Abstract
Purpose
Delirium is common after cardiac surgery, and it is associated with short- and long-term consequences, including cognitive decline. Identification of patients who are vulnerable to delirium might allow for early implementation of delirium-prevention strategies in older adults undergoing surgery. Brain MRI findings provide insight into structural brain changes that may identify vulnerable patients. The purpose of this study was to examine the association between brain MRI characteristics potentially associated with delirium vulnerability and the development of postoperative delirium in a nested cohort of patients undergoing cardiac surgery.
Methods
We identified 79 cardiac surgery patients who had brain MRI imaging after cardiac surgery, as part of an ongoing randomized trial evaluating the efficacy of blood pressure management based on cerebral autoregulation monitoring versus standard management for improving neurological outcomes. Cerebral lateral ventricular size, cortical sulcal width, and white matter hyperintensities (WMH) on brain MRI scans were graded on a validated 0 to 9 scale, and categorized into tertiles. New ischemic lesions were characterized as present or absent. Delirium was assessed using a validated chart-review. Neuropsychological testing performed before surgery was used to establish preoperative cognitive baseline. Multivariable logistic regression was used to assess the independent association between MRI characteristics and postoperative delirium.
Findings
Twenty-eight of 79 (35.4%) patients developed postoperative delirium. Patients with delirium had higher unadjusted ventricular size (median 4 vs. 3, p=0.003), and there was a trend towards higher sulcal sizes and WMH grades. Increasing tertiles of ventricular size (Odds Ratio [OR] 3.59; 95% Confidence Interval [CI] 1.59–8.12; p=0.002) and sulcal size (OR 2.15; 95%CI 1.13–4.12; p=0.02) were associated with postoperative delirium, with a trend for tertiles of WMH grade (OR 1.91; 95%CI 0.99–3.68; p=0.05). In multivariable models adjusted for logistic EuroSCORE, baseline cognitive status, bypass time, and any postoperative complication, each tertile of ventricular size was associated with increased odds of postoperative delirium (OR 3.23 per tertile increase in ventricular size; 95%CI 1.21–8.60; p=0.02). There were no differences in odds of delirium by tertiles of sulcal grade, tertiles of white matter grade, or presence of new ischemic lesions, in adjusted models.
Implications
Increased brain ventricular size was independently associated with delirium after cardiac surgery. These results suggest that cerebral atrophy may contribute to increased vulnerability for postoperative delirium. Baseline brain MRIs may be useful in identifying cardiac surgery patients at high risk for postoperative delirium, who might benefit from targeted perioperative approaches to prevent delirium.
Keywords: cardiac surgery, postoperative delirium, white matter hyperintensities, sulcal width, ventricular size
Introduction
Delirium is as an acute confusional state that is common in hospitalized older adults.1 The incidence of delirium after surgery has been reported to be between 4–65%,2 with the highest incidences generally reported after hip fracture surgery3 and cardiac surgery.4,5 Although once thought to be transient and self-limiting, it is now recognized that delirium is associated with increased morbidity,6 mortality,7 institutionalization,8 and cognitive decline.9,10 Thus, increasing efforts have focused on characterizing the pathophysiology of postoperative delirium, and in particular, identifying vulnerable older adults who might benefit from targeted delirium-prevention strategies.
In patients undergoing cardiac surgery, the characteristics most strongly associated with postoperative delirium reflect cerebral pathology, including prior stroke,11 baseline cognitive impairment,5,12 and depression.5 Since these brain measures are somewhat non-specific, better characterization of brain structure and function, through modalities such as brain magnetic resonance imaging (MRI) scans, may yield insights into the pathophysiology of delirium and also identify vulnerable patients at highest risk for postoperative delirium so that anesthetic approaches could be modified.13
Despite the potential of MRI findings as biomarkers, association between brain MRI findings and risk for postoperative delirium have not been consistent.14 Multiple cerebral infarcts15 and white matter hyperintensities16–18 (WMH) have been associated with postoperative delirium in some studies. Similarly, cerebral atrophy has been associated with postoperative delirium in heterogeneous patient populations.19,20 However, weaknesses of prior studies include use of poorly validated delirium assessments, imprecise characterization of MRI findings, lack of accounting for potentially confounding variables, and heterogeneous patient populations. Recently, a prospective well-done study in non-cardiac surgery patients addressed many limitations of prior studies and found no difference in cerebral atrophy or WMH by delirium status after surgery.21 However, it is unclear if these findings would be generalizable to patients undergoing cardiac surgery, because the pathophysiology of delirium in cardiac surgery patients may be different due to underlying patient characteristics (such as high prevalence of cerebrovascular disease) and different surgical insults (such as cardiopulmonary bypass and associated inflammatory burden).
In the present study, we hypothesized that specific brain MRI characteristics—ventricular and sulcal size, WMH, and new ischemic lesions—would be associated with postoperative delirium in patients undergoing cardiac surgery.
Patients and Methods
The study procedures met with the approval of the Institutional Review Board and were performed after receiving individual written informed consents.
Patients
This study was a prospective observational study, nested in an ongoing multi-year randomized controlled trial study evaluating the association between cerebral blood flow autoregulation22 and brain injury after cardiac surgery (registration: www.clinicaltrials.gov NCT 00981474). The objectives of this study were not the primary outcome of the trial, so this is a secondary data analysis. Patients underwent surgery between October 2009 and August 2012. The primary inclusion criterion was primary or re-operative coronary artery bypass graft (CABG) and/or valve or aortic surgery that required cardiopulmonary bypass with a high risk for neurologic complications (stroke or encephalopathy) as determined by a Johns Hopkins risk score >0.1.23 Exclusion criteria were renal failure requiring dialysis, non-English speaking, contraindications to MRI (e.g. pacemaker) and emergency surgery. As part of the main randomized controlled trial, patients were randomized 1:1 to blood pressure targets during cardiopulmonary bypass based on measures of cerebral autoregulation vs. standard of care targets. Data on postoperative outcomes (major morbidity/mortality, acute kidney injury, and delirium) in a subset of these patients has been reported separately.24,25 During the time period of the nested cohort study, 1337 patients were screened, of which 777 (58.1%) did not meet enrollment criteria, 246 (18.4)% were not approached for logistical reasons (such as staff availability on weekends or emergent procedure), 134 (10.0%) declined participation, and 180 (13.5%) were enrolled. Of the enrolled patients, 101 did not complete MRI (25 refused, 20 were unable to schedule, 17 had retained epicardial pacemaker leads or other metal identified with screening head x-rays, 10 were unable to tolerate the MRI procedure, 10 had scans that were unavailable or uninterpretable for analysis, 9 were discharged early, 7 died, and 3 withdrew) leaving a total of 79 patients for analysis. A consort diagram is included as Appendix A.
Perioperative Care
Patients received standard institutional monitoring including radial arterial blood pressure. General anesthesia was induced and maintained with midazolam (0.15 mg kg−1), fentanyl (5–20 μg kg−1) and isoflurane, with pancuronium or vecuronium for muscle relaxation. Cardiopulmonary bypass was with a non-occlusive roller pump, a membrane oxygenator, and the circuit included a ≤40-μm arterial line filter. Non-pulsatile flow was maintained between 2.0 and 2.4 L/min m−2. Patients were managed using alpha-stat pH management, and rewarming was based on institutional standards with a goal of maintaining nasopharyngeal temperature < 37°C. Postoperative sedation was with propofol 20 – 75 μg kg−1 min−1 until appropriate for tracheal extubation or for 24 hours after surgery. Patients requiring > 24 hours of mechanical ventilation received fentanyl and midazolam.
Brain MRI Scans
Patients underwent brain MRI scans generally within 5–8 days after surgery (range 2–23 days). All MRI imaging was performed on a 3 Tesla Siemens MRI research scanner (Munich, Germany), and the following MRI sequences were obtained and utilized in the analysis: T1-weighted, T2/FLAIR (fluid attenuation inversion recovery), and diffusion-weighted imaging (DWI). Cerebral ventricular size was assessed on a 0 to 9 scale by comparison to eight reference images with successively increasing lateral ventricular size, ranging from normal (grade 1) to severe (grade 8) enlargement. These methods were previously developed and validated for the Cardiovascular Health Study.26 Patients with ventricles smaller than grade 1 received grade 0, while patients with ventricles larger than grade 8 received grade 9. Similarly, cerebral sulcal widening and WMH were assessed on a 0 to 9 scale of increasing severity by comparison with references images as previously described.26 Acute ischemic lesions on MRI were determined by presence of any increased signal on the DWI sequence with corresponding low signal on the ADC maps. All images were assessed by a board-certified neurologist (RF), who was blinded to the patients’ delirium assessment. A second investigator (MB), also blinded to the patients’ delirium assessment, reviewed randomly selected images from 20% of the sample. Both reviewers were masked to all patient characteristics, including delirium status, neuropsychological testing, and randomization status. A weighted kappa statistic was calculated to assess inter-rater agreement (κ=0.69 for WMHs, κ=0.62 for sulcal widening, and κ=0.63 for ventricular size).
Delirium and Neuropsychological Assessment
Delirium was assessed using a validated chart-review method.27 Because clinical judgment was required, a research assistant trained in formal delirium assessment by one of the authors (KN, a psychiatrist) performed all the chart abstractions using a chart-based instrument. The abstracter was masked to MRI findings and postoperative neuropsychological tests. According to the methodology of Inouye et al. (as specifically outlined in Appendix 1 of the reference),27 the abstractor searched the medical record for any mention of key terms, with evidence of acute onset or change, to support a diagnosis of delirium. Key terms and pertinent evidence were based on the following question: ―Is there any evidence from the chart of acute confusional state (e.g. delirium, mental status change, inattention, disorientation, hallucinations, agitation, inappropriate behavior, etc.)?” All sections of the medical record were searched, including but not limited to progress notes, nursing notes, social work notes, physical/occupational therapy notes, and consultant notes. For each possible episode of delirium, the abstractor recorded the source of information, time of onset, and a verbatim description of the episode. A three-person panel (KN, CB, LM) with training in formal delirium assessment and active involvement in delirium research reviewed the chart abstractions and determined the final diagnosis of delirium. Both the research assistant who abstracted data and the participants in the delirium consensus panel were masked to randomization status, MRI results, and neurocognitive test results.
Neuropsychological testing was performed 1–3 days before surgery. The tests assessed a number of cognitive domains known to be associated with vascular disease, and thus potentially affected in patients undergoing cardiac surgery.28,29 The testing battery consisted of the Rey Auditory Verbal Learning Test,30 a test of verbal learning and memory; Rey Complex Figure Test,31 a test of visuospatial ability and executive function; Controlled Oral Word Association Test,32 a test of executive functions; Symbol Digit Modalities Test,33 a test of psychomotor speed and attention control; Trail Making B,34 a test of visuomotor speed, attention, and executive functions; and the Grooved Pegboard Test,35 a measurement of fine motor dexterity and speed.
Covariates and other variables were recorded from the anesthesia preoperative or intraoperative record and from other clinical documentation by research staff.
Data Analysis
Statistical analyses were conducted using Stata Statistical Software: Release 12. (College Station, TX: StataCorp LP)
Brain MRI ventricular size grade, cerebral sulcal grade, and WMH grade were non-normally distributed and were thus categorized into tertiles. New ischemic lesion(s) were considered as a binary (yes/no) variable. The primary outcome was any episode of delirium, considered as a binary variable.
Several important covariates were examined for inclusion in final regression models. To estimate baseline cognition, a composite cognitive Z-score was calculated using prior methodology from the individual cognitive test scores.36 Briefly, individual neuoropsychological test scores were converted to Z-scores, using the mean and standard deviation of the baseline scores from the control group from the randomized parent study as the reference. After each individual cognitive test score was converted to a Z-score, the scores were combined into an average Z-score, calculated from the average of the non-missing individual test Z-scores. Finally, the average Z-scores were re-normalized using the control group’s mean and standard deviation to develop a composite cognitive Z-score outcome. Postoperative complications were prospectively assessed, and a composite comorbidity outcome was defined as new clinically diagnosed stroke, new dialysis requirement, sepsis, mechanical ventilation >48 hours, inotropic medications > 24 hours, new intra-aortic balloon pump insertion, cardiac arrest, or reoperation due to bleeding.
Analysis
We estimated our sample would have 86% power with an alpha of 0.05 to detect an odds ratio of 2.3, based on increasing tertile of ventricular size and adjusted for other variables in the model. Baseline patient and surgical characteristics were compared using t-tests for normally distributed variables, rank sum tests for non-normally distributed variables, and chi squared tests and Fisher’s exact tests for categorical variables. Logistic regression models were used to assess the association between brain MRI characteristics and postoperative delirium. For all analyses, p-values <0.05 were considered significant. Covariates to include in the final adjusted regression model were chosen a priori, as well as based on subsequent comments by reviewers, and included logistic EuroSCORE (which includes age, type of surgery, and history of prior cardiac surgery), composite cognitive z-score, total cardiopulmonary bypass time, and a composite of any complication. We also conducted several sensitivity analyses, in which we added the following variables to the final model: (1) age and previous cardiac surgery, (2) surgical procedure, (3) and individual postoperative complications (mechanical ventilation>48 hours, inotrope>24 hours, postoperative stroke, new intra-aortic balloon pump insertion, cardiac arrest, and reoperation for bleeding). In all models, we also assessed whether randomization affected the outcomes, and found no differences in inferences, so we did not include this in the final models presented.
Results
There were no significant differences in patient or surgical characteristics between the 79 patients included in this study (who underwent MRI scanning) and patients in the parent study who did not undergo MRI scanning, as shown in Appendix B. In particular, the average age of participants without MRI (71.4±8.6) was similar to the age of participants with MRI scans (70.1±7.8; p=0.29), as was the incidence of postoperative delirium (26.7% vs. 35.4%; p=0.23 respectively).
The characteristics of patients in this cohort are shown in Table 1. The average age of patients was 70.1±7.8 years, and there was a high prevalence of hypertension, coronary artery disease, history of smoking, and diabetes. When stratified by median ventricular size on brain MRI (Table 1), patients with ventricular grade >3 (median value) were older, more likely to have had prior cardiac surgery, had longer cardiopulmonary bypass times, more mechanical ventilation > 48 hours, and more intra-aortic balloon pumps inserted. Differences in patients according to sulcal size, white matter volume, and the presence or absence of new ischemic lesions are shown in Appendices C, D, and E respectively. Significantly, patients with sulcal size and white matter grade greater than median values were older than patients with corresponding MRI characteristics less than median values.
Table 1.
Patient and surgical characteristics by median ventricular grade (larger grade indicating larger ventricles)
| Ventricle grade ≤3 (n=44) |
Ventricle grade >3 (n=35) |
All Patients (n=79) |
P- value |
|
|---|---|---|---|---|
| Age (years), mean±SD | 67.9±7.3 | 72.8±7.7 | 70.1±7.8 | 0.005 |
| Female, n (%) | 15 (34.1) | 7 (20.0) | 22 (27.9) | 0.17a |
| Race, n (%) | 0.40b | |||
| White | 36 (81.8) | 27 (77.1) | 63 (79.8) | |
| Black | 6 (13.6) | 4 (11.4) | 10 (12.7) | |
| Other | 2 (4.5) | 4 (11.4) | 6 (7.6) | |
| Baseline Comorbidities | ||||
| Stroke, n (%) | 2 (4.6) | 4 (11.4) | 6 (7.6) | 0.40b |
| Transient ischemic attack, n (%) | 6 (13.6) | 2 (5.7) | 8 (10.1) | 0.29 b |
| COPD or asthma, n (%) | 5 (11.4) | 9 (25.7) | 14 (17.7) | 0.14 b |
| Ever smoker, n (%) | 22 (50.0) | 22 (62.9) | 44 (55.7) | 0.25a |
| Coronary artery disease, n (%) | 34 (77.3) | 32 (91.4) | 66 (83.5) | 0.13 b |
| Hypertension, n (%) | 34 (77.3) | 32 (91.4) | 66 (83.5) | 0.13 b |
| Congestive heart failure, n (%) | 5 (11.6) | 6 (17.2) | 11 (13.9) | 0.52 b |
| Atrial fibrillation, n (%) | 8 (18.2) | 6 (17.2) | 14 (17.7) | 0.90 a |
| Myocardial infarction, n (%) | 13 (29.6) | 13 (37.2) | 26 (32.9) | 0.48 a |
| Diabetes mellitus, n (%) | 25 (56.8) | 15 (42.9) | 40 (50.6) | 0.22 a |
| Previous cardiac surgery, n (%) | 1 (2.3) | 7 (20.0) | 8 (10.1) | 0.02 b |
| Left ventricular ejection fraction, median (IQR) | 55 (48–60) | 55 (45–60) | 55 (45–60) | 0.96 |
| Logistic EuroSCORE, median (IQR) | 3.7 (2.4–5.8) | 6.4 (3.9–14.6) | 4.6 3–10.7) | 0.002 |
| Cognitive z-score, median (IQR) | 0.40 (−0.39–0.88) | −0.22 (−0.72–0.56) | 0.02 (−0.69–0.76) | 0.12 |
| Medications | ||||
| Aspirin, n (%) | 34 (77.3) | 31 (88.6) | 65 (82.3) | 0.24 b |
| Diuretics, n (%) | 16 (36.6) | 11 (31.4) | 27 (34.2) | 0.65 a |
| Nitrates, n (%) | 8 (18.2) | 9 (25.7) | 17 (21.5) | 0.42 a |
| Anti-lipidemics, n (%) | 29 (65.9) | 20 (57.1) | 49 (62.0) | 0.43 a |
| Beta-adrenergic receptor blockers, n (%) | 28 (63.6) | 19 (54.3) | 47 (59.5) | 0.40 a |
| Calcium channel blockers, n (%) | 8 (18.8) | 10 (28.6) | 18 (22.8) | 0.28 a |
| Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, n (%) | 13 (29.6) | 14 (40.0) | 27 (34.2) | 0.33 a |
| Surgery, n (%) | 0.20 b | |||
| CABG | 31 (70.5) | 19 (54.3) | 50 (63.3) | |
| Valve | 5 (11.4) | 5 (14.3) | 10 (12.7) | |
| CABG + Valve | 5 (11.4) | 10 (28.6) | 15 (19.0) | |
| Other | 3 (6.8) | 1 (2.9) | 4 (5.2) | |
| Cardiopulmonary bypass time (min), mean±SD | 101±42 | 122±53 | 110±48 | 0.055 |
| Cross-clamp time (min), mean±SD | 64 ±25 | 75±32 | 69±29 | 0.09 |
| Intraoperative fentanyl (mcg), med (IQR) | 1425 (1250–2000) | 1350 (900–1750) | 1400 (1100–1750) | 0.23 |
| Intraoperative midazolam (mg), med (IQR) | 11.5 (10–20) | 15 (10–20) | 14 (10–20) | 0.11 |
| Postoperative Complications | ||||
| Stroke, n (%) | 3 (6.8) | 1 (2.9) | 4 (5.1) | 0.63 b |
| Inotropic drug > 24 hours, n (%) | 3 (6.8) | 6 (17.1) | 9 (11.4) | 0.17 b |
| New Intra-aortic Balloon Pump, n (%) | 0 (0) | 4 (11.4) | 4 (5.1) | 0.04 b |
| Mechanical ventilation >48 hours, n (%) | 1 (2.7) | 6 (17.2) | 7 (8.9) | 0.04 b |
| Sepsis, n (%) | 0 (0) | 0 (0) | 0 (0) | N/A |
| Cardiac Arrest, n (%) | 2 (4.6) | 0 (0) | 2 (2.5) | 0.50 b |
| Reoperation due to bleeding, n (%) | 0 (0) | 1 (2.9) | 1(1.3) | 0.44 b |
| New dialysis, n (%) | 0 (0) | 0 (0) | 0 (0) | N/A |
| Composite,c n (%) | 8 (18.2) | 9 (25.7) | 17 (21.5) | 0.42 a |
Abbreviations: COPD: chronic obstructive pulmonary disease; EuroSCORE: European System for Cardiac Operative Risk Evaluation; CABG: coronary artery bypass graft surgery
Chi squared test for significance
Fishers exact test for significance
Composite complications include stroke, inotropic drug>24 hours, new intra-aortic balloon pump, mechanical ventilation>48 hours, sepsis, new dialysis requirement, cardiac arrest, or reoperation due to bleeding.
Twenty-eight (35.4%) patients developed postoperative delirium. Characteristics of patients with and without delirium are shown in Table 2. Patients with delirium were older, had more baseline asthma or chronic obstructive pulmonary disease and more prior cardiac surgery compared to patients without delirium. In the postoperative period, patients with delirium had a higher incidence of composite postoperative complications compared to patients without delirium. Specifically, patients with delirium had a higher incidence of mechanical ventilation >48 hours, inotropic drug >24 hours, and new intra-aortic balloon pump insertion. There was no difference in incidence of stroke in patients with vs. without delirium, and no patients developed sepsis or a new need for dialysis.
Table 2.
Patient and surgical characteristics by delirium status
| No delirium (n=51) |
Delirium (n=28) |
P-value | |
|---|---|---|---|
| Age (years), mean±SD | 68.4±7.3 | 73.1 ±8.0 | 0.01 |
| Female, n (%) | 15 (29.4) | 7 (25.0) | 0.80 a |
| Race, n (%) | 0.78 a | ||
| White | 41 (80.4) | 22 (78.6) | |
| Black | 7 (13.7) | 3 (10.7) | |
| Other | 3 (5.9) | 3 (10.7) | |
| Baseline Comorbidities | |||
| Stroke, n (%) | 4 (7.8) | 2 (7.1) | 1.00 b |
| Transient ischemic attack, n (%) | 6 (11.8) | 2 (7.1) | 0.71 b |
| COPD or asthma, n (%) | 5 (9.8) | 9 (32.1) | 0.03 b |
| Ever smoker, n (%) | 27 (52.9) | 17 (60.7) | 0.51 a |
| Coronary artery disease, n (%) | 43 (84.3) | 23 (82.1) | 1.00 b |
| Hypertension, n (%) | 41 (80.4) | 25 (89.3) | 0.36 b |
| Congestive heart failure, n (%) | 7 (13.7) | 4 (14.3) | 1.0 b |
| Atrial fibrillation, n (%) | 8 (15.7) | 6 (21.4) | 0.52 a |
| Myocardial infarction, n (%) | 13 (25.5) | 13 (46.4) | 0.06 a |
| Diabetes mellitus, n (%) | 29 (56.9) | 11 (39.3) | 0.14 a |
| Previous cardiac surgery, n (%) | 2 (3.9) | 6 (21.4) | 0.02 b |
| Left ventricular ejection fraction, median (IQR) | 55 (45–60) | 55 (48–55) | 0.46 |
| Logistic EuroSCORE, median (IQR) | 4.2 (2.4–6.4) | 7.5 (3.3–17.3) | 0.01 |
| Cognitive z-score, median (IQR) | 0.41 (−0.43 – 0.89) | −0.27 (−0.88 – 0.35) | 0.03 |
| Medications | |||
| Aspirin, n (%) | 41 (80.4) | 24 (85.7) | 0.76 b |
| Diuretics, n (%) | 16 (31.4) | 11 (39.3) | 0.48 a |
| Nitrates, n (%) | 10 (19.6) | 7 (25.0) | 0.58 a |
| Anti-lipidemics, n (%) | 29 (56.9) | 20 (71.4) | 0.20 a |
| Beta-adrenergic receptor blockers, n (%) | 28 (54.9) | 19 (67.9) | 0.26 a |
| Calcium channel blockers, n (%) | 11 (21.6) | 7 (25.0) | 0.73 a |
| Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, n (%) | 16 (31.4) | 11 (39.3) | 0.48 a |
| Surgery, n (%) | 0.06 b | ||
| CABG | 35 (68.6) | 15 (53.6) | |
| Valve | 6 (11.8) | 4 (14.3) | |
| CABG + Valve | 7 (13.7) | 8 (28.6) | |
| Other | 3 (5.9) | 1 (3.6) | |
| Cardiopulmonary bypass time (min), mean±SD | 104±43 | 121±55 | 0.13 |
| Cross-clamp time (min), mean±SD | 67±29 | 72±29 | 0.52 |
| Intraoperative fentanyl (mcg), med (IQR) | 1325 (1100–1750) | 1550 (950–1800) | 0.89 |
| Intraoperative midazolam (mg), med (IQR) | 13 (10–20) | 14.5 (10–20) | 0.59 |
| Postoperative Complications | |||
| Stroke, n (%) | 1 (2.0) | 3 (10.7) | 0.13 b |
| Inotropic drug > 24 hours, n (%) | 2 (3.9) | 7 (25.0) | 0.008 b |
| New Intra-aortic Balloon Pump, n (%) | 0 (0) | 4 (14.3) | 0.01 b |
| Mechanical ventilation >48 hours, n (%) | 0 (0) | 7 (25.0) | <0.001 b |
| Sepsis, n (%) | 0 (0) | 0 (0) | N/A |
| Cardiac Arrest, n (%) | 0 (0) | 2 (7.1) | 0.12 b |
| Reoperation due to bleeding, n (%) | 1 (2.0) | 0 (0) | 1.0 b |
| New dialysis, n (%) | 0 (0) | 0 (0) | N/A |
| Composite complications,c n (%) | 4 (7.8) | 13 (46.4) | <0.001 |
Abbreviations: COPD: chronic obstructive pulmonary disease; EuroSCORE: European System for Cardiac Operative Risk Evaluation; CABG: coronary artery bypass graft surgery
Chi squared test for significance
Fishers exact test for significance
Composite complications include stroke, inotropic drug>24 hours, new intra-aortic balloon pump, mechanical ventilation>48 hours, sepsis, new dialysis requirement, cardiac arrest, or reoperation due to bleeding.
Brain MRI scans were obtained at a median of 6 days (IQR 5–8) after surgery. Median ventricular grade was 3 (IQR 3–4), median sulcal grade was 3 (IQR 2–5), and median white matter grade was 2 (IQR 1–3). New ischemic lesions were present in 44 (55.7%) patients. As shown in Table 3, patients with delirium had significantly higher (unadjusted) ventricular size, and a trend towards higher sulcal grade and WMH grade (both p=0.05), compared to patients without delirium.
Table 3.
Characteristics of Brain MRI Scans by Delirium Status.
| No Delirium (n=51) |
Delirium (n=28) |
P-value | |
|---|---|---|---|
| Ventricular size grade, median (IQR) | 3 (3–4) | 4 (3–4.5) | 0.003 |
| Sulcal size grade, median (IQR) | 3 (2–4) | 3.5 (3–5) | 0.05 |
| White matter hyperintensity grade, median (IQR) | 2 (1–3) | 3 (1–4) | 0.05 |
| New ischemic lesions, n (%) | 26 (51.0%) | 18 (64.3%) | 0.26 |
Abbreviations: MRI: magnetic resonance imaging; IQR: interquartile range
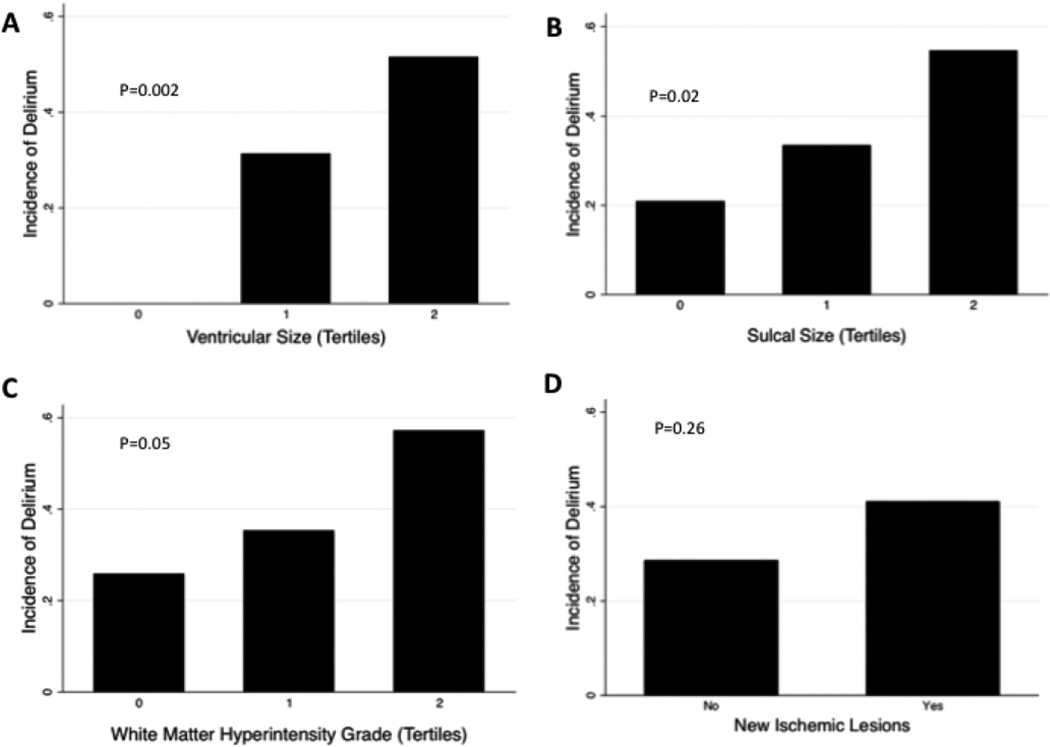
As shown in Tables 4 and 5, in unadjusted models, each increasing tertile of ventricular size, sulcal size, and WMH grade (trend only), but not the presence of new ischemic lesions, was associated with an increased risk of postoperative delirium. The dose-response relationships between postoperative delirium and brain MRI characteristics are further shown in the Figure. In models adjusted for logistic EuroSCORE, baseline cognitive status, cardiopulmonary bypass time, and presence of any postoperative complication, each increasing tertile of ventricular size was associated with increased odds of postoperative delirium (OR 3.23 per tertile increase in ventricular size, 95% CI 1.21–8.60; p=0.02). (Table 4) There were no differences in the odds of postoperative delirium by tertiles of sulcal size, tertiles of WMH grade, or presence of new ischemic lesions in the adjusted models. (Table 5). We also conducted sensitivity analyses in which we included (1) age and history of previous cardiac surgery, (2) the type of surgical procedure, (3) individual complications (in lieu of a composite variable), and (4) number of units of blood products transfused and found that the inferences for all adjusted models were unchanged.
Table 4.
Unadjusted and Adjusted Odds of Delirium by Tertile of Ventricular Size on Brain MRI Scans
| Unadjusted | Adjusteda | |||||
|---|---|---|---|---|---|---|
| Odds Ratio |
95% CI | P-value | Odds Ratio |
95% CI | P-value | |
| Ventricular size (tertile) | 3.59 | 1.59–8.12 | 0.002 | 3.23 | 1.21–8.60 | 0.02 |
| Logistic EuroSCORE | 1.13 | 1.04–1.22 | 0.002 | 1.08 | 0.98–1.19 | 0.14 |
| Baseline cognitive z-score | 0.58 | 0.35–0.96 | 0.03 | 0.69 | 0.38–1.26 | 0.22 |
| Cardiopulmonary bypass time (min) | 1.01 | 1.00–1.02 | 0.14 | 0.99 | 0.98–1.01 | 0.42 |
| Composite Complication | 10.2 | 2.88–35.99 | <0.001 | 12.2 | 2.56–58.4 | 0.002 |
Abbreviations: MRI: magnetic resonance imaging; EuroSCORE: European System for Cardiac Operative Risk Evaluation;
Adjusted for all characteristics presented in the table
Composite complications include stroke, inotropic drug>24 hours, new intra-aortic balloon pump, mechanical ventilation>48 hours, sepsis, new dialysis requirement, cardiac arrest, or reoperation due to bleeding.
Table 5.
Unadjusted and Adjusted Odds of Delirium by Additional Characteristics of Brain MRI Scans
| Unadjusted | Adjusteda | |||||
|---|---|---|---|---|---|---|
| Odds Ratio |
95% CI | P-value | Odds Ratio |
95% CI | P-value | |
| Sulcal size (tertile) | 2.15 | 1.13–4.12 | 0.02 | 1.62 | 0.74–3.53 | 0.23 |
| White matter hyperintensity grade (tertile) | 1.91 | 0.99–3.68 | 0.05 | 1.28 | 0.54–3.06 | 0.58 |
| New ischemic lesions | 1.73 | 0.67–4.67 | 0.26 | 2.25 | 0.69–7.29 | 0.18 |
Abbreviations: MRI: magnetic resonance imaging
Adjusted for logistic EuroScore, cognitive z-score, cardiopulmonary bypass time, and any complication
Figure.
Graph of delirium incidence according to brain MRI scan characteristics.
(For tertile of ventricular size, tertile 0=grade 0–2, tertile 1=grade 3, tertile 2= grade 4–9. For tertile of sulcal size, tertile 0=grade 0–2, tertile 1=grade 3–4, tertile 2=grade 5–9. For tertiles of WMH, tertile 0=grade 0–1, tertile 1=grade 2–3, tertile 2=grade 4–9.
Discussion
In this study, we found that tertile of ventricular size was independently associated with postoperative delirium. Although tertiles of both sulcal size and severity of WMH were associated (or trended to association) with postoperative delirium in unadjusted models, neither of these parameters, nor the presence of new ischemic lesions, were independently associated with postoperative delirium.
Brain MRI has emerged as a powerful tool to investigate structural brain abnormalities with high spatial resolution and may be particularly useful in prediction of surgical outcomes (including delirium), since it is objective and widely available.13 Our findings of a relationship between brain ventricular volume size and delirium is consistent with previous reports from studies of patients admitted to medical and non-cardiac surgical intensive care units.37 Enlarged brain ventricular size reflects cerebral atrophy, and thus our data support the hypothesis that neuronal loss may predispose patients to delirium after a precipitating insult like cardiac surgery.38 However, in contrast to the results of prior studies,16–18 in our study, WMH volumes were not independently associated with postoperative delirium. There may be several reasons for these different results. First, prior studies have generally not accounted for postoperative complications and baseline cognitive status. Postoperative complications may be an important confounding variable since they are associated both with baseline patient disease severity and with postoperative delirium (p<0.001 in adjusted models in our data). Similarly, few studies have adjusted for baseline cognitive status, although baseline cognitive status is highly associated with postoperative delirium and also with cerebral atrophy and WMH. In this study, we were particularly interested if brain MRI characteristics would be associated with postoperative delirium even after accounting for cognitive status, so we did account for cognition in our models. In our study, it is noteworthy that severity of WMH trended towards association with postoperative delirium in univariate analysis, but was not independently associated with postoperative delirium, suggesting either the importance of confounding variables or that our study may have been underpowered to detect this particular adjusted association. Additionally, measurement of WMH was not identical to prior studies, and so misclassification error may have contributed to the discrepancy in results.
Recently, a well-done study in non-cardiac surgery patients found no association of WMH or global brain volumes with postoperative delirium.21 However, the mechanism of delirium may be different for patients undergoing cardiac surgery for several reasons. First, patients undergoing cardiac surgery have high baseline morbidity—including a high prevalence of cerebrovascular and cardiovascular disease and high illness severity scores. Additionally, cardiac surgery involves exposure to cardiopulmonary bypass, which results in heightened inflammation, changes in cerebral blood flow, and cerebral emboli. Thus, our results demonstrate that decreases in ventricular size may be most important in patients with cardiac and cerebrovascular risk factors undergoing cardiac surgery.
Our findings highlight that traditional assessments of older adults in the preoperative period may miss important pathology that would inform postoperative management. Indeed, the American Geriatrics Society and American College of Surgeons recently partnered to develop guidelines for the preoperative assessment of older adults,39 and the recommendations move beyond traditional organ-based approaches towards evaluation of cognitive, functional, nutritional, and frailty status, among others. For patients at high risk for delirium based on preoperative assessment, tailored perioperative strategies are crucial, and general principles include avoidance of precipitating agents, such as benzodiazepines, emphasis on mobilization, screening for the development of delirium, and consideration of avoiding excessive depth of anesthesia.40,41 Although brain imaging is not practical in all surgical patients, it is noteworthy that in this study ventricular size was linearly associated with baseline cognitive performance. Thus, although brain MRI scans may not be available on all patients, formal cognitive assessment may shed insight into important brain pathology and inform postoperative management. However, formal cognitive assessment in the preoperative period is not widely practiced.42 Nevertheless, with a growing appreciation of the importance of preventing delirium and cognitive decline, formal neuroimaging may play a future role in risk stratification of patients or targeting vulnerable patients for perioperative optimization.
Our finding also highlight that postoperative complications are strongly associated with the development of postoperative delirium, with the implication that postoperative events may play an important role in the pathogenesis of delirium. As an example, transition-state models examining corticosteroid administration in the ICU have demonstrated an increased risk of delirium in the 24 hours following steroid administration.43 However, many validated prediction models for delirium after cardiac surgery only consider baseline characteristics5 or characteristics present at ICU admission44 and do not incorporate important postoperative information. Our results raise the possibility that prevention of postoperative complications may also be an important delirium-prevention strategy, although further research needs to be done.
Strengths of this study include the use of validated delirium assessments and objective scales to characterize brain MRI findings, rigorous assessment of baseline cognitive status and covariates, and a focus on cardiac surgery patients. There are several limitations to this study. Very importantly, brain MRI scans were obtained in the postoperative period and thus do not formally precede the incidence of delirium. Thus, it is possible that delirium contributed to postoperative MRI changes. However, ventricular size and sulcal width are considered relatively static and robust features that change slowly over relatively long periods of time.45 Similarly, our WMH grading accounted for and excluded new ischemic lesions seen on DWI, ensuring that graded WMH reflected pre-surgical status. Second, we used a rigorous validated method of delirium assessment; however, we did not conduct in-person assessments and compared to the well-known Confusion Assessment Method46 sensitivity of our method has been shown to be 74% and specificity 83%.27 Thus, misclassification error may be present. Our delirium assessment was likely more sensitive for hyperactive delirium (which is clinically recognizable), compared to hypoactive delirium (which may not be as clinically apparent).27 Outcomes after both hypoactive and hyperactive delirium have been shown to be poor,47 so identifying patients with hypoactive delirium is important for future studies. Our study size was small, so we were limited in the number of covariates that could be considered; thus, our results could be explained by residual confounding, although we used multiple models as a sensitivity analysis. Finally, only 44% of enrolled patients underwent MRI. Although patient characteristics were generally similar between these patient groups, this limits the generalizability of our results and may introduce selection bias.
Conclusions
Results from this study demonstrate that increased ventricular size may be independently associated with delirium after cardiac surgery. These results imply that patients with increased ventricular size on preoperative imaging may benefit from targeted delirium-prevention strategies. Further study is needed to confirm these findings in a cohort with in-person delirium assessments that is sensitive to hypoactive delirium.
Acknowledgments
The authors thank Gayane Yenokyan PhD for statistical support.
Funding
This work was supported by NIH (RO3 AG042331), the Jahnigen Career Development Award, the Research Career Development Core of the Johns Hopkins University Claude D. Pepper Older Americans Independence Center, NIA P30AG021334; the International Anesthesia Research Society, and the Johns Hopkins Clinician Scientist Award (CB); NIH (RO1 HL092259) (CH). and an institutional NIH KL2 grant from the Johns Hopkins Institute for Clinical and Translational Research (ICTR) (KL2TR001077) (RF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
There are no conflicts of interest. For unrelated studies, Karin Neufeld has received research support from Ornim Medical, and Charles Hogue has received research support from Covidien, Inc. and served on the advisory board for Ornim Medical.
Contributor Information
Charles Brown, Zayed 6208, 1800 Orleans St., Baltimore MD 21287, United States of America, cbrownv@jhmi.edu, 410 955 7519.
Roland Faigle, Phipps 484, 600 N Wolfe Street, Baltimore MD 21287, United States of America, rfaigle1@jhmi.edu.
Lauren Klinker, Zayed 6208, 1800 Orleans St., Baltimore MD 21287, United States of America, lklinke2@jhmi.edu.
Mona Bahouth, 466 Phipps, 600 N. Wolfe St., Baltimore MD 21205, United States of America, mbahout1@jhmi.edu.
Laura Max, Zayed 6208, 1800 Orleans St., Baltimore MD 21287, United States of America, lmax2@jhmi.edu.
Andrew LaFlam, Zayed 6208, 1800 Orleans St., Baltimore MD 21287, United States of America, alaflam20@gmail.com.
Karin J. Neufeld, Osler 320, 600 N. Wolfe St., Baltimore MD 21287, United States of America, kneufel2@jhmi.edu.
Kaushik Mandal, Zayed 7107, 1800 Orleans St., Baltimore MD 21287, United States of America, kmandal2@jhmi.edu.
Rebecca Gottesman, Phipps 446D, 600 North Wolfe Street, Baltimore, MD 21287, rgottesm@jhmi.edu.
Charles Hogue, Zayed 6208, 1800 Orleans St., Baltimore MD 21287, United States of America, chogue2@jhmi.edu.
References
- 1.Inouye S, Westendorp R, Sazynski J. Delirium in elderly people. Lancet. 2014;383:911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudolph JL, Marcantonio E. Postoperative delirium: acute change with long-term implications. Anesth Analg. 2011;112:1202–1211. doi: 10.1213/ANE.0b013e3182147f6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sieber F, Zakriya K, Gottschalk A, et al. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proc. 2010;85:18–26. doi: 10.4065/mcp.2009.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ottens TH, Dieleman JM, Sauër A-MC, et al. Effects of dexamethasone on cognitive decline after cardiac surgery: a randomized clinical trial. Anesthesiology. 2014;121(3):492–500. doi: 10.1097/ALN.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 5.Rudolph JL, Jones RN, Levkoff SE, et al. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation. 2009;119(2):229–236. doi: 10.1161/CIRCULATIONAHA.108.795260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin B, Buth K, Arora R, Baskett R. Delirium as a predictor of sepsis in post-coronary artery bypass grafting patients: a retrospective cohort study. Crit Care. 2010;14:R171. doi: 10.1186/cc9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gottesman R, Grega M, Bailey M, et al. Delirium after coronary artery bypass graft surgery and late mortality. Ann Neurol. 2010;67:338–344. doi: 10.1002/ana.21899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCusker J, Cole M, Dendukuri N, Belzile E, Primeau F. Delirium in older medical inpatients and subsequent cognitive and functional status: a prospective study. CMAJ. 2001;165(5):575–583. [PMC free article] [PubMed] [Google Scholar]
- 9.Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367(1):30–39. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacLullich AMJ, Beaglehole A, Hall RJ, Meagher DJ. Delirium and long-term cognitive impairment. Int Rev Psychiatry. 2009;21(1):30–42. doi: 10.1080/09540260802675031. [DOI] [PubMed] [Google Scholar]
- 11.Kazmierski J, Kowman M, Banach M, et al. Preoperative predictors of delirium after cardiac surgery: a preliminary study. Gen Hosp Psychiatry. 2006;28(6):536–538. doi: 10.1016/j.genhosppsych.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Veliz-Reissmüller G, Torres HA, van der Linden J, Lindblom D, Jönhagen ME. Pre-operative mild cognitive dysfunction predicts risk for post-operative delirium after elective cardiac surgery. Aging Clin Exp Res. 2007;19(3):172–177. doi: 10.1007/BF03324686. [DOI] [PubMed] [Google Scholar]
- 13.Alsop DC, Fearing MA, Johnson K, Sperling R, Fong TG, Inouye SK. The role of neuroimaging in elucidating delirium pathophysiology. J Gerontol A Biol Sci Med Sci. 2006;61(12):1287–1293. doi: 10.1093/gerona/61.12.1287. [DOI] [PubMed] [Google Scholar]
- 14.Soiza RL, Sharma V, Ferguson K, Shenkin SD, Seymour DG, MacLullich AMJ. Neuroimaging studies of delirium: A systematic review. J Psychosom Res. 2008;65:239–248. doi: 10.1016/j.jpsychores.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 15.Otomo S, Maekawa K, Goto T, Baba T, Yoshitake A. Pre-existing cerebral infarcts as a risk factor for delirium after coronary artery bypass graft surgery. Interact Cardiovasc Thorac Surg. 2013;17(5):799–804. doi: 10.1093/icvts/ivt304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatano Y, Narumoto J, Shibata K. White-Matter Hyperintensities Predict Delirium After Cardiac Surgery. Am J Geriatr Psychiatry. 2013;21(10):938–945. doi: 10.1016/j.jagp.2013.01.061. [DOI] [PubMed] [Google Scholar]
- 17.Shioiri A, Kurumaji A, Takeuchi T, Matsuda H, Arai H, Nishikawa T. White Matter Abnormalities as a Risk Factor for Postoperative Delirium Revealed by Diffusion Tensor Imaging. Am J Geriatr Psychiatry. 2012;18(8):743–753. doi: 10.1097/JGP.0b013e3181d145c5. [DOI] [PubMed] [Google Scholar]
- 18.Root JC, Pryor KO, Downey R, et al. Association of pre-operative brain pathology with post-operative delirium in a cohort of non-small cell lung cancer patients undergoing surgical resection. Psychooncology. 2013;22(9):2087–2094. doi: 10.1002/pon.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koponen H, Hurri L, Stenbäck U, Riekkinen PJ. Acute confusional states in the elderly: a radiological evaluation. Acta Psychiatr Scand. 1987;76(6):726–731. doi: 10.1111/j.1600-0447.1987.tb02946.x. [DOI] [PubMed] [Google Scholar]
- 20.Figiel GS, Coffey CE, Djang WT, Hoffman G, Doraiswamy PM. Brain magnetic resonance imaging findings in ECT-induced delirium. J Neuropsychiatry Clin Neurosci. 1990;2(1):53–58. doi: 10.1176/jnp.2.1.53. [DOI] [PubMed] [Google Scholar]
- 21.Cavallari M, Hshieh TT, Guttmann CRG, et al. Brain atrophy and white-matter hyperintensities are not significantly associated with incidence and severity of postoperative delirium in older persons without dementia. Neurobiol Aging. 2015;36:2122–2129. doi: 10.1016/j.neurobiolaging.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ono M, Brady K, Easley RB, et al. Duration and magnitude of blood pressure below cerebral autoregulation threshold during cardiopulmonary bypass is associated with major morbidity and operative mortality. J Thorac Cardiovasc Surg. 2014;147:483–489. doi: 10.1016/j.jtcvs.2013.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKhann GM, Grega MA, Borowicz LM, et al. Encephalopathy and stroke after coronary artery bypass grafting: incidence, consequences, and prediction. Arch Neurol. 2002 Sep;59(9):1422–1428. doi: 10.1001/archneur.59.9.1422. [DOI] [PubMed] [Google Scholar]
- 24.Ono M, Arnaoutakis GJ, Fine DM, et al. Blood Pressure Excursions Below the Cerebral Autoregulation Threshold During Cardiac Surgery are Associated With Acute Kidney Injury. Crit Care Med. 41(2):464–471. doi: 10.1097/CCM.0b013e31826ab3a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown CH, Morrissey C, Ono M, et al. Impaired olfaction and risk of delirium or cognitive decline after cardiac surgery. J Am Geriatr Soc. 2015;63(1):16–23. doi: 10.1111/jgs.13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manolio TA, Kronmal RA, Burke GL, et al. Magnetic resonance abnormalities cardiovascular disease in older adults. The Cardiovascular Health Study. Stroke. 1994;25(2):318–327. doi: 10.1161/01.str.25.2.318. [DOI] [PubMed] [Google Scholar]
- 27.Inouye SK, Leo-Summers L, Zhang Y, Bogardus ST, Leslie DL, Agostini JV. A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc. 2005;53(2):312–318. doi: 10.1111/j.1532-5415.2005.53120.x. [DOI] [PubMed] [Google Scholar]
- 28.van Dijk D, Keizer AM, Diephuis JC, Durand C, Vos LJ, Hijman R. Neurocognitive dysfunction after coronary artery bypass surgery: A systematic review. J Thorac Cardiovasc Surg. 2000;120(4):8–8. doi: 10.1067/mtc.2000.108901. [DOI] [PubMed] [Google Scholar]
- 29.Stump DA. Selection and clinical significance of neuropsychologic tests. Ann Thorac Surg. 1995;59(5):1340–1344. doi: 10.1016/0003-4975(95)00108-w. [DOI] [PubMed] [Google Scholar]
- 30.Powell J, Cripej L, Dodril lC. Assessment of brain impairment with the Rey Auditory-Verbal Learning Test. Arch Clin Neuropsychol. 1991;6:241–249. [PubMed] [Google Scholar]
- 31.Meyers JE, Meyers KR. Rey complex figure test under four different administration procedures. Clin Neuropsychol. 1995;9(1):63–67. [Google Scholar]
- 32.Lezak M. Neuropsychological Assessment. New York: Oxford University Press; 1983. [Google Scholar]
- 33.Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. New York: Oxford University Press; 1998. [Google Scholar]
- 34.Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery. Tucson, AZ: Neuropsychology Press; 1985. [Google Scholar]
- 35.Costa LD, Vaughan HG, Jr, Levita E, et al. Purdue Pegboard as a predictor of the presence and laterality of cerebral lesions. J Consult Psychol. 1963;27:133–137. doi: 10.1037/h0040737. [DOI] [PubMed] [Google Scholar]
- 36.Selnes O, Grega M, Borowicz L, Jr, et al. Cognitive outcomes three years after coronary artery bypass surgery: a comparison of on-pump coronary artery bypass graft surgery and nonsurgical controls. Ann Thorac Surg. 2005;79:1201–1219. doi: 10.1016/j.athoracsur.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 37.Gunther ML, Morandi A, Ely EW. Pathophysiology of Delirium in the Intensive Care Unit. Crit Care Clin. 2008;24(1):45–65. doi: 10.1016/j.ccc.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210–220. doi: 10.1038/nrneurol.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chow WB, Ko CY, Rosenthal RA, Esnaoloa NF. [Accessed July 2, 2015];ACS NSQIP/AGS Best practice guidelines: optimal preoperative assessment of the geriatric surgical patient. doi: 10.1016/j.jamcollsurg.2012.06.017. https://www.facs.org/~/media/files/quality%20programs/nsqip/acsnsqipagsgeriatric2012guidelines.ashx. [DOI] [PubMed]
- 40.Brown C. Delirium in the cardiac surgical ICU. Curr Opin Anesthesiol. 2014;27:117–122. doi: 10.1097/ACO.0000000000000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.American Geriatric Society Expert Panel on Postoperative Delirium in Older Adults. Postoperative Delirium in Older Adults: Best Practice Statement from the American Geriatrics Society. J Am Coll Surg. 2015;220(2):136–148. doi: 10.1016/j.jamcollsurg.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 42.Crosby G, Culley D, Hyman BT. Preoperative cognitive assessment of the elderly surgical patient: A call for action. Anesthesiology. 2011;114:1265–1268. doi: 10.1097/ALN.0b013e31821b1bc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schreiber MP, Colantuoni E, Bienvenu OJ, et al. Corticosteroids and Transition to Delirium in Patients With Acute Lung Injury. Crit Care Med. 2014;42(6):1480–1486. doi: 10.1097/CCM.0000000000000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Afonso A, Scurlock C, Reich D, et al. Predictive Model for Postoperative Delirium in Cardiac Surgical Patients. Semin Cardiothorac Vasc Anesth. 2010;14(3):212–217. doi: 10.1177/1089253210374650. [DOI] [PubMed] [Google Scholar]
- 45.LeMay M. Radiologic changes of the aging brain and skull. Am J Roentgenol. 1984;143:383–389. doi: 10.2214/ajr.143.2.383. [DOI] [PubMed] [Google Scholar]
- 46.Inouye S, van Dyck C, Alessi C, Balkin S, Siegal A, Horwitz R. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 47.Stransky M, Schmidt C, Ganslmeier P, et al. Hypoactive delirium After cardiac surgery as an independent risk factor for prolonged mechanical ventilation. J Cardiothorac Vasc Anesth. 2011;25(6):968–974. doi: 10.1053/j.jvca.2011.05.004. [DOI] [PubMed] [Google Scholar]