Abstract
Alzheimer’s disease (AD), a progressive neurodegenerative disorder, is linked to oxidative stress, altered amyloid precursor protein (APP) proteolysis, tau hyperphosphorylation and the accumulation of amyloid-β (Aβ) plaques and neurofibrillary tangles (NFT). A growing body of evidence suggests that mitochondrial dysfunction can be a key promoter of all of these pathologies and predicts that restoration of mitochondrial function might be a potential therapeutic strategy for AD. Therefore, in the present study, we tested the beneficial effect of a nutraceutical formulation Nutrastem II (Nutra II), containing NT020 (a mitochondrial restorative and antioxidant proprietary formulation) and pyrroloquinolinequinone (PQQ, a stimulator of mitochondria biogenesis) in 5XFAD transgenic mice. Animals were fed Nutra II for 12 weeks, starting at 3 months of age, after which behavioral and neuropathological endpoints were determined. The data from behavioral test batteries clearly revealed that dietary supplementation of Nutra II effectively ameliorated the motor deficiency and cognitive impairment of 5XFAD mice. In addition, Nutra II also protected mitochondrial function in 5XFAD mice brain, as evidenced by declined ROS levels and membrane hyperpolarization, together with elevated ATP levels and respiratory states. Interestingly, while Nutra II treatment only slightly reduced soluble Aβ42 levels, this formulation significantly impacted tau metabolism, as shown by reduced total and phosphorylated tau levels of 5XFAD mouse brain. Taken together, these preclinical findings confirm that mitochondrial function may be a key treatment target for AD and that Nutra II should be further investigated as a potential candidate for AD therapy.
Keywords: Neuroscience
1. Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder linked to oxidative stress, altered amyloid precursor protein (APP) proteolysis, abnormal tau processing and the accumulation of amyloid-β (Aβ) plaques and neurofibrillary tangles [1, 2, 3, 4]. The intracellular accumulation of Aβ as well as other AD-associated proteins, including tau and proteolytic products of ApoE4, can lead to mitochondrial dysfunction, the main source of reactive oxidative species (ROS) generation [5, 6]. Further evidence suggests that the mitochondrial dysfunction and the subsequent oxidative stress may augment the production and aggregation of Aβ as well as facilitate the phosphorylation of tau, thus forming a vicious cycle that promotes the initiation and progression of AD [7]. Mitochondria may therefore have a central role in AD pathogenesis and restoration of the impaired mitochondrial function might be a rationale therapeutic strategy for AD [8, 9].
Our laboratory has screened 25 natural compounds for their ability to restore mitochondrial function in neuroblastoma cells expressing Swedish mutant APP and identified two compounds, epigallocatechin 3-gallate (EGCG) and luteolin, as top mitochondrial restorative compounds [10]. As an in vivo confirmation, EGCG restored mitochondrial respiratory rates, mitochondrial membrane potential, ROS production and ATP in APPPS1 mice. These results support previous studies showing that EGCG, the most abundant catechin in green tea, decreases amyloidogenic APP proteolysis, Aβ plaques and cognitive dysfunction in AD mice [11, 12]. In light of recent failures in clinical trials of more than a dozen single-target antiamyloidogenic drugs for the treatment of AD, recent studies have focused on the potential effectiveness of nutritional supplements, or nutraceuticals, with multiple therapeutic targets [12, 13, 14, 15]. NT-020, a propriety blend of blueberry, green tea extract, carnosine and vitamin D3 [16, 17], has previously been shown to reduce cognitive decline in aged rats and humans, by enhancing mitochondrial function, as well as enhancing neural stem cell proliferation and reducing oxidative injury [17, 18, 19]. In the present study, we tested the therapeutic effectiveness of NT-020 on AD-related mitochondrial dysfunction, neuropathology and behavioral impairment in the 5XFAD transgenic mouse model [20]. In addition, since AD presents with a more advanced stage of cognitive decline compared with aging alone, we aimed to further enhance the potency of NT-020 by the addition of pyrroloquinonine (PQQ), a stimulator of mitochondrial biogenesis [21, 22], yielding Nutra II.
2. Materials and methods
All experiments were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of South Florida, College of Medicine. All animals were housed in environmentally controlled conditions (12-h light/dark cycle at 21 °C) and provided food and water ad libitum. Transgenic female 5XFAD mice (Tg6799 line) were purchased from the Jackson Laboratory (Bar Harbor, ME). These mice co-express and co-inherit FAD mutant forms of human APP (the Swedish mutation: K670N, M671L; the Florida mutation: I716V; the London mutation: V717I) and PS1 (M146L; L286V) transgenes under transcriptional control of the neuron-specific mouse Thy-1 promoter [20]. These mice generate Aβ42 almost exclusively, with amyloid deposition beginning at 2 months of age and impaired memory at 4–5 months of age. Female 5XFAD mice have been reported to have a median life span of 24+ months [23] and we lost no mice over the course of study. A total of 19 female 5XFAD mice were fed Nutra II enriched food and 11 female 5XFAD and 10 female WT mice (B6SJLF1/J) were fed control diet (Tekland Global 18% Protein Rodent Diet, 2018, Harlan Laboratories, Madison, WI) between 3 and 6 months of age, in order to treat them during the early development of AD. Behavioral analyses were then performed to evaluate motor and cognitive performance, followed by euthanization and analysis of AD pathology and mitochondrial function (Fig. 1). To enrich food with Nutra II, the contents of 1 capsule of NutraStem Cardio (NutraTherapeutics, Tampa, FL), containing 450 mg proprietary blend (green tea leaf extract, blueberry lowbush powder, L-carnosine, Vitablue® wild blueberry extract), 1,000 IU vitamin D3 and 20 mg BioVin® grape seed extract), and 1 capsule of PQQ Caps (Life Extension, Ft Lauderdale, FL), containing 10 mg PQQ, was mixed with 1000 g chow (Tekland 2018). This translates into 72 mg Proprietary Blend, 160 IU vitamin D3, 3.2 mg Biovin and 1.6 mg PQQ per kg per day or 1.8 mg Proprietary Blend, 4 IU vitamin D3, 0.08 mg Biovin and 0.04 mg PQQ per day per mouse.
Fig. 1.
Schematic diagram of experimental design and procedure.
2.1. Behavioral analysis
Previous studies have indicated that a concurrence of motor and cognitive impairments occurs in both AD patients and rodent models [24, 25]. Therefore, behavioral analysis was performed to assess these impairments as well as general anxiety levels in Nutra II treated and untreated 5XFAD mice, as described previously [26, 27, 28]. These analyses included the rotarod test (RT), open field task (OFT), elevated-plus maze (EPM), contextual and cued fear conditioning (FCT), Morris water maze (MWM) and Y maze (YM).
2.1.1. Rotarod test (RT)
The sensorimotor performance of 5XFAD mice were first determined using a rotarod test. Mice were positioned on the rod (diameter 3.6 cm) of the equipment (Rotarod 7650 accelerating model; UgoBasile, Biological Research Apparatus, Varese, Italy) to gauge differences in balance and motor coordination. The rod on the apparatus was set at 1.0 rpm and mice were placed, 5 at a time, each in an assigned location on the rod. The rod was allowed to steadily accelerate up to 40.0 rpm over a 3-min session. Mice were then removed and allowed to rest for 30 min until returning to the remaining 2 sessions of the test, yielding a total of 3 trials. Evaluation was made by monitoring latency to fall and maximal rotation rate before the mouse fell down. A soft pad was placed under the equipment as a precautionary measure against physical damages from falls.
2.1.2. Open field test (OFT)
The open field was used as a standard test of general activity. Animals were monitored for 10 min in a 40 cm square open field with video tracking software (ANY-Maze®, Stoelting, Wood Dale, IL, USA) and under moderate lighting. General activity levels were evaluated by determining the total distance traveled and average speed. Anxiety levels were assessed by the pattern of exploration in the open field (center vs. periphery) as well as frequency of rearing, defined as the mouse standing upright on the hind legs, with the forepaws not touching any surface.
2.1.3. Elevated-plus maze (EPM)
Anxiety and locomotor activity were further assessed through the EPM. The EPM consisted of two well-lit open arms (35 cm) facing each other and two enclosed arms (30.5 cm) also facing each other. Each arm was attached to a common center platform (4.5 cm square) and elevated 40 cm off the floor. The mouse was placed in the center platform and allowed to explore the maze for 5 min. Video tracking software measured movement in each section (ANY-Maze®). The number of closed and open arm entries, total distance traveled and average speed was recorded.
2.1.4. Fear conditioning test (FCT)
Fear conditioning test was used to assess memory formation that is especially suited for proper hippocampal functioning. Animals were placed in the fear conditioning apparatus (Panlab, Spain) for 2 min. Then, a 30 s acoustic conditioned stimulus (CS; 80 dB tone) was delivered and a 0.5 mA shock unconditioned stimulus (US) was applied to the floor grid during the last 2 s of the CS. Training consisted of two CS-US pairings, with a 1.5 min interval between each. The mice were placed in the chamber and monitored for freezing behavior (i.e., motionless position for at least two consecutive seconds) to the context 24 h after training (no shocks or conditioned stimulus given). Immediately after the contextual test, mice were placed into a novel context and exposed to the CS for 3 min (cued fear conditioning). Learning and memory were assessed by measuring freezing behavior.
2.1.5. Morris water maze (MWM)
The Morris hidden-platform water maze consisted of a circular pool (1.38 m diameter) filled with nontoxic opaque water at room temperature and divided into four equal quadrants (Q1–Q4) with an escape platform (10 cm diameter) hidden beneath the water (3 cm) in the center of Q1. The environment surrounding the pool was decorated with eye-catching visual cues to aid the mice in orientating themselves with respect to the pool. Each mouse was subjected to four training trials a day over a 3-day period. Each trial began by placing the mouse into a different quadrant and allowing it to swim freely for a maximum of 60 s. The same quadrant start pattern was used each day. After swimming to the platform, or being guided to the platform if the mouse was unable to locate the platform within 60 s, the animal was allowed to remain on the platform for 30 s before starting the next trial. The latency for each animal to locate the platform in all four trials was recorded by video tracking software (ANY-Maze®). A 60 s probe trial was performed on the fourth day to determine memory retention. For this single trial, the submerged platform was removed and each mouse was placed into the quadrant opposite to the quadrant that formerly contained the platform in acquisition testing. The number of annulus crossings, the percentage of time spent in each quadrant and average swim speed was determined from the videotapes.
2.1.6. Y-maze (YM)
This task was used to assess basic mnemonic processing by spontaneous percent alternation and exploratory activity by total number of arm choices of mice placed into a black Y-maze. The arms of this maze were 21 cm-long and 4 cm-wide with 40 cm-high walls. Each mouse was placed in one of the arms and allowed one five-minute trial of free exploration of the three alleys in the maze. The number of total arm choices and sequence of arm choices were recorded. Alternation percentage is defined by the proportion of arm choices that differ from the last two choices. For instance, if a mouse selected the following sequence of arm choices (1, 2, 3, 1, 3, 1, 2, 1), the total number of alternation opportunities would be six (total entries minus two) and the percentage alternation would be 50% (three of six). Before each trial, the interior of the maze was sprayed with a diluted vinegar solution to erase any scent cues.
2.2. Mitochondria functional analysis
2.2.1. Isolation of brain mitochondria
Mice were anesthetized with isofluorane and transcardially perfused with ice-cold physiological saline containing heparin (10 U/ml). After euthanasia, brains were isolated and quartered using a mouse brain slicer (Muromachi Kikai, Tokyo, Japan). The first and second anterior quarters were homogenized for Western blot analysis of amyloid and tau pathology and analysis of mitochondrial function and the third and fourth posterior quarters were used for immunohistochemistry. Brain mitochondrial isolation was performed using a standard procedure [10, 29]. Briefly, brain regions of interest were quickly removed on ice and then placed in an ice cold glass Dounce homogenizer containing five volumes of isolation buffer (215 mM mannitol, 75 mM sucrose, 0.1% BSA, 1 mM EGTA, 20 mM HEPES (Na +), pH 7.2). Following homogenization, a low-speed spin (1,300 × g for 5 min) to remove unbroken cells and nuclei was performed. The supernatant was carefully placed in fresh tubes, topped off with isolation buffer, and spun down again at 13,000 × g for 10 min. The supernatant was discarded and the resultant mitochondrial pellets were resuspended in 500 μL of isolation buffer without EGTA. The final mitochondrial pellet suspended in isolation buffer without EGTA yielded a final protein concentration of approximately 10 mg/mL using a BCA protein assay kit and immediately stored on ice. All mitochondrial results were normalized to mg protein. For all mitochondrial analyses, the brain areas of interest from 2 mice of the same genotype were combined to form a single homogenate that was then assayed in triplicate.
2.2.2. Respiratory states
The respiratory function of mitochondria was measured using a miniature Clark type oxygen electrode (Strathkelvin MT200A chamber). 100 μg (0.3 mg/ml final concentration) of mitochondria were suspended in a sealed, constantly stirred and thermostatically controlled chamber at 37 C containing 350 μL of respiration buffer (125 mM KCl, 1 mM MgCl2, 2 mM KH2PO4, 5 mM pyruvate, 2.5 mM malate, 500 μM EGTA, 20 mM HEPES, pH 7.0). State III respiration was assessed by the addition of 200 μM ADP. State IV respiration was achieved by addition of 1 μM oligomycin. Maximal respiration (state V) was assessed by addition of 1 μM FCCP. The respiratory control ratio (RCR) was determined by dividing the rate of oxygen consumption for state III or V by that of state IV and was consistently 4.0 or greater for the WT mice.
2.2.3. Reactive oxygen species (ROS)
Mitochondrial ROS production was measured following incubation of isolated mitochondria with 25 μM 2′,7′-dichlorodihydrofluorescein diacetate (DCF) for 20 min and then the DCF fluorescence (excitation filter 485/20 nm, emission filter 528/20 nm) was read as previously described [30]. In short, 100 μg (0.8 mg/ml final concentration) of isolated mitochondria were added to 120 μL of KCl-based respiration buffer (see above) with 5 mM pyruvate and 2.5 mM malate added as respiratory substrates and 25 μM DCF. Mitochondrial ROS production was determined in the absence or presence 0.5% ethanol to increase ROS production and ensure measurements were within the range of the indicator.
2.2.4. Membrane potential
A 200 μM stock solution of JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide) was made using DMSO as the solvent. The assay buffer contained mitochondrial isolation buffer with the addition of 5 mM pyruvate and 5 mM malate. 150 μL of assay buffer and 20 μL (1.2 mg/ml final concentration) of mitochondria were added to the wells of a 96 well black, clear bottom microplate (Corning) followed by the addition of 1 μM JC-1 and mixed gently. The microplate was covered with aluminum foil and left at room temperature for 20 min before reading. Fluorescence (excitation 530/25 nm, emission 590/35 nm) was then measured.
2.2.5. ATP levels
ATP was determined in isolated mitochondria using CellTiterGlo® (Promega, Madison, WI). For these experiments, ATP standard curves were run in the range of 0.5 to 50 μM. ATP levels from the mitochondrial samples were recorded as relative fluorescence activity and translated into ATP concentrations using the calibration curves.
2.3. AD pathology
2.3.1. Immunohistochemistry (IHC)
Brains were fixed in 4% paraformaldehyde in PBS at 4 °C overnight and routinely processed in paraffin. Five coronal sections were prepared with a 100-μm interval and a thickness of 25–30 μm for each brain by microtome or cryostat sectioning [12, 31]. IHC staining was conducted according to the manufacturer’s protocol using a Vectastain ABC Elite kit (Vector Laboratories, Burlingame, CA) coupled with the diaminobenzidine reaction using a biotinylated human Aβ17–24 monoclonal antibody (4G8; 1:200, Covance Research Products, Emeryville, CA). Normal mouse or rabbit serum (isotope control) or 0.1 M PBS (pH 7.4) was used instead of primary or secondary antibody or ABC reagent as a negative control.
2.3.2. Western blotting (WB)
Briefly, for WB analysis, brain homogenates were prepared in cell lysate buffer and proteins were separated using 10% bicine/tris gel, transferred to 0.2 μm nitrocellulose membranes (Bio-Rad, Hercules, CA) and visualized using standard immunoblotting protocol. All antibodies were diluted in Tris-buffered saline (TBS) containing 5% (w/v) non-fat dry milk. Blots were developed using the Luminol reagent (Thermo Fisher Scientific) and densitometric analysis was performed as described previously [12] using a Fluor-S MultiImager with Quantity One software (Bio-Rad). Primary antibodies used included p-tau231 (PHF1, Abcam® Cambridge, MA), p-tau181 (AT270, Thermo Fisher Scientific, IL, USA), total tau46 (Sigma Aldrich, St Louis, MO), 6E10 (sAPPα; Covance, Emeryville, CA, USA), pAb751/770 (holo APP; EMD Biosciences, La Jolla, CA), 82E1 (Aβ, β-CTF) and anti-actin antibody (Sigma Aldrich).
2.4. Statistical analysis
The 5XFAD mice were assigned to control and Nutra II treatment groups randomly. Investigators determining mitochondrial function were blinded from animal group assignments. Remaining investigations of cognitive and motor behavior, Aβ burden and tau pathology were unblinded. All data were presented as mean ± SD. Comparison between groups was performed by Student’s t test or ANOVA followed by LSD or Bonferroni post hoc test. A P value of <0.05 was considered statistically significant. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS), release 18.0 (IBM, Armonk, NY).
3. Results
3.1. Behavioral analysis
3.1.1. Nutra II improves motor coordination, spontaneous locomotor activity and learning in 5XFAD mice
In the present study, we determined if a combination of NT020 and PQQ, called Nutra II, reduces motor, cognitive and mitochondrial dysfunction in the 5XFAD mouse model. Total body weights did not differ between WT and 5XFAD mice with or without dietary treatment with Nutra II (Fig. 2). The accelerating rotarod test, evaluating coordination and motor skill acquisition, suggested that untreated 5XFAD mice spent less time on the rod and achieved a lower maximal rate compared to WT mice, indicating impairments in motor learning and coordination (Fig. 3). Notably, these impairments were reversed by Nutra II dietary supplementation.
Fig. 2.

Total body weight did not differ between 5XFAD mice treated with Nutra II in comparison with untreated 5XFAD and WT mice - 5XFAD mice were treated orally with chow containing Nutra II for 12 weeks starting at 3 months of age or fed normal chow. WT (B6SJLF1/J) mice fed normal chow served as control.
Fig. 3.
Nutra II improves motor learning and coordination in 5XFAD mice as determined using the rotarod task - 5XFAD mice untreated spent less time on the rod (lower latency to fall) and achieved a lower maximal rate compared to WT mice, indicating impairment in motor learning and coordination. Notably, this impairment was significantly reversed by Nutra II treatment. Nutra II also significantly reversed impairments in total average latency to fall and maximal rate achieved in 5XFAD mice (*P < 0.05 versus untreated).
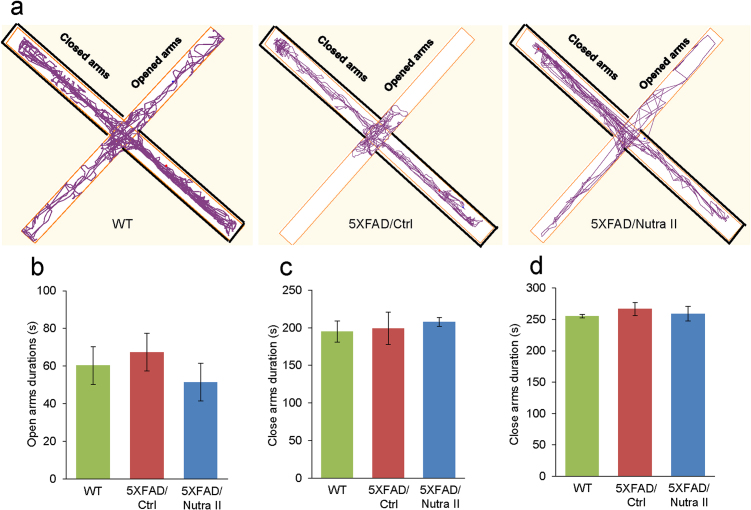
In addition, 5XFAD mice exhibited reduced locomotor and exploratory activity, as evidenced by lower total distance traveled, average speed and rearing frequency in a novel environment of the open field task (Fig. 4a). Likewise, 5XFAD mice exhibited lower total distance traveled, average speed as well as total, open and closed arms entries in the elevated plus maze task (Fig. 4c, d). Notably, this hypoactivity was reversed by Nutra II treatment. No significant differences were observed in the time spent in open and closed arms of the EPM test (Fig. 5), as well as the time spent in the central and peripheral zones of the open field task (Fig. 4b), of 5XFAD compared with WT mice, indicating no change in anxiety level.
Fig. 4.
Nutra II improves spontaneous locomotor activity in 5XFAD mice as determined using the open field task and elevated plus maze test - 5XFAD mice untreated exhibited reduced locomotor and exploratory activity in comparison with WT mice, as evidenced by reduced total distance traveled, average speed and rearing frequency in a novel environment (a). However, 5XFAD mice did not exhibit increased anxiety compared with WT mice, as determined by similar amounts of time in the central and peripheral zones (b). The hypoactivity observed in 5XFAD mice was reversed with Nutra II treatment (*P < 0.05 versus untreated). The reduced spontaneous locomotor activity in 5XFAD mice was confirmed by the reduced total distance traveled, average speed (c) and open, closed and total arms entries in elevated plus maze (d). This reduced activity was reversed with Nutra II treatment (**P < 0.01). Notably, 5XFAD mice did not exhibit increased anxiety, evidenced as similar proportion of time spent in the open and closed arms in comparison with WT mice.
Fig. 5.
Nutra II increased exploratory behavior as assessed by elevated plus maze task - Diagrams show representative tracings of exploratory behavior in WT and 5XFAD mice treated with Nutra II or left untreated in the elevated plus maze task. All mice showed similar durations in open arms and closed arms.
3.1.2. Nutra II improves cognitive function in 5XFAD mice
To assess hippocampal-dependent learning and memory, 5XFAD and WT control mice were tested in the hidden-platform Morris water maze and Y maze tasks. With the Morris water task, untreated 5XFAD mice displayed an increase in escape latency to find the hidden platform during training between trials 8 and 12 compared to WT mice (Fig. 6a). This increase was reversed after treatment with Nutra II. Likewise, during the probe trial, untreated 5XFAD mice displayed a decreased time spent in the target quadrant compared with WT mice, which again was reversed with Nutra II treatment (Fig. 6c). Consistent with rotarod test, EPM and OF test, 5XFAD mice also showed a declined motor ability in MWM test, as indicated by a gradually decreased swimming speed (Fig. 6b). As expected, this motor deficiency can be ameliorated by Nutra II treatment. In the Y maze test, levels of spontaneous alternation were significantly lower in untreated 5XFAD mice compared with WT mice, and this impairment was reversed by Nutra II treatment (Fig. 6d). These results further support a role for Nutra II to improve hippocampal-dependent learning and memory in 5XFAD mice. Latency for first exit from the starting arm was increased in untreated 5XFAD mice compared with WT mice, confirming a reduced exploratory and risk assessment behavior in this AD mouse model (Fig. 6e). This deficiency of risk assessment behavior was unaltered by Nutra II treatment.
Fig. 6.
Nutra II improves hippocampal-dependent learning and memory - The cognitive function of 5XFAD mice was assessed using Morris water maze (a-c), Y-maze (d-e) and fear conditioning test (f-g). In the Morris water maze test, 5XFAD mice untreated displayed an increased latency to find the platform (a), decreased swimming speed (b) and a decreased time spent in the target quadrant compared with WT mice (c), which was reversed with Nutra II treatment (**P < 0.01 versus untreated). Consistent with Morris water maze test, levels of spontaneous alternation in Y-maze were significantly lower in untreated 5XFAD mice compared with WT mice, and this impairment was reversed with Nutra II treatment (*P < 0.05). The impaired cognitive function and the reversal effect of Nutra II were also assessed by using fear conditioning test. 5XFAD mice treated with Nutra II showed increased levels of freezing in response to both conditioned stimulus or context compared to 5XFAD untreated mice (*P < 0.05).
Due to the declined motor activity in 5XFAD mice, hippocampal-dependent associative learning and memory were further assessed using contextual and cued fear conditioning test, a classic behavioral task independent of motor activity. Animals were trained with a standard two-shock protocol and freezing to the context (hippocampal dependent) or to the conditioned stimulus in a novel context (hippocampal and amygdala dependent) was used as an index of memory formation. When placed in the novel environment and presented with the conditioned stimulus (cued test) 24 h following training, 5XFAD mice showed a significant decrease in freezing compared to WT mice (p < 0.01, Fig. 6f). Likewise, 5XFAD mice showed a decrease in freezing when placed in the context 24 h (context test) following training compared to WT mice (Fig. 6g). These results indicate that the genetic impairments in 5XFAD mice lead to a limitation in both hippocampal- and amygdala-dependent learning. However, 5XFAD mice treated with Nutra II showed similar levels of freezing in response to either conditioned stimulus or context as that observed in WT mice. Therefore, Nutra II can reverse the learning impairment observed in this AD mouse model.
3.2. Amyoid-β and Tau pathology
While WB analysis indicates that Nutra II treatment did not significantly decrease the levels of Aβ (p = 0.08) or β-CTF (p > 0.05) in the brain of 5XFAD mice (Fig. 7a, b), the ELISA data suggested a slight decrease in soluble Aβ42 production (p < 0.05, Fig. 7c). Likewise, 12-weeks dietary supplement with Nutra II slightly ameliorated the deposition of amyloid plaques in those important brain regions for memory, including hippocampus (H), entorhinal cortex (EC) and retrosplenial cortex (RSC), as determined by immunohistochemistry (Fig. 7d). Consistently, Nutra II increased α-cleavage of APP, as evidenced by significantly increased sAPPα level (P < 0.05, Fig. 7a, b). In addition, Nutra II treatment also reduced the levels of both total and phosphorylated tau protein (Fig. 8). Therefore, the improvement of working memory elicited by Nutra II seems related to the slightly decreased levels of Aβ, β-CTF, amyloid plaques and tau, and to the significantly enhanced sAPPα levels.
Fig. 7.
Nutra II slightly decreases levels of Aβ, β-CTF, and Aβ plaque deposition in 5XFAD mice - After Nutra II treatment, 5XFAD mice were sacrificed for analysis of holo APP, sAPPα, β-CTF, Aβs and β-actin as control in brain homogenates using WB analysis. Untreated 5XFAD mice were also sacrificed for analysis as control. Representative WB shows holo APP, as determined by pAb751/770, sAPPα, as determined by 6E10, β-CTF and Aβ, as determined by 82E1, and β-actin, as determined by β-actin specific antibodies in triplicate (a). Full non-adjusted images of WB shown in Fig. S1 (Fig. S1.pdf). The band density was calculated by using Image J (b). Aβ1-40/42 concentrations in mouse brain homogenates from Nutra II treated and untreated 5XFAD mice were also determined by ELISA (c). Aβ plaques in hippocampal (H), entorhinal cortex (EC) and retrosplenial cortex (RSC) was examined by immunohistochemistry staining using 4G8 (d).
Fig. 8.
Nutra II reduces levels of total and phosphorylated tau in 5XFAD mice - After Nutra II treatment, 5XFAD mice were sacrificed for analysis of phosphorylated tau, total tau and β-actin as control in brain homogenates using WB analysis. Untreated 5XFAD and WT mice were also sacrificed as controls. Representative WB shows PHF1, as determined by p-tau231, total tau, as determined by tau46, and β-actin as determined by β-actin specific antibodies in duplicate (WT) or triplicate (5XFAD/Ctrl and 5XFAD/Nutra II, a). Full non-adjusted images of WB shown in Fig. S2 (Fig. S2.pdf). PHF1 immunoreactivity was increased in 5XFAD mice in comparison with WT mice and PHF1 and total tau immunoreactivities were reduced after Nutra II treatment in 5XFAD mice in comparison with untreated 5XFAD mice (a, upper panel). Representative WB shows phospho-tau (AT270), as determined by p-tau181, and β-actin in Nutra II treated and untreated 5XFAD mice in triplicate, confirming that Nutra II reduces phospho-tau in this AD mouse model (c). The bands density were calculated using Image J (b & d).
3.3. Mitochondrial function
In order to determine if Nutra II could improve mitochondrial function in the brains of 5XFAD mice, brain mitochondria were isolated from WT and untreated and Nutra II-treated 5XFAD mice. The respiratory rates (indexed by State III − V), ROS production, mitochondrial membrane potential (MMP) and mitochondrial ATP levels were determined. 5XFAD mice exhibited increased brain ROS levels, which was accompanied by significant mitochondrial membrane hyperpolarization (Table 1). Nutra II reversed these elevations of ROS and MMP hyperpolarization, while elevating ATP and respiratory rates. Therefore, the improved locomotor and cognitive performance observed in 5XFAD mice after Nutra II treatment is likely related to elevated brain ATP levels and respiratory rates, together with reduced ROS levels.
Table 1.
Mitochondrial function in WT and 5XFAD mice fed normal or Nutra II containing chow.
| WT | 5XFAD | 5XFAD + Nutra II | |
|---|---|---|---|
| ATP | 1.00 ± 0.32 | 1.20 ± 0.20 | 1.39 ± 0.37 |
| ROS | 1.00 ± 0.36 | 1.23 ± 0.53 | 1.02 ± 0.35 |
| State III | 1.00 ± 0.12 | 1.33 ± 0.11 | 1.34 ± 0.34 |
| State IV | 1.00 ± 0.44 | 1.49 ± 0.49 | 2.01 ± 1.16 |
| State V | 1.00 ± 0.20 | 1.23 ± 0.32 | 1.64 ± 0.72 |
| State III/IV | 1.00 ± 0.12 | 0.89 ± 0.07 | 0.55 ± 0.19*† |
| State V/IV | 1.00 ± 0.19 | 0.82 ± 0.21 | 0.63 ± 0.13* |
| Pol | 5.20 ± 1.34 | 19.63 ± 7.66* | 12.24 ± 6.64 |
| Depol | 1.00 ± 0.18 | 1.76 ± 0.46* | 1.32 ± 0.44 |
| Pol/Depol | 1.00 ± 0.20 | 2.58 ± 0.55* | 2.05 ± 0.88 |
ATP equals adenosine triphosphate, ROS equals reactive oxygen species, States III, IV and V equal respiratory states and Pol and Depol equals mitochondrial polarization and depolarization, respectively. The data was analyzed in two groups with different values for control WT mice between groups, particularly for RCR. Since the relative values were similar between groups after normalization to the WT mice (controls), the data were combined after normalization to the corresponding WT mice, except for Pol which was normalized to mean WT Depol value. RCR values for the WT mice (State III/IV and V/IV) were 16.43 ± 1.51 and 16.43 ± 2.43 for Group 1 and 4.84 ± 0.40 and 3.94 ± 0.53 for Group 2, respectively. RCR values in untreated 5XFAD mice were similar to WT mice, but in the Nutra II treated 5XFAD mice RCR values decreased to 6.50 ± 0.72 and 9.18 ± 1.31 for Group 1 and 3.45 ± 0.28 and 2.72 ± 0.15 for Group 2. Asterisk indicates P < 0.05 compared with WT. Dagger indicates P < 0.05 compared with 5XFAD untreated (Mean ± SD).
4. Discussion
In the present study, Nutra II, recently developed as a nutraceutical combination of NT-020 and PQQ, improved cognitive function in 5XFAD transgenic mice, as determined by Morris water maze, Y maze and fear conditioning tests. In addition, Nutra II improved motor coordination, locomotor activity and exploratory activity as determined by rotorod, open field and elevated plus maze tests. The ability of Nutra II to improve motor coordination was also confirmed in our studies by hind limb clasping observation (data not shown). These behavioral improvements were paralleled with a significantly enhanced accumulation of sAPPα, slight decreases of Aβ/β-CTF production and amyloid plaques formation, and a significantly reduced accumulation of total and phosphorylated tau in 5XFAD mouse brain. Nutra II also increased mitochondrial function in the brain, as evidenced by elevations of ATP levels and maximal respiratory rates, together with reduced ROS accumulation and mitochondrial membrane hyperpolarization. Together these results indicate that Nutra II has beneficial effects on multiple therapeutic targets in the brain, improving mitochondrial function, reducing tau accumulation and enhancing nonamyloidogenic sAPPα production, thereby improving motor ability and cognitive functions.
Previous epidemiological studies show that diets rich in colorful fruits and vegetables that are high in polyphenols or flavonoids may reduce the risk of developing neurodegenerative diseases, such as cognitive impairment, dementia, Parkinson disease, or Alzheimer disease [12, 13, 14, 15]. Functionally impaired 20 mo old Fisher rats orally treated with NT020, a proprietary formulation of blueberry, green tea extract, carnosine, and vitamin D3, exhibited reduced cognitive impairment, measured by the Morris water maze task, compared with that of age-matched untreated rats [17]. In addition, NT020 reduced microglial activation, while increasing cell proliferation and neurogenesis, in the dentiate gyrus (DG) and subventricular zone (SVZ) of the hippocampus in the aged rats, two known stem cell niches in the brain. Therefore, NT020 can reduce cognitive impairment, possibly by exerting anti-inflammatory and neurogenic actions related to enhanced proliferation of neural stem cells. In vitro studies indicate that NT020 stimulates the proliferation of human stem cells derived from bone marrow, bone marrow-derived CD34+ cells and CD133+ progenitor cells derived from peripheral blood [16]. NT020 also reduced the oxidative stress-induced apoptosis of microglia cells and neurons in vitro and cultured bone marrow cells removed from mice given NT020 orally for 2 weeks exhibited a dose-related reduction of oxidative stress induced cell death. Therefore, the beneficial actions of NT020 are due in part to enhanced stem cell proliferation and survival in the face of oxidative stress.
Since AD presents with a more advanced stage of cognitive impairment compared with aging alone, we aimed to enhance the beneficial action of NT020 for the treatment of AD by the addition of PQQ. PQQ has been proposed as an important redox catalyst, growth factor and antioxidant defense in bacteria, plants and animals [21, 32]. PQQ is considered to be an important nutrient having anti-inflammatory, antioxidative and neuroprotective effects [21, 22]. In animals models, PQQ attenuates neuronal cell death associated with stroke, spinal cord injury and traumatic brain injury [33, 34], by decreasing iNOs expression, protecting NMDA receptor function, decreasing lesion or infarct size and increasing axon density [35, 36]. In neural stem and progenitor cells, PQQ increases the antioxidant enzymes SOD, catalase and glutathione peroxidase [37]. PQQ can protect human neuroblastoma SH-SY5Y cells against Aβ-induced neurotoxicity [38] and prevent α-synuclein amyloid fibril formation. PQQ can also increase mitochondrial biogenesis and function, potentially by activation of PGC-1α, ras, DJ-1, Nrf2 and ERK signaling, increasing mitochondrial gene expression and reducing mitochondrial ROS levels [21, 22, 39, 40]. However, it is unlikely that PQQ acts by increasing PGC-1α levels alone, since overexpression of PGC-1α exacerbates amyloid and tau deposition in AD mice [41]. In the present study, a loss of mitochondrial function in 5XFAD mice was evidenced primarily by mitochondrial membrane hyperpolarization, which can be due to enhanced glucose metabolism. Nutra II reversed this membrane hyperpolarization, in part by increasing mitochondrial inner membrane permeability to protons, evidenced by increased state IV respiration and reduced state III/IV and V/IV ratios. This uncoupling potentially reduced ROS production since there normally is a direct relationship between ROS production and membrane potential. In a previous study [10], EGCG and luteolin restored mitochondrial function in APPPS1 mice at 9.5 mo of age by reducing mitochondrial ROS levels and elevating ATP levels, RCR and membrane potential. The major difference between these studies is that ATP levels, RCR and membrane potential were reduced in the APPPS1 mice but not reduced in the present study, possibly reflecting a less advanced stage of AD.
In conclusion, Nutra II is proposed as an effective treatment for AD, reducing cognitive and locomotor impairment in the 5XFAD mouse model. These improvements can be due, in part, to enhanced sAPPα levels, reduced tauopathy and reduced mitochondrial oxidative stress. In the 5XFAD mouse model, mitochondria-dependent oxidative stress can lead to a loss of mitochondrial function and induce tau pathology, which in turn impairs cognitive and motor ability. In addition, the treatment with Nutra II was well tolerated. Since Nutra II is composed of natural ingredients, it should be considered a safe and effective treatment for AD in humans. Further studies should determine the effectiveness of Nutra II for reducing cognitive and locomotor impairment while reducing AD-associated pathology and mitochondrial dysfunction at various advanced stages of AD using various doses and feeding regimens. In addition, future studies should assess the effects of other compounds which enhance mitochondrial function on AD pathology and behavioral impairment, in order to confirm the importance of mitochondrial functional restoration in AD treatment.
Declarations
Author contribution statement
Darrell Sawmiller: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Song Li: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Takashi Mori, Vedad Delic: Performed the experiments; Analyzed and interpreted the data.
Ahsan Habib: Performed the experiments.
David Rongo: Wrote the paper.
Patrick Bradshaw, R Douglas Shytle: Conceived and designed the experiments.
Cyndy Sanberg, Paula Bickford: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Jun Tan: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the National Institutes of Health (NIH)/NCCIH (R01AT007411, J.T.); NIH/NIA (R21AG049477, J.T.); and the Silver Endowment (USF). J.T. holds the Sliver Chair in Developmental Neurobiology. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Competing interest statement
The authors declare the following conflict of interests: Cyndy Sanberg is a paid employee of Saneron CCEL Therapeutics.
Additional information
No additional information is available for this paper.
Acknowledgements
The assistance of the following individuals in performing mitochondrial function assays is greatly appreciated: Sandra Zivkovic, Tam-Anh Phan, Crupa Kurien, Stephen Bell, Christian Reynes, Yumeng Zhang and Vinh Ben Dinh.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Smith C.D. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc. Natl. Acad. Sci. U S A. 1991;88(23):10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goedert M. Tau protein and the neurofibrillary pathology of Alzheimer's disease. Ann. N Y Acad. Sci. 1996;777:121–131. doi: 10.1111/j.1749-6632.1996.tb34410.x. [DOI] [PubMed] [Google Scholar]
- 3.Golde T.E., Eckman C.B., Younkin S.G. Biochemical detection of Abeta isoforms: implications for pathogenesis, diagnosis, and treatment of Alzheimer's disease. Biochim. Biophys. Acta. 2000;1502(1):172–187. doi: 10.1016/s0925-4439(00)00043-0. [DOI] [PubMed] [Google Scholar]
- 4.Selkoe D.J. The role of APP processing and trafficking pathways in the formation of amyloid beta-protein. Ann. N Y Acad. Sci. 1996;777:57–64. doi: 10.1111/j.1749-6632.1996.tb34401.x. [DOI] [PubMed] [Google Scholar]
- 5.Casley C.S. Beta-amyloid inhibits integrated mitochondrial respiration and key enzyme activities. J. Neurochem. 2002;80(1):91–100. doi: 10.1046/j.0022-3042.2001.00681.x. [DOI] [PubMed] [Google Scholar]
- 6.Chang S. Lipid- and receptor-binding regions of apolipoprotein E4 fragments act in concert to cause mitochondrial dysfunction and neurotoxicity. Proc. Natl. Acad. Sci. U S A. 2005;102(51):18694–18699. doi: 10.1073/pnas.0508254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y., Zhao B. Oxidative stress and the pathogenesis of Alzheimer's disease. Oxid. Med. Cell. Longev. 2013;2013 doi: 10.1155/2013/316523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 9.Moreira P.I. Mitochondrial dysfunction is a trigger of Alzheimer's disease pathophysiology. Biochim. Biophys. Acta. 2010;1802(1):2–10. doi: 10.1016/j.bbadis.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Dragicevic N. Green tea epigallocatechin-3-gallate (EGCG) and other flavonoids reduce Alzheimer's amyloid-induced mitochondrial dysfunction. J. Alzheimers Dis. 2011;26(3):507–521. doi: 10.3233/JAD-2011-101629. [DOI] [PubMed] [Google Scholar]
- 11.Rezai-Zadeh K. Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008;1214:177–187. doi: 10.1016/j.brainres.2008.02.107. [DOI] [PubMed] [Google Scholar]
- 12.Rezai-Zadeh K. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J. Neurosci. 2005;25(38):8807–8814. doi: 10.1523/JNEUROSCI.1521-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joseph J.A., Shukitt-Hale B., Casadesus G. Reversing the deleterious effects of aging on neuronal communication and behavior: beneficial properties of fruit polyphenolic compounds. Am. J. Clin. Nutr. 2005;81(Suppl. 1):313S–316S. doi: 10.1093/ajcn/81.1.313S. [DOI] [PubMed] [Google Scholar]
- 14.Arts I.C., Hollman P.C. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005;81(Suppl. 1):317S–325S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 15.Darvesh A.S. Oxidative stress and Alzheimer's disease: dietary polyphenols as potential therapeutic agents. Expert Rev. Neurother. 2010;10(5):729–745. doi: 10.1586/ern.10.42. [DOI] [PubMed] [Google Scholar]
- 16.Bickford P.C. Nutraceuticals synergistically promote proliferation of human stem cells. Stem Cells Dev. 2006;15(1):118–123. doi: 10.1089/scd.2006.15.118. [DOI] [PubMed] [Google Scholar]
- 17.Acosta S. NT-020, a natural therapeutic approach to optimize spatial memory performance and increase neural progenitor cell proliferation and decrease inflammation in the aged rat. Rejuvenation Res. 2010;13(5):581–588. doi: 10.1089/rej.2009.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Small B.J. Nutraceutical intervention improves older adults' cognitive functioning. Rejuvenation Res. 2014;17(1):27–32. doi: 10.1089/rej.2013.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bachstetter A.D. Spirulina promotes stem cell genesis and protects against LPS induced declines in neural stem cell proliferation. PLoS One. 2010;5(5) doi: 10.1371/journal.pone.0010496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oakley H. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J. Neurosci. 2006;26(40):10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rucker R., Chowanadisai W., Nakano M. Potential physiological importance of pyrroloquinoline quinone. Altern. Med. Rev. 2009;14(3):268–277. [PubMed] [Google Scholar]
- 22.Misra H.S., Rajpurohit Y.S., Khairnar N.P. Pyrroloquinoline-quinone and its versatile roles in biological processes. J. Biosci. 2012;37(2):313–325. doi: 10.1007/s12038-012-9195-5. [DOI] [PubMed] [Google Scholar]
- 23.Rae E.A., Brown R.E. The problem of genotype and sex differences in life expectancy in transgenic AD mice. Neurosci. Biobehav. Rev. 2015;57:238–251. doi: 10.1016/j.neubiorev.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Buchman A.S., Bennett D.A. Loss of motor function in preclinical Alzheimer's disease. Expert. Rev. Neurother. 2011;11(5):665–676. doi: 10.1586/ern.11.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jawhar S. Motor deficits, neuron loss, and reduced anxiety coinciding with axonal degeneration and intraneuronal Abeta aggregation in the 5XFAD mouse model of Alzheimer's disease. Neurobiol. Aging. 2012;33(1):e29–e40. doi: 10.1016/j.neurobiolaging.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 26.Wang C. Free radical scavenging activity and neuroprotective potentials of D138, one Cu(II)/Zn(II) Schiff-base complex derived from N,N'-bis(2-hydroxynaphthylmethylidene)-1,3-propanediamine. Neurochem. Res. 2014;39(9):1834–1844. doi: 10.1007/s11064-014-1392-1. [DOI] [PubMed] [Google Scholar]
- 27.Li S. Swedish mutant APP-based BACE1 binding site peptide reduces APP beta-cleavage and cerebral Abeta levels in Alzheimer's mice. Sci. Rep. 2015;5 doi: 10.1038/srep11322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers J.T. CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J. Neurosci. 2011;31(45):16241–16250. doi: 10.1523/JNEUROSCI.3667-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dragicevic N. Mitochondrial amyloid-beta levels are associated with the extent of mitochondrial dysfunction in different brain regions and the degree of cognitive impairment in Alzheimer's transgenic mice. J. Alzheimers Dis. 2010;20(Suppl. 2):S535–S550. doi: 10.3233/JAD-2010-100342. [DOI] [PubMed] [Google Scholar]
- 30.Brown M.R., Geddes J.W., Sullivan P.G. B rain region-specific, age-related, alterations in mitochondrial responses to elevated calcium. J. Bioenerg. Biomembr. 2004;36(4):401–406. doi: 10.1023/B:JOBB.0000041775.10388.23. [DOI] [PubMed] [Google Scholar]
- 31.Mori T. Tannic Acid Is a Natural beta-Secretase Inhibitor That Prevents Cognitive Impairment and Mitigates Alzheimer-like Pathology in Transgenic Mice. J. Biol. Chem. 2012;287(9):6912–6927. doi: 10.1074/jbc.M111.294025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stites T.E., Mitchell A.E., Rucker R.B. Physiological importance of quinoenzymes and the O-quinone family of cofactors. J. Nutr. 2000;130(4):719–727. doi: 10.1093/jn/130.4.719. [DOI] [PubMed] [Google Scholar]
- 33.Jensen F.E. The putative essential nutrient pyrroloquinoline quinone is neuroprotective in a rodent model of hypoxic/ischemic brain injury. Neuroscience. 1994;62(2):399–406. doi: 10.1016/0306-4522(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L. The neuroprotective effect of pyrroloquinoline quinone on traumatic brain injury. J. Neurotrauma. 2012;29(5):851–864. doi: 10.1089/neu.2011.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aizenman E. Interaction of the putative essential nutrient pyrroloquinoline quinone with the N-methyl-D-aspartate receptor redox modulatory site. J. Neurosci. 1992;12(6):2362–2369. doi: 10.1523/JNEUROSCI.12-06-02362.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirakawa A. Pyrroloquinoline quinone attenuates iNOS gene expression in the injured spinal cord. Biochem. Biophys. Res. Commun. 2009;378(2):308–312. doi: 10.1016/j.bbrc.2008.11.045. [DOI] [PubMed] [Google Scholar]
- 37.Guan S. Pyrroloquinoline quinone against glutamate-induced neurotoxicity in cultured neural stem and progenitor cells. Int. J. Dev. Neurosci. 2015;42:37–45. doi: 10.1016/j.ijdevneu.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J.J., Zhang R.F., Meng X.K. Protective effect of pyrroloquinoline quinone against Abeta-induced neurotoxicity in human neuroblastoma SH-SY5Y cells. Neurosci. Lett. 2009;464(3):165–169. doi: 10.1016/j.neulet.2009.08.037. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Q. Neuroprotective effects of pyrroloquinoline quinone against rotenone injury in primary cultured midbrain neurons and in a rat model of Parkinson's disease. Neuropharmacology. 2016;108:238–251. doi: 10.1016/j.neuropharm.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Q. Involvement of ERK1/2 pathway in neuroprotective effects of pyrroloquinoline quinine against rotenone-induced SH-SY5Y cell injury. Neuroscience. 2014;270:183–191. doi: 10.1016/j.neuroscience.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 41.Dumont M. PGC-1alpha overexpression exacerbates beta-amyloid and tau deposition in a transgenic mouse model of Alzheimer's disease. FASEB J. 2014;28(4):1745–1755. doi: 10.1096/fj.13-236331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.