITING PROCESS
Narrative writing is a complex process that involves various stakeholders. Figure 1 represents stakeholders involved in NWP. The process begins with template finalization which is a crucial step in the NWP as it avoids subjectivity. It is advisable to write sample narratives for all possible criteria, which usually ranges between three and five narratives; however, this may vary based on the total number of narratives for that study and the team's preference. Finalized sample narratives need to be clean, concise, and appropriate, as they form the basis for drafting further batches. Thus, an agreement with all the stakeholders/reviewers on the content/template of sample narratives is desirable. Finally, the compiled batch of narratives is shared with the respective stakeholders before publishing. For the purpose of audit trail, all versions of the narrative document should be stored in the repository/document management system. Figure 2 represents the generic process for handling any NWP.
Figure 1.
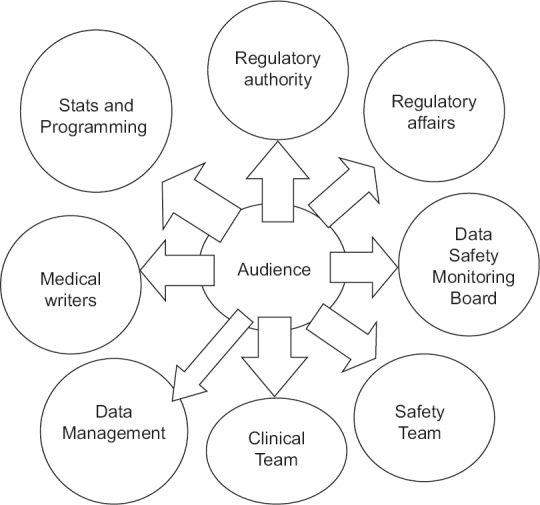
Stakeholders involved in narrative writing process
Figure 2.
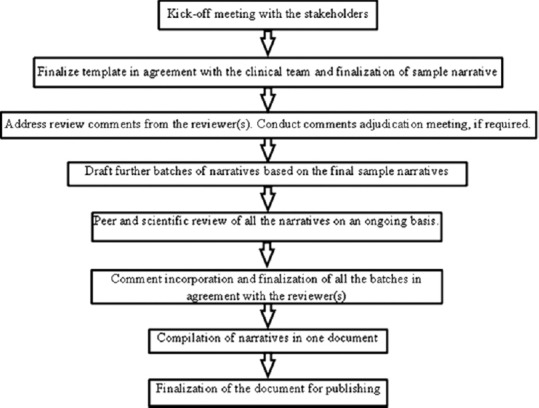
Process steps for handling any narrative writing project
CHALLENGES IN NARRATIVE WRITING PROCESS
Of the several challenges encountered while drafting narratives, the most commonly observed challenges are (a) large number of resources required for any given project, (b) maintaining consistency in the narratives due to multiple resources working on the same project, (c) requirement of quick turnaround time and timely delivery with high quality, (d) not having standardize data sources/patient profiles for most of the programs, (e) different types of data sources, (f) high variability in template finalization before and after sample narratives due to differences in opinion of the reviewers, (g) change in reviewers during the intermediate/later stages of the project, (h) delay in review cycles, and (i) not receiving timely clinical team consensus on the addressed comments and pending queries.
Mitigation strategies/tips
Mitigation strategies/tips for the aforesaid challenges include (a) excellent project management skills for efficient resource utilization, (b) automation of narratives, (c) standardization of data sources/patient profiles throughout the process, (d) finalization of template before starting sample narratives, in consensus with the clinical team, (e) finalization of sample narratives before dispatching future batches, (f) rigorous follow-ups and proactive communication with all the stakeholders (g) letting everyone know the impact of delay on timelines, (h) standardization of the review process, and (i) regular calls with clinical team to address review comments or pending queries.
EFFECTIVE WAYS TO EXECUTE NARRATIVE WRITING PROCESS
Review of data sources
Before drafting the sample narratives, it is prerequisite to review the data sources (patient profiles or listings) for all the necessary information as per the agreed narrative template/content and protocol requirements. Every data point in the template should be mapped against the patient profile. Missing/discrepant data, if any, should be highlighted on an immediate basis, and its inclusion or deletion in the narrative needs to be justified.
Data validation
Data validation/data quality control (QC) process is of prime importance to all the stakeholders as it helps in generating a high-quality narrative document(s) and is an integral part of the entire project management. Narratives that are generated with the aid of automation tool needs validation of data against the data sources to ensure quality. The lead writer should randomly perform data QC of automated narratives to confirm data accuracy and credibility. Any discrepancies should be fixed immediately, so as to avoid such errors in the future batches.
Quality calibration
Quality calibration is a paramount step to ensure consistency within the narratives in a batch and among the team members so as to meet reviewer(s) expectations. Usually, quality calibration should be performed on a quarterly or biannual basis. It also helps in tracking inter- and intra-variability while performing QC of the document. Quality calibration involves assigning the same narrative for QC to different QCers and to the same QCer at different time points, and checking inter and intra-QCer variability against the master QCed narrative.
AUTOMATION OF NARRATIVES
In the current digital era, revolution in technology has brought modernization in health-care industry to effectively operate the process in an efficient and convenient way that significantly reduces the cost of pharmaceutical organizations. Automation in narrative writing is a current trend that every pharmaceutical industry is adopting to provide quality and consistency within the narratives batch. The tool which helps in creating automated narratives, extracts relevant information for all the identified patients in desired format/template. This tool is very helpful for studies with large volume of narratives (usually >150).
The key features of autogenerated narratives are:
Consistency in narratives
High-quality narratives with minimal/no error
Preformatted and editable narratives generation, which gives flexibility to modify based on the need
Decrease turnaround time for overall narratives
Ability to generate graphs and bar diagrams
Maintain audit trail and version control at each stage.
Medical review
Medical and scientific review of the narratives is a must, even if the narratives are generated with an automation tool. Narratives should make sense from a scientific perspective; hence a thorough medical and scientific review is desirable to conclude event(s) summary.
A medical reviewer possesses in-depth knowledge of the therapeutic area and usually verifies medical plausibility of the event/s, inclusion/exclusion of the content from a scientific perspective, safety data reported in the narrative, and flow and alignment of contents according to the applicable regulatory requirements.
PROJECT MANAGEMENT
Project management plays a vital role in effectively managing the NWP, considering that the quality and timely submission is paramount to success. Tracking the total number of narratives, name of resources, and other activities are critical in projects involving a large number of patient narratives. MS Excel can be used as a tracking tool for managing large volume of narratives and is particularly useful if all stakeholders are able to adapt each other's processes. This may not be applicable in instances, where it records confidential information such as time spent per narrative for budgetary purposes.[3] Effective project management mainly involves the following activities:
Resource management
Managing resources during narrative writing projects is critical and usually depends on submission timelines. Adequate allocation of resources in each project also depends on duration of the project, total number of narratives, availability of resources, and their therapeutic area expertise.
Narrative tracking sheet
The narrative tracking sheet is a tool which includes list of patients requiring narratives and their criteria (death, SAE, AE leading to study drug discontinuation, and event of special interest). This tool may be used to capture few other details such as author, reviewer, and dates.
Resource utilization tracker
Resource utilization tracker lists down all the ongoing studies and percent utilization and free capacity of resource(s) in each project.
Master tracker
This tracker may include all the ongoing, completed, and planned studies with detailed description of each.
Quality dashboard
It is very important to maintain quality of each narrative and to track the quality in a quantitative manner; quality tracker should be in place. Generally, quality scoring sheet is used to calculate major and minor errors of narratives to meet quality parameters as defined by customer. Study wise and writer wise error trend analysis may be performed with the help of this tracker.
A FEW TIPS FOR NARRATIVE WRITING
Always use hard space and hard hyphens for days, dates, and laboratory values to avoid splitting on next page or next line except at the beginning of sentence (hard hyphen: ctrl + shift + hyphen; hard space: ctrl + shift + space)
Be consistent while using patient or subject throughout the narrative, as per agreement with clinical team
Tagging the narrative template against the information available in data sources which helps the QCer/reviewer to trace the information easily
Always use generic names for concomitant medications or AE treatment medications. Exception: For combination drugs, generic drug name should be provided along with trade name in bracket, for example, lisinopril-hydrochlorothiazide (Zestoretic)
Always use preferred terms (PT) for the event and medical history/ongoing condition. The verbatim term should only be used if there are same PTs for two different conditions (e.g., PT: pain in extremity should be reported as pain in hands or pain in legs)
Self QC by the author is very important step forFirst Time Right approach.
CONCLUSION
This article focuses on ways to overcome challenges encountered during the NWP, and the best practices to ensure high quality and timely submission of narrative documents to the regulatory authorities. Automation in narrative writing is an emerging approach, and most of the pharmaceutical companies are adapting it to be resource, cost, and time efficient.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.International Conference on Harmonization of Technical Requirements for Registration of Pharmaceutical for Human Use: ICH Harmonized Tripartite Guidel. Structure and Content of Clinical Study Reports E3. Step 4 Version. [Last updated on 1995 Nov 30; Last cited on 2016 Jul 05]. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E3/E3_Guideline.pdf .
- 2.Reviewer Guidance Conducting a Clinical Safety Review of a New Product Application and Preparing a Report on the Review. [Last updated on 2005 Feb 25; Last cited on 2016 Jul 05]. Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm072974.pdf .
- 3.Moores Y. Patient Safety Narratives – Clinical Trials: Medical Writing and Patient Safety Narratives. Drug Development and Delivery. 2015. May, [Last accessed on 2016 Apr 01]. Available from: http://www.drug-dev.com/Main/Back-Issues/PATIENT-SAFETY-NARRATIVES-Clinical-Trials-Medical-921.aspx .


