Abstract
Purpose
To assess 12-month outcomes and safety of clinical magnetic resonance (MR)–guided focused ultrasound (US) treatments of uterine leiomyomas.
Materials and Methods
Between March 2005 and December 2009, 150 women with symptomatic uterine leiomyomas were clinically treated with MR-guided focused US at a single institution; 130 patients completed treatment and agreed to have their data used for research purposes. Patients were followed through retrospective review of medical records and phone interviews conducted at 3-, 6-, and 12-month intervals after treatment to assess additional procedures and symptom relief. Outcome measures and treatment complications were analyzed for possible correlations with the appearance of the tumors on T2-weighted imaging.
Results
The cumulative incidence of additional tumor-related treatments 12 months after MR-guided focused US was 7.4% by the Kaplan–Meier method. At 3-, 6-, and 12-month follow-up, 86% (90 of 105), 93% (92 of 99), and 88% (78 of 89) of patients reported relief of symptoms, respectively. No statistically significant correlation between tumor appearance on T2-weighted imaging and 12-month outcome was found. Treatment-related complications were observed in 17 patients (13.1%): 16 patients had minor complications and one had a major complication (deep vein thrombosis). All complications were resolved within the 12-month follow-up period.
Conclusions
MR-guided focused US is a noninvasive treatment option that can be used to effectively and safely treat uterine leiomyomas and delivers significant and lasting symptom relief for at least 12 months. The incidence of additional treatment during this time period is comparable with those in previous reports of uterine artery embolization.
Uterine leiomyomas are benign tumors of the uterus that are estimated to occur in 20%–80% of reproductive-age (1,2). Uterine leiomyomas can be asymptomatic, but can also cause significant symptoms, including pelvic pain, pressure, menorrhagia, dysmenorrhea, or urinary frequency. Leiomyoma-related symptoms account for approximately 30%–70% of hysterectomies in the United States (3–5). Myomectomy and uterine artery embolization are less invasive uterus-sparing alternatives to hysterectomy available to women who wish to retain their uteri (6–8).
Magnetic resonance (MR)–guided focused ultrasound (US) is a treatment technique in which a US beam is guided to selectively ablate tissues within its focus, in parallel with MR imaging used for anatomic visualization, beam guidance, real-time thermometry, and postprocedural assessment (9). In October 2004, the United States Food and Drug Administration approved the ExAblate 2000 MR-guided focused US device (InSightec, Haifa, Israel) for treatments of uterine leiomyomas. The MR-guided focused US treatments are performed on an outpatient basis, and, other than introduction of intravenous and urinary bladder catheters, are essentially noninvasive with minimal and uncommon associated risks (10–14).
Since 2005, all clinical MR-guided focused US treatments of uterine leiomyomas at our institution have been performed with the ExAblate 2000 system (13–15). In this report, we describe our initial clinical experience with this modality in practice by considering coarse measures of treatment outcome: the incidence of additional treatments after MR-guided focused US treatments, self-reported symptom relief, and treatment-related complications. This retrospective review is limited to 12 months after treatment. The self-reported relief measurements presented here are based on self-assessments performed as part of clinical care after treatment.
MATERIALS AND METHODS
Patient Sample
The present study describes the results of a retrospective study of 150 women with symptomatic uterine leiomyomas who were clinically treated with MR-guided focused US at a single institution between March 2005 and December 2009. For each patient, the type and extent of leiomyoma-related symptoms, together with abdominal MR imaging examination, were used to determine whether MR-guided focused US was an appropriate clinical treatment option. The details of patient screening performed in our practice have been described previously by Hesley et al (14). The patients described here were not asked to consent to any research protocol and were each treated on an individual basis with a technique that was believed would provide an effective relief of their leiomyoma-related symptoms. Patient data were retrospectively recorded and analyzed after institutional review board approval was obtained. Patients who denied access to their medical records for research purposes (n = 14) were excluded from the analysis.
MR-guided Focused US Treatments
Treatments were performed with an ExAblate 2000 device (InSightec) integrated with a Signa MR scanner (GE Healthcare, Milwaukee, Wisconsin). Patients were positioned prone on a modified MR table containing an embedded high-power, MR-compatible, phased-array US transducer. Before treatment, T2-weighted fast spin-echo MR images of the patient were acquired in three anatomic planes for the purpose of treatment planning and lesion targeting. During the treatment, uterine leiomyoma tissues were selectively targeted and ablated through a series of individual US pulses (hereafter referred to as sonications). A typical 3-hour treatment session consisted of 60–100 sonications. Sonication energies were continually adjusted to achieve treatment temperatures sufficient for tissue ablations, and both were recorded for future analysis. After treatment, gadolinium contrast medium was injected intravenously and T1-weighted fast spoiled gradient-recalled images were obtained for assessment and measurement of the volume of ablated (ie, nonperfused) tumor tissues. Detailed descriptions of the ExAblate 2000 device and MR-guided focused US treatments can be found elsewhere (14,15).
Patient Follow-up
Patients were interviewed by phone at 3-, 6-, and 12-month intervals after MR-guided focused US treatments to assess symptom relief and whether additional procedures had been performed. Symptom improvement was self-reported based on percentages as follows: 0%–10%, “insignificant”; 11%–40%, “moderate”; 41%–70%, “considerable”; and 71%–100%, “excellent” symptom relief. Additionally, patients were asked to provide a more detailed description of change in their pretreatment symptoms (eg, menorrhagia, pain, or bulk-related symptoms). Patients were asked to report any additional procedures performed for relief of leiomyoma-related symptoms. The complete medical records and treatment images were also reviewed to identify unreported treatments and complications.
Tumor Classification and Volume Measurements
Image analysis was performed in consensus between two radiologists and a medical physicist experienced in uterine leiomyoma imaging and MR-guided focused US treatments. The volumetric measurements were performed with Vitrea 2.2 segmentation software (version 3.0; Vital Images, Minnetonka, Minnesota). Anatomic pretreatment T2-weighted MR images were used to measure tumor volumes. The cumulative volume of all tumors within the uterus (ie, total tumor load) and the volume of the dominant (ie, largest) tumor were recorded. T1-weighted images with gadolinium contrast agent, acquired upon completion of treatment, were used to measure ablated tumor tissues (Fig 1). The nonperfused volume (summed for all ablated tumors) and nonperfused volume ratio (ie, nonperfused volume divided by total tumor load and rendered as a percentage) were recorded.
Figure 1.
Example of posttreatment T1-weighted images of ablated uterine leiomyomas with gadolinium contrast. The non-enhancing areas (arrows) correspond to nonperfused tissues resulting from MR-guided focused US ablation.
The classification of uterine leiomyomas according to their T2 signal intensity was similar to that introduced by Funaki et al (16), with an additional category reflecting variable tumor heterogeneity: “dark with minimal heterogeneity” (ie, signal intensity lower than that of myometrium, < 25% heterogeneous), “dark with substantial heterogeneity” (ie, signal intensity lower than that of myometrium, > 25% heterogeneous), “isointense” (ie, signal intensity equal to that of myometrium), and “bright” (ie, signal intensity greater than that of myometrium). For each patient, the T2-weighted imaging category of the dominant leiomyoma was recorded (Fig 2).
Figure 2.
Representative images for each of the four tumor enhancement categories according to their appearance on T2-weighted imaging: 1, dark with minimal heterogeneity; 2, dark with substantial heterogeneity; 3, isointense; and 4, bright.
Statistical Methods
Separate one-way analysis of variance models were fit to compare the mean posttreatment nonperfused volume ratios, sonication energies, and treatment temperatures between the T2-weighted imaging enhancement groups. In the presence of statistically significant differences, pairwise comparisons between the groups were evaluated with a Tukey “Studentized” range test. The mean symptom relief was compared between the respondents to any two interview periods with a two-sided paired t test. Time-to-event methodologies were used to evaluate the event of additional treatment for continued uterine leiomyoma–related symptoms, taking into account the varying duration of follow-up between the time of the initial MR-guided focused US procedure and the date of the additional treatment. The duration of follow-up was censored at the date of procedure that was performed for other reasons or at the date of last contact for patients without subsequent procedures. The Kaplan–Meier method was used to estimate the cumulative incidence of subsequent procedures performed for continued leiomyoma-related symptoms (17). Cox proportional-hazards models were fit to evaluate factors associated with the need for a subsequent procedure (18). Associations were summarized by using the hazard ratio and corresponding 95% CI. All calculated P values were two-sided, and P values lower than .05 were considered to indicate a significant difference. Analyses were performed with SAS software (version 9.1; SAS, Cary, North Carolina).
RESULTS
Patient Characteristics
Between March 2005 and December 2009, a total of 150 women were scheduled for clinical MR-guided focused US treatment of symptomatic uterine leiomyomas at a single institution; 14 patients completed treatment but denied use of their data for research purposes. Summary of patient demographics, symptoms, and leiomyoma characteristics are provided in Table 1. Among the remaining 136 patients, the prescribed treatment was not completed in six (Table 2), yielding a treatment completion rate of 95.6%. The results presented henceforth are based on the group of remaining 130 patients who completed treatment; 71 were treated in a single 3-hour session and 59 were treated in two sessions performed on two consecutive days. Among those treated in two sessions are three patients in whom the prescribed treatment was modified: one treatment was interrupted because of equipment problems and the patient had to come back for another session 5 months later; and in two cases, subcutaneous edema was observed on completion of the first treatment session and had resolved by the time of the second treatment session, at 8 and 12 months, respectively.
Table 1.
Patient, Symptom, and Uterine Leiomyoma Characteristics (N = 130)
| Characteristic | Value |
|---|---|
| Age (y) | |
| Mean ± SD | 45.6 ± 5.5 |
| Range | 31.9–58.5 |
| Race* | |
| White | 75 (87.2) |
| Black | 5 (5.8) |
| Native American/Asian/other | 6 (7.0) |
| Not disclosed or unknown | 44 |
| Out of state residency | 82 (63.1) |
| Symptom | |
| Bleeding | 98 (75.4) |
| Bulk | 88 (67.7) |
| Pain | 33 (25.4) |
| Number of leiomyomas | |
| 1 | 50 (38.5) |
| 2–3 | 39 (30.0) |
| ≥ 4 | 41 (31.5) |
| Dominant leiomyoma location | |
| Submucosal | 14 (10.8) |
| Intramural | 86 (66.1) |
| Subserosal | 22 (16.9) |
| Transmucosal | 8 (6.2) |
| Dominant leiomyoma diameter (cm) | |
| Mean ± SD | 8.4 (2.9) |
| Median | 8 |
| Interquartile range | 6.4–9.7 |
| Total tumor volume (cm3) | |
| Mean ± SD | 350.3 ± 307.2 |
| Median | 243.3 |
| Interquartile range | 154.3–467.1 |
| Total tumor volume per patient | |
| < 100 cm3 | 21 (16.2) |
| 101–200 cm3 | 34 (26.1) |
| 201–300 cm3 | 22 (16.9) |
| 301–400 cm3 | 11 (8.5) |
| 401–500 cm3 | 15 (11.5) |
| > 500 cm3 | 27 (20.8) |
Note.—Values in parentheses are percentages.
Percentages are among patients who disclosed race.
Table 2.
Details of Incomplete MR-guided Focused US Treatments
| Case | Description | Follow-up |
|---|---|---|
| 1 | Could not tolerate sonications (pain); treatment aborted after six sonications. | Lost to follow-up |
| 2 | After 15 sonications, patient reported abdominal discomfort; subcutaneous fat edema identified, session terminated. | Hysterectomy 3 mo later |
| 3 | First session completed; patient could not tolerate prone position and test sonications during second session; treatment session aborted before dose sonications performed. | Myomectomy 5 mo later |
| 4 | First session completed; during second session, patient could not tolerate prone position; session aborted before dose sonications performed. | Emergency hysterectomy for infarcted tumor 6 wk later |
| 5 | First session completed; second session delayed for 6 mo (subcutaneous fat edema); patient could not tolerate prone position; treatment aborted before sonications. | Lost to follow-up |
| 6 | First session performed with poor results; patient declined to receive second session. | Lost to follow-up |
Tumor Volume, Imaging Appearance, Nonperfused Volume Ratio, and Treatment Parameters
The mean tumor load per patient was 350.3 cm3 ± 307.2 (median, 243.3 cm3; range, 18–1,845 cm3). Based on the dominant tumor per patient, tumors were classified as follows: 71 (54.6%) dark with minimal heterogeneity, 44 (33.9%) dark with substantial heterogeneity, six (4.6%) isointense, and nine (6.9%) bright.
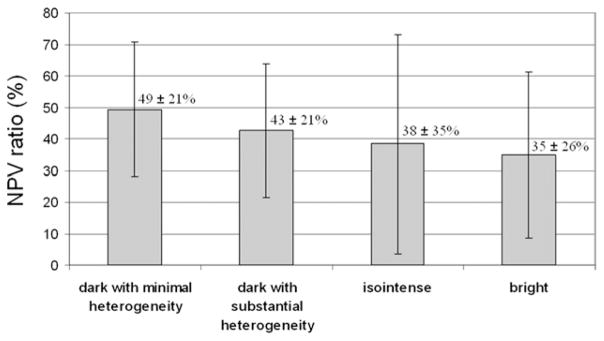
The mean nonperfused volume ratio immediately after treatment was 45.4% ± 22.5 (median, 42.7%; range, 0.2%–100%). The ratio was not significantly different among the T2-weighted imaging appearance categories (one-way analysis of variance, P = .17; Fig 3).
Figure 3.
Posttreatment nonperfused tumor volume ratios corresponding to different T2-weighted imaging appearance categories (mean ± SD). The differences were not found to be statistically significant.
The average values of sonication energies and resulting treatment temperatures among four T2-weighted imaging appearance groups are listed in Table 3. Using the Tukey Studentized range test to control for multiple comparisons, all pairwise comparisons attained statistical significance (P < .05) except between dark with minimal heterogeneity and isointense (sonication energy), dark with minimal heterogeneity and dark with substantial heterogeneity, and isointense and bright (treatment temperature).
Table 3.
Sonication Energies and Treatment Temperatures for Patient Groups Based on T2-weighted Imaging Appearance
| Group | Sonication Energy (J)*
|
Treatment Temperature (°C)†
|
||||
|---|---|---|---|---|---|---|
| Mean ± SD | Median | Range | Mean ± SD | Median | Range | |
| Dark | ||||||
| Minimal heterogeneity | 2,302 ± 710 | 2,200 | 417–4,200 | 69.2 ± 9.8 | 69 | 38–105 |
| Substantial heterogeneity | 2,489 ± 688 | 2,400 | 374–4,080 | 69.4 ± 9.7 | 69 | 43–140 |
| Isointense | 2,253 ± 471 | 2,160 | 1,280–3,412 | 65.7 ± 7.5 | 66 | 49–91 |
| Bright | 2,645 ± 624 | 2,400 | 621–3,840 | 66.4 ± 9.2 | 66 | 41–102 |
| Overall | 2,403 ± 695 | 2,366 | 374–4,200 | 68.9 ± 9.7 | 68 | 38–140 |
Based on an F-test from a one-way analysis of variance model, the mean sonication energy level was significantly different among the four groups (P < .001). On the Tukey Studentized range test to control for the multiple comparisons, all pairwise comparisons still attained significance (P < .05) except dark with minimal heterogeneity versus isointense.
Mean temperature was significantly different among the four groups (P < .001). With the Tukey Studentized range test to control for multiple comparisons, all pairwise comparisons still attained significance (P < .05) except for dark with minimal heterogeneity versus dark with substantial heterogeneity and isointense versus bright.
Treatment-related Complications
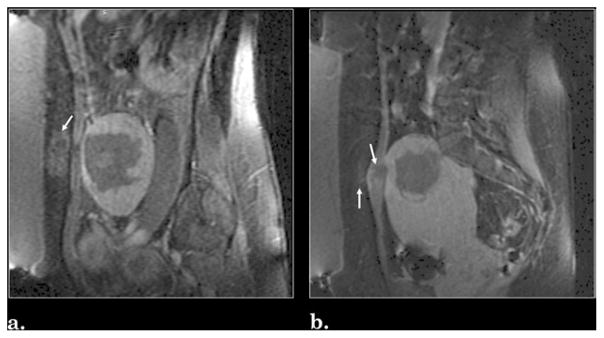
Among 130 patients treated with MR-guided focused US, complications were observed in a total of 17 (13.1%). Mild abdominal edema was noted in 11 patients (8.5%): eight (6.2%) had subcutaneous fat edema, two (1.5%) had simultaneous subcutaneous fat and abdominal muscle edema, and one (0.8%) had simultaneous subcutaneous fat edema and skin erythema (Fig 4). Lower back discomfort associated with treatment was reported by five patients (3.8%): three reported pain and two reported sciatica (involving numbness and temporary decrease in strength). Attempts were made to follow any patients with complications more closely, but the exact time to resolution of symptoms was uncertain because the patients were unable to clearly recall when their discomfort resolved. Therefore, the time of phone contact to document resolution was used. In all five cases of lower back discomfort, symptoms continued to improve and were resolved by the time of the 12-month interview. One major complication occurred (0.8%) in the form of a deep vein thrombosis, which was subsequently treated with anticoagulation therapy. With exception of the case of deep vein thrombosis, all treatment-related complications were minor and were resolved with over-the-counter pain relievers.
Figure 4.
Example of treatment complications as seen in contrast-enhanced T1-weighted MR images: (a) subcutaneous abdominal fat edema and (b) simultaneous subcutaneous fat and rectus muscle edema. Regions of edema are indicated with arrows.
Additional Procedures after MR-guided Focused US
A total of eight patients had an additional procedure for continued uterine leiomyoma–related symptoms within 1 year after their MR-guided focused US treatment: seven underwent hysterectomy and one underwent endometrial ablation. An additional three patients had their leiomyomas removed incidentally at the time of a surgery performed for other conditions: one woman underwent hysterectomy during surgery for an ovarian cyst, one underwent myomectomy during surgery for a pancreatic tumor, and one underwent hysterectomy when her gynecologist was not able to successfully perform a Papanicolaou test. The latter patient had reported 85% improvement in her leiomyoma symptoms after MR-guided focused US.
Among the remaining 119 patients, 92 had at least 1 year of follow-up after their MR-guided focused US procedure. At 6, 9, and 12 months after MR-guided focused US, the cumulative incidences of alternative treatment (based on all 130 patients) were 0%, 2.6%, and 7.4%, respectively.
Patients with a lower nonperfused volume ratio tended to be more likely to have an additional treatment within 1 year, but this difference did not reach significance (Cox model, P = .11; hazard ratio, 1.34 per a 10-unit decrease in nonperfused volume ratio; 95% CI, 0.94–1.93).
With just eight events of additional treatment, and given the small sample sizes in the isointense and bright T2-weighted imaging appearance groups, we had limited statistical power to detect a difference in this outcome among the different T2-weighted imaging appearance categories (Cox model, P = .56).
Symptom Relief
At 3 months, 105 patients were available for interview: 90 (85.7%) reported symptom improvement and 14 (13.3%) reported no symptom relief. One patient (1%) reported worsening of her symptoms (feeling of vertigo in addition to menorrhagia and back pain). This patient was subsequently lost to follow-up. At 6 months, 99 patients were available for interview: 92 (92.9%) reported overall symptom improvement and seven reported no relief. At 12 months, 81 patients were available for interview, and an additional eight received additional treatments for continued uterine leiomyoma–related symptoms (these cases were therefore considered treatment failures). In this group of 89 patients, 78 (87.6%) reported overall symptom improvement and 11 (12.4%) reported no relief of their symptoms (including the eight patients who received additional treatments).
Percentage symptom relief scores at 3-, 6-, and 12-month follow-up periods are shown in Table 4. Among the 43 patients who provided a percentage estimate of symptom relief at the 3- and 6-month follow-up intervals, the 6-month ratings were slightly more favorable (mean ± SD, 74% ± 23 vs 68% ± 23; P = .093, two-sided paired t test). Similarly, among the 37 patients who provided a percentage estimate of symptom relief at the 3- and 12-month follow-up intervals, the 12-month ratings were slightly more favorable (77% ± 22 vs 68% ± 23; P = .081).
Table 4.
Symptom Relief after MR-guided Focused US Treatment
| Interval (mo) | No of. Pts.* | Symptom Relief
|
|||
|---|---|---|---|---|---|
| Insignificant | Moderate | Considerable | Excellent | ||
| 3 | 63 | 1 (1.6) | 8 (12.7) | 18 (28.6) | 36 (57.1) |
| 6 | 74 | 2 (2.7) | 11 (14.9) | 14 (18.9) | 47 (63.5) |
| 12 | 70 | 1 (1.4) | 6 (8.6) | 12 (17.1) | 51 (72.9) |
Note.—Values in parentheses are percentages.
Number of interviewees who provided percentage relief scores at each follow-up interval.
Of the 14 patients who reported no symptom relief at the 3-month follow-up interval, 12 reported improvement at the 6-month interval, one reported no change, and one was scheduled to undergo hysterectomy. Of these 12 patients, nine continued to report improvement by 12 months, one underwent hysterectomy for continued tumor symptoms, one underwent hysterectomy for other reasons, and one lacked 12-month follow-up.
Relief of Baseline Symptoms, T2-weighted Imaging Appearance, and Nonperfused Volume
Of the 130 patients, 72 (55.4%) had more than one of the three symptom categories (menorrhagia, bulk, and pain; Table 1). Symptom improvement at 3, 6, or 12 months had no significant relationship with pretreatment symptoms, and we were unable to find any consistent patterns that enabled us to draw any conclusions.
No clear trend was observed in terms of a relationship between T2-weighted imaging appearance and symptom relief, and the numbers were too sparse to achieve any statistical significance. Data on leiomyoma appearance on T2-weighted imaging relative to outcomes at 12 months are shown in Table 5.
Table 5.
Treatment Outcomes at 12 Months Based on T2-weighted Imaging Appearance
| Group | No. of Pts.* | Overall Symptom Relief
|
||
|---|---|---|---|---|
| Improved | No Change | Additional Procedures | ||
| Dark | ||||
| Minimal heterogeneity | 48 | 43 (90) | 2 (4) | 3 (6) |
| Substantial heterogeneity | 33 | 29 (88) | — | 4 (12) |
| Isointense | 4 | 4 (100) | — | — |
| Bright | 4 | 2 (50) | 1 (25) | 1 (25) |
Note.—Differences are not statistically significant. Values in parentheses are percentages.
Among 89 patients who provided relief information at 12-month interview.
No consistent relationship could be found between nonperfused volume ratio immediately after treatment and self-reported relief (as a percentage), as reflected by the Pearson correlation coefficients of −0.10, 0.21, and 0.12 at 3, 6, and 12 months, respectively. It is important to keep in mind, however, that the patients who underwent alternative treatment between 6 and 12 months did not continue to report values for percentage relief and were therefore not considered in the determination of the correlation estimate for the 12-month interval.
DISCUSSION
Several groups have published reports on follow-up after MR-guided focused US treatment (19–21). Funaki et al (19) reported 24-month follow-up of 91 women and described leiomyoma shrinkage, reduction of symptom severity score, and rate of additional procedures. Lenard et al (20) reported 12-month follow-up of 66 women involved in a prospective multicenter clinical trial (including assessment of leiomyoma shrinkage and reduction of symptom severity score). LeBlang et al (21) investigated uterine leiomyoma shrinkage in 80 women 6 months after MR-guided focused US.
In the present work, we present follow-up data collected in phone interviews from 130 women treated clinically with MR-guided focused US at a single institution between March 2005 and December 2009. Our treatment completion rate was 95.6%, with lack of completion largely related to discomfort from lying in the prone position inside the MR scanner. We found MR-guided focused US to be a safe procedure: with exception of one case of deep vein thrombosis, no major adverse events were recorded.
The cumulative incidence of additional leiomyoma-related surgical treatments, such as hysterectomy or myomectomy, was 7.4% at 12 months after treatment. This is well within the range of values reported for uterine artery embolization (2.9%–10.6% at 1 y) (7,22,23). Funaki et al (19) reported a cumulative 4.4% incidence of additional procedures at 12 months after MR-guided focused US and suggested that the incidence is dependent on T2-weighted imaging appearance, being highest (26.9%) in cases of bright enhancing tumors. The additional procedure rates reported here could indeed suggest a trend (5.1% rate in cases of dark enhancement with minimal heterogeneity, 10.9% in cases of dark enhancement with substantial heterogeneity; 0% in isointense cases, and 14.3% in bright enhancement cases), but, because of the relatively low number of bright enhancing leiomyomas, this finding was not statistically significant.
Importantly, the reported percent symptom relief was high (Table 4) and lasting; among women who were interviewed at 3 and 12 months, only approximately 11% reported a change to a lower relief category. The average score of symptom relief trended up between 3 and 12 months, although the increase was not statistically significant.
The work of Lenard et al (19), LeBlang et al (20), and Funaki et al (21) suggests that nonperfused volume ratios and clinical outcomes are reduced in patients with bright-enhancing uterine tumors. Our clinical experience demonstrated that higher sonication energies were required in bright-enhancing tumors to achieve therapeutic temperature increases. With our patient-specific treatment plans, we did not observe any statistically significant difference in non-perfused volume ratio or clinical outcome based on T2-weighted imaging appearance (Fig 3, Table 5). The relationship between the nonperfused volume ratio at the time of the procedure and long-term symptom relief (ie, > 12 mo) is a subject of ongoing investigation.
There are several limitations to the present study. Data on symptom relief are self-reported and were obtained via phone interviews. The lack of a validated symptom questionnaire is also a limitation of the study. Other limitations arise from the non–research-related nature of the patient cohort and associated difficulties in obtaining data (eg, retrospective study design, follow-up limited to 12 months). However, because there are limited published longitudinal data on treatment outcomes after MR-guided focused US, we believe these limitations do not overshadow the main conclusions of the study. Since the study period, we have incorporated the validated Uterine Fibroid Symptom and Quality of Life questionnaire as part of our routine clinical evaluation and are conducting 36-month follow-up of all subjects.
In conclusion, our data demonstrate MR-guided focused US to be an effective technique to noninvasively and safely treat uterine leiomyomas that delivers significant and lasting symptom relief. Women typically experience only minimal discomfort during or immediately after the procedure and were able to return to their normal daily activities the following day. Symptom relief occurs relatively soon after the procedure and is likely to increase and persist during a 12-month period.
Acknowledgments
The authors acknowledge Joel P. Felmlee, PhD, for useful discussions, and Patty L. Johnson for her invaluable contribution to data collection.
Footnotes
From the SIR 2010 Annual Meeting.
E.A.S. is a clinical trial investigator for InSightec (Haifa, Israel) through the National Institutes of Health and National Institute of Child Health and Human Development. None of the other authors have identified a conflict of interest.
References
- 1.Kjerlulff KH, Erickson AB, Langerberg PW. Chronic gynecologic conditions reported by US women: findings from the National Health Interview Survey, 1984 to 1992. Am J Public Health. 1996;86:195–196. doi: 10.2105/ajph.86.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill CR, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 3.Graves EJ, Kozak LJ. National hospital discharge survey: annual summary, 1996. Vital Health Stat. 1999;140:i–iv. 1–46. [PubMed] [Google Scholar]
- 4.Wilcox LS, Koonin LM, Pokras R, et al. Hysterectomy in the United States, 1988–1990. Obstet Gynecol. 1994;83:549–555. doi: 10.1097/00006250-199404000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Broder MS, Kanouse DE, Mittman BS, Bernstein SJ. The appropriateness of recommendations for hysterectomy in Southern California. Obstet Gynecol. 2000;95:199–205. doi: 10.1016/s0029-7844(99)00519-0. [DOI] [PubMed] [Google Scholar]
- 6.Verkauf BS. Myomectomy for fertility enhancement and preservation. Fertil Steril. 1992;58:1–15. doi: 10.1016/s0015-0282(16)55128-0. [DOI] [PubMed] [Google Scholar]
- 7.Spies JB, Asher SA, Roth AR, Kim J, Levy EB, Gomez-Jorge J. Uterine artery embolization for leiomyomata. Obstet Gynecol. 2001;98:29–34. doi: 10.1016/s0029-7844(01)01382-5. [DOI] [PubMed] [Google Scholar]
- 8.Lumdsen MA. Embolization versus myomectomy versus hysterectomy: which is best, when? Hum Reprod. 2002;17:253–259. doi: 10.1093/humrep/17.2.253. [DOI] [PubMed] [Google Scholar]
- 9.Cline HE, Hynynen K, Watkins RD, et al. Focused US system for MR imaging-guided tumor ablation. Radiology. 1995;194:731–737. doi: 10.1148/radiology.194.3.7862971. [DOI] [PubMed] [Google Scholar]
- 10.Tempany CM, Stewart EA, McDannold NJ, et al. MR imaging – guided focused ultrasound surgery of uterine leiomyomas: a feasibility study. Radiology. 2003;226:897–905. doi: 10.1148/radiol.2271020395. [DOI] [PubMed] [Google Scholar]
- 11.Stewart EA, Gedroyc WM, Tempany C, et al. Focused ultrasound treatment of uterine fibroid tumors: safety and feasibility of a noninvasive thermoablative technique. Am J Obstet Gynecol. 2003;189:48–54. doi: 10.1067/mob.2003.345. [DOI] [PubMed] [Google Scholar]
- 12.Stewart EA, Rabinovici J, Tempany CM, et al. Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertil Steril. 2006;85:22–29. doi: 10.1016/j.fertnstert.2005.04.072. [DOI] [PubMed] [Google Scholar]
- 13.Hesley GK, Felmlee JP, Gebhart JB, et al. Noninvasive treatment of uterine fibroids: early Mayo Clinic experience with magnetic resonance imaging guided focused ultrasound. Mayo Clin Proc. 2006;81:936–942. doi: 10.4065/81.7.936. [DOI] [PubMed] [Google Scholar]
- 14.Hesley GK, Gorny KR, Henrichsen TL, et al. A clinical review of focused ultrasound ablation with magnetic resonance guidance. An option for treating uterine fibroids. Ultrasound Q. 2008;24:131–139. doi: 10.1097/RUQ.0b013e31817c5e0c. [DOI] [PubMed] [Google Scholar]
- 15.Gorny KR, Hangiandreou NJ, Hesley GK, et al. MR guided focused ultrasound: technical acceptance measures for a clinical system. Phys Med Biol. 2006;51:3155–73. doi: 10.1088/0031-9155/51/12/011. [DOI] [PubMed] [Google Scholar]
- 16.Funaki K, Fukunishi H, Fanaki T, et al. Magnetic resonance-guided focused ultrasound surgery for uterine fibroids: relationship between the therapeutic effects and signal intensity on preexisting T2-weighted magnetic resonance images. Am J Obstet Gynecol. 2007;196:184.el–184.e6. doi: 10.1016/j.ajog.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 18.Cox DR. Regression models and life-tables (with discussion) J R Stat Soc Ser B. 1972;34:187–220. [Google Scholar]
- 19.Funaki K, Fukunishi H, Sawada K. Clinical outcomes of magnetic resonance-guided ultrasound surgery for uterine myomas: 24 month follow-up. Ultrasound Obstet Gynecol. 2009;34:584–589. doi: 10.1002/uog.7455. [DOI] [PubMed] [Google Scholar]
- 20.Lenard ZM, McDannold NJ, Fennessy FM, et al. Uterine leiomyomas: MR imaging-guided focused ultrasound surgery—imaging predictors of success. Radiology. 2008;249:187–194. doi: 10.1148/radiol.2491071600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeBlang SD, Hoctor K, Steinberg FL. Leiomyoma shrinkage after MRI-guided focused ultrasound treatment: report of 80 patients. AJR Am J Roentgenol. 2010;194:274–280. doi: 10.2214/AJR.09.2842. [DOI] [PubMed] [Google Scholar]
- 22.Marret H, Cottier JP, Alonso AM, et al. Predictive factors for fibroids recurrence after uterine artery embolization. Br J Obstet Gynaecol. 2005;112:461–465. doi: 10.1111/j.1471-0528.2004.00487.x. [DOI] [PubMed] [Google Scholar]
- 23.Spies JB, Myers ER, Worthington-Kirsch R, et al. The FIBROID registry: symptom and quality-of-life status 1 year after therapy. Obstet Gynecol. 2005;106:1309–1318. doi: 10.1097/01.AOG.0000188386.53878.49. [DOI] [PubMed] [Google Scholar]