Abstract
Bisphenol A (BPA) is an endocrine disrupting chemical that is ubiquitous in wild and built environments. Due to variability in study design, the disruptive effects of BPA have proven difficult to experimentally replicate. This study was designed to assess the disruptive actions of dietary BPA exposure, while carefully controlling for known confounders. Parental CD1 mice were acclimated to defined diet containing BPA (0.03, 0.3, 3, 30, or 300 ppm) or 17α-ethinyl estradiol (EE; 0.0001, 0.001, and 0.01 ppm) and bred to produce progeny (F1) that were maintained through adulthood on the same diet as the parents. In F1 females, uterine weights were increased in all EE and the 30-ppm BPA-exposure groups, demonstrating model sensitivity and estrogen-like actions of BPA. In BPA-exposed females, no treatment-related differences were observed in parental reproductive function, or in the timing of puberty and metabolic function in female offspring. In F1 males, modest changes in body weight, adiposity and glucose tolerance, consistent with improved metabolic function, were observed. Associated with increased prolactin and increased circulating testosterone levels, balanopreputial separation was accelerated by 0.03 and 3.0 ppm BPA and anogenital distance at postnatal day 21 was increased in males by 0.03 ppm BPA. Sperm counts were also increased with 3.0 ppm BPA exposures. Overall, BPA was found to have modest, sex specific endocrine disruptive effects on a variety of end points below the established no observed adverse effect level. The dose response characteristics for many of the effects were nonmonotonic and not predictable from high-dose extrapolations.
Keywords: Bisphenol A, diabetes, endocrine disruptor, estrogen, metabolic effects, obesity, prolactin
Introduction
Bisphenol A (BPA) metabolites are present in the urine of more than 90% of humans tested.1,2 First characterized as estrogenic in 1936, BPA has since become one of the highest volume industrial chemicals used worldwide.3,4 Monomeric BPA is now primarily used for the manufacture of polycarbonate plastics and epoxy resins. Although stable and inactive as a polymer, water-soluble monomeric BPA can leach from the polymer coatings of food and beverage packaging, polycarbonate plastics, and epoxy resins into consumed food and liquids, especially upon exposure to elevated temperature and acidic conditions.5–7 As a result, consumption of contaminated foods and beverages is considered the major source of human exposure to BPA. Although BPA is rapidly eliminated following phase 2 metabolism, humans are exposed throughout their entire life span to doses that might affect normal endocrine functions.8–10
Numerous experimental animal studies have assessed the effects of BPA exposure in vivo. Some of those studies found that BPA exposure was associated with alterations in reproductive development and function in both male and female rodents.11–13 However, the results of other studies showed minimal or no effects on reproductive end points.14,15 Further, the results from some animal studies suggest that pre-or perinatal BPA exposure was associated with weight gain or increased adipose mass.11,16,17 In contrast, no changes in metabolic parameters resulting from perinatal or whole-life BPA exposures were detected in other studies.14,15,18 Unfortunately, differences in experimental design have made it difficult to compare results across independent studies performed in different laboratories. The use of developmental or whole-life exposures, differing routes of administration, different species and strains, the presence or absence of phytoestrogen and/or xenoestrogen contaminants in food and water, and differences in end point analysis have contributed to significant intrastudy variability. This variability has resulted in an unclear picture of the magnitude of the harmful endocrine disrupting effects associated with BPA exposures. Drawing upon the high level of scrutiny leveled at past studies, the present study was designed to assess the effects of toxicological- and human-relevant oral BPA exposures in vivo, while removing uncontrolled sources of endocrine-disrupting chemicals (EDCs) and metabolic or endocrine confounders. To ensure that the endocrine-disrupting effects observed were caused by BPA without interference from uncontrolled contaminating EDCs, known sources of EDC contamination in the laboratory environment, including those in animal housing, drinking water, and diet were eliminated. The administration of BPA via oral consumption of diet was chosen to mimic the major route of exposure in humans. Similarly, exposure during critical periods of fetal, perinatal, and postnatal development and maturation allowed for ethological, circadian, and metabolic variations that occur during different stages of life similar to the variability in exposure believed to occur in human populations.19 At the cost of increasing experimental variability, the outbred CD1 mouse strain was used to include genetic variability of sensitivity, and to allow comparison with many previous murine studies of BPA exposure. A broad range of end points associated with reproductive development and function (e.g., onset of puberty, fertility and fecundity, and altered reproductiveorgan weights) and with metabolic function (body weight, composition, plasma lipid levels, and glucose tolerance) were assessed and compared. The results of this study demonstrated a high sensitivity for detection of estrogen-like activity in male and female CD1 mice while maintaining a human-relevant exposure paradigm at doses of BPA below the established no observed adverse effect level (NOAEL) and extending to levels that closely approximate the estimated human oral exposure levels.
Materials and Methods
Animals
All animal procedures were performed in accordance with protocols approved by the University of Cincinnati Institutional Animal Care and Use Committee and followed recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. Animals were maintained on a 14-hour light and 10-hour dark cycle. Diet and drinking water were provided ad libitum. Animals were housed in single-use polyethylene cages that are certified BPA-free (Innovive, San Diego, California) with Sani-chip bedding (Irradiated Aspen Sani-chip; PJ Murphy Forest Products Corp, Montville, New Jersey) to avoid mycoestrogen-contaminated corncob bedding.20 Sterile drinking water with oxidizable organics reduced to <1% of source was generated by a dedicated water purification system (Millipore Rios 16 with ELIX UV/Progard 2, Billerica, Massachusetts) and dispensed from polyethylene water bottles.
Exposure
Mice were maintained on assigned dietary exposure from arrival until necropsy. Food consumption was measured weekly. To eliminate phytoestrogens present in milled and soy-protein-based diets, animals were fed a defined phytoestrogen-free diet (Product #D1010501, Research Diets, Inc, New Brunswick, New Jersey) either unsupplemented (control) or supplemented with BPA (2,2-bis(4-hydroxyphe-nyl)propane; CAS No. 80-05-7; Lot 11909; USEPA/NIEHS standard) or 17α-ethinyl estradiol (EE; 1,3,5(10)-estratrien-17α-ethinyl-3,17β-diol; CAS No. 57-63-6; Batch No. H923; Steraloids Inc, Newport, Rhode Island) that was homogenously incorporated into chow at desired final concentrations (BPA: 0.03–300 ppm resulting in a dose range of 0.004–40 mg/kg/d; or EE: 0.0001–0.01 ppm, resulting in a dose range of 0.00002–0.001 mg/kg/d).21,22 Additionally, the metabolic energy of the diet was 3.63 kcal/g to eliminate uterotrophic effects resulting from high energy density.21,23 All nutritional details related to defined diet #D1010501 may be found in Kendziorski et al (2012).21
Breeding
A total of 410 (205 each sex) Hsd: ICR mice (CD1; designated the P0 generation) age 6 to 7 weeks were received from Harlan Laboratories, Inc (Indianapolis, Indiana). Males and females were assigned a coded study number and randomly divided equally among 9 exposure groups, and each group housed 5 per cage with the same sex for 2 weeks prior to mating. During this acclimation period, general health, body weight, food consumption, and vaginal cytology were monitored. Following acclimation, males and females were paired and single housed for breeding, and observed daily for copulation plug no more than 3 hours into the light-on cycle. Assessment of vaginal cytology was stopped upon observation of a copulation plug. Males were removed upon observation of a copulation plug or after 2 weeks without observed copulation. Females were then observed twice daily for any health-related outcomes, signs of pregnancy, and for parturition (designated postnatal day 0 or PND 0). Offspring (designated F1) were separated from dams at PND 21 and group housed by sex 5 per cage. In cases of more than 5 males or 5 females per litter, same sex littermates were evenly distributed between 2 cages. Soiled bedding from nonstudy males exposed to the same assigned diet was added to the F1 female cages biweekly to stimulate normal sexual maturation.
Sperm Quality
Sperm quality was assessed by calculating total sperm numbers and percentage motile sperm in P0 males following 6 to 8 weeks of exposure and in the F1 males at final necropsy (~PND 90). Right cauda epididymis was excised, weighed, and macerated in 0.5 mL Tris-buffered Ringer solution (pH 7.4) using sterile surgical scissors and then filtered through a 40 μm nylon strainer and diluted to a total volume of 6 mL in Tris-buffered Ringer solution. Progressively motile and nonmotile sperm were manually counted via microscopic examination with a hemocytometer by an observer blinded to treatment, with counts independently confirmed by a second blinded observer. All procedures were performed at 37°C.
Growth and Maturation
Body weights were measured weekly from arrival for P0 or from PND 1 for offspring. Beginning on PND 15, females were examined daily no later than 3 hours into the light-on cycle for vaginal opening (VO) as previously described.24 Progression of estrous cycles was monitored immediately following observation of VO by microscopic assessment of the cellular morphology from samples collected by daily vaginal lavage with saline.21 Beginning on PND 20, males were observed for bala-nopreputial separation (BPS). Anogenital distance (AGD) was measured as the distance from the superior edge of the external genitalia to the inferior edge of the anus by a handheld digital caliper precise to 0.03 mm (Fisherbrand Traceable Digital Caliper, Thermo Fisher Scientific, Inc, Waltham, Massachusetts).
Plasma Hormone and Phytoestrogen Levels
Steroid hormone levels were measured by high-performance liquid chromatography/tandem mass spectrometry (HPLC-MS/MS; NMS Labs, Willow Grove, Pennsylvania). At necropsy, endogenous 17β-estradiol was measured during estrus (detection limit 2 pg/mL) and the total testosterone was measured from adult males (detection limit 2 ng/dL). The plasma concentrations of phytoestrogens S-eqoul, daidzein, and genistein for F1 animals were measured by reverse phase HPLC-MS/MS with a detection limit of 1 ng/mL, as previously described.22,25
Plasma prolactin (PRL) was measured via an Nb2 lymphoma bioassay as previously described.26 Briefly, serum starved Nb2 cells were plated in 96-well plates (20 000 cells/ well) and incubated with human PRL (National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Maryland) in triplicate, or a plasma aliquot from submandibu-lar vein collection at PND 36 in duplicate. After 3 days, cell numbers were determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method. The amount of PRL in plasma was calculated from a standard curve, with a detection limit of 0.5 ng/mL.
Necropsy
Necropsy was performed after approximately 14 weeks on dietary exposures for P0 mice (~120–140 days old), and beginning on PND 90 for the F1 offspring. Approximately half of the animals were euthanized by CO2 asphyxiation and perfused via cardiac puncture into the left ventricle with 10% neutral-buffered formalin (Polysciences, Inc, Warrington, Pennsylvania). Tissues were postfixed with multiple changes of 10% neutral-buffered formalin or phosphate-buffered 5% paraformaldehyde and then embedded in paraffin for histological analysis. The remaining animals were euthanized by rapid cervical dislocation. Tissues were rapidly excised and weighed after being cleaned of excess connective tissue and fat, and blotted free of excess fluids. Prostates were dissected away from seminal vesicles and bladder, leaving ventral, lateral, dorsal, and anterior lobes attached to the urethra. Blood was collected from the ascending vena cava using a heparinized syringe and plasma separated in polypropylene tubes by centrifugation at 1000g. Plasma was eluted and both fractions were stored at −80°C.
Glucose Tolerance Test
Glucose tolerance tests were performed after a 14-hour fast. Blood glucose was determined with a handheld glucometer (Truetrack by Nipro Diagnostics, Inc, Fort Lauderdale, Florida). Blood from the saphenous vein (5 μL) was applied directly to the glucose strip to measure fasting levels of blood glucose. Glucose (1.5 mg glucose/g body weight) was immediately administered by intraperitoneal (ip) injection with blood glucose determinations made at 15, 30, 60, and 120 minutes postinjection.
Plasma Lipids
Plasma lipids were determined in samples collected from the saphenous vein of 14-hr fasted mice by the University of Cincinnati Mouse Metabolic Phenotyping Core using individual colorimetric assays. Triglycerides were measured using the glycerol phosphate oxidase—4-chlorophenol, 4-aminophenazone peroxidase method (Randox Laboratories, Ltd, Crumlin, Co Antrim, United Kingdom), phospholipids were measured by the choline oxidase—N-ethyl-N-(2-hydroxy-3-sulfopropyl)-3,5-dimethoxyaniline sodium salt method (Code No. 433-36201; Wako Diagnostics, Richmond, Virginia), nonesterified fatty acids were measured using the acyl-CoA synthetase—acyl-CoA oxidase method (Code No. 999-34691; Wako Diagnostics), and total cholesterol was measured using the Thermo Scientific Infinity Cholesterol assay (Cat. No. TR13421; Fisher Diagnostics, Fisher Scientific Company, LLC, Middletown, Virginia).
Body Composition
Total body composition was assessed in live, unanesthetized mice by nuclear magnetic resonance (NMR; EchoMRI; Echo Medical Systems, Houston, Texas) at the University of Cincinnati Mouse Metabolic Phenotyping Center supported by NIH grant DK059630 (http://mousephenotype.uc.edu/UC/). Total body analysis revealed absolute amounts of total fat tissue, lean tissue, and water. Individuals were measured in duplicate, and values were considered acceptable if they differed from one another by less than 10%. The proportion of perigonadal fat mass in relation to whole body fat mass (percentage perigonadal fat) was calculated as an indication of abdominal adipose deposition.
Statistical Analysis
All measures and end point assessments were made by an observer blinded to treatments, litter, and sex when appropriate. The statistical unit used was the litter or breeding pair for all analyses. For end points from F1 animals, the mean value for each sex within the litter was determined and used for analysis. All statistical analyses for differences in values compared to control were made independently for BPA and EE.27 Variation due to litter size was accounted for by considering the number of pups in a litter as a covariate in analysis of covariance for body weight. To avoid the influence of extreme litter size on end point sensitivity, litters with less than 6 pups were excluded from analysis of effects in offspring.28
In cases where variances among groups were unequal, data were transformed by taking a natural log (ln) of each data point after which ln-transformed data were analyzed. Outliers were identified using Grubb’s test. Analysis of weight data was performed using one-way analysis of variance followed by Dunnett Multiple Comparison post hoc test with GraphPad Prism v5 software (GraphPad Prism, GraphPad Software, Inc, La Jolla, California), or when appropriate, by analysis of covariance using SyStat 13 (Systat Software, Inc, Chicago, Illinois). Analysis of timed data was performed using the Kaplan-Meier method and estimates of food consumption per mouse per day were analyzed by linear regression (GraphPad Prism). Unless otherwise noted, data are presented as mean ± standard deviation (SD). A minimal level of statistical significance for differences in values among or between groups was considered P < 0.05.
Results
Food Consumption and Exposure
The mass of food consumed was measured for P0 females while they were housed individually during gestation and for the F1 offspring after weaning. From food consumption data, the daily-caloric intake and exposure dosage were calculated (Supplementary Table 1). Previous study revealed that CD1 mice were extremely averse to consuming chow containing 0.1 ppm EE.21 Here, female P0 mice in the 0.01-ppm EE-exposure group consumed fewer calories per day compared to those in the control group (P < 0.05). Caloric intake was significantly greater in F1 males of the 30-ppm BPA and 0.0001-ppm EE groups (P < 0.005). Female offspring showed no dose-related differences in caloric intake. The absence of exposures to potentially estrogenic isoflavones was confirmed for each exposure group with no detectable S-eqoul, daidzein, and genistein present in serum (data not shown).
Across exposure groups, the daily intake of BPA was below the defined NOAEL of 50 mg/kg/d29 in the 300-ppm BPA group (40.0 ± 14.2 mg/kg/d), approximated the established reference dose of 50 μg/kg/d30 for the 0.3-ppm BPA group (43.89 ± 17.22 μg/kg/d), and extended into a low-dose range of 4.11 ± 1.67 μg/kg/d for the 0.03-ppm BPA group. Circulating BPA concentrations were not measured because numerous well-designed studies have defined the pharmacokinetic profile of oral BPA.8,9,31,32 Extrapolation from previous results indicates that circulating aglycone BPA in 3-ppm BPA group would reach approximately 0.5 ng/mL (~2 × 10−9 mol/L). Human data suggest that circulating concentrations of total BPA range from >1.3 to 5.7 × 10−9 mol/L, with aglycone BPA estimated at 1000-fold less than total BPA.19,32 These estimates indicate that the range of doses tested here reflects the upper limit of normal human exposures.6
Fertility and Fecundity
Based on previously published findings demonstrating that consumption of diets containing 0.1 and 1.3 ppm EE resulted in complete loss of fecundity in CD1 mice,21 0.01 ppm EE was chosen as the high control-dose for estrogen-like effects. Across the dose ranges of BPA and EE, no dose-related changes in fertility or fecundity were observed. There were no differences in mating index, days to copulation, fertility index, or gestation length for any of the BPA- or EE-exposure groups. In all exposure groups, the litter size and proportion of males per litter were similar and did not deviate from expected normal values for the CD1 strain (Supplementary Table 2).
Body Weights
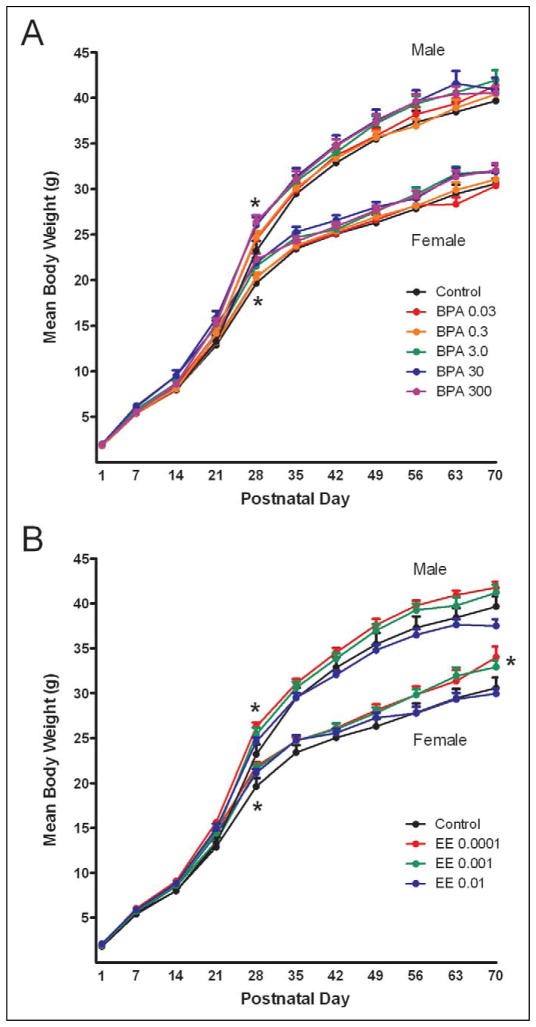
Repeated measures analysis of covariance indicated a significant effect of diet and PND on body weight for both females and males (P < 0.0001; Figure 1). The covariate effect of litter size on body weight was significant from PND 1 through PND 35 for females (P < 0.01) and from PND 1 through PND 42 for males (P < 0.02). No differences in body weights were observed in either males or females at PND 1. In females, analysis of covariance for individual PNDs indicated a significant difference at PND 28, where the 3.0-, 30-, and 300-ppm BPA and the 0.0001-ppm EE group weights were significantly greater than control (P < 0.0001). After PND 35, analysis of variance indicated a significant effect of exposure on body weight at PND 70 with the mean weight of females in the 0.0001-ppm EE group significantly increased (P ≤ 0.018).
Figure 1.
Assessment of F1 body weights from PND 1 to PND 70 with BPA (A) or EE (B) exposure (ppm). Body weights were collected weekly from PND 1 until end of study for all F1 mice. A significant increase in body weight for 3.0, 30, and 300 ppm BPA-exposed groups (P < 0.0001) and 0.0001 ppm EE (P < 0.0001)-exposed groups was detected in comparison to control at PND 28 for both males and females. Females exposed to 0.0001 ppm EE weighed significantly more than controls at PND 70 (P = 0.018). For all time points n > 12 litters; for PND 1 to 53, n > 15 litters. Data are presented as group mean ± SEM. *Indicates a significant effect of exposure by ANCOVA (Females PND 1–35 and males PND 1–42) or by ANOVA (P < 0.05). PND indicates postnatal day; BPA, Bisphenol A; EE, 17α-ethinyl estradiol; ANCOVA, analysis of covariance; ANOVA, analysis of variance; SEM, standard error of the mean.
For males, analysis of differences on individual PNDs showed a significant effect of exposure at PND 28. The mean weights in the 3.0-, 30-, and 300-ppm BPA and 0.0001-ppm EE groups were increased compared to control (P < 0.0001). No differences were observed in male body weights after PND 28, nor were there any differences in total body fat.
Organ Weights
The mean liver weight of the females in the 0.3, 3.0, or 30 ppm BPA- and all EE-exposure groups were significantly less than those of controls (P < 0.002). No differences were observed in the weights of the perigonadal fat pad, cardiac fat pad, kidney, spleen, heart, or brain (Table 1). In males, there were no dose-related differences in the weights of liver, kidney, spleen, heart, brain, testis, or prostate. However, there was a significant increase in the weight of the cardiac fat pad with 30 ppm BPA and 0.001 ppm EE exposure, and a decrease in perigonadal fat pad weight with 3.0 ppm BPA and 0.01 ppm EE exposures (P < 0.02; Table 2).
Table 1.
F1 Female Organ Weights (g).
| Control
|
Bisphenol A
|
17α-Ethinyl Estradiol
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.0 ppm | 0.03 ppm | 0.3 ppm | 3.0 ppm | 30 ppm | 300 ppm | 0.0001 ppm | 0.001 ppm | 0.01 ppm | |
| P. Fat | 1.08 ± 0.52 (15) | 1.06 ± 0.47 (15) | 1.02 ± 0.52 (17) | 0.95 ± 0.34 (18) | 1.12 ± 0.42 (14) | 0.92 ± 0.25 (16) | 1.12 ± 0.41 (16) | 0.94 ± 0.33 (17) | 0.86 ± 0.35 (16) |
| C. Fat | 0.09 ± 0.03 (14) | 0.08 ± 0.02 (16) | 0.09 ± 0.01 (16) | 0.09 ± 0.02 (18) | 0.10 ± 0.02 (14) | 0.09 ± 0.02 (16) | 0.09 ± 0.02 (16) | 0.09 ± 0.01 (16) | 0.08 ± 0.02 (16) |
| Liver | 1.70 ± 0.50 (10) | 1.62 ± 0.25 (10) | 1.56 ± 0.36a (15) | 1.58 ± 0.33a (17) | 1.51 ± 0.21a (12) | 1.73 ± 0.29 (16) | 1.53 ± 0.29a (15) | 1.65 ± 0.29a (15) | 1.44 ± 0.20a (14) |
| Kidney | 0.19 ± 0.03 (10) | 0.18 ± 0.02 (10) | 0.18 ± 0.03 (15) | 0.19 ± 0.03 (17) | 0.19 ± 0.02 (12) | 0.19 ± 0.02 (16) | 0.19 ± 0.03 (15) | 0.18 ± 0.02 (15) | 0.18 ± 0.03 (13) |
| Spleen | 0.13 ± 0.05 (10) | 0.11 ± 0.02 (10) | 0.12 ± 0.03 (15) | 0.13 ± 0.04 (16) | 0.12 ± 0.03 (12) | 0.13 ± 0.03 (16) | 0.11 ± 0.02 (15) | 0.12 ± 0.02 (15) | 0.12 ± 0.01 (13) |
| Heart | 0.18 ± 0.02 (10) | 0.18 ± 0.01 (10) | 0.17 ± 0.03 (15) | 0.18 ± 0.03 (17) | 0.19 ± 0.03 (12) | 0.17 ± 0.02 (16) | 0.18 ± 0.02 (15) | 0.18 ± 0.02 (15) | 0.18 ± 0.02 (14) |
| Brain | 0.51 ± 0.01 (11) | 0.51 ± 0.02 (15) | 0.51 ± 0.04 (13) | 0.52 ± 0.02 (12) | 0.51 ± 0.04 (12) | 0.52 ± 0.02 (13) | 0.51 ± 0.02 (13) | 0.51 ± 0.02 (14) | 0.51 ± 0.02 (9) |
| Uterus | 0.14 ± 0.02 (12) | 0.17 ± 0.05 (12) | 0.14 ± 0.04 (17) | 0.17 ± 0.05 (15) | 0.18 ± 0.06 (15) | 0.16 ± 0.05 (16) | 0.18 ± 0.05a (15) | 0.18 ± 0.05a (16) | 0.20 ± 0.04a (15) |
Abbreviations: P. fat, perigonadal fat pad; C. fat, cardiac fat pad; ANCOVA, analysis of covariance; ANOVA, analysis of variance; SD, standard deviation.
Note: Values represent group means ± SD (n).
Significantly different from control by ANCOVA or ANOVA where appropriate (P < 0.05).
Table 2.
F1 Male Organ Weights (g).
| Control
|
Bisphenol A
|
17α-Ethinyl Estradiol
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.0 ppm | 0.03 ppm | 0.3 ppm | 3.0 ppm | 30 ppm | 300 ppm | 0.0001 ppm | 0.001 ppm | 0.01 ppm | |
| P. Fat | 0.95 ± 0.39 (16) | 0.83 ± 0.27 (14) | 1.19 ± 0.48 (15) | 0.65 ± 0.20a (17) | 0.94 ± 0.56 (12) | 0.63 ± 0.19 (15) | 0.81 ± 0.25 (16) | 0.73 ± 0.24 (17) | 0.56 ± 0.27a (16) |
| C. Fat | 0.08 ± 0.02 (15) | 0.08 ± 0.02 (15) | 0.09 ± 0.02 (13) | 0.08 ± 0.02 (16) | 0.11 ± 0.04a (12) | 0.08 ± 0.03 (15) | 0.09 ± 0.01 (15)) | 0.10 ± 0.03b (18 | 0.08 ± 0.02 (16) |
| Liver | 2.26 ± 0.34 (16) | 2.24 ± 0.35 (14) | 2.30 ± 0.20 (13) | 2.23 ± 0.36 (16) | 2.23 ± 0.26 (10) | 2.21 ± 0.31 (11) | 2.47 ± 0.26 (15) | 2.23 ± 0.16 (13) | 2.13 ± 0.22 (12) |
| Kidney | 0.29 ± 0.04 (16) | 0.30 ± 0.03 (15) | 0.30 ± 0.05 (14) | 0.31 ± 0.03 (16) | 0.31 ± 0.04 (11) | 0.29 ± 0.04 (12) | 0.31 ± 0.03 (16) | 0.30 ± 0.02 (15) | 0.29 ± 0.02 (14) |
| Spleen | 0.12 ± 0.03 (16) | 0.12 ± 0.02 (15) | 0.11 ± 0.03 (13) | 0.13 ± 0.02 (16) | 0.13 ± 0.03 (11) | 0.11 ± 0.02 (12) | 0.12 ± 0.02 (16) | 0.12 ± 0.02 (15) | 0.13 ± 0.03 (14) |
| Heart | 0.23 ± 0.03 (17) | 0.23 ± 0.03 (14) | 0.24 ± 0.03 (14) | 0.23 ± 0.02 (16) | 0.26 ± 0.04 (11) | 0.25 ± 0.03 (12) | 0.24 ± 0.02 (15) | 0.24 ± 0.01 (14) | 0.23 ± 0.03 (14) |
| Brain | 0.51 ± 0.03 (15) | 0.52 ± 0.02 (11) | 0.51 ± 0.02 (13) | 0.52 ± 0.02 (13) | 0.50 ± 0.04 (6) | 0.51 ± 0.02 (13) | 0.53 ± 0.02 (14) | 0.51 ± 0.01 (13) | 0.51 ± 0.02 (11) |
| Testis | 0.13 ± 0.02 (16) | 0.13 ± 0.01 (13) | 0.13 ± 0.01 (14) | 0.14 ± 0.01 (16) | 0.13 ± 0.01 (12) | 0.14 ± 0.02 (11) | 0.14 ± 0.02 (16) | 0.13 ± 0.02 (14) | 0.13 ± 0.02 (13) |
| Prostate | 0.15 ± 0.04 (15) | 0.15 ± 0.02 (11) | 0.14 ± 0.03 (13) | 0.16 ± 0.02 (14) | 0.14 ± 0.02 (6) | 0.16 ± 0.02 (14) | 0.16 ± 0.02 (14) | 0.15 ± 0.02 (11) | 0.14 ± 0.02 (10) |
| Epid | 0.02 ± 0.00 (10) | 0.02 ± 0.00 (9) | 0.02 ± 0.01 (7) | 0.02 ± 0.00 (11) | 0.02 ± 0.00 (8) | 0.02 ± 0.00 (9) | 0.02 ± 0.00 (10) | 0.02 ± 0.00 (9) | 0.02 ± 0.00 (9) |
Abbreviations: P. fat, perigonadal fat pad; C. fat, cardiac fat pad; Epid, cauda epididymis; ANCOVA, analysis of covariance; ANOVA, analysis of variance; SD, standard deviation.
Note: Values represent group means ± SD (n).
Significantly different from control by ANCOVA or ANOVA where appropriate (P < 0.05).
Reproductive Maturity
Overall, the timing of VO and first cornification was not altered by exposure (Table 3). It is notable, however, that for 3 litters in the 0.3-ppm BPA exposure group the average time to VO was exceptionally early, occurring before PND 16. Balanopreputial separation was significantly accelerated in males in the 0.03 and 3.0-ppm BPA exposure groups and in the 0.001-ppm EE exposure groups (P < 0.02), with 50% of males progressing through BPS by PND 27.4, 27.8, and 27.4, respectively. In contrast, 50% of control males progressed through BPS on PND 29.4 (Table 3).
Table 3.
Reproductive Maturation and Anogenital Distance.
| Control
|
Bisphenol A
|
17α-Ethinyl Estradiol
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.0 ppm | 0.03 ppm | 0.3 ppm | 3.0 ppm | 30 ppm | 300 ppm | 0.0001 ppm | 0.001 ppm | 0.01 ppm | ||
| BPS | 29.5 ± 1.9 | 27.6 ± 0.8a | 28.4 ± 1.5 | 27.8 ± 0.8 a | 28.1 ± 0.6 | 28.1 ± 0.6 | 28.2 ± 0.6 | 27.2 ± 1.4a | 28.5 ± 0.8 | |
| VO | 25.5 ± 2.7 | 26.1 ± 2.6 | 26.9 ± 2.2 | 26.9 ± 2.3 | 26.1 ± 2.2 | 26.2 ± 2.8 | 26.7 ± 3.0 | 26.5 ± 1.7 | 25.8 ± 1.4 | |
| Cornification | 29.7 ± 3.0 | 30.5 ± 2.1 | 31.1 ± 2.4 | 30.1 ± 3.2 | 30.5 ± 1.1 | 30.1 ± 1.7 | 31.0 ± 3.3 | 29.9 ± 2.3 | 29.8 ± 2.3 | |
| AGD (mm) | ||||||||||
| PND 14 | F | 5.34 ± 0.52 | 5.42 ± 0.43 | 5.65 ± 0.72 | 5.66 ± 0.72 | 5.99 ± 0.61 | 5.89 ± 0.72 | 5.84 ± 0.63 | 5.63 ± 0.59 | 5.88 ± 0.60 |
| M | 6.86 ± 0.56 | 7.13 ± 0.60 | 6.92 ± 0.69 | 7.16 ± 0.79 | 7.44 ± 0.63 | 7.43 ± 0.70 | 7.45 ± 0.52 | 7.07 ± 0.46 | 7.53 ± 0.79 | |
| PND 21 | F | 7.31 ± 0.94 | 7.65 ± 0.54 | 7.39 ± 0.86 | 7.44 ± 0.57 | 7.80 ± 0.52 | 7.69 ± 0.53 | 8.00 ± 5.89 | 7.81 ± 0.69 | 7.74 ± 0.75 |
| M | 10.24 ± 1.29 | 11.27 ± 0.91a | 10.88 ± 1.01 | 11.12 ± 0.96 | 11.36 ± 1.14a | 11.48 ± 0.99a | 11.57 ± 0.96a | 11.44 ± 1.17a | 11.19 ± 1.57 | |
Abbreviations: BPS, postnatal day of balanopreputial separation; VO, postnatal day of vaginal opening; cornification, postnatal day of first vaginal cornification; AGD, anogenital distance; ANCOVA, analysis of covariance; ANOVA, analysis of variance; SD, standard deviation.
Note: Values represent group mean ± SD (n ≥ 16 litters).
Significantly different from control by Kaplan-Meier (BPS, VO, and cornification) or ANOVA (AGD) analysis (P < 0.05).
Metabolic End Points
Plasma lipids and body composition were determined to identify exposure-induced effects on lipid metabolism and storage (Table 4). While differences in body weight were not detected, the proportion of perigonadal adipose mass (an indicator of abdominal adipose accumulation) was significantly less in males exposed to BPA 3.0 and 300 ppm and in 0.0001 and 0.001 ppm EE (Table 4; P < 0.02). No differences were observed for body composition or proportion of perigonadal adipose mass in females.
Table 4.
Metabolic Changes in Response to BPA and EE Exposure.
| Control
|
Bisphenol A
|
17α-Ethinyl Estradiol
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.0 ppm | 0.03 ppm | 0.3 ppm | 3.0 ppm | 30 ppm | 300 ppm | 0.0001 ppm | 0.001 ppm | 0.01 ppm | ||
| Minimum sample size | F | 5 | 6 | 8 | 9 | 6 | 8 | 10 | 10 | 8 |
| M | 9 | 8 | 8 | 8 | 7 | 9 | 10 | 6 | 7 | |
| Triglycerides | F | 138.4 ± 44.4 | 187.4 ± 23.6 | 155.3 ± 38.6 | 164.9 ± 36.4 | 164.6 ± 45.1 | 138.1 ± 34.4 | 121.1 ± 27.8 | 153.5 ± 43.7 | 153.9 ± 45.4 |
| M | 108.4 ± 55.5 | 155.4 ± 68.5 | 131.5 ± 37.9 | 194.6 ± 36.3a | 159.2 ± 60.8 | 148.0 ± 44.6 | 113.6 ± 66.4 | 162.2 ± 37.7 | 115.7 ± 35.7 | |
| Total cholesterol | F | 136.7 ± 42.5 | 124.2 ± 33.2 | 123.9 ± 45.2 | 118.8 ± 26.0 | 139.0 ± 48.5 | 124.5 ± 25.1 | 121.9 ± 33.1 | 133.8 ± 26.1 | 144.9 ± 50.3 |
| M | 144.1 ± 46.4 | 170.0 ± 35.8 | 146.4 ± 37.7 | 176.2 ± 27.8 | 189.7 ± 35.9a | 175.6 ± 44.8 | 161.3 ± 27.5 | 187.7 ± 39.4a | 165.0 ± 37.1 | |
| Phospholipids | F | 194.4 ± 53.0 | 196.8 ± 42.0 | 178.9 ± 44.1 | 171.0 ± 25.1 | 213.3 ± 55.5 | 197.9 ± 36.8 | 173.3 ± 29.9 | 202.8 ± 47.2 | 224.1 ± 35.5 |
| M | 210.9 ± 49.6 | 264.5 ± 52.6a | 200.5 ± 21.7 | 238.1 ± 20.4 | 272.5 ± 41.6a | 278.5 ± 48.6a | 234.3 ± 46.3 | 295.2 ± 35.9a | 268.1 ± 46.8 | |
| NEFA | F | 1.5 ± 0.3 | 2.2 ± 0.5a | 1.9 ± 0.3 | 2.0 ± 0.2 | 2.0 ± 0.4 | 1.8 ± 0.3 | 1.9 ± 0.4 | 1.8 ± 0.3 | 1.4 ± 0.3 |
| M | 1.4 ± 0.5 | 1.9 ± 0.3a | 1.8 ± 0.4a | 1.9 ± 0.1a | 1.8 ± 0.3a | 1.7 ± 0.3 | 1.4 ± 0.4 | 1.6 ± 0.1 | 1.2 ± 0.3 | |
| % Body fat | F | 12.0 ± 5.6 | 14.3 ± 3.4 | 13.8 ± 4.6 | 13.7 ± 5.7 | 15.9 ± 7.5 | 13.4 ± 4.4 | 13.5 ± 6.2 | 15.3 ± 5.0 | 13.8 ± 3.4 |
| M | 10.4 ± 5.1 | 9.8 ± 2.4 | 16.1 ± 5.4 | 11.4 ± 5.3 | 12.0 ± 6.9 | 11.5 ± 2.1 | 12.8 ± 4.5 | 14.6 ± 5.3 | 8.9 ± 3.1 | |
| % Perigonadal fat | F | 24.2 ± 5.6 | 28.5 ± 4.5 | 28.4 ± 8.4 | 21.4 ± 10.2 | 25.9 ± 10.3 | 29.0 ± 6.4 | 26.9 ± 4.7 | 21.6 ± 5.6 | 22.8 ± 5.9 |
| M | 26.7 ± 11.6 | 28.6 ± 7.1 | 23.1 ± 10.3 | 13.5 ± 3.7a | 17.3 ± 6.4 | 15.8 ± 2.7a | 16.3 ± 4.4a | 14.8 ± 2.3a | 18.9 ± 4.6 | |
Triglycerides, Total Cholesterol and Phospholipids measured in mg/dl; NEFA - non-esterified fatty acids (mEq/L).
% Body fat (g/g) = fat mass (from NMR; g) / Body wt (g) × 100.
% Perigonadal Fat (g/g) = perigonadal fat mass (g) / total body fat mass (from NMR; g) × 100.
Significantly different from Control by ANOVA (p < 0.05) (n≥6 individuals).
Non-esterified fatty acids were significantly elevated in females from the 0.03-ppm BPA group and in males exposed to 0.03, 0.3, 3.0, and 30 ppm BPA (P < 0.05). Males had elevated triglycerides in the 3.0-ppm BPA exposure group (P ≤ 0.024), elevated cholesterol at 30-ppm BPA and 0.001 ppm EE (P < 0.05), and elevated PLs with exposure to 0.03, 30 and 300 ppm BPA and 0.001 ppm EE (P < 0.008).
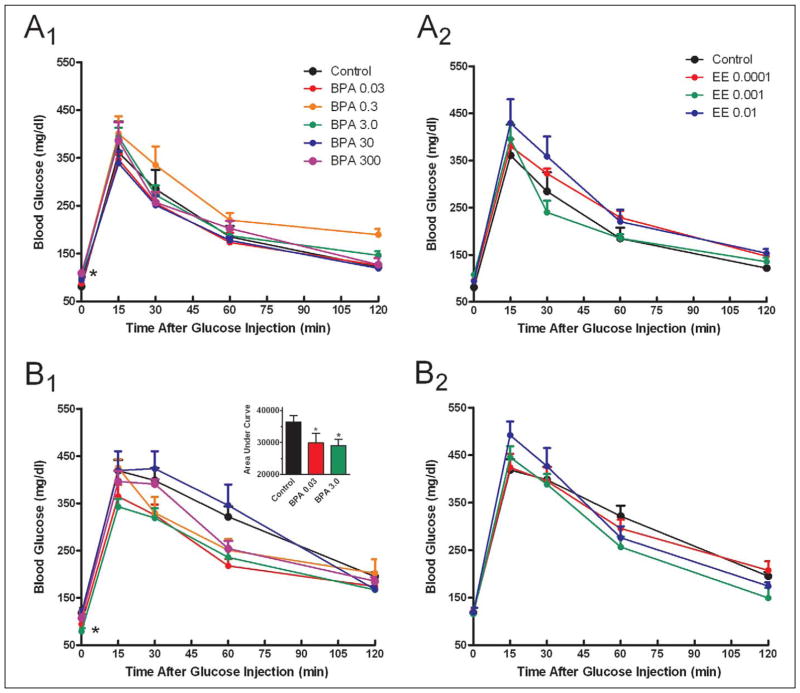
A significant increase in fasting blood glucose was observed in females exposed to 300 ppm BPA (Figure 2A). Males showed the opposite effect in that fasting blood glucose was decreased in the 3.0-ppm BPA group, and the area under the curve for glucose tolerance testing was significantly less than controls for the 0.03-and 3-ppm BPA groups (Figure 2B; 2B1 inset; P < 0.05).
Figure 2.
Assessment of glucose tolerance. Glucose tolerance tests were performed using an ip injection of 1.5 mg/kg glucose in females (A1, A2) and males (B1, B2) after a 14-hour fasting period with either BPA (A1, B1) or EE (A2, B2) exposure. Females exposed to 300 ppm BPA had elevated fasting blood glucose compared with control, while males exposed to 3 ppm BPA had decreased fasting blood glucose (P < 0.05). Area under the curve was significantly decreased in males exposed to 0.03 and 3.0 ppm BPA compared to control (B1 inset; P < 0.05). Data indicate group mean ± SEM. For all exposures n ≥ 6. * indicates a significant difference from control by ANOVA (P < 0.05). BPA indicates Bisphenol A; EE, 17α-ethinyl estradiol; SEM, standard error of the mean.
Hormone-Sensitive Tissues
Sperm quality
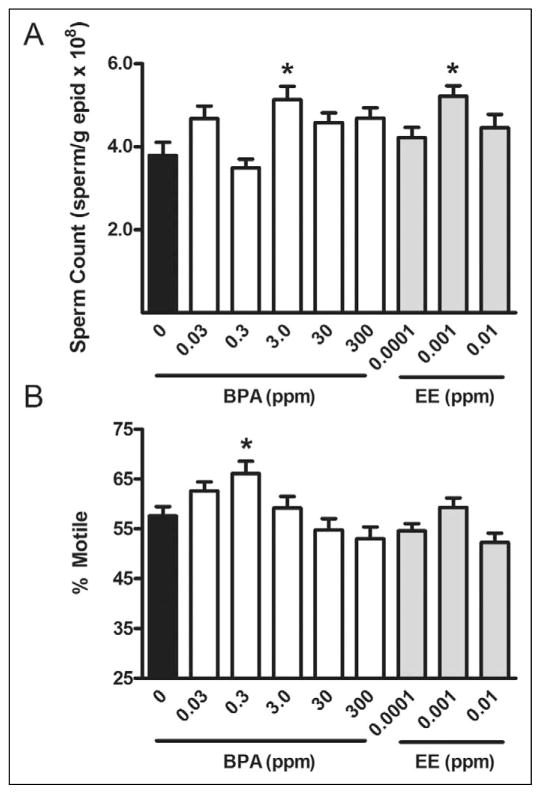
At the time of necropsy, sperm counts in the P0 0.03- and 0.3-ppm BPA exposure groups were approximately 23% higher than in the control group (P < 0.0001; Figure 3). No changes were observed in P0 sperm counts or motility for any other doses of BPA or EE. In F1 males, the total number of sperm for the 3.0-ppm BPA exposure group and 0.001-ppm EE exposure groups were also increased compared to control (P < 0.011), with sperm motility (% motile) increased in the 0.3-ppm BPA group (P ≤ 0.0022; Figure 3). There was no association between sperm counts and testis weights, nor any dose-related effect on cauda epididymis weight (Table 2).
Figure 3.
Assessment of sperm quality in F1 males. Sperm count normalized to epididymal weight (A) and % motile sperm (B) were analyzed as markers of sperm quality in F1 males. Total sperm counts were higher in 3.0 ppm BPA-exposed and 0.001 ppm EE-exposed mice (P < 0.011), while the percentage of motile sperm was increased in the 0.3 ppm BPA-exposure group compared to control (P = 0.0022). Values represent group mean ± SEM. For all exposures, n ≥ 8. *Indicates significant difference from control by ANOVA (P < 0.05). BPA indicates Bisphenol A; EE, 17α-ethinyl estradiol; ANOVA, analysis of variance; SEM, standard error of the mean.
Uterotrophic response
Uterine weights were significantly greater in all three EE-exposure groups compared with control (P ≤ 0.0051; Table 1). BPA also increased uterine weight in the 30-ppm exposure group (P ≤ 0.0136). In the 3.0-ppm BPA group, an increase in uterine weight that approached significance was also observed (P = 0.0750). There were no differences in plasma estradiol in control or BPA-exposed females during estrus (data not shown).
Anogenital distance
An increase in AGD was observed in males from the 0.03-, 30-, and 300-ppm BPA and 0.0001- and 0.001-ppm EE exposure groups at PND 21 (P < 0.05). Female AGD was not altered by any dietary exposure (Table 3).
Hormone Levels in Males
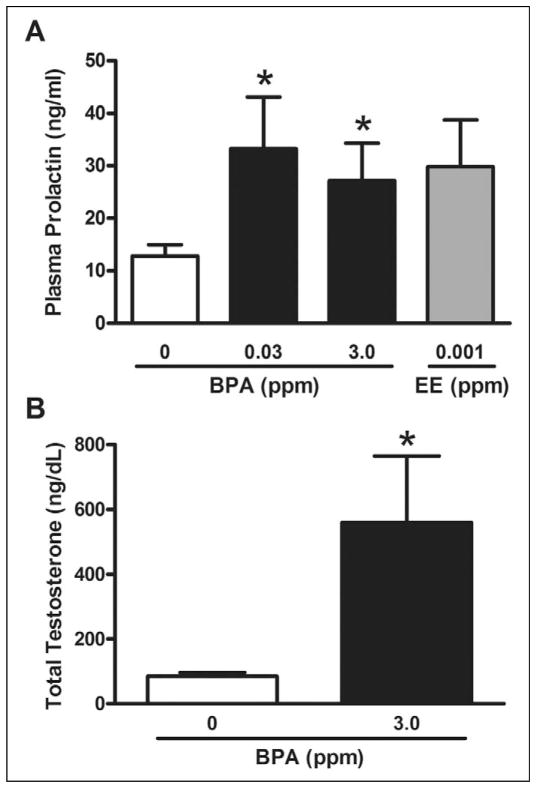
Given the association between hyperprolactinemia and accelerated puberty in males,33 plasma PRL was assayed at PND 36 for control and exposure groups with early BPS (Figure 4A). A significant increase in plasma PRL was observed in groups exposed to 0.03 and 3.0 ppm BPA (P < 0.05), with mean difference for the 0.001-ppm EE group approaching significance (P = 0.078). Mean circulating PRL in affected BPA groups ranged from 13 ng/mL to greater than 75 ng/mL, while control levels ranged from approximately 3 to 20 ng/mL. Because the increased sperm counts in the 3.0 BPA-exposed males could result from increases in androgens, we measured serum androgen levels. Total testosterone was found to be approximately 6.8-fold greater in the 3.0-ppm BPA exposure group compared to control (P ≤ 0.015; Figure 4B).
Figure 4.
Assessment of circulating concentrations of (A) prolactin (ng/mL) at PND 36 and (B) total testosterone (ng/dL) at PND 70 to 85 in F1 males. Prolactin was significantly elevated compared to controls in 0.03 and 3.0 ppm BPA groups (P < 0.05) and approached significance in the 0.001 ppm EE (P = 0.078). Total testosterone was significantly elevated with 3.0 ppm BPA exposure (P = 0.015), with *indicating significantly different from control by t test (P < 0.05). PND indicates postnatal day; BPA, Bisphenol A; EE, 17α-ethinyl estradiol.
Discussion
Bisphenol A is an estrogen-like EDC able to mimic or antagonize the normal function of endogenous estrogens both in vitro and in vivo. Independent replication of consistently reproducible endocrine-disrupting actions however, has proven difficult in experimental animals. While all studies have limitations, studies investigating BPA actions are deeply scrutinized and have been criticized as flawed for numerous limitations including the use of inappropriate animal models, the lack of a positive control for estrogenic activity, laboratory contamination, limited statistical power, and using exposure paradigms that do not reflect human exposure. Drawing upon the knowledge gained from past studies, the experimental design used here attempted to control for known variables and confounders that were previously identified as experimental limitations of past studies. By assessing a human relevant exposure paradigm and controlling for environmental factors known to influence results of EDC studies, it was possible to assess the endocrine disrupting effects of BPA exposure in male and female CD1 mice with decreased variability and increased sensitivity. For example, the estrogen-responsive uterotrophic effects observed in females with dietary 30 ppm BPA and 0.0001 to 0.01 ppm EE is a clear indication that exposure to these chemicals can elicit an estrogen-like response in females.34 The observed increase in uterine weight also indicates that exposure to BPA at doses 100-fold below the previously defined NOAEL29 induces an estrogen-like response in the uterus of CD1 mice.
Reproductive effects of BPA in primagravida CD1 females were previously observed only at concentrations of BPA above the highest dose used in this study.12,15,21 In agreement with those previous studies, no effects on fecundity, latency to mating, or duration of pregnancy were observed in dams exposed to BPA or EE. Additionally, there were no indications of exposure-related affects upon litter size or sex ratio. As a whole, those results, and those of previous studies, constitute reproducible and compelling evidence that exposures to low doses of BPA or EE below 0.01 ppm do not alter fecundity in exposed dams, nor sex determination of the resulting offspring.
In male offspring, exposure to both BPA and EE caused androgen-like phenotypic responses in perinatal AGD and early appearance of an external indication of puberty onset (Table 3). Those findings suggest that BPA and EE exposures result in increased testosterone production early in life.35,36 While the idea that estrogens cause increased androgen-like effects may appear paradoxical, it is well established that hyperprolactinemia can cause a prolonged increase in testicular testosterone production causing an increase in circulating testosterone levels.37 Because estrogens and BPA directly stimulate PRL secretion by rapid signaling mechanisms and also increase PRL release by reducing dopamine’s inhibitory effects on the anterior pituitary,37–39 we determined whether androgen-like effects were associated with increased levels of circulating PRL at puberty (PND 36) and increased testosterone in adults (~PND 90) from affected EE (PRL: 0.001 ppm EE) and BPA (PRL: 0.03 and 3.0 ppm BPA; T: 3.0 ppm BPA) exposure groups (Figure 4). Increased PRL was found concurrent with early reproductive maturation, and testosterone was significantly elevated in the 3.0 ppm BPA-exposed males. These findings support the hypothesis that BPA and EE have estrogen-like effects on pituitary release of PRL resulting in an associated elevation of testosterone levels that could cause the observed increase in male AGD and an early onset of puberty. Increased sperm counts were also observed in the affected 3.0 ppm BPA-exposure group, a finding also suggestive of increased testicular testosterone. Those findings conflict with interpretations from a human study suggesting that high levels of BPA were associated with decreased semen quality,40 however, other studies failed to find any evidence of an association between BPA exposures and decreased semen quality41,42
Bisphenol A has also been described as an “obesogen” able to increase adipose mass and disrupt metabolic function.16 Increased abdominal adipose deposition has been associated with the loss of glucose tolerance and is a risk factor for developing metabolic disease.43 No effect on weight or adiposity was detected in females, and the detected decrease in male abdominal adiposity with BPA exposure (Table 4) contrasts with some previous studies that suggest BPA increases adiposity.16,17 Similarly, other dietary exposure studies have failed to detect increases in adiposity as a result of BPA exposure,15,18 though additional metabolic effects might be expected in older animals.44 The evidence for early life, male-specific BPA influences on metabolic responses was further supported by the observed improvement in glucose tolerance and lower baseline fasting blood glucose in BPA-exposed males. The effects of BPA on insulin secretion and tolerance were not measured, however, leaving open the possibility that the improvement in glucose tolerance could result from compensatory hyperinsulinemia, which can lead to the development of noninsulin-dependent diabetes mellitus or metabolic syndrome as previously reported after 6 months in mice.44 The observed changes in this phenotype appear to be a decrease in the degree of sexual dimorphism between males and females, with males apparently becoming more female like in their responses to glucose challenge (Figure 2). A previous study demonstrated that early life exposure to BPA did not affect glucose tolerance in either males or females when exposed prenatally to BPA.18 This difference is considered likely to reflect differences in exposure paradigm and suggests that the observed difference in glucose tolerance in males was at least in part due to the presence of BPA concurrent with the glucose tolerance testing.
In humans, an association has been identified between higher urinary BPA concentrations and metabolic diseases; though no causative evidence exists connecting BPA and any metabolic disease.45 Here an elevation in plasma lipids by BPA and EE exposure in males indicates a modest disruption of lipid metabolism without altering total adiposity (Table 4). Both BPA and EE were, however, found to alter the distribution of adipose to subcutaneous rather than abdominal stores in male mice, suggesting that the males treated with BPA became more female-like in their fat distribution as well as glucose tolerance. However, sexually dimorphic differences in lipid metabolism were not affected in a similar manner. Although the metabolic differences between males and females are the result of complex developmental and homeostatic influences in multiple tissues, the results of this study suggest that BPA acts in an estrogen-like manner to affect metabolic function and adipose deposition in males while having minimal effects in females.
The high sensitivity of hormone systems, differences in the expression of receptors, and interactions with other hormones can result in nonmonotonic dose response relationships for endocrine-sensitive end points.46,47 Some toxicological assessments of BPA actions have disregarded the low-dose/ nonmonotonic effects as “spurious” and irrelevant even though they are likely the result of endocrine disruption. In this study, a number of effects were observed at both low and high doses of BPA, while others appear to follow classical sigmoidal log concentration–response relationships. Anogenital distance and sperm counts appeared to follow a U-shaped dose response with significant effects observed at very low and high doses, and no significant effect at an intermediate dose of BPA (Table 3, Figure 3). Whereas changes in organ weights and sperm motility in males appear to follow an inverted U-shaped dose response in that only doses in the middle of the range, but not the high or low ends, caused significant differences (Tables 1 and 2, Figure 3). It is well established that estrogens act through multiple receptors, which in some cases have opposing effects and also act via direct nuclear receptor activity and by rapid signaling mechanisms, and that the concerted actions of those mechanisms contribute to the observed concentration-dependent effect.48 For example, rapid signaling effects of 17β-estradiol, BPA, and other environmental estrogens in neurons and cardiac myocytes are characterized by nonmonotonic dose response relationships in vivo and in vitro.2,49 Those effects arise by the activation of opposing signaling mechanisms mediated by multiple receptor systems that regulate counterbalancing stimulatory and inhibitory actions. Such responses are highly replicable and represent the complexity of endogenous hormone regulation and endocrine disruption. It is likely that greater complexity is associated with many physiological responses and that nonmonotonic response are common for many, if not most, complex physiological phenotypes related to endocrine disruption.
Further, interactions within and across hormone systems, such as the hypothalamic–pituitary–gonadal (HPG) axis, can contribute to resulting phenotypes and increase the complexity associated with interpretation of EDC effects. In some cases, it will be difficult to predict the complex systemic interactions of hormones responsible for a given result because physiological responses and phenotypes are regulated by the interplay of a myriad of hormonal and developmental signals. For example, here it was found that an androgen-like phenotypic effect was likely mediated by BPA and EE exposures causing an increase in PRL secretion and an associated increase in testosterone levels. Those findings highlight the value of examining the effects of a wide range of doses on a variety of interrelated end points.
In conclusion, BPA exposure below the previously established NOAEL for rodents had a variety of detectable disruptive actions on hormonally regulated phenotypes. Responses to BPA generally followed nonmonotonic dose-response relationships with efficacious doses spanning 10- to 100-fold concentration range. We interpret the nonmonotonic dose-response relationships to have resulted from multiple levels of molecular regulation, including negative feedback regulation, tissue- and sex-specific effects, and the differential effects of multiple receptor subtypes. All of these regulatory mechanisms play critical roles in coordinating development and the physiological processes regulated by hormones. In males, a paradoxical androgen-like response, possibly due to a BPA-induced elevation in PRL, was observed for some end points, while for other end points males demonstrate a more female-like phenotype in response to BPA exposure, suggesting a combination of organizational effects and direct hormone disruption. All responses in males can be explained by agonist or partial-agonist activities of BPA action via estrogen-like modes of action. Combined with the uterotrophic response observed in females orally exposed to 30 ppm BPA (5.5 mg/kg/d), the results of this study indicate clearly that dietary BPA exposure is able to disrupt endogenous estrogen activity in CD1 mice.
Supplementary Material
Supplementary Table 1. Estimated Caloric Intake and Dosage
Supplementary Table 2. Measures of Fertility and Fecundity
Acknowledgments
The authors thank Dr Kenneth D. Setchell for analysis of phytoestrogen content in plasma; Dr Randy J. Seeley for assistance with metabolic end points; and Dr Heather B. Patisaul for valuable discussion and critical review of the manuscript.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health [R01 ES015145, RC2 ES018765, T32 ES016646].
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005;113(4):391–395. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zsarnovszky A, Le HH, Wang HS, Belcher SM. Ontogeny of rapid estrogen-mediated extracellular signal-regulated kinase signaling in the rat cerebellar cortex: potent nongenomic agonist and endocrine disrupting activity of the xenoestrogen bisphenol A. Endocrinology. 2005;146(12):5388–5396. doi: 10.1210/en.2005-0565. [DOI] [PubMed] [Google Scholar]
- 3.Dodds EC, Lawson W. Synthetic estrogenic agents without the phenanthrene nucleus. Nature. 1936:137. [Google Scholar]
- 4.Chapin RE, Adams J, Boekelheide K, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res B Dev Reprod Toxicol. 2008;83(3):157–395. doi: 10.1002/bdrb.20147. [DOI] [PubMed] [Google Scholar]
- 5.Le HH, Carlson EM, Chua JP, Belcher SM. Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicol Lett. 2008;176(2):149–156. doi: 10.1016/j.toxlet.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FAO/WHO. Toxicological and Health Aspects of Bisphenol A: Report of Joint FAO/WHO Expert Meeting and Report of Stakeholder Meeting on Bisphenol A. World Health Organization; 2011. [Google Scholar]
- 7.Cooper JE, Kendig EL, Belcher SM. Assessment of bisphenol A released from reusable plastic, aluminium and stainless steel water bottles. Chemosphere. 2011;85(6):943–947. doi: 10.1016/j.chemosphere.2011.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doerge DR, Twaddle NC, Vanlandingham M, Brown RP, Fisher JW. Distribution of bisphenol A into tissues of adult, neonatal, and fetal Sprague-Dawley rats. Toxicol Appl Pharmacol. 2011;255(3):261–270. doi: 10.1016/j.taap.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Doerge DR, Twaddle NC, Vanlandingham M, Fisher JW. Pharmacokinetics of Bisphenol A in neonatal and adult CD-1 mice: Inter-species comparisons with Sprague-Dawley rats and rhesus monkeys. Toxicol Lett. 2011;207(3):298–305. doi: 10.1016/j.toxlet.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Prins GS, Ye SH, Birch L, Ho SM, Kannan K. Serum bisphenol A pharmacokinetics and prostate neoplastic responses following oral and subcutaneous exposures in neonatal Sprague-Dawley rats. Reprod Toxicol. 2011;31(1):1–9. doi: 10.1016/j.reprotox.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akingbemi BT, Sottas CM, Koulova AI, Klinefelter GR, Hardy MP. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology. 2004;145(2):592–603. doi: 10.1210/en.2003-1174. [DOI] [PubMed] [Google Scholar]
- 12.Cabaton NJ, Wadia PR, Rubin BS, et al. Perinatal exposure to environmentally relevant levels of bisphenol A decreases fertility and fecundity in CD-1 mice. Environ Health Perspect. 2011;119(4):547–552. doi: 10.1289/ehp.1002559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newbold RR, Jefferson WN, Padilla-Banks E. Prenatal exposure to bisphenol A at environmentally relevant doses adversely affects the murine female reproductive tract later in life. Environ Health Perspect. 2009;117(6):879–885. doi: 10.1289/ehp.0800045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tinwell H, Haseman J, Lefevre PA, Wallis N, Ashby J. Normal sexual development of two strains of rat exposed in utero to low doses of bisphenol A. Toxicol Sci. 2002;68(2):339–348. doi: 10.1093/toxsci/68.2.339. [DOI] [PubMed] [Google Scholar]
- 15.Tyl RW, Myers CB, Marr MC, et al. Two-generation reproductive toxicity study of dietary bisphenol A in CD-1 (Swiss) mice. Toxicol Sci. 2008;104(2):362–384. doi: 10.1093/toxsci/kfn084. [DOI] [PubMed] [Google Scholar]
- 16.Miyawaki J, Sakayama K, Kato H, Yamamoto H, Masuno H. Perinatal and postnatal exposure to bisphenol A increases adipose tissue mass and serum cholesterol level in mice. J Atheroscler Thromb. 2007;14(5):245–252. doi: 10.5551/jat.e486. [DOI] [PubMed] [Google Scholar]
- 17.Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect. 2001;109(7):675–680. doi: 10.1289/ehp.01109675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryan KK, Haller AM, Sorrell JE, Woods SC, Jandacek RJ, Seeley RJ. Perinatal exposure to bisphenol A and the development of metabolic syndrome in CD-1 mice. Endocrinology. 2010;151(6):2603–2612. doi: 10.1210/en.2009-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teeguarden JG, Calafat AM, Ye X, et al. Twenty-four hour human urine and serum profiles of bisphenol A during high-dietary exposure. Toxicol Sci. 2011;123(1):48–57. doi: 10.1093/toxsci/kfr160. [DOI] [PubMed] [Google Scholar]
- 20.Markaverich B, Mani S, Alejandro MA, et al. A novel endocrine-disrupting agent in corn with mitogenic activity in human breast and prostatic cancer cells. Environ Health Perspect. 2002;110(2):169–177. doi: 10.1289/ehp.02110169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kendziorski JA, Kendig EL, Gear RL, Belcher SM. Strain specific induction of pyometra and differences in immune responsiveness in mice exposed to 17alpha-ethinyl estradiol or the endocrine disrupting chemical bisphenol A. Reprod Toxicol. 2012;4(1):22–30. doi: 10.1016/j.reprotox.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thigpen JE, Setchell KD, Padilla-Banks E, et al. Variations in phytoestrogen content between different mill dates of the same diet produces significant differences in the time of vaginal opening in CD-1 mice and F344 rats but not in CD Sprague-Dawley rats. Environ Health Perspect. 2007;115:1717–1726. doi: 10.1289/ehp.10165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thigpen JE, Haseman JK, Saunders H, et al. Dietary factors affecting uterine weights of immature CD-1 mice used in uterotrophic bioassays. Cancer Detect Prev. 2002;26(5):381–393. doi: 10.1016/s0361-090x(02)00122-8. [DOI] [PubMed] [Google Scholar]
- 24.Thigpen JE, Haseman JK, Saunders HE, Setchell KD, Grant MG, Forsythe DB. Dietary phytoestrogens accelerate the time of vaginal opening in immature CD-1 mice. Comp Med. 2003;53:607–615. [PubMed] [Google Scholar]
- 25.Brown NM, Setchell KD. Animal models impacted by phytoestrogens in commercial chow: implications for pathways influenced by hormones. Lab Invest. 2001;81(5):735–747. doi: 10.1038/labinvest.3780282. [DOI] [PubMed] [Google Scholar]
- 26.Zinger M, McFarland M, Ben-Jonathan N. Prolactin expression and secretion by human breast glandular and adipose tissue explants. J Clin Endocrinol Metab. 2003;88(2):689–696. doi: 10.1210/jc.2002-021255. [DOI] [PubMed] [Google Scholar]
- 27.Haseman JK, Bailer AJ, Kodell RL, Morris R, Portier K. Statistical issues in the analysis of low-dose endocrine disruptor data. Toxicol Sci. 2001;61(2):201–210. doi: 10.1093/toxsci/61.2.201. [DOI] [PubMed] [Google Scholar]
- 28.Palmer AK, Ulbrich BC. The cult of culling. Fundam Appl Toxicol. 1997;38(1):7–22. doi: 10.1006/faat.1997.2319. [DOI] [PubMed] [Google Scholar]
- 29.NTP U S. Carcinogenesis Bioassay of Bisphenol A (CAS No. 80-05-7) in F344 Rats and B6C3F1 Mice (Feed Study) Natl Toxicol Program Tech Rep Ser. 1982;215:1–116. [PubMed] [Google Scholar]
- 30.EPA, U.S. [Accessed April 9, 2012];Integrated Risk Information System Oral RfD Summary: Bisphenol A. http://www.epa.gov/iris/subst/0356.htm.
- 31.Sieli PT, Jasarevic E, Warzak DA, et al. Comparison of serum bisphenol A concentrations in mice exposed to bisphenol A through the diet versus oral bolus exposure. Environ Health Perspect. 2011;119(9):1260–1265. doi: 10.1289/ehp.1003385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fisher JW, Twaddle NC, Vanlandingham M, Doerge DR. Pharmacokinetic modeling: prediction and evaluation of route dependent dosimetry of bisphenol A in monkeys with extrapolation to humans. Toxicol Appl Pharmacol. 2011;257(1):122–136. doi: 10.1016/j.taap.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 33.Aguilar R, Bellido C, Sanchez-Criado JE, Aguilar E. Mechanisms of precocious puberty induced in male rats by pituitary grafts. J Reprod Fertil. 1988;83(2):879–883. doi: 10.1530/jrf.0.0830879. [DOI] [PubMed] [Google Scholar]
- 34.Harris HA, Katzenellenbogen JA, Katzenellenbogen BS. Characterization of the biological roles of the estrogen receptors, ERalpha and ERbeta, in estrogen target tissues in vivo through the use of an ERalpha-selective ligand. Endocrinology. 2002;143(11):4172–4177. doi: 10.1210/en.2002-220403. [DOI] [PubMed] [Google Scholar]
- 35.Kawashima K, Nakaura S, Nagao S, Tanaka S, Kuwamura T. Quantitative evaluation of virilizing activity of steroids by measuring morphological changes in urogenital region of rats. Endocrinol Jpn. 1975;22(5):439–444. doi: 10.1507/endocrj1954.22.439. [DOI] [PubMed] [Google Scholar]
- 36.Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial separation as an external sign of pubertal development in the male rat. Biol Reprod. 1977;17(2):298–303. doi: 10.1095/biolreprod17.2.298. [DOI] [PubMed] [Google Scholar]
- 37.Takeyama M, Nagareda T, Takatsuka D, Uchida K, Wakabayashi K, Matsumoto K. Effects of hyperprolactinemia on activities of 17-hydroxylase, 17 beta-ol-dehydrogenase and 5 alpha-reductase in neonatally grafted and host testes in mice. J Steroid Biochem. 1985;22(6):837–842. doi: 10.1016/0022-4731(85)90294-8. [DOI] [PubMed] [Google Scholar]
- 38.Steinmetz R, Brown NG, Allen DL, Bigsby RM, Ben-Jonathan N. The environmental estrogen bisphenol A stimulates prolactin release in vitro and in vivo. Endocrinology. 1997;138(5):1780–1786. doi: 10.1210/endo.138.5.5132. [DOI] [PubMed] [Google Scholar]
- 39.Wozniak AL, Bulayeva NN, Watson CS. Xenoestrogens at picomolar to nanomolar concentrations trigger membrane estrogen receptor-alpha-mediated Ca2+ fluxes and prolactin release in GH3/B6 pituitary tumor cells. Environ Health Perspect. 2005;113(4):431–439. doi: 10.1289/ehp.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li DK, Zhou Z, Miao M, et al. Urine bisphenol A (BPA) level in relation to semen quality. Fertil Steril. 2011;95(2):625–630. doi: 10.1016/j.fertnstert.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 41.Meeker JD, Ehrlich S, Toth TL, et al. Semen quality and sperm DNA damage in relation to urinary bisphenol A among men from an infertility clinic. Reprod Toxicol. 2010;30:532–539. doi: 10.1016/j.reprotox.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mendiola J, Jorgensen N, Andersson AM, et al. Are environmental levels of bisphenol A associated with reproductive function in fertile men? Environ Health Perspect. 2010;118(9):1286–1291. doi: 10.1289/ehp.1002037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schalch DS, Kipnis DM. Abnormalities in carbohydrate tolerance associated with elevated plasma nonesterified fatty acids. J Clin Invest. 1965;44(12):2010–2020. doi: 10.1172/JCI105308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Batista TM, Alonso-Magdalena P, Vieira E, et al. Short-term treatment with bisphenol A leads to metabolic abnormalities in adult male mice. PLoS One. 2012;7(3):e33814. doi: 10.1371/journal.pone.0033814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carwile JL, Michels KB. Urinary bisphenol A and obesity: NHANES 2003–2006. Environ Res. 2011;111(6):825–830. doi: 10.1016/j.envres.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Melnick R, Lucier G, Wolfe M, et al. Summary of the National Toxicology Program’s report of the endocrine disruptors low-dose peer review. Environ Health Perspect. 2002;110(4):427–431. doi: 10.1289/ehp.02110427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vandenberg LN, Colborn T, Hayes TB, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33(3):378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Belcher SM. Rapid signaling mechanisms of estrogens in the developing cerebellum. Brain Res Rev. 2008;57(2):481–492. doi: 10.1016/j.brainresrev.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Belcher SM, Chen Y, Yan S, Wang HS. Rapid estrogen receptor-mediated mechanisms determine the sexually dimorphic sensitivity of ventricular myocytes to 17beta-estradiol and the environmental endocrine disruptor bisphenol A. Endocrinology. 2012;153(2):712–720. doi: 10.1210/en.2011-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Estimated Caloric Intake and Dosage
Supplementary Table 2. Measures of Fertility and Fecundity