Abstract

In this work, the chemical and thermal stability of a primary amine-functionalized ion-exchange resin (Lewatit VP OC 1065) is studied in view of the potential options of regenerating this sorbent in a CO2 removal application. The adsorbent was treated continuously in the presence of air, different O2/CO2/N2 mixtures, concentrated CO2, and steam, and then the remaining CO2 adsorption capacity was measured. Elemental analysis, BET/BJH analysis, Fourier transform infrared spectroscopy, and thermogravimetric analysis were applied to characterize adsorbent properties. This material was found to be thermally and hydrothermally stable at high temperatures. However, significant oxidative degradation occurred already at moderate temperatures (above 70 °C). Temperatures above 120 °C lead to degradation in concentrated dry CO2. Adding moisture to the concentrated CO2 stream improves the CO2-induced stability. Adsorbent regeneration with nitrogen stripping is studied with various parameters, focusing on minimizing the moles of purge gas required per mole of CO2 desorbed.
1. Introduction
The concentration of CO2 in the atmosphere has increased by some 100 ppm from the 1700s to 2005 and is nowadays above 400 ppm.1 Electricity production and transportation were estimated to contribute around 68% of total CO2 emission in the U.S. in 2014, produced by burning of fossil fuel such as coal, natural gas, and petroleum.2 These types of fuel cannot be replaced by biofuel or carbon neutral fuel in large scale in the near future. Under these circumstances, carbon capture and storage (CCS) becomes important to halt the CO2 emission rate. In the report released by National Technology Laboratory in 2010, CCS technologies would add around 83% and 44% to the cost of electricity (COE) for a new pulverized coal plant (PC) and new natural gas combined cycle power plants (NGCC) respectively.3 For years, efforts have been made to reduce the costs of carbon dioxide capture.4−7 Amine scrubbing using aqueous amine solutions is so far the most commercial and well-developed technology for postcombustion CO2 capture.8 However, it is still an expensive technology since heating up the liquid water is energy intensive. Dry sorbent processes are seen as the possible next generation method for CO2 capture.9
Supported-amine based adsorbents (SASs) are assumed to follow similar reaction pathways as aqueous phase amines but require less energy as they avoid energy required to heat up the bulk of water. Supported-amine sorbents contain amine functional groups (such as primary amine and secondary amine) on a solid supports. The bindings of the functional groups to the support materials can be very different and is based on their preparation methods.10 SASs are said to possess other advantages over aqueous amine solvents such as faster sorption kinetics, higher CO2 capacity, higher stability, and higher resistance to contaminants, and being less corrosive and more environmentally friendly.5,10,11 The development of amine based sorbents is still in its early stage compared to aqueous amine solvents. Until now, most studies on SASs emphasize modifying the sorbent materials for a higher uptake capacity of CO2.9 Since 2010, the literature shows an increasing number of studies on other important aspects such as kinetics,12−15 process design and optimization,16−20 and sorbent stability.21−27 Among these, the topic of stability is very important since it determines the lifetime of the sorbent, which is not inexpensive. The lifetime of the sorbent is closely related to the number of allowable cycles for the adsorbent. The higher this number of cycles, the lower the cost of the adsorbent per unit of CO2 produced:
| 1 |
The stability of the SASs can be affected by multiple factors such as operating temperature and the presence of O2, CO2, and steam, based on previous studies.21−35 These factors relate to either the adsorption feed gas (e.g., flue gas or air) or to the regeneration stripping gas. It is crucial that for each sorbent the effects of these different factors on the sorbent are identified and quantified before scaling-up the process.
Depending on the application, oxygen will be present in different concentrations in the feed gas. For CO2 removal from more concentrated feeds (such as biogas), air could even be considered for use as stripping gas and hence be present during regeneration. Bollini et al.30 and Heydari-Gorji et al.29 reported in 2011 separately the long-term effect of air at high temperature on grafted primary, secondary, and tertiary monoamines as well as on mixed-amine materials containing both primary and secondary amines. Interestingly, both studies found that primary and tertiary amines show superior oxidative stability compared to secondary amines on the studied supported amines. Subsequently, Heydari-Gorji et al.23 studied supported amines based on SBA-15 impregnated with linear polyethylenimine (PEI). It was found that the materials were deactivated severely in CO2 free air. In contrast, the oxidative stability of the adsorbents was improved in the presence of moisture and CO2. This is due to the fast reaction between CO2 and solid amines, leading to the formation of carbamate and bicarbonate, which protect the material from oxygen attack. The practical implication of this finding may be limited, since adding CO2 to the regeneration gas will result in a reduction of the CO2 working capacity. Based on aforementioned studies, it is clear that the oxidative stability of the studied SASs is related to both the state of the amines and the gas conditions.
Using pure CO2 in a thermal swing regeneration of the adsorbents can be relevant when aiming to produce a stream of pure CO2 without requiring downstream separation. With CO2 at elevated temperature, the formation of urea groups on SASs was reported by Drage et al.21 based on the observation of an increase in sorbent weight when the temperature was higher than 140 °C under a flow of pure CO2 on a PEI impregnated silica sorbent. The weight increases corresponding to a secondary product formed, which could not adsorb CO2 and was identified to contain urea linkages. Sayari et al.22 found CO2-induced deactivation in amine-containing material even at temperatures as low as 50 °C in the presence of pure CO2. The urea was confirmed to be responsible for the deactivation by 13C CP MAS NMR and DRIFT. Remarkably, they demonstrated that the urea can be completely inhibited and regenerated by adding very little moisture. Subsequently in 2011, Sayari et al.28 published another paper on the effect of state of amine in urea formation. They observed that primary monoamine rather than secondary monoamine are deactivated in pure CO2 at 55 °C for adsorption and 120 °C for regeneration in a purge flow of N2 over 60 cycles. The difference in the stability of the different amines was associated with isocyanate, an intermediate when forming urea. This isocyanate can be produced from dehydration of carbamic acid formed only from primary amine. Mixed amines, containing primary and secondary amines, can form isocyanate from the presence of primary amine, which further reacts with primary or secondary amine to form urea. Later, in 2012, an extended study on the mechanism of urea formation was published by the same group.24 In this study, the stability of a wide variety of mesoporous silica-grafted and impregnated amine sorbents was investigated in the presence of CO2. Both in adsorption and in desorption, the samples were exposed to a dry, pure CO2 stream during adsorption at 50 or 100 °C and desorption at 130–160 °C. The total time of CO2 exposure in both adsorption and desorption is 30 h. All materials except for secondary monoamine deactivated significantly, for more than 50% capacity decrease in these 30 h, attributed to the formation of urea based on two mechanisms. One is the formation of open-chain urea, which can only be formed from primary amine. The other mechanism is to form cyclic urea. This is the only mechanism for urea formation between secondary multiamines such as linear polyethylenimine (PEI). Didas et al.27 evaluated different pathways to form urea by DFT calculations and discovered that the one producing isocyanate as intermediate is the lowest-energy route. This finding indicates that primary amines are more likely to form urea, in line with the finding by Sayari et al.28
Steam is relatively cheap and widely available and used in industrial operations.36 When using steam to regenerate the adsorbent, it is simple to separate the regenerated CO2 from the product gas via condensation of water. Solid amines obtained on silica-type support through different preparation methods were shown to be completely regenerated through steam stripping under mild conditions.34 However, the supports of these adsorbents, amorphous silica, can be problematic during long-term steam treatment. The hydrothermal stability of silica supported amine solid sorbents has been investigated by Li, Jones, et al.35 They investigated different classes of amine sorbents, supported with silica mesocellular foam (MCF) by flowing steam in the presence of air or nitrogen at 106–120 °C for 24 h. The degradation of these amine sorbents is because structural collapse, which is supported by a reduction in surface area and pore volume. For this reason, the same group switched from MCF silica to alumina for further research.37 Three years later, in 2013, Hammache, Pennline, et al.32 investigated the impact of steam on sorbent of PEI impregnated with silica. After 5 h exposing to steam at 105 °C, a decrease of 12% in CO2 uptake was measured. A reduction in the surface area and pore volume on the sorbent was observed after steam treatment, which is in line with the observations from Li et al.35 One intriguing finding from Hammache et al.32 is that no structural destruction was identified from SEM and BET on bare SiO2 supports. Based on their study, they postulated that the decreases in texture properties are attributed to a reagglomeration of the amines, resulting in a partial blockage of the pores, thereby limiting CO2 access.
In summary of literature findings, the stability of amine based sorbents are related to the natures of amine, the types of support, and the preparation methods. Specifically with regard to the types of support, the effect of O2, CO2, and H2O have been mainly explored on the materials with silica-type supports,21,22,26,30,38 alumina-type supports,33,37 or cellulose.25,39 Recently another type of supported amine sorbents, amine functionalized ion exchange resins (and specifically Lewatit VP OC 1065), was investigated for CO2 removal applications in our research group. This material is a polystyrene based ion-exchange resin (IER), functionalized with a primary amine.40 It is demonstrated that this IER exhibits high CO2 equilibrium capacity, fast kinetics,20 and high tolerance of water.41,42 Yet there is little information on the stability of this IER. An initial study of the stability of this IER has been done by the group of Kitchin, measuring the performance decay in the presence of air at 120 °C.43 According to their results, the CO2 adsorption capacity reduces dramatically after 7 days of continuous treatment. But the temperature as high as 120 °C would not be necessary for the regeneration using air as sweep gas. So far, there is no comprehensive study on the stability of this IER.
The objective of this paper is to study the stability of this IER over variable conditions during long-term exposure, thereby varying specifically the O2, CO2, and H2O partial pressure in the feed gas and operating over a wide temperature range. The deactivation results can be used to extrapolate the sorbent lifetime. Furthermore, regeneration experiments in inert gas under varying flow rate, and temperature will be studied using a lab-scale fixed bed reactor.
2. Experimental Section
2.1. Material
The sorbent material used in this study was obtained from Lanxess. It is a commercial adsorbent contains polystyrene–divinylbenzene copolymer functionalized with aminomethylene groups.44 The external morphology was measured by scanning electron microscopy (SEM) named JEOL JSM 6010LA; the result is shown in Figure 1 displaying the porous structure of the sample. The averaged pore volume, surface area, and pore radius of the IER are 0.2 cm3/g, 25 m2/g, and 38 nm, respectively. The adsorbent is spherical-bead like with diameter between 0.3 and 1 mm. The molar concentration of N is 7.5 mol/kg, measured by Alesi et al.45 via energy-dispersive X-ray spectroscopy (EDS).
Figure 1.
SEM graph of Lewatit VP OC 1065.
2.2. Continuous Exposure to O2-, CO2-, N2- and H2O-Containing Gases
The stability of the IER under the conditions of continuous exposure to O2-, CO2-, N2-, and steam-containing gas was examined in a continuous flow setup, presented in Figure S1 in the Supporting Information (SI). A test tube DURAN GL 14 (13 mm diameter and a 100 mm height) was loaded with 1 g of the adsorbent. The test tube was put into a heating block with temperature controller. Degradation experiments were performed at the temperature range of 50–200 °C with different gas compositions, obtained by mixing technical-air, pure N2, and pure CO2. To exclude a possible impact of CO2 during the oxidative degradation tests, a larger “guard bed” filled with the same IER was connected upstream of the test tube to obtain CO2 free air. A bubbling humidifier filled with deionized water was connected prior to the test tube and mixed with dry air and dry concentrated CO2 to investigate the moisture effect. Two three-way valves were connected to let the gas mixture either bypass or pass through the “guard bed” and/or the humidifier. Prior to each experiment, the IER was first heated up to 100 °C for 1 h in N2 to desorb any preadsorbed CO2 and H2O. A separate test showed that by this pretreatment the sorbent capacity was not affected negatively. After this desorption step, the conditions in the test tube were adjusted to the measurement temperature, before switching to the gas to be tested; dry air, CO2-free (CF) air, CO2/O2 (0–42% CO2, 12% or 21% O2, both as dry and as humidified gas), N2, or CO2/N2 (80% or 100% CO2). The oxidative stability was tested at different temperatures in the range of 50–120 °C up to 72 h of exposure. Particularly, an extensively long measurement of 18 days dry air exposure was carried out at 80 °C for inquiring more information on the effect of oxygen after 72 h. Apart from the effect of oxygen, the CO2-induced stability was measured under the flow of 80 vol % and 100% CO2/N2 at 120 and 150 °C up to 7 days. Additionally, the thermal effect was measured in pure nitrogen at 150 °C for 72 h. After the long-term exposure treatment, the material was then cooled at lab temperature under flowing N2 and then collected for further analysis of the remaining CO2 adsorption capacity. Steam stability was studied in small assembled lab-scale setup, where 1 g of the adsorbent was loaded in a Büchner funnel with filter paper underneath. Under the funnel, a three neck flask filled with deionized water was applied as steam generator. The other two mouths of the boiling flask were connected to the supply of water and the thermometer. The supply of water compensates the loss of steam but did not affect the water boiling. A watch glass was used to cover the funnel. The thermocouple was connected to a hot plate below the boiling flask. By regulation of the power of the hot plate, intensive boiling water can be generated. In this way, the setup produced a continuous flow of saturated water vapor at ambient pressure, passing the adsorbent particles. In advance to the steam exposure, the IER has been treated in N2 at 100 °C for 60 min then moved to the setup for treating in steam continuously for 48 h. Subsequently, the CO2 adsorption capacity was measured and compared with that of the fresh adsorbent.
2.3. Adsorbent Characterization after the Degradation Experiments
A NETZSCH STA 449 F3 Jupiter thermogravimetric analyzer (TGA) was used to evaluate the CO2 adsorption uptake of the IER before and after the degradation experiments. The CO2 uptake was measured twice for each sample, then the average value was shown in the Results and Discussion section. A typical run in TGA consists of preheating the sample in flowing N2 at 100 °C for 1 h to remove the preadsorbed CO2 and moisture, then cooling to 40 °C before switching to a gas mixture of 15% CO2/N2 for 3 h of adsorption. Elemental analysis (Carlo Erba EA 1100 CHNS, EA) was used to determine any change in chemical composition of the IER after continuous exposure treatment. Prior to the elemental analysis, the sample was again pretreated in flowing nitrogen at 100 °C for 1 h to obtain a CO2-unloaded sample. Approximately 20 mg of ground sample was placed into the machine for one measurement, which takes 6 min. Surface areas and total pore volumes of the sample were measured to identify the changes in the morphology of the IER after continuous exposure in O2-containing gas. The results were estimated from N2 physisorption data obtained by measurements performed on a Micromeritics Tristar apparatus at 77 K. Prior to physisorption analysis, the sample was degassed at 150 °C for at least 10 h. Surface areas were estimated by the Brunauer–Emmett–Teller (BET) equation. The pore size distribution and the pore volume were determined from the nitrogen desorption branch using the Barrett–Joyner–Halenda (BJH) method. Fourier transform infrared (FTIR) spectroscopy analysis was used to determine the changes in the nature of amine of the IER as a result of exposure to O2- and CO2-containing gases. Measurements were taken on a Bruker IR Tensor 27 at an optical cell temperature of 200 °C, and spectra were recorded in the range of 400–4000 cm–1.
2.4. Thermal Swing Desorption of CO2
The regeneration of the sorbent material was measured in separate fixed-bed (16 mm ID, 500 mm long) setup. A schematic is shown in Figure S2 in the SI. The setup was equipped with other apparatuses such as three mass flow controllers, a water bath, and an infrared CO2 gas analyzer. The CO2 concentration in the inlet gas was controlled by mixing a flow of high purity (grade 5.0) N2 and high purity (grade 5.0) CO2. The flow rates were controlled using two BROOKS mass flow controllers. The CO2 analyzer (LI-COR LI840A) was used to monitor the CO2 concentration in the outlet gas of the fixed-bed reactor (detection range 0–2 mol %). In the adsorption process, a JULABO F32 water bath was used to control the temperature of the reactor. In the regeneration process, an electric heating spiral, which was wound around the column, was used to control the temperature to reach the maximum temperature of 150 °C. Typically, around 5 g of dried IER was loaded in the reactor during the fixed bed desorption tests. Prior to the adsorption, the reactor was first heated up to 100 °C for 60 min to completely desorb the preadsorbed CO2 and water. Then, 1.5 vol % CO2/N2 gas stream passed through the column at 40 °C until the concentration of CO2 in the outlet equaled the concentration in the inlet. Subsequently, temperature swing desorption by N2 stripping was conducted by using N2 as sweep gas to the reactor at flow rates in the range of 0.50 to 2.50 L/min at different temperatures in the range of 80 to 120 °C. The product gas of the regeneration was brought to a nondispersive infrared (NDIR) CO2 analyzer for quantification.
3. Results and Discussion
3.1. Thermal Effect and the Effect of Oxygen on the Sorbent
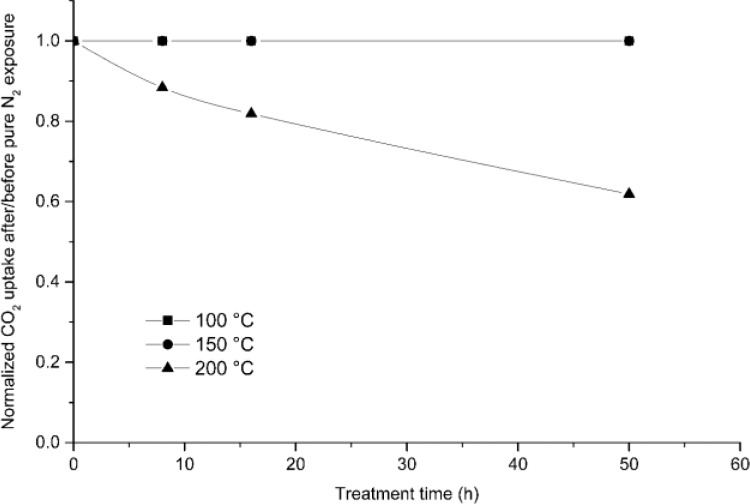
The thermal and oxidative stability of the IER was measured in pure N2 and O2 containing gases at different temperatures. The adsorbent material displays thermal stability when the temperature is below 150 °C, as shown in Figure 2. The sorbent has been tested at 100 and 150 °C; the curves displayed in Figure 2 are straight and overlapping, indicating that there is no degradation after 50 h at both temperatures. Yet the sorbent is degraded severely when temperature is ramped up further to 200 °C. It is found that 39% of the CO2 uptake capacity is lost after continuous exposure in N2 at 200 °C for a time span of 50 h. However, for this IER a regeneration temperature of 150 °C seems sufficiently high to allow for complete regeneration, and there is no clear need to increase the temperature beyond this level. Furthermore, this result can be regarded as blank test to compare with the results in the succeeding sections.
Figure 2.
Normalized CO2 adsorption uptake capacity (evaluated at 15 vol % CO2, 40 °C) of the IER after long-term exposure to N2 at temperatures of 100, 150, and 200 °C.
To evaluate the impact of oxygen during adsorption or regeneration, experiments were done in the setup described in the section 2.2. The experiments were carried out using a continuous flow of dry air in the temperature range of 50 to 120 °C, which was deemed as practical thermal swing operating window. Figure 3 shows the CO2 adsorption capacity of the adsorbent after treatment, normalized by their adsorption capacity before treatment, and plotted as a function of the treatment duration. According to the results, the CO2 capacity of the IER is affected by the oxygen when the temperature is high. The IER displays a dramatic decrease in CO2 uptake capacity when the temperature is above 80 °C, whereas it seems stable (for the time span evaluated) at 50 °C. The CO2 uptake reduces by as much as 30.2%, 46.7%, and 80.5% of its original capacity, when treated at temperatures of 80, 100 and 120 °C, respectively, for 72 h. On the other hand, the adsorbent material does not seem to be progressively degraded by the oxygen-containing gas by the continuous exposure at the lower temperature conditions (50–70 °C). The CO2 adsorption capacity decreases less than 10% at 70 °C after 72 h air exposure. It is noteworthy that the rate of losing capacity from 80 to 120 °C is faster at the beginning and decreases with progressing time. The degradation rate increases with temperature; it seems that a kinetic effect is a dominant factor in the degradation mechanism. To examine the effect of air in a prolonged condition (more than 72 h), a longer measurement was done at 80 °C for 432 h, resulting in an additional 31% of CO2 capacity losses (hence, to a total capacity loss of 61% in 432 h). To study the thermal effect separately from that of oxygen, the IER was also treated at elevated temperature in the presence of pure N2. A minor 5% decrease in CO2 adsorption capacity was found in the IER after being treated in pure N2 for 72 h at 150 °C, ruling out the thermal effect as main contributor to capacity loss. It was therefore concluded that the main reason for the capacity decrease observed was oxidative degradation. Earlier studies demonstrated that primary amines are, among the amines, the most stable ones to oxidative degradation.29,30 The amine sorbents in both cited studies are supported with silica-type material. Our results are in line with an earlier publication of Hallenbeck et al., who found 79% CO2 capacity loss after continuous exposure in air at 120 °C for 7 days using the same material.43
Figure 3.
Effect of temperature in dry air exposure on the CO2 adsorption capacity (evaluated at 15 vol % CO2, 40 °C) as a function of treatment time.
In a further experiment, the effect of the oxygen concentration on the degradation rate was investigated. The experimental work was carried out by mixing the air with nitrogen to a gas mixture with a reduced oxygen concentration of 12 vol %, as verified by micro-GC analysis. A series of experiments was carried out by treating the sorbent in 12% O2 at 80, 100, and 120 °C for 72 h. According to the results in Figure 4, the reduction of oxygen concentration slows down the degradation rate for the sorbent in all cases. After 72 h, the CO2 capacity during treatment with 12% O2 decreases by 9%, 42%, and 72% at 80, 100, and 120 °C, respectively, versus 30.2%, 46.7%, and 80.5% reduction in air at the same temperatures. Decreasing the concentration of oxygen to 12% reduced the degradation most significantly at 80 °C. At higher temperatures the differences between runs with air and the gas with 12% O2 is much smaller. The results show that oxidative degradation at high temperature is difficult to avoid if oxygen is present. It is therefore essential to avoid as much as possible the presence of oxygen during the regeneration process.
Figure 4.
Normalized CO2 adsorption capacity (evaluated at 15 vol % CO2, 40 °C), normalized by fresh sorbent capacity under the same conditions, after treating the sample with 12% and 21% O2 (balance N2) at the temperature of 80, 100, and 120 °C. At 100 °C, the IER was treated under two additional conditions: (a) 12% O2 and 42% CO2; (b) 12% O2, 42% CO2 and 2847 Pa water.
Subsequently, the effect of CO2 on oxidative degradation of the IER was studied. From the work by Heydari-Gorji et al.23 it appears that the oxidative stability of supported amines is significantly improved in the case of humid gases containing both O2 and CO2. Experiments were carried out using 12%:42%:46% O2/CO2/N2 at 100 °C and atmospheric pressure for both dry and humidified purge gases, with a partial pressure of water of 2847 Pa. As shown in Figure 4, this IER exhibits 30% and 24% uptake capacity losses after exposure to dry and wet CO2 containing gases at 100 °C after 72 h. This is significantly less compared to 42% CO2 capacity losses at the same oxygen concentration without CO2. Hence, indeed, the oxidative stability increases in the presence of humidified gases containing both CO2 and O2 under mild conditions, probably because of their rapid conversion to carbamate and bicarbonate that protect the amine group from degradation by oxygen.23 However, we found that the reduction in O2 degradation is not as large as in the cited study. When oxygen is present during regeneration at elevated temperatures, some degradation seems unavoidable.
Apart from the study of oxidative stability of the IER at high temperatures, also the sorbent oxidative stability at relative low temperature was studied. In this test, 2 g of the sample was exposed to ambient (indoor) air at 20–25 °C, at 30–60% relative humidity for three months. The sample was then collected and its remaining capacity for CO2 was measured every month. The results (Figure S3 in SI) show there is no significant capacity loss after three months of exposure. The oxygen only has detrimental effect on the IER at high temperature (above 70 °C in this study), but exposure to oxygen at ambient conditions (e.g., during CO2 air capture or in flue gas) is not leading to significant degradation rates. Since only continuous exposure is tested here, additional work with multiple adsorption/desorption cycles is recommended for confirmation and for evaluation of sorbent lifetime during cyclic operation.
3.2. Sorbent Characterization before and after Oxidative Degradation
On the basis of the results in the section 3.1, the loss of CO2 capacity during regeneration is found to be more related to oxidative degradation rather than to thermal degradation effects. Several chemical reaction pathways involved in oxidative degradation of amines have been proposed in literature. In these proposed reactions, the reaction products can be categorized as in either gas31 or solid phase.38 If the loss of amine functionality leads to gaseous degradation products, there would be a corresponding change in the nitrogen content of the sorbent. For this reason, elemental analysis (EA) and sorbent structure characterization techniques (BET and BJH, SEM) were applied to characterize the material. Besides, FTIR analysis was applied to identify possible newly formed species in the solid state. The degraded sample used in this section was obtained after treatment in dry air at 120 °C for 72 h.
The elemental composition of the IER before and after oxidative degradation is shown in Table 1. The experiments were repeated three times resulting in nitrogen loadings of 6.82 mol/kg for the fresh sample and 5.25 mol/kg for the degraded sample, a reduction of 23%. Meanwhile, the (dry) CO2 adsorption uptake drops from 2.15 to 0.42 mol/kg, hence a reduction of more than 80%. Since in the absence of water two moles of amine bind to one mole of CO2 in dry conditions theoretically, the decrease in CO2 adsorption capacity is much larger than the decrease in N-content. Therefore, the decrease of the N-content is not the main reason (nor the main characteristic in analysis) for the reduction of CO2 uptake.
Table 1. Mass-Based Elemental Composition and CO2 Capacity of Lewatit VP OC 1065 before and after Degradation Experiments in Dry Air at 120°C for 72 h.
| Adsorbent | % C | % H | % N | % O | N loading (mol/kg) | CO2 uptakea (mol CO2/kg IER) |
|---|---|---|---|---|---|---|
| fresh_01 | 81.04 | 8.38 | 9.53 | 1.05 | 6.81 | 2.15 |
| fresh_02 | 80.48 | 8.23 | 9.46 | 1.83 | 6.76 | 2.15 |
| fresh_03 | 80.57 | 8.29 | 9.64 | 1.50 | 6.89 | 2.15 |
| degraded_01 | 79.06 | 7.01 | 7.24 | 6.68 | 5.17 | 0.42 |
| degraded_02 | 80.00 | 6.99 | 7.44 | 5.57 | 5.31 | 0.42 |
| degraded_03 | 79.53 | 7.01 | 7.36 | 6.10 | 5.26 | 0.42 |
CO2 capacity was measured at 40 °C for 15 vol % CO2 in N2 at atmospheric pressure
Oxidative degraded species still present in the solid sorbent may lead to CO2 adsorption capacity decreases for two reasons. First, the newly formed species may accumulate in the pores, potentially leading to pore blocking, which should be reflected by morphology changes in the degraded material. To analyze this, the surface area, pore volume, and pore radius were measured as shown in Table 2. The results show the surface area of the fresh and degraded sample to be similar. The pore volume decreases somewhat from 0.20 to 0.16 cm3/g accompanied by a reduction of pore diameter from 38 to 32 nm. However, the CO2 uptake losses, as large as 80%, exceed the 20% reduction in the pore volume and the minor change in area. The minor change in morphology is further confirmed by SEM. The surface pore volume of the degraded adsorbent declines somewhat but cannot completely explain the extreme reduction in the capacity for CO2. The results of SEM can be found in Figure S4 in the Supporting Information. Thus, the structural morphology changes only contribute slightly, if at all, to the losses of the CO2 uptake. Second, the amine functional group is altered, forming new species that are incapable of capturing CO2. For this purpose, it is important to examine the functional groups present in the sorbent after oxidative degradation.
Table 2. N2 Physisorption Characterization of Lewatit VP OC 1065 before and after Oxidative Degradation in Dry Air at 120°C for 72 h.
| fresh IER | degraded IER | |
|---|---|---|
| BET surface area (m2/g) | 24.8 | 23.4 |
| BJH pore volume (cm3/g) | 0.20 | 0.16 |
| BJH pore diameter (nm) | 38 | 32 |
Figure 5 shows the FTIR spectra of IER before and after exposure to 120 °C for 72 h in dry air. All the samples have been desorbed in a flow of N2 at 100 °C for 1 h in advance to measuring the FTIR spectra, in order to eliminate the C=O signal due to carbamate. After treatment in oxidizing conditions, it was found that the intensity of the bands in the ranges of 1350–1480, 1600, 2850–3000, and 3300–3400 cm–1, which belong to the alkane C–H bending, amide N–H deformation of primary amine, C–H stretching, and N–H stretching, decrease.38,46 After oxidative degradation, the decrease of the intensity of C–H band is more pronounced than that of the N–H stretching bond. Similar changes of a reduction of FTIR peak intensity of the band in C–H stretching range can be observed in studies with other supported amine sorbents, such as AEAPDMS-NFC25 and TP600S,38 treated for 15 h in humid air at 90 °C and for 12 h at 100 °C in air, respectively. Furthermore, a new peak in the range of 1660–1680 cm–1 was observed after oxidative degradation, which is consistent with the findings of other studies in the field of oxidative degradation.29,38 However, different types of species were related to this range according to different papers. Calleja et al.47 found an additional peak at 1667 cm–1 on their amine grafted SBA-15 after drying in air at 110 °C for 85 h and associated this with C=N species. Meanwhile, Srikanth et al.38 tested one type of SAS with SiO2 supported on TEPA, which lost 55% of its original CO2 adsorption capacity after exposure in air at 100 °C for 12 h, exhibiting an extra peak at 1670 cm–1 upon degradation. It was proposed in Srikanth’s study that there are two species corresponding to this peak. One is nitrite (N=O) formed by oxidation of the primary amine. The other is carbonyl C=O resulting from amide species, of which the band is overlapping with the nitrites N=O band. In the present work, both the NH2 and the CH2 spectra decline, which may point to the formation of species in line with Srikanth’s study. The formation of nitrite and amide is in agreement with the increased oxygen content, see Table 1. In summary, the decrease of intensity in the ranges of 1350–1480, 1600, 2850–3000, and 3300–3400 cm–1 together with the increase in the range of 1660–1680 cm–1 clearly shows the change on the surface groups in the degraded sorbent.
Figure 5.
IR absorbance spectra before and after exposure to dry air at 120 °C for 72 h.
3.3. CO2-Induced Degradation
Using CO2 as purge gas for regeneration can be a relevant condition when pure CO2 is targeted as product. Due to the adsorption equilibrium, the required regeneration temperature when using pure CO2 to desorb the sorbent is much higher than for nitrogen stripping. According to the result of Alessi and Kitchin,45 the resin used in this study can regenerate completely under 1 atm of CO2 at 200 °C. However, the thermal stability turns out to be a problem at this temperature for the IER studied here. Based on the results on thermal stability, as shown in Figure 2, the maximum temperature of continuous CO2 exposure was found to be around 150 °C. We therefore evaluated the sorbent stability under 0.8 atm CO2 at 120 °C and at 1 atm of CO2 at 150 °C, as can be seen in Figure 6. By comparing of the results at 120 and 150 °C, we found that sorbent degradation increases with temperature and with the partial pressure of CO2. The loss of CO2 capacity is around 9% at 120 °C under continuous 80% CO2 exposure for 72 h. The degradation for (repeated) short periods of exposure was not tested, but this is probably best tested in a multicycle duration test. The samples after the 72 h continuous treatment were analyzed by FTIR, see Figure 7. The results of FTIR show that the deactivated sample develops a peak at 1670 cm–1 and the intensity of this peak increases with the increased extent of degradation. The developed peak lays in the same range with the FTIR result of fresh urea, which points toward the formation of urea after treatment in concentrated CO2.
Figure 6.
CO2 adsorption capacity after treatment in dry 80% CO2/N2 at 120 °C and 100% dry CO2 at 150 °C, normalized by the fresh sorbent capacity.
Figure 7.
IR spectra for IER after treatment in dry 80% CO2 at 120 °C and 100% dry CO2 at 150 °C for 72 h conditions, as well as for fresh urea and undegraded IER sample. Samples of IER were pretreated at 100 °C in flowing N2 for 1 h then cooled.
From the literature, it was found that primary amines are more likely to form urea than secondary and tertiary amines in the presence of CO2 since the intermediate species in urea formation, isocyanate, is only produced from primary amines.28 Surprisingly, this IER shows less tendency to be deactivated by CO2 to form urea in comparison with other SASs, reported in literature: the CO2 uptake loss of PEI-423/600/1800-MM,21 PEI-SBA-15,23 and MCM-41-s-pMono22 after exposure in pure CO2 for 1 h at 130 °C, for 10 h in 5% CO2 at 105 °C, and at 55 °C in pure CO2 for 30 h, respectively, are all above 20%. The treatment conditions for the amine based sorbents mentioned above were significantly less severe compared to the conditions in this study. This distinguished stability in concentrated CO2 of the IER studied here may originate from the manufacturing method and, hence, is probably more related to the amine–support interaction than to the type of amine. This is illustrated by an earlier study,22 where the adsorption capacity loss of PEI-MCM-41 prepared by impregnation decreased by 41% after CO2 exposure at 105 °C for 22 cycles while the capacity loss was as high as 45% for MONO-MCM-41, a grafting material, treating under the same conditions but for 40 cycles.
The sorbent was also tested for degradation under CO2 exposure after humidifying the gas at the dew point of water at 23 °C. The experiments were conducted under a flow of 80% CO2/N2 at 120 and 150 °C for 72 h in both dry and wet conditions. Based on the results shown in Table 3, the sorbent treated with humidified gas degraded less compared with the IER treated under dry conditions. In an additional experiment, the material was treated first in a flow of pure dry CO2 at 150 °C for 72 h, then under a flow of N2 containing 0.6% RH for 24 h without altering the temperature. The CO2 uptake of the fresh sample, the sample treated in dry CO2, and the sample post-treated in wet N2 is 2.15, 1.66, and 1.80 mol/kg, respectively. Hence, there is a recovery of 7% of the CO2 capacity of the fresh IER due to post-treatment in wet N2. These findings demonstrate that the CO2-induced deactivation of sorbent is reduced but not completely recovered by either using humidification of the feed or postprocessing hydrolysis. As opposed to the findings in this study, Sayari et al. indicated that the formed urea from grafted propyl amine can be completely recovered under a stream of N2 containing 0.15% RH and 200 °C.22 For this SAS, however, it is difficult to reproduce the condition since thermal degradation occurs at 200 °C. In other studies, urea formed was recovered in SBA-15PL-60023 and MONO-MCM-4122 by adding moisture at a dew point of 20 °C to the CO2 stream at 75 °C (6% RH) and 105 °C (2% RH). The experiments in this study were carried out at lower RH, which may have contributed to the incomplete prevention of urea formation or recovery by hydrolysis. In an attempt to improve the prevention of urea formation, the temperature of the water column saturator was increased from 23 to 60 °C, but the resulting increase in RH did not improve the results. In conclusion, it seems advisable to control the temperature below 120 °C to avoid CO2-induced degradation, which is important in view of sorbent regeneration.
Table 3. CO2 Uptake for IER after Exposure to Dry Concentrated CO2, Wet Concentrated CO2, and Post-Processing in Humidified N2.
| q_CO2a adsorption uptake (mol/kgIER) |
||
|---|---|---|
| condition | dry | wet |
| 120 °C, 72 h, 80%b | 1.97 | 2.10 (2 vol % H2O) |
| 150 °C, 72 h, 80%b | 1.69 | 1.81 (1.8 vol % H2O) |
| 150 °C, 72 h, 100%c | 1.66 | 1.80 (1.8 vol % H2O) |
q_CO2 = 2.15 for the undegraded IER, measured at 40 °C under 15% CO2/N2.
Concentrated CO2 streams were humidified using a water column controlled at 23 °C.
Degraded sample was collected, then treated in a flow of N2 at 150 °C containing 0.6% RH for 24 h.
When using pure CO2 at ambient pressure as the purge medium at this temperature, thermodynamics limits the working capacity of CO2. The proposed mode of application is to reduce the CO2 partial pressure during regeneration by using steam or reducing the system pressure. Steam can be simply separated by condensation and has the accompanying advantage that it inhibits partly the formation of urea. For such a steam-assisted regeneration approach, it is necessary to check the effect of steam itself on sorbent stability, which is presented in the following section.
3.4. The Influence of Water Vapor on Sorbent Degradation
The effect of continuous exposure to steam on the uptake of CO2 in the IER, in comparison to other supported amine sorbents, in shown in Figure 8. As illustrated in the figure, no significant capacity loss for this IER was observed after 48 h of exposure. For nearly all other studies on steam stability of supported amines, a significant CO2 capacity decrease was observed. The differences are related to both the choice of the amine and the choice of supports, which are silica-based or γ-alumina based in the cited studies. When comparing the performances of these latter two supports, γ-alumina displays better stability toward steam exposure than mesoporous silica SBA-15.37 The poor steam stability of the mesoporous silica is owing to structure collapse and hydrolysis, which dramatically decrease the final CO2 capacity. On the other hand, the hydrolysis of γ-alumina forming boehmite does not result in significant loss of CO2 uptake.33 The formation of boehmite occurs within 12 h of steam exposure and resulted in merely 12% CO2 uptake loss, while amine leaching decreases 50% of sorbent CO2 loading capacity when the exposure to steam continued from 12 to 24 h. Apart from the choice of support, steam stability can also be affected by the preparation method of the sorbent. A sorbent MCF-HAS, which was prepared by in situ polymerization, lost 6% of capacity after N2/steam exposure at 106 °C for 24 h, whereas 19% of the capacity was lost for a sorbent MCF-PEI, which was synthesized by physical impregnation.35 The instability of these mesocellular foam (MCF) silica supported amines in steam is ascribed to both structure destruction of the supports and amine degradation. To sum up, both the choice of the support and the preparation method of the sorbent have significant influence on the steam stability of the solid amine sorbents. The good steam stability of the IER studied in this work is therefore enabled by the good hydrothermal stability of the sorbent structure and the C–C chemical bonding of the amine groups to the polymeric backbone of the sorbent, avoiding leaching of the functional amine group, and hence a much better hydrothermal stability than Si–O–C bonds.48
Figure 8.
Impact of continuous steam exposure on the normalized CO2 capacity for the studied IER and for other supported amine sorbents.32,33,35,37
3.5. Regeneration Studies Using Nitrogen Stripping
Regeneration of CO2 loaded sorbent by nitrogen stripping does not degrade the IER when the temperature is below 150 °C and is in that sense cost-efficient. For optimizing cost efficiency, the amount of (inert) stripping gas during regeneration should be minimized. In this study, the amount of inert stripping gas required during regeneration will be investigated using nitrogen, but results will hold for other inert gases, most likely including water vapor. The economy of nitrogen stripping is affected by the choice of temperature and flow. To assess the effect of temperature and flow on the IER, experiments were done at 100 °C with flow rates of 0.50–2.50 L min–1. At a fixed flow rate of 1.00 L min–1, the temperature was varied between 80 and 120 °C. The results of varying the flow rate are shown in Figure S5 in the SI, showing that the rate of regeneration increases with increasing flow rate of the stripping gas. Among the flows studied, the lowest flow rate of 0.50 L min–1 results in the most time-consuming regeneration process, requiring double the amount of time than for the flow rate of 1.00 L min–1 to regenerate 80% of CO2 from the IER. The reason for this is probably that the adsorption equilibrium limits the regeneration rate of CO2 at low flow rates, due to the higher concentration of CO2 inside the reactor during regeneration. Therefore, next to a temperature swing, it is important to maintain a certain flow to flush out the regenerated CO2 (partial pressure swing). To measure the effect of temperature on the nitrogen stripping at constant flow rate, the experiments were carried out at a flow rate of 1.00 L min–1 at temperatures in the range of 80–120 °C. The results are shown in Figure S6 in the SI. The results show that the regeneration rate of CO2 increases with increasing temperature. The regeneration takes much longer time at 80 °C than at the other four temperatures. This is because the adsorption equilibrium capacity noticeably limits the desorption rate at 80 °C. At higher temperatures the position of the adsorption equilibrium is much more favorable for desorption. Hence, for a given flow rate a certain minimum temperature is necessary to maintain a fast regeneration rate of CO2.
Operating at various flows and temperatures leads to different costs related to nitrogen use and to energy consumption for raising the temperature. The cost of purge (N2) for sorbent regeneration is related to the amount of purge (N2) required per amount of CO2 desorbed. The ratio of the amount of N2 required to desorb per amount of CO2 (factor F, in mol of N2/mol of CO2) is calculated using eq 2.
| 2 |
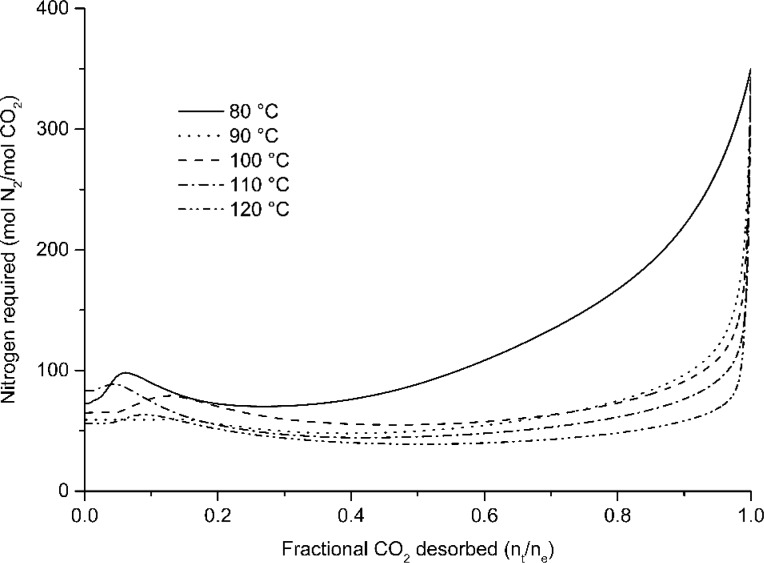
In this equation, n (mol) represents the total amount of CO2 that can be desorbed, while a and ta (s) represent the fraction of CO2 actually desorbed and the time required to reach that fraction. The fractional CO2 desorbed is defined by the ratio of CO2 desorbed at time ta (s) to the maximum total amount of CO2 desorbed during the regeneration. Symbols ϕN2 (L/s) and Vm (L/mol) represent flow of the nitrogen and the standard volume of 1 mol of gas at the desorption temperature. Figure 9 shows the relation between the fractional CO2 desorbed and the value of F. From this figure, it is clear that regeneration of the sorbent beyond 95% requires enormous amounts of purge gas. Furthermore, when the fractional CO2 desorbed is less than 90%, there is a decrease in the value of factor F as the flow is decreased. Although the regeneration time is maximal under 0.50 L min–1, it consumes the least amount of nitrogen to regenerate the same amount of CO2 in the first 90% regeneration.
Figure 9.
Effect of the flow on nitrogen required vs fractional CO2 desorbed at 100 °C in the range of 0.50–2.50 L min–1.
The choice of fractional regeneration of CO2 is also important as it determines the working capacity of the process. Working capacity also determines the energy consumption for regeneration in the form of sensible heat per unit of CO2. We estimated the purchase cost of the sorbent and cost of the nitrogen required at and 13 €/kg and 2 €/ton, respectively. The time of adsorption, 100% working capacity, and lifetime of the sorbent are assumed to be 1 h, 1.5 mol/kg,41 and 3 years, respectively. The results of the calculation of the costs related to sorbent and nitrogen use for regeneration at 100 °C and at 95% fractional CO2 desorbed are shown in Table 4 for the experimental flow rates studied. The results show that the cost of the purge overweighs the cost of the IER. The minimum cost of considering both the nitrogen and the sorbent occurs at minimum value of F. The idea is to decrease F by increasing the temperature, as this is favorable for reversing the adsorption equilibrium. The value of F needs to decrease to 65 to reach a total price less than 100 €/tonCO2.
Table 4. Moles of Nitrogen, Time Required to Desorb 95% of the adsorbed CO2, and the Corresponding Cost of Nitrogen and Sorbent per Amount of CO2 Captured over a Range of Flows at 100 °C.
| flow rate (L min–1) | F95 (N2/CO2) (mol/mol) | t95 (s) | cost of purge (€/tonCO2) | cost of IER (€/tonCO2) | total cost (€/tonCO2) |
|---|---|---|---|---|---|
| 0.50 | 125 | 2442 | 159 | 13.9 | 173 |
| 1.00 | 110 | 1071 | 140 | 10.8 | 151 |
| 1.50 | 118 | 768 | 150 | 10.2 | 160 |
| 2.00 | 119 | 583 | 152 | 9.7 | 162 |
| 2.50 | 130 | 530 | 166 | 9.7 | 176 |
The influence of temperature on the F factor is shown in Figure 10. The results show that the value of F decreases with increase of the temperature. The increase of temperature will result in a higher cost for the thermal energy to the increase the temperature from adsorption conditions (here taken at 40 °C) to the regeneration temperature. The economic analysis is estimated based on the energy consumption of heating the IER, nitrogen, and heat of reaction. The results are shown in Table 5. The heat capacity of the IER and the N2 are assumed to be 1.5 and 1.04 kJ/(kg·K).20 The calculation of the sensible heat does not consider any heat integration. At an increased regeneration temperature, the increment of the sensible energy required for the sorbent is compensated by a reduction in the energy consumed by the purge, since less purge gas is required. Both the value of F and the temperature affect the energy consumption of the purge. The energy consumption of the purge is always larger than either the energy of the sorbent or the reaction energy. Fortunately, this energy is also easier to recover if heat integration is implemented.
Figure 10.
Effect of the temperature on nitrogen required vs fractional CO2 desorbed under 1.00 L min–1 in the temperature range of 80–120 °C.
Table 5. Moles of Nitrogen, Time Required to Desorb 95% of the Adsorbed CO2, and the Corresponding Energy in the Regeneration at the Temperature of 80–120 °C under the Flow of 1.00 L min–1.
| temp (°C) | F95 (N2/CO2) (mol/mol) | t95 (s) | energy for IER (GJ/tonCO2) | energy for N2 (GJ/tonCO2) | reaction energy41 (GJ/tonCO2) | total (GJ/tonCO2) |
|---|---|---|---|---|---|---|
| 80 | 267 | 2603 | 1.0 | 7.1 | 1.7 | 9.8 |
| 90 | 121 | 1174 | 1.2 | 4.0 | 1.7 | 6.9 |
| 100 | 110 | 1071 | 1.4 | 4.4 | 1.7 | 7.5 |
| 110 | 92 | 897 | 1.7 | 4.3 | 1.7 | 7.7 |
| 120 | 69 | 669 | 1.9 | 3.7 | 1.7 | 7.3 |
In summary, a first indication of the required amount of stripping gas to regenerate the sorbent and per ton of CO2 regenerated is now obtained. When using a stripping gas, the cost of the purge medium is essential for determining the process economics. Even at a low, fictive price of 2 €/ton N2, the cost of nitrogen as stripping gas is much more than the cost of the thermal energy required. A cheaper purge medium and further process optimization is required to regenerate the sorbent. Steam stripping may be a better option in this regard as it enables sorbent regeneration and (pure) CO2 production. However, the presence of water in the process may have an influence on desorption kinetics for some types of supported amine sorbents.32,49 Further study on the effect of steam in terms of desorption kinetics is required and ongoing.
4. Conclusion
In this work, we have evaluated the thermal and chemical stability of Lewatit VP OC 1065 in view of the potential strategies of regenerating this sorbent in CO2 removal application. The effect of long-term continuous exposure to air, different O2/CO2/N2 mixtures, concentrated CO2, and steam on the CO2 adsorption uptake is investigated at different temperatures and exposure time for both dry and wet conditions. In view of the degradation observed, sorbent regeneration should be carried out in absence of oxygen when operating above 70 °C and at temperatures below 150 °C to avoid thermal degradation. If the partial pressure of CO2 approaches 1 bar, the maximum temperature should not be higher than 120 °C to avoid urea formation. Humidity was unable to completely prevent urea formation nor to reverse it. The application of steam or water vapor, however, did not negatively affect the sorbent capacity. Regeneration of IER by nitrogen stripping, as inert gas, has been evaluated in terms of required amount of purge gas at different flow rates and temperatures and for different degrees of sorbent regeneration. Nitrogen stripping is not an attractive option in practical application since it is too expensive. Considering the stability of the sorbent and the low cost of steam makes steam stripping a promising method for sorbent regeneration. Still, optimization of regeneration is required taking into account actual prices for utilities and sorbent costs, as well as heat integration.
Acknowledgments
This research was carried out within the EU MIRACLES project (www.miraclesproject.eu) and has received funding from the European Union’s Seventh Framework Program for research; technological development and demonstration under Grant Agreement No. 613588.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.iecr.6b04645.
Schematic of the adsorbent treatment setup, schematic of the adsorbent adsorption and regeneration setup, long-term oxidative degradation at ambient condition, SEM of fresh and degraded IER, effect of flow and temperature on adsorbent regeneration kinetics (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Raupach M. R.; Marland G.; Ciais P.; Le Quere C.; Canadell J. G.; Klepper G.; Field C. B. Global and regional drivers of accelerating CO2 emissions. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 10288–10293. 10.1073/pnas.0700609104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inventory of U.S. greenhouse gas emissions and sinks: 1990–2014; EPA 430-R-16-002; U.S. Environmental Protection Agency, April 15, 2016, 1990–2014; p 78.
- Cost and Performance Baseline for Fossil Energy Plants Vol. 1: Bituminous Coal and Natural Gas to Electricity. NETL; 2010.
- Bhown A. S.; Freeman B. C. Analysis and Status of Post-Combustion Carbon Dioxide Capture Technologies. Environ. Sci. Technol. 2011, 45, 8624–8632. 10.1021/es104291d. [DOI] [PubMed] [Google Scholar]
- Duke M. C.; Ladewig B.; Smart S.; Rudolph V.; Diniz da Costa J. C. Assessment of postcombustion carbon capture technologies for power generation. Front. Chem. Eng. China 2010, 4, 184–195. 10.1007/s11705-009-0234-1. [DOI] [Google Scholar]
- D’Alessandro D. M.; Smit B.; Long J. R. Carbon Dioxide Capture: Prospects for New Materials. Angew. Chem., Int. Ed. 2010, 49, 6058–6082. 10.1002/anie.201000431. [DOI] [PubMed] [Google Scholar]
- Kenarsari S. D.; Yang D.; Jiang G.; Zhang S.; Wang J.; Russell A. G.; Wei Q.; Fan M. Review of recent advances in carbon dioxide separation and capture. RSC Adv. 2013, 3, 22739–22773. 10.1039/c3ra43965h. [DOI] [Google Scholar]
- Mazari S. A.; Si Ali B.; Jan B. M.; Saeed I. M.; Nizamuddin S. An overview of solvent management and emissions of amine-based CO2 capture technology. Int. J. Greenhouse Gas Control 2015, 34, 129–140. 10.1016/j.ijggc.2014.12.017. [DOI] [Google Scholar]
- Dutcher B.; Fan M.; Russell A. G. Amine-Based CO2 Capture Technology Development from the Beginning of 2013-A Review. ACS Appl. Mater. Interfaces 2015, 7, 2137–2148. 10.1021/am507465f. [DOI] [PubMed] [Google Scholar]
- Samanta A.; Zhao A.; Shimizu G. K. H.; Sarkar P.; Gupta R. Post-Combustion CO2 Capture Using Solid Sorbents: A Review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. 10.1021/ie200686q. [DOI] [Google Scholar]
- Goto K.; Yogo K.; Higashii T. A review of efficiency penalty in a coal-fired power plant with post-combustion CO2 capture. Appl. Energy 2013, 111, 710–720. 10.1016/j.apenergy.2013.05.020. [DOI] [Google Scholar]
- Bollini P.; Brunelli N. A.; Didas S. A.; Jones C. W. Dynamics of CO2 Adsorption on Amine Adsorbents. 1. Impact of Heat Effects. Ind. Eng. Chem. Res. 2012, 51, 15145–15152. 10.1021/ie301790a. [DOI] [Google Scholar]
- Bollini P.; Brunelli N. A.; Didas S. A.; Jones C. W. Dynamics of CO2 Adsorption on Amine Adsorbents. 2. Insights Into Adsorbent Design. Ind. Eng. Chem. Res. 2012, 51, 15153–15162. 10.1021/ie3017913. [DOI] [Google Scholar]
- Monazam E. R.; Shadle L. J.; Siriwardane R. Equilibrium and absorption kinetics of carbon dioxide by solid supported amine sorbent. AIChE J. 2011, 57, 3153–3159. 10.1002/aic.12516. [DOI] [Google Scholar]
- Serna-Guerrero R.; Sayari A. Modeling adsorption of CO2 on amine-functionalized mesoporous silica. 2: Kinetics and breakthrough curves. Chem. Eng. J. 2010, 161, 182–190. 10.1016/j.cej.2010.04.042. [DOI] [Google Scholar]
- Zhao W.; Zhang Z.; Li Z.; Cai N. Continuous CO2 Capture in Dual Fluidized Beds Using Silica Supported Amine. Energy Procedia 2013, 37, 89–98. 10.1016/j.egypro.2013.05.088. [DOI] [Google Scholar]
- Hoffman J. S.; Hammache S.; Gray M. L.; Fauth D. J.; Pennline H. W. Parametric study for an immobilized amine sorbent in a regenerative carbon dioxide capture process. Fuel Process. Technol. 2014, 126, 173–187. 10.1016/j.fuproc.2014.04.027. [DOI] [Google Scholar]
- Zhang W.; Liu H.; Sun C.; Drage T. C.; Snape C. E. Capturing CO2 from ambient air using a polyethyleneimine–silica adsorbent in fluidized beds. Chem. Eng. Sci. 2014, 116, 306–316. 10.1016/j.ces.2014.05.018. [DOI] [Google Scholar]
- Schöny G.; Zehetner E.; Fuchs J.; Pröll T.; Sprachmann G.; Hofbauer H. Design of a bench scale unit for continuous CO2 capture via temperature swing adsorption—Fluid-dynamic feasibility study. Chem. Eng. Res. Des. 2016, 106, 155–167. 10.1016/j.cherd.2015.12.018. [DOI] [Google Scholar]
- Veneman R.; Hilbers T.; Brilman D. W. F.; Kersten S. R. A. CO2 capture in a continuous gas–solid trickle flow reactor. Chem. Eng. J. 2016, 289, 191–202. 10.1016/j.cej.2015.12.066. [DOI] [Google Scholar]
- Drage T. C.; Arenillas A.; Smith K. M.; Snape C. E. Thermal stability of polyethylenimine based carbon dioxide adsorbents and its influence on selection of regeneration strategies. Microporous Mesoporous Mater. 2008, 116, 504–512. 10.1016/j.micromeso.2008.05.009. [DOI] [Google Scholar]
- Sayari A.; Belmabkhout Y. Stabilization of Amine-Containing CO2 Adsorbents: Dramatic Effect of Water Vapor. J. Am. Chem. Soc. 2010, 132, 6312–6314. 10.1021/ja1013773. [DOI] [PubMed] [Google Scholar]
- Heydari-Gorji A.; Sayari A. Thermal, Oxidative, and CO2-Induced Degradation of Supported Polyethylenimine Adsorbents. Ind. Eng. Chem. Res. 2012, 51, 6887–6894. 10.1021/ie3003446. [DOI] [Google Scholar]
- Sayari A.; Heydari-Gorji A.; Yang Y. CO2-Induced Degradation of Amine-Containing Adsorbents: Reaction Products and Pathways. J. Am. Chem. Soc. 2012, 134, 13834–13842. 10.1021/ja304888a. [DOI] [PubMed] [Google Scholar]
- Gebald C.; Wurzbacher J. A.; Tingaut P.; Steinfeld A. Stability of Amine-Functionalized Cellulose during Temperature-Vacuum-Swing Cycling for CO2 Capture from Air. Environ. Sci. Technol. 2013, 47, 10063–10070. 10.1021/es401731p. [DOI] [PubMed] [Google Scholar]
- Ahmadalinezhad A.; Sayari A. Oxidative degradation of silica-supported polyethylenimine for CO2 adsorption: insights into the nature of deactivated species. Phys. Chem. Chem. Phys. 2014, 16, 1529–1535. 10.1039/C3CP53928H. [DOI] [PubMed] [Google Scholar]
- Didas S. A.; Zhu R.; Brunelli N. A.; Sholl D. S.; Jones C. W. Thermal, Oxidative and CO2 Induced Degradation of Primary Amines Used for CO2 Capture: Effect of Alkyl Linker on Stability. J. Phys. Chem. C 2014, 118, 12302–12311. 10.1021/jp5025137. [DOI] [Google Scholar]
- Sayari A.; Belmabkhout Y.; Da’na E. CO2 Deactivation of Supported Amines: Does the Nature of Amine Matter?. Langmuir 2012, 28, 4241–4247. 10.1021/la204667v. [DOI] [PubMed] [Google Scholar]
- Heydari-Gorji A.; Belmabkhout Y.; Sayari A. Degradation of amine-supported CO2 adsorbents in the presence of oxygen-containing gases. Microporous Mesoporous Mater. 2011, 145, 146–149. 10.1016/j.micromeso.2011.05.010. [DOI] [Google Scholar]
- Bollini P.; Choi S.; Drese J. H.; Jones C. W. Oxidative Degradation of Aminosilica Adsorbents Relevant to Postcombustion CO2 Capture. Energy Fuels 2011, 25, 2416–2425. 10.1021/ef200140z. [DOI] [Google Scholar]
- Bedell S. A.; Worley C. M.; Darst K.; Simmons K. Thermal and oxidative disproportionation in amine degradation—O2 stoichiometry and mechanistic implications. Int. J. Greenhouse Gas Control 2011, 5, 401–404. 10.1016/j.ijggc.2010.03.005. [DOI] [Google Scholar]
- Hammache S.; Hoffman J. S.; Gray M. L.; Fauth D. J.; Howard B. H.; Pennline H. W. Comprehensive Study of the Impact of Steam on Polyethyleneimine on Silica for CO2 Capture. Energy Fuels 2013, 27, 6899–6905. 10.1021/ef401562w. [DOI] [Google Scholar]
- Sakwa-Novak M. A.; Jones C. W. Steam Induced Structural Changes of a Poly(ethylenimine) Impregnated gamma-Alumina Sorbent for CO2 Extraction from Ambient Air. ACS Appl. Mater. Interfaces 2014, 6, 9245–9255. 10.1021/am501500q. [DOI] [PubMed] [Google Scholar]
- Li W.; Choi S.; Drese J. H.; Hornbostel M.; Krishnan G.; Eisenberger P. M.; Jones C. W. Steam-Stripping for Regeneration of Supported Amine-Based CO2 Adsorbents. ChemSusChem 2010, 3, 899. 10.1002/cssc.201000131. [DOI] [PubMed] [Google Scholar]
- Li W.; Bollini P.; Didas S. A.; Choi S.; Drese J. H.; Jones C. W. Structural Changes of Silica Mesocellular Foam Supported Amine-Functionalized CO2 Adsorbents Upon Exposure to Steam. ACS Appl. Mater. Interfaces 2010, 2, 3363–3372. 10.1021/am100786z. [DOI] [PubMed] [Google Scholar]
- Shah I. K.; Pre R.; Alappat B. J. Steam Regeneration of Adsorbents: An Experimental and Technical Review. Chem. Sci. Trans. 2013, 2 (4), 1078–1088. 10.7598/cst2013.545. [DOI] [Google Scholar]
- Chaikittisilp W.; Kim H.-J.; Jones C. W. Mesoporous Alumina-Supported Amines as Potential Steam-Stable Adsorbents for Capturing CO2 from Simulated Flue Gas and Ambient Air. Energy Fuels 2011, 25, 5528–5537. 10.1021/ef201224v. [DOI] [Google Scholar]
- Srikanth C. S.; Chuang S. S. C. Spectroscopic Investigation into Oxidative Degradation of Silica-Supported Amine Sorbents for CO2 Capture. ChemSusChem 2012, 5, 1435–1442. 10.1002/cssc.201100662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebald C.; Wurzbacher J. A.; Tingaut P.; Zimmermann T.; Steinfeld A. Amine-Based Nanofibrillated Cellulose As Adsorbent for CO2 Capture from Air. Environ. Sci. Technol. 2011, 45, 9101–9108. 10.1021/es202223p. [DOI] [PubMed] [Google Scholar]
- Tirio A. P.Process and apparatus for carbon dioxide capture via ion exchange resins. Patent US 20119988550 A1, 2011.
- Veneman R.; Frigka N.; Zhao W.; Li Z.; Kersten S.; Brilman W. Adsorption of H2O and CO2 on supported amine sorbents. Int. J. Greenhouse Gas Control 2015, 41, 268–275. 10.1016/j.ijggc.2015.07.014. [DOI] [Google Scholar]
- Smal I. M.; Yu Q.; Veneman R.; Fränzel-Luiten B.; Brilman D. W. F. TG-FTIR Measurement of CO2-H2O co-adsorption for CO2 air capture sorbent screening. Energy Procedia 2014, 63, 6834–6841. 10.1016/j.egypro.2014.11.717. [DOI] [Google Scholar]
- Hallenbeck A. P.; Kitchin J. R. Effects of O2 and SO2 on the Capture Capacity of a Primary-Amine Based Polymeric CO2 Sorbent. Ind. Eng. Chem. Res. 2013, 52, 10788–10794. 10.1021/ie400582a. [DOI] [Google Scholar]
- Product and manufacturer information - Lewatit VP OC 1065; LANXESS, edition 2011-12-21.
- Alesi W. R.; Kitchin J. R. Evaluation of a Primary Amine-Functionalized Ion-Exchange Resin for CO2 Capture. Ind. Eng. Chem. Res. 2012, 51, 6907–6915. 10.1021/ie300452c. [DOI] [Google Scholar]
- Bruice P. Y.Organic Chemistry, 7th ed.; Pearson: Upper Saddle River, NJ, 2014. [Google Scholar]
- Calleja G.; Sanz R.; Arencibia A.; Sanz-Pérez E. S. Influence of Drying Conditions on Amine-Functionalized SBA-15 as Adsorbent of CO2. Top. Catal. 2011, 54, 135–145. 10.1007/s11244-011-9652-7. [DOI] [Google Scholar]
- Wang J.; Huang L.; Yang R.; Zhang Z.; Wu J.; Gao Y.; Wang Q.; O’Hare D.; Zhong Z. Recent advances in solid sorbents for CO2 capture and new development trends. Energy Environ. Sci. 2014, 7, 3478–3518. 10.1039/C4EE01647E. [DOI] [Google Scholar]
- Hahn M. W.; Steib M.; Jentys A.; Lercher J. A. Mechanism and Kinetics of CO2 Adsorption on Surface Bonded Amines. J. Phys. Chem. C 2015, 119, 4126–4135. 10.1021/jp512001t. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.