Naturally occurring melanins possess unique physicochemical properties that are potentially useful in a broad range of biomedical and industrial applications. They block ionizing radiation,[1] act as antimicrobial agents,[2] antioxidants,[3] metal chelating agents,[4,5] and strengthen exoskeletons.[6] But natural melanins are difficult to manipulate due to their highly crosslinked, heterogeneous nature.[6] Synthetic melanin-like materials have been designed with more controllable properties by mimicking biological melanin production. Mammalian melanogenesis is initiated by tyrosinase oxidation of tyrosyl or o-diphenolic substrates (e.g., dopamine).[6] Biopolymers substituted with these substrates can be used to easily fabricate biomaterials through controlled oxidation. For example, tyramine-dextran conjugates can be used as injectable scaffolds for tissue engineering.[7] Poly(ethylene glycol) (PEG)-catechol conjugates can function as encapsulants in pancreatic islet cell transplantation.[8] Recently, melanin-like nanoparticles surface grafted with PEG were shown to be biocompatible free radical scavengers.[3] Much of this work has mimicked melanin production in animals. Little attention has been given to non-mammalian or unnatural melanogenesis in the design of materials. The use of synthetic chemistry is an intriguing way to produce melanin-like materials with novel or enhanced properties. Here we describe the synthesis and characterization of synthetic hydrogels with melanin-like features. Their unique chemical properties were exploited to design surfaces with biochemically and spatially controlled cell adhesion, and to encapsulate living cells.
Gallic compounds are plant metabolites derived from gallic acid that are ubiquitous in nature and commonly consumed as part of a human diet.[9,10] These compounds are not substrates for biological melanogenesis due to their inhibition of tyrosinases.[11,12] While investigating gallate substituted poly(ethylene glycol)s (PEGs) we determined that these compounds could be oxidized to brown pigmented hydrogels (see Supporting Information Video). To produce these compounds, PEG-amines were end substituted with gallate groups through a three step reaction scheme (See Experimental Section). When exposed to sodium periodate, PEG-gallate acted as a macromer, resulting in the rapid formation of a brown gel (see Supporting Information Video). Gelation was achieved using equimolar or excess amounts of periodate to gallate. This redox initiated gelation follows the same principles as natural melanogenesis. Both periodate and tyrosinase oxidation of o-diphenols can be used to synthesize the o-quinones which then crosslink to melanins through autoxidation.[13,14] Similarly, gallic compounds are oxidized by periodate to o-quinones but not tyrosinases, which they are known to inhibit.[15] Mono-, bi-, tetra-, and octafunctional macromers were generated to study gelation. No gel was obtained when monofunctional PEG-gallates were used or at macromer concentrations below 1.25 wt%.
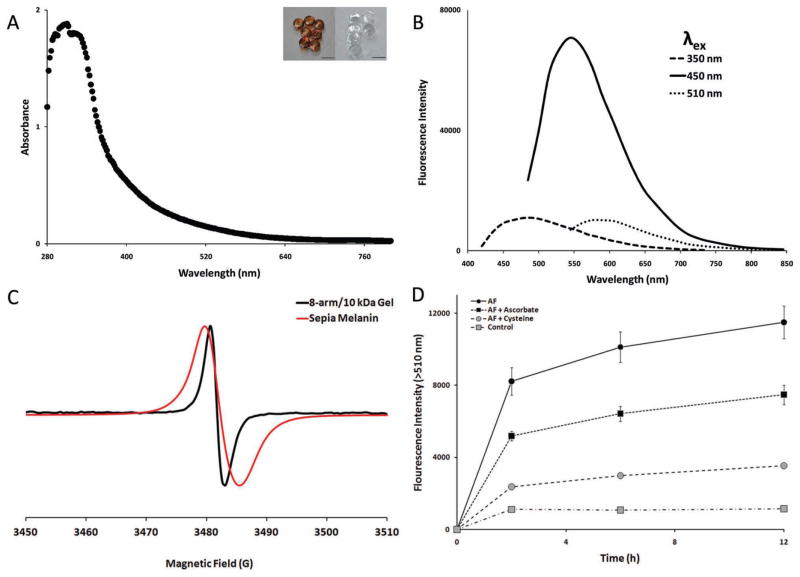
The hydrogels were characterized by UV/Vis, fluorescence, and electron paramagnetic resonance (EPR) spectroscopy to determine their similarity to natural melanins. Melanins absorb light broadly at wavelengths from ultraviolet to red, with peak absorbance in the UV region.[16] Likewise, the hydrogels absorbed broadly with a peak at 300 nm (Figure 1A). Melanin is also susceptible to bleaching by certain oxidizing agents.[17] The hydrogels were easily bleached transparent by brief exposure to sodium hypochlorite (Figure 1A inset). The hydrogels also autofluoresced with peak emission at 545 nm (Figure 1B). These values fall within the emission range for melanins in human skin.[18] Another distinguishing feature natural melanins is paramagnetism, which is due to the presence of stable free radicals.[19] This ability to act as radical trap has led others to investigate melanin-like nanomaterials as therapeutic antioxidants.[3] The electron paramagnetic resonance (EPR) spectrum for the hydrogels confirmed the presence of organic radicals: a single peak was observed absent of hyperfine coupling with a g-factor of 2.0032 (Figure 1C) This supports the presence of an irregular, crosslinked polymer network with mixed bonding arrangements and radicals localized to single quinone residues.[19] Compared to sepia melanin the hydrogels had a sharper absorbance peak. This suggests that the hydrogels possess a more narrow range of bonding arrangement than animal melanins.
Figure 1.
Characterization of melanin-like properties (A) Characteristic absorbance spectrum for PEG-gallate derived gels. (B) Time course of AF-gel adduct formation. Fluorescence at t = 0 was subtracted at all time points. Each data point represents the mean ± SEM (n = 6). Lines are included to guide the eye. (C) Gel excitation-emission properties at three λex maxima. (D) EPR spectrum for hydrogels and sepia melanin. (inset) Melanic gel drops formed from 7.5 μL of 20 wt% 8-arm/10 kDa macromer solution and equal volume of 0.16M NaIO4 before (left) and after (right) soaking gel drops in 10% Clorox bleach for 5 minutes. Scale bars = 4 mm.
The presence of stable, presumably gallotype quinones within the polymer network allowed for the introduction of nucleophilic compounds, via Michael addition.[20] Galloquinones are known to act as electrophilic Michael acceptors,[15] but little is known about the reactivity of galloquinone derived melanins. Quinoid electrophilicity within the gels was investigated using 5-aminofluorescein (AF), a fluorescein derivative that undergoes a dramatic increase in fluorescence when it’s amine is bound covalently.[21] AF coupling to hydrogels was monitored over time by measuring fluorescence emission (Figure 1D). Control gels (grey squares) without AF showed weak fluorescence emission that did not increase significantly over time. In the presence of AF (black circles), emission increased steadily for up to 12 hours, after which no significant increase in fluorescence was detected. This was moderately inhibited when gels were also bathed in sodium ascorbate (black squares). Ascorbate prevents adduct formation by reducing quinones to unreactive phenols.[16] Cysteine was also effective at inhibiting conjugation (grey circles); probably by competitive inhibition of amine adduct formation.
PEG substituted o-diphenols are known to coat a wide variety of surfaces due to strong hydrogen bonding.[22,23] Aqueous PEG-gallate solutions readily adsorbed onto tissue culture treated polystyrene (TCPS) in the form of coherent thin liquid films. This behavior was not observed on untreated polystyrene, glass, gold, or PTFE surfaces. The vacuum plasma treatment of polystyrene for cell culture use may produce an optimal combination of aromatic and hydrogen bonding sites for gallate groups. When these adsorbates were dried and exposed to aqueous periodate a stable thin gel film coated resulted. Quinoid reactivity within these films offered a facile way to covalently bind cell adhesion peptides. Gel coated TCPS microwells were rendered cell adhesive after being bathed in a solution of peptides containing the arginine-glycine-aspartic acid (RGD) (see Experimental Section). This sequence is ubiquitous in the extracellular matrix of mammalian cells and is known to facilitate cell adhesion onto synthetic substrates.[24] The viability of cells stably seeded on gel surfaces after washing was used to assay peptide coupling.
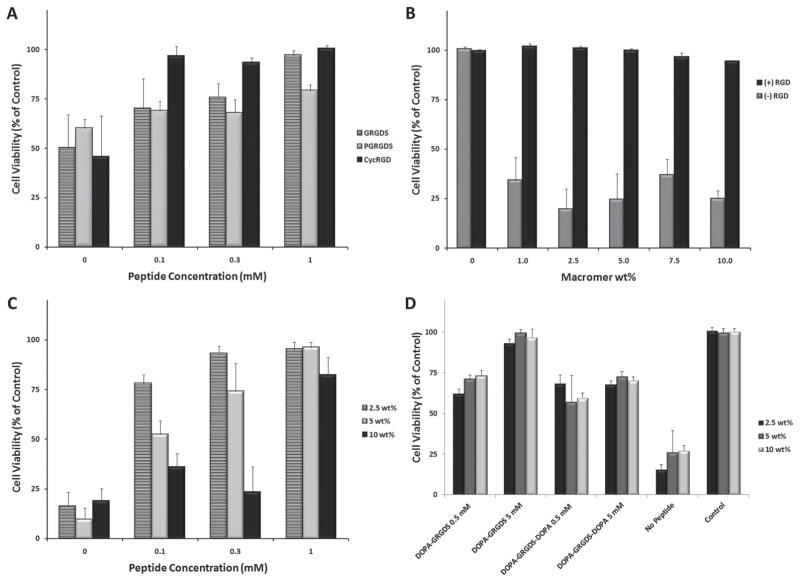
Unmodified hydrogels supported cell attachment poorly or not at all. Soaking the gels with 1 mM solutions of RGD-containing peptides for >7 h at 37 °C was enough to facilitate the adhesion of HeLa, A549, and human mesenchymal stem cells (MSCs). (Figure 2) The reactivity of the gels towards peptides was stable over time. Delaying the exposure of gel films to 1mM GRGDS peptide for 24, 48, or 96 h post gelation had no significant effect on cell adhesion. A cyclized peptide (cycRGD) supported cell attachment and spreading better and at lower concentrations than linear peptides.(Figure 2A) Cyclized RGD sequences are known to have higher affinity for integrins than their linear counterparts.[24] The viability of MSCs was measured after 10 days on gels pre-treated with a heterobifunctional peptide, GRGDSPC. MSC spreading was best on gels treated with this peptide, possibly due to its ability to form gel adducts at both termini. Cell viability was greatly influenced by the concentration of coating solution at lower peptide concentrations of 0–0.3 mM. (Figure 2C) The use of an 8-arm/10 kDa, 2.5 wt% coating solution resulted in adhesive gels at all peptide concentrations, whereas higher amounts of peptide were required to achieve similar viability at higher gel weight fractions. The density of adhesive ligands on a substrate is known to have a non-linear effect on cell motility.[25]
Figure 2.
The viability of cells stably seeded on peptide treated hydrogel thin films measured with an MTS assay. (A) 48 h viability of HeLa cells seeded on 8-arm/10 kDa gels as a function of ligand type and concentration. (B) 72 h viability of A549 cells on 8-arm/20 kDa gels treated with cycRGD as a function of macromer concentration. (C) 10 day viability of MSCs on 8-arm/10 kDa gels treated with GRGDSPC. (D) 72 h viability of HeLa cells seeded on 8-arm/10 kDa gels with DOPA modified peptides. All data is expressed as mean + standard deviation (n = 5).
In some applications the introduction of biological cues during gelation may be preferred (e.g., high throughput screening or cell encapsulation). Cell adhesive peptides modified with terminal L-3,4-dihydroxyphenylalanine (DOPA) residues were used to functionalize gels in situ. When these peptides were dissolved in macromer solutions before gelation, the resulting thin gel films were cell adhesive to HeLa cells.(Figure 2D) But when MSCs were encapsulated within hydrogel droplets the addition of DOPA-GRGDS during gelation had a negative effect on cell survival. (Supporting Information Figure 2) The observed cytotoxicity may have been caused by the DOPA moiety, DOPA quinone, or the mixed melanin resulting from both DOPA and galloquinones.
The selective surface adsorption of PEG-gallate macromers and fast gelation was exploited to develop a removable and flexible substrate for printing nanomaterials on top or both sides of the gel. The selected nanomaterials were zeolites L, a transparent porous aluminosilicate in which it is possible to entrap fluorescent dyes or small molecules in order to visualize them. Furthermore the zeolites can be functionalized with biomolecules and we have covalently bound RGD to favor cell adhesion. Self-assembled monolayers of zeolite L-type microcrystals can be patterned on functionalized glass substrates in order to spatially control cell adhesion.[26] A hydrogel substrate is an attractive alternative due to mechanical properties that are more tunable and better approximate biological tissue. In addition to supporting stable microcontact printed zeolite L monolayers, the soft pliable nature of melanic hydrogel thin films also allowed for bilateral micropatterning. In order to obtain the double printing and to transfer the layer on the soft gel we have proceeded as follows. RGD or amine functionalized zeolite monolayers were printed onto glass or TCPS supported hydrogel thin films (see Experimental Section) using a PDMS stamp and incubated for 12 hours. Zeolite micropatterns were found to be stable upon washing and able to support the spatially controlled HeLa cell attachment.(Figure 3) Amine functionalized zeolites formed more stable patterns than those functionalized with hydroxyl groups, presumably due to adduct formation. On glass surfaces the hydrogel films could be removed and printed on the opposite side to act as a bilateral microprinted scaffold. The microcontact printing and the gel imprinted on both side with the zeolites is illustrated in the various steps in Figure 3A. The functionalization of the zeolites monolayer with RGD allows cell adhesion only on the stripes formed by the zeolites, (Figure 3 B) while the insertion into the gel of the GRGDS peptide favor the cell adhesion also on the gel and the loss of the pattern (Figure 3C). Finally the formation of a double printing on both side of the gel results in the adhesion of the cells on both side (Figure 3D). This technique may be useful for engineering hierarchical or multilayered living tissue constructs.
Figure 3.
Microcontacting of zeolites on melanic hydrogel thin films. (A) Method for bilateral microcontact printing on removal thin films. (B) Micropatterned red fluorescent amino functionalized zeolite monolayers on hydrogels support HeLa cell (DAPI) attachment. (C) Spatially controlled cell attachment is lost when gels are pretreated with GRGDS peptide. (D) Bilateral RGD functionalized zeolite printing supports cell attachment on both sides of hydrogel films. Scale bars = 400 μm
To our knowledge, the use of gallic macromers as building blocks for hydrogels has not previously been reported. The branched o-diphenolic structure of PEG-gallate conjugates allowed them to undergo a type of bio-inspired melanogenesis upon oxidation. The resulting hydrogels possessed properties similar to naturally occurring melanins yet were derived from non-melanogenic precursors. While natural melanins are difficult to manipulate, a hydrogel with melanin-like properties may provide straightforward methods for incorporation of these functionalities into biomaterials. The reactivity of the hydrogels towards nucleophiles allows them to controllably incorporate a wide range or combinatorial mixtures of biologically compounds. The rapid gelation kinetics of PEG-gallates may present challenges in fabricating bulk hydrogel constructs, such as scaffold for tissue engineering. But this feature was useful in designing reactive surfaces and substrates for microfabrication.
Experimental Section
Materials
Monofunctional, 2-arm, 4-arm, and 8-arm PEG-NH2 was purchased from JenKem Technology USA Inc. (Allen, TX). Tris(benzyloxy) benzoic acid (TBBA) was purchased from TCI America (Portland, OR). Diisopropylcarbodiimide (DIC), N-hydroxysuccinimide (NHS), Palladium black, gallic acid, Folin-Ciocalteu reagent, sodium L-ascorbate, and all organic solvents were purchased from Sigma-Aldrich (St. Louis, MO). Hydrogen gas was procured from Airgas (Piscataway, NJ). RPMI-1640, phosphate buffered saline (PBS), Hank’s Balanced Salt Solution (HBSS), heat inactivated fetal bovine serum (FBS), and antibiotics were purchased from Invitrogen (Carlsbad, CA). HeLa and A549 cells were purchased from American Type Culture Collection (Manassas, VA).
PEG-Gallate Synthesis
Amine terminated linear and branched PEGs were end substituted with benzyl protected gallate groups through carbodiimide coupling followed by deprotection over a palladium catalyst. Fourier transform IR spectroscopy was used to confirm amide bond formation (Supplemental Information). In a typical reaction, TBBA (242 mg, 0.55 mmoles), NHS (63 mg, 0.55 mmoles), and DIC (69 mg, 0.55 mmoles) were reacted in dichloromethane (2 mL) for 2 h to form a TBBA-NHS ester. PEG-NH2 in dichloromethane (2 mL) was then added at a TBBA-NHS/NH2 ratio of 1.1. Triethylamine was added at a 5-fold molar excess. The reaction was stirred overnight at room temperature. The solvent was removed by rotary evaporation and the product dissolved in 9:1 dimethylformamide/formic acid. This product was deprotected over Pd black, under H2, at equimolar amounts of palladium and benzyl groups, for 24 hours at 40 °C. The palladium was removed by passing the mixture through a chromatography column packed with cotton, sand, and celite, followed by passing the mixture through a 0.2 μm teflon syringe filter. Full deprotection was confirmed by 1H NMR which showed the disappearance of–CH2-O- shifts at 5.0 and 5.1 ppm. The solvent was removed by rotary evaporation and the product was reconstituted in a 10 mM aqueous sodium ascorbate solution with 5 μM EDTA. The solution was dialyzed twice against 10mM sodium ascorbate, followed by a final two hour dialysis against Millipore water. The solutions were then flash frozen in liquid nitrogen and the product isolated by lyophilization. A Folin-Ciocalteu assay[27] was used to confirm the presence of gallate termini (Supplemental Information)
Aminofluorescein Coupling
In U-bottom transparent 96-well microplate wells, 5 μL of 20 wt% 8-arm/20 kDa PEG-gallate was mixed with an equal volume of 0.16 M aq. NaIO4 and gelled for two minutes. The gels were washed for 5 minutes in 200 μL water 3x, followed by a wash in PBS. To each well, 200 μL of 0.25 mM aminofluorescein in PBS was added with or without 10 mM sodium ascorbate or L-cysteine. Fluorescence was measured from the bottom of each well at various time points using a Wallac 1420 VICTOR2 multilabel counter (Perkin-Elmer, Norwalk CT)
Cell Culture
HeLa and A549 cells were maintained in RPMI-1640 with L-glutamine, penicillin/streptomycin, and 10% heat inactivated FBS. Frozen vials of hMSCs were obtained from the Center for the Preparation and Distribution of Adult Stem Cells (medicine.tamhsc. edu/irm/msc-distribution.html) that supplies standardized preparations of MSCs enriched for early progenitor cells under the auspices of an NIH/NCRR grant (P40 RR 17447-06). hMSCs at passage 3 were used for all experiments. hMSCs were maintained in αMEM with L-glutamine, penicillin/streptomycin, and 20% FBS.
2D and 3D Cell Studies
In tissue culture treated polystyrene 96-well microplate wells, 20–50 μL of macromer solution was added then quickly removed. The wells were allowed to air dry, then filled with 0.1 mL 0.1 M aq. NaIO4. After 2 min, the supernatant was aspirated off and wells were washed 3x with 200 μL Ultrapure water (Invitrogen), and once with PBS. To each well, 15 μL of peptide solution in PBS or PBS alone was added and the plates were incubated overnight at 37 °C. Each well was washed 3x with PBS before being seeded with cells. The peptide sequences GRGDS and GRGDSPC were obtained from American Peptide Company, Inc (Sunnyvale, CA). The sequences PGRGDS, DOPA-GRGDS, DOPA-GRGDS-DOPA, and cyclized GSSKGGGCRGDC were synthesized by the Swanson Biotechnology Center of the Koch Institute at MIT.
Before encapsulation MSCs cell suspensions were centrifuged for 5 minutes at 500 rcf. Cell pellets were rinsed twice with 0.85% w/v saline. PEG-gallate macromers with or without DOPA-GRGDS were dissolved in saline and used to re-suspend cells. A 7.5 μL droplet of the suspension was added to 2 mm wells on PTFE printed glass slides (Electron Microscopy Sciences, Hatfield, PA). An equal volume of 15 mM NaIO4 in saline was placed on top and the mixture was allowed to gel for 2 minutes. The droplets were bathed in 10 mL of HBSS three times before being placed in cell culture media. After 3 days droplets were rinsed twice in HBSS for 5 minute then exposed to HBSS with 2 μM calcein AM and 4 μM ethidium homodimer (LIVE/DEAD Viability/Cytotoxicity Kit for mammalian cells, Invitrogen Corp.) for 45 minutes before imaging with an EVOS fluorescence microscope (AMG, Bothell, WA).
Supplementary Material
Acknowledgments
This research was sponsored by the Armed Forces Institute of Regenerative Medicine award number W81XWH-08-2-0034. The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702–5014 is the awarding and administering acquisition office. The content of the study does not necessarily reflect the position or the policy of the Government, and no official endorsement should be inferred. This work was also supported by a European Research Council Advanced grant award n. 2009-247365 (L.D.C and N.S.K), a UNCF-Merck postdoctoral fellowship (O.Z.F), and Royal Society of Chemistry’s Journals Grants for International Authors (L.D.C). Grants from the NSF also provided instrument support to the DCIF at MIT (CHE-9808061, DBI-9729592). The authors would like to thank Juan Fuentes, Long Le, and Chao Xue for their help in evaluating gelation methods.
Footnotes
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Omar Z. Fisher, David H. Koch Institute for Integrative Cancer Research, MIT Room 76-661, 77 Mass Ave, Cambridge, MA 02139-4307, USA
Benjamin L. Larson, Harvard-MIT Division of Health Sciences and Technology, MIT Room 76-661, 77 Mass Ave, Cambridge, MA 02139-4307, USA
Paulina S. Hill, David H. Koch Institute for Integrative Cancer Research, MIT Room 76-661, 77 Mass Ave, Cambridge, MA 02139-4307, USA
David Graupner, David H. Koch Institute for Integrative Cancer Research, MIT Room 76-661, 77 Mass Ave, Cambridge, MA 02139-4307, USA.
Mai-Thi Nguyen-Kim, David H. Koch Institute for Integrative Cancer Research, MIT Room 76-661, 77 Mass Ave, Cambridge, MA 02139-4307, USA.
Nermin Seda Kehr, Physikalisches Institut and CeNTech, Westfälische Wilhelms-Universität Münster, Heisenbergstrasse 11, 48149 Münster, Germany.
Prof. Luisa De Cola, Physikalisches Institut and CeNTech, Westfälische Wilhelms-Universität Münster, Heisenbergstrasse 11, 48149 Münster, Germany
Prof. Robert Langer, David H. Koch Institute for Integrative Cancer Research, MIT Room 76-661, 77 Mass Ave, Cambridge, MA 02139-4307, USA. Harvard-MIT Division of Health Sciences and Technology, MIT Room 76-661, 77 Mass Ave, Cambridge, MA 02139-4307, USA. Department of Chemical Engineering, Massachusetts Institute of Technology, MIT Room 76-661, 77 Mass Ave, Cambridge, MA 02139-4307, USA
Prof. Daniel G. Anderson, David H. Koch Institute for Integrative Cancer Research, MIT Room 76-661, 77 Mass Ave, Cambridge, MA 02139-4307, USA. Harvard-MIT Division of Health Sciences and Technology, MIT Room 76-661, 77 Mass Ave, Cambridge, MA 02139-4307, USA. Department of Chemical Engineering, Massachusetts Institute of Technology, MIT Room 76-661, 77 Mass Ave, Cambridge, MA 02139-4307, USA
References
- 1.Dadachova E, Casadevall A. Curr Opin Microbiol. 2008;11:525. doi: 10.1016/j.mib.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burtt EH, Schroeder MR, Smith LA, Sroka JE, McGraw KJ. Biol Lett. 2011;7:214. doi: 10.1098/rsbl.2010.0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ju KY, Lee Y, Lee S, Park SB, Lee JK. Biomacromolecules. 2011;12:625. doi: 10.1021/bm101281b. [DOI] [PubMed] [Google Scholar]
- 4.Howell RC, Schweitzer AD, Casadevall A, Dadachova EA. Nucl Med Biol. 2008;35:353. doi: 10.1016/j.nucmedbio.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hung YC, Sava VM, Juang CL, Yeh TC, Shen WC, Huang GWS. J Ethnopharmacol. 2002;79:75. doi: 10.1016/s0378-8741(01)00358-0. [DOI] [PubMed] [Google Scholar]
- 6.Nicolaus RA, Piattelli M, Fattorusso E. Tetrahedron. 1964;20:1163. doi: 10.1016/s0040-4020(01)98983-5. [DOI] [PubMed] [Google Scholar]
- 7.Jin R, Teixeira LSM, Dijkstra PJ, Zhong ZY, van Blitterswijk CA, Karperien M, Jan FJ. Tissue Eng Part A. 2010;16:2429. doi: 10.1089/ten.TEA.2009.0764. [DOI] [PubMed] [Google Scholar]
- 8.Brubaker CE, Kissler H, Wang LJ, Kaufman DB, Messersmith PB. Biomaterials. 2010;31:420. doi: 10.1016/j.biomaterials.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang CS, Lambert JD, Sang SM. Arch Toxicol. 2009;83:11. doi: 10.1007/s00204-008-0372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung KT, Wong TY, Wei CI, Huang YW, Lin Y. Crit Rev Food Sci Nutr. 1998;38:421. doi: 10.1080/10408699891274273. [DOI] [PubMed] [Google Scholar]
- 11.Kubo I, Kinst-Hori I, Nihei K, Soria F, Takasaki M, Calderon JS, Cespedes CL. Z Naturforsch C. 2003;58:719. doi: 10.1515/znc-2003-9-1022. [DOI] [PubMed] [Google Scholar]
- 12.Kim YJ. Biol Pharm Bull. 2007;30:1052. doi: 10.1248/bpb.30.1052. [DOI] [PubMed] [Google Scholar]
- 13.Jimenez-Atienzar M, Cabanes J, Gandia-Herrero F, Garcia-Carmona F. Biochem Biophys Res Commun. 2004;319:902. doi: 10.1016/j.bbrc.2004.05.077. [DOI] [PubMed] [Google Scholar]
- 14.Lee BP, Dalsin JL, Messersmith PB. Biomacromolecules. 2002;3:1038. doi: 10.1021/bm025546n. [DOI] [PubMed] [Google Scholar]
- 15.Quideau S, Feldman KS, Appel HM. J Org Chem. 1995;60:4982. doi: 10.1021/jo961043u. [DOI] [PubMed] [Google Scholar]
- 16.Verrax F, Delvaux M, Beghein N, Taper H, Gallez B, Calderon PB. Free Radic Res. 2005;39:649. doi: 10.1080/10715760500097906. [DOI] [PubMed] [Google Scholar]
- 17.Korytowski W, Sarna T. J Biol Chem. 1990;265:12410. [PubMed] [Google Scholar]
- 18.RichardsKortum R, SevickMuraca E. Annu Rev Phys Chem. 1996;47:555. [Google Scholar]
- 19.Blois MS, Maling JE, Zahlan AB. Biophys J. 1964;4:471. doi: 10.1016/s0006-3495(64)86797-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penalver MJ, Rodriguez-Lopez JN, Garcia-Molina F, Garcia-Canovas F, Tudela J. Anal Biochem. 2002;309:180. doi: 10.1016/s0003-2697(02)00312-3. [DOI] [PubMed] [Google Scholar]
- 21.Munkholm C, Parkinson DR, Walt DR. J Am Chem Soc. 1990;112:2608. [Google Scholar]
- 22.Zurcher S, Wackerlin D, Bethuel Y, Malisova B, Textor M, Tosatti S, Gademann K. J Am Chem Soc. 2006;128:1064. doi: 10.1021/ja056256s. [DOI] [PubMed] [Google Scholar]
- 23.Park SY, Lee H, Lee KD, Pyo KB, Lee H. Langmuir. 2010;26:3790. doi: 10.1021/la904909h. [DOI] [PubMed] [Google Scholar]
- 24.Amedee J, Verrier S, Pallu S, Bareille R, Jonczyk A, Meyer J, Dard M. Biomaterials. 2002;23:585. doi: 10.1016/s0142-9612(01)00145-4. [DOI] [PubMed] [Google Scholar]
- 25.Rehfeldt F, Engler AJ, Discher DE. Biophys J. 2007:636A. [Google Scholar]
- 26.Kehr NS, Riehemann K, El-Gindi J, Schafer A, Fuchs H, Galla HJ, De Cola L. Adv Funct Mater. 2010;20:2248. [Google Scholar]
- 27.Ainsworth EA, Gillespie KM. Nat Prrefotocols. 2007;2:875. doi: 10.1038/nprot.2007.102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.