Abstract
Although treatment with stem/progenitor cells is a promising approach to heart disease, enthusiasm for cell therapy has been dampened by the inconsistent, modest, borderline, or undetectable benefits reported in clinical trials (all of which have used one dose of cells)1,2,3,4. As a result, clinical translation has not occurred (no cell-based therapy is close to being approved for heart disease), and a rising tide of skepticism has bedeviled the field,5, 6 leading some critics even to question whether clinical studies should continue. Here I propose that a major reason for the modest, borderline, or disappointing results is the administration of only one dose of cells, which causes the benefits of cell therapy to be underestimated. Iargue that just as most pharmacologic agents are ineffective when given once but can be highly effective when given repeatedly, so a cell product may be ineffective, or modestly effective, when given as a single treatment, but may turn out to be quite efficacious if given repeatedly. This concept constitutes a major paradigm shift, with potentially vast implications for the entire field of reparative medicine.
Keywords: Stem cells, progenitor cells, cardiac repair, cardiac regeneration, cell therapy
Subject Terms: Cell Therapy, Stem Cells
Rationale for repeated cell administrations
The efficacy of cell therapyis limited by the poor engraftment of the cells, which disappear rapidly after transplantation. For example, following administration of c-kitPOS cardiac progenitor cells (CPCs) in mice, rats, and pigs, the number of cells remaining in the heart declines precipitously to very low values;1,2,7,8 e.g., in the mouse heart, <8% of the CPCs present immediately after transplantation remain 1 week later, and after 35 days this number falls to <3%.8 Despite this, administration of CPCs improves LV function and the improvement is long-lasting (at least 1 year).1,2,7,8 Rapid disappearance of transplanted cells has been observed with most, if not all, other cell types,1,2 indicating that poor engraftment is a universal problem and a major factor that limits the efficacy of essentially all types of cells tested heretofore. Although much effort has focused on enhancing cell engraftment via pharmacologic or genetic manipulations, the effects of these maneuverson cell retention have generally been less than impressive:no matter how cells are “preconditioned” or engineered, the vast majority do not persist in the heart.1,2,9,10 Consequently, our grouphaspursuedan alternative strategy: we have sought to overcome poor engraftment by administering repeated cell doses.11,12
Remarkably, this intuitive strategy had not been carefully tested. Almost all preclinical studies conducted to date have based their assessment of efficacy on the outcome of one cell administration. In the clinical arena, no trial has used multiple doses, which could be a reason for the borderline or disappointing results.1,3,4 It seems self-evident that since the myocardial content of transplanted cells declines rapidly after adoptive transfer, irrespective of which cell type is used,1,2,7,8,9,10,11,12,13 injecting a cell product only once cannot be an adequate test of the efficacy of that product. For the full therapeutic effects to become apparent, repeated doses are necessary to replace the cells that disappear.
Repeated cell administrations in rodents
Our results in rodents indicate that repeated cell therapy is much more effective than single–dose therapy.11,12 When rats with chronic ischemic cardiomyopathy (old myocardial infarction [MI]) were given three doses of c-kitPOS CPCs 35 days apart, each dose produced a similar increase in LV function, so that the total cumulative improvement was approximately triple that observed after one dose.11 The multiple-dose group also exhibited less fibrosis in the noninfarcted region. In a subsequent study in mice with old MI, we found that three doses of cardiac mesenchymal cells (MSCs), given 14 days apart, produced a significantly greater improvement in LV function and myocardial fibrosis in the noninfarcted region compared with one dose.12 Thus, the repeated-treatment paradigm is not restricted to c-kit POS CPCs or to rats,11 but applies to other cell types (CMCs) and species (mice).12 In both studies,11,12 engraftment of transplanted cells was minimal (myocytes attributable to differentiation of transplanted cells were <1% of the total myocyte population), indicating that the beneficial effects of repeated cell administrations were underlain by paracrine actions. Given that virtually all types of cells appear to promote cardiac repair via paracrine mechanisms,2,13 there is no obvious reason to postulate that c-kitPOS CPCs or CMCs should be unique in the requirement that multiple doses must be given for a full reparative effect to occur. It seems more likely that the observations made with these cells would be applicable to other cell types as well.
Conceptual framework of repeated cell administrations
Our studies11,12 suggest that repeated administrations of cells are more efficacious because they producerepetitive bursts of extracellular vesicle (EV) or other paracrine factor release, which results in cumulative paracrine actions. According to this conceptual framework (Figure), the 1st administration of cells results in a “spike” in myocardial cell content, which peaks within hours and then declines precipitously in the ensuing days (A). Transplanted cells impart their salubrious effects not by engrafting, but by releasing EVs (or other paracrine factors) into the surrounding tissue, with a burst of secretion immediately after transplantation followed by a sustained low-level release over the following weeks (B). Subsequent cell administrations result in analogous “spikes” in myocardial cell content (A) and bursts of EV release (B), each of which produces additional functional improvement in a cumulative fashion (C). It should be noted, however, that transplanted cells do not disappear completely (Fig. 1A). For example, small numbers of exogenous c-kitPOS CPCs persistas long as 1 year after transplantation.7 Furthermore, the number of c-kitPOS CPCs remaining in the heart at 35 days after the end of the treatment protocol was higher after three doses than after one dose, although the difference was not statistically significant.11 Whether the long-term persistence of these small numbers of cells is necessary for the beneficial functional effects to persist is unknown. It is also unknown whether the progressive buildup in the myocardial content of exogenous cells after multiple doses (solid red area in Fig. 1A) is important for the cumulative beneficial effects of repeated therapy and, if so, whether the mechanism involves a cumulative, sustained increase in EV releaseby exogenous cells that persist in the long term (solid blue area in Fig. 1B).
Figure 1. Conceptual paradigm of repeated cell therapy.
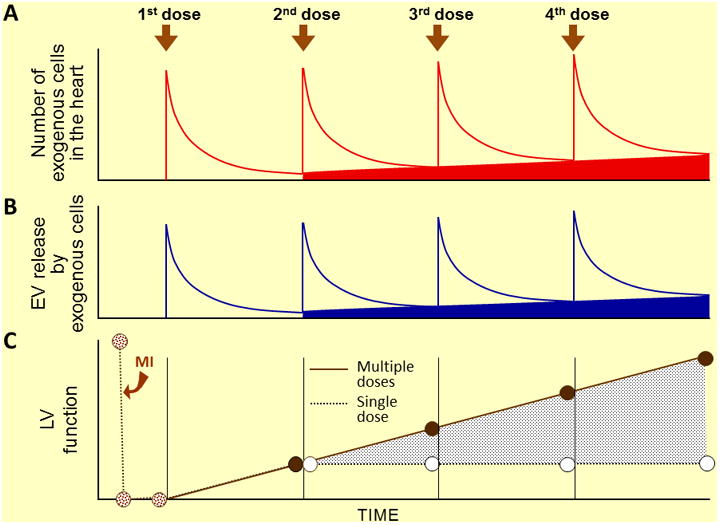
A. Following cell administration, the number of transplanted cells in the myocardium falls rapidly8 but a small number persist for many weeks (at least 1 year).7 Repeated cell doses produce a cumulative increase in the number of transplanted cells that persist long-term in the myocardium (red area).11,12 Each cell dose produces a burst of EV release (B), which is responsible for the cumulative therapeutic effects (C). The cumulative increase in long-lasting exogenous cells (red area) may be associated with a cumulative increase in sustained EV release by these cells (blue area). Panels A and C are based on experimental data11,12; panel B is speculative.
Why do we expect one dose of cells to be sufficient?
With rare exceptions, no one expects one dose of drugs to bring about the desired outcome; why, then, is one dose of cells expected to be enough, given that cells (like drugs) are cleared from the body? There are multiple reasons for this. When research on cardiac regeneration began, it was widely believed that transplanted cells would engraft and differentiate into cardiac cells; in this scenario, it seemed logical to assume that greater efficacy could be achieved simply by increasing the number of cells (more transplanted cells = more regenerated myocardium), with no need for repeated treatment. We now know that the vast majority of transplanted cells disappear quickly, regardless of the number that is administered1,2,7,8,9,10,11,12 Another reason is that administering more than one dose of cells to rodents is difficult because the stress of repeated thoracotomies is associated with prohibitively high mortality and because most Animal Committees would not approve such protocols. We have recently developed a new technique that enables repeated cell administrations to be performed percutaneously by advancing a needle into the LV cavity, without thoracotomy; this method is safe and effective.11,12 In human studies, the use of repeated treatments has been hindered by a multitude of regulatory issues primarily related to the novel nature of the product (cells) and the lack of preclinical experience. Now that boththe safety of cell transplantation and the ephemeralpresence of transplanted cells in the recipient heart are appreciated, we are well poised to abandon the somewhat irrational and naïve belief that one dose of cells is sufficient to achieve the desired effect.
Future directions
The superiority of repeated treatments over single treatments needs to be verified with cell types other than c-kitPOS CPCs and CMCs. Importantly, every effort should be made to translate the repeated-treatment paradigm to humans. For that to occur, many issues need to be elucidated. For example, does this paradigm apply to large animals? What arethe optimal number and frequency of cell doses? Can the therapeutic effects of multiple doses be recapitulated by a single, large dose containing an equivalent number of cells? In addition, the mechanism of action of repeated treatments needs to be clarified. It will be important to identify the paracrine factor (s) involved and to determine whether repeated injections of EVs mimic the effects of repeated cell injections.
Implications of the repeated-treatment paradigm
Despite being disarmingly simple, repeated dosing has the potential to be a disruptive advance that may fundamentally transform the entire field of cell therapy. If this concept is applicable to most cell types, the current paradigm of cell therapy would change dramatically, with far-reaching implications for both preclinical and clinical studies. The translational potential of this idea is enormous.
The notion that repeated cell administrations are markedly more effective than a single administration implies that after a single dose, the therapeutic benefits of cell therapy will be underestimated. However, over the past 15 years virtually all clinical trials and almost all preclinical investigations of cell therapy have used a single dose.1,2,3,4 If one dose is not sufficient to evaluate efficacy, then the conclusions of these studies, particularly those that have reported “negative” results, could be questioned because the benefits of the treatment may have been underestimated or even completely overlooked. Disquietingly, an entire body of literature (almost all studies conducted to date) may have to be reconsidered. Were previous investigations “negative” because the product did not work or because the treatment protocol was inadequate? How many times was a therapeutic effect missed because of the use of a single treatment? Is it possible that conclusions achieved heretofore regarding lack of efficacy of cell products were wrong? Has the “potency” of these products really been assessed?
Even more importantly, the concept that multiple cell doses are necessary for a full therapeutic effect to be manifest means that future studies should adopt protocols that incorporate repeated administrations. This is a major departure from current approaches in cell-based therapies, one that affects profoundly the design of preclinical and clinical studies alike. In both cases, protocols based on a single treatment should no longer be considered adequate to assess the therapeutic value of the cell product.
Conclusions
The rapid disappearance of transplanted cells from the host tissue is a major impediment to the success of cell therapy. Given that cells, like drugs, are cleared soon after administration, expecting one dose of cells to repair the heart is no more reasonable than expecting one dose of antibiotics to cure an infectious disease. The universal adherence to a single-dose paradigm may have led to significant underestimation of the therapeutic effects of most, if not all, cell types tested heretofore, and may be responsible for the borderline or disappointing results obtained in clinical trials.
Repeated dosing has the potential to be a disruptive advance that may revolutionize cell therapy. By replenishing cells with repeated treatments, the loss of transplanted cells can be alleviated in a manner that is simpler, more effective, more broadly applicable, more practical, and probably safer than the use of pharmacologic or genetic manipulations of the cell product. Repeated administration of cells is clinically relevant and eminently feasible in patients: once cells have been expanded, they can be frozen and stored for subsequent injection, which could be repeated at periodic intervals until the desired therapeutic effect is achieved. Given that single-dose cell therapy has proven to be very safe1,2,3,4 and that cells are cleared quickly, repeated doses are likely to be safe as well.
The field of cell therapy is at a crossroads. After almost two decades of intense efforts, conclusive evidence of benefit in patients with heart disease is still lacking. Clearly, the viability of this field will depend on whether therapeutic strategies are developed that are demonstrably and reproducibly effective. Shifting from the old single-dose paradigm of the past 20 years to a new, multiple-dose paradigm may be the criticalcatalyst that is needed to achieve this goal.
Acknowledgments
Sources of Funding: Supported by NIH Grants P01 HL078825 and UM1 HL113530.
Footnotes
Disclosures: None.
References
- 1.Sanganalmath SK, Bolli R. Cell therapy for heart failure: A comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–834. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keith MC, Bolli R. “String theory” of c-kit (pos) cardiac cells: A new paradigm regarding the nature of these cells that may reconcile apparently discrepant results. Circ Res. 2015;116:1216–1230. doi: 10.1161/CIRCRESAHA.116.305557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher SA, Doree C, Mathur A, Martin-Rendon E. Meta-analysis of cell therapy trials for patients with heart failure. Circ Res. 2015;116:1361–77. doi: 10.1161/CIRCRESAHA.116.304386. [DOI] [PubMed] [Google Scholar]
- 4.Gyöngyösi M, Wojakowski W, Lemarchand P, Lunde K, Tendera M, Bartunek J, et al. Meta-Analysis of Cell-based CaRdiacstUdiEs (ACCRUE) in patients with acute myocardial infarction based on individual patient data. Circ Res. 2015;116:1346–60. doi: 10.1161/CIRCRESAHA.116.304346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahara M, Santoro F, Chien KR. Programming and reprogramming a human heart cell. EMBO J. 2015;34:710–738. doi: 10.15252/embj.201490563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerbin KA, Murry CE. The winding road to regenerating the human heart. Cardiovasc Pathol. 2015;24:133–140. doi: 10.1016/j.carpath.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang XL, Li Q, Rokosh G, Sanganalmath SK, Chen N, Ou Q, Stowers H, Hunt G, Bolli R. Long-term outcome of administration of c-kit (pos) cardiac progenitor cells after acute myocardial infarction: Transplanted cells do not become cardiomyocytes, but structural and functional improvement and proliferation of endogenous cells persist for at least one year. Circ Res. 2016;118:1091–1105. doi: 10.1161/CIRCRESAHA.115.307647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong KU, Guo Y, Li QH, Cao P, Al-Maqtari T, Vajravelu BN, Du J, Book MJ, Zhu X, Nong Y, Bhatnagar A, Bolli R. C-kit+ cardiac stem cells alleviate post-myocardial infarction left ventricular dysfunction despite poor engraftment and negligible retention in the recipient heart. PLoS One. 2014;9:e96725. doi: 10.1371/journal.pone.0096725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai C, Guo Y, Teng L, Nong Y, Tan M, Book MJ, Zhu X, Wang XL, Du J, Wu WJ, Xie W, Hong KU, Li Q, Bolli R. Preconditioning human cardiac stem cells with an ho-1 inducer exerts beneficial effects after cell transplantation in the infarcted murine heart. Stem Cells. 2015;33:3596–3607. doi: 10.1002/stem.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li QH, Guo YR, Ou QH, Wu WJ, Zhu XP, Luo L, Li J, Bolli R. Expression of heme oxygenase-1 in cardiac stem cells enhances the efficacy of cell therapy for attenuating left ventricular dysfunction and remodeling after myocardial infarction. Circulation. 2012;126:A9534. [Google Scholar]
- 11.Tokita Y, Tang XL, Li Q, Wysoczynski M, Hong KU, Nakamura S, Wu W, Xie W, Li D, Hunt G, Ou Q, Stowers H, Bolli R. Repeated administrations of cardiac progenitor cells are markedly more effective than a single administration: A new paradigm in cell therapy. Circ Res. 2016 doi: 10.1161/CIRCRESAHA.116.308937. Epub 2016/07/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo Y, Wysoczynski M, Non Y, Tomlin A, Zhu X, Gumpert A, Nasr M, Muthusamy S, Li H, Book M, Khan A, Hong K, Li Q, Bolli R. Repeated doses of cardiac mesenchymal cells are therapeutically superior to a single dose in mice with old myocardial infarction. Basic Res Cardiol. doi: 10.1007/s00395-017-0606-5. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodgkinson CP, Bareja A, Gomez JA, Dzau VJ. Emerging concepts in paracrine mechanisms in regenerative cardiovascular medicine and biology. Circ Res. 2016;118:95–107. doi: 10.1161/CIRCRESAHA.115.305373. Epub 2016/02/04. [DOI] [PMC free article] [PubMed] [Google Scholar]


