Abstract
Oligomeric proanthocyanidins (OPACs) are potent and renewable natural bioactives possible to be refined into chemically standardized mixtures for biological applications. Herein, we found that multiscale interactions of OPACs with the dentin matrix create tight biointerfaces with hydrophobic methacrylate adhesives on wet surfaces. An enriched mixture of OPACs, with a known phytochemical profile, was produced from grape seed crude extract (Vitis vinifera; enriched grape seed extract [e-GSE]) and applied to dentin matrices to determine changes to the mechanical properties and biodegradability of the dentin matrix and favorable resin adhesion mechanisms. Methods included a 3-point flexural test, quantification of hydroxyproline (collagen solubilization), static and dynamic nanomechanical analyses, resin-dentin microtensile bond strength, and micropermeability at the adhesive interface. The e-GSE-modified dentin matrix exhibited remarkably low collagen solubilization and sustained the bulk elastic properties over 12 mo. Tan δ findings reveal a more elastic-like behavior of the e-GSE-modified dentin matrix, which was not affected by H-bond destabilization by urea. Dentin-methacrylate biointerfaces with robust and stable adhesion were created on e-GSE-primed dentin surfaces, leading to a dramatic decrease of the interfacial permeability. Standardized OPAC mixtures provide a new mechanism of adhesion to type I collagen–rich tissues that does not rely on hydrophilic monomers. The bioadhesion mechanism involves physicochemical modifications to the dentin matrix, reduced tissue biodegradation, and bridging to methacrylate resins.
Keywords: collagen, biointerfaces, nanoindentation, bond strength, extracellular matrix, polyphenols
Introduction
Type I collagen accounts for 30% to 40% of the human body and is found in structural organs such as bones and teeth. Type I collagen is the main constituent of the dentin organic matrix, and its mechanical properties are determined by its composition and complex hierarchical structure (Orgel et al. 2006; Bertassoni, Marshall, and Swain 2012). The dentin matrix has high clinical relevance in daily dental practice for the placement of adhesive restorations, accounting for an estimated 123 million annual placements of dental restorations in the United States alone (American Dental Association 2007).
Secondary caries and margin breakdown are the most frequent causes of failed adhesive restorations (Astvaldsdottir et al. 2015; Nedeljkovic et al. 2015). Despite numerous advances in dental restorative materials, degradation of the adhesive interface still occurs as a mutual process involving the adhesive resin and dentin (Chiaraputt et al. 2008; Castellan et al. 2013; Leme et al. 2015). The mechanism of adhesion relies on the formation of a hybrid zone, with micromechanical interlocking between the resin and the collagen-rich dentin matrix. The degradation of unprotected collagen at the resin-dentin adhesive interface by activation of host-derived enzymes is also associated with early failure of resin composite restorations (Mazzoni et al. 2012). Therefore, the stability of the underlying tissue is key for the durability of such adhesive joints and, hence, the service life of the restoration.
Biomimetic strategies to enhance the physicochemical properties of dentin and, thus, the interfaces between the tooth and the artificial material are a focus of investigation in our group. Such strategies involve the mediation of exogenous collagen cross-links to enhance the mechanical properties of the tissue (Bedran-Russo et al. 2012; Vidal, Aguiar, et al. 2014). The use of plant-derived proanthocyanidins that can be extracted from waste products of renewable resources has drawn wide attention to various fields of research and applications (He et al. 2008) from environmental and health perspectives. A size exclusion model can be applied for the dentin biomodification ability of proanthocyanidins (Vidal, Leme, et al. 2014), identifying medium-size oligomeric proanthocyanidins (OPACs) as the most promising bioactive principles (Phansalkar et al. 2015). The presence of a catechol moiety in catechins, the monomeric unit of proanthocyanidins, makes OPACs attractive as a new bioadhesive, since catechol-functionalized molecules have inspired adhesion studies in medical and materials engineering involving a variety of organic, inorganic, and metallic surfaces (Lee et al. 2007; Ryu et al. 2011; Jenkins et al. 2013; Zhang et al. 2014; Maier et al. 2015).
A series of studies were carried out to characterize interactions of enriched OPACs with the dentin matrix and the role of OPAC mixtures at establishing stable dentin-resin bonds. The key hypotheses for the present study were as follows: 1) treatment with an enriched OPAC mixture enhances the bulk (macroscale) and surface (nanometer scale) mechanical properties of the dentin matrix; 2) an enriched OPAC mixture mediates stable collagen cross-links and reduces the long-term degradation of dentin matrices; and 3) an enriched OPAC mixture exhibits potent bioadhesive properties, resulting in enhanced bond strength and sealing of the resin-dentin interface.
Materials and Methods
Preparation of Monomeric and Oligomeric-Rich Proanthocyanidin Mixtures
Enriched grape seed extract (e-GSE) was prepared from a crude extract of the seeds of Vitis vinifera by a 2-phase partitioning procedure (see Appendix), resulting in the exclusion of most of the higher-order oligomeric and polymeric proanthocyanidins, as reported previously (Phansalkar et al. 2015).
Tissue Bulk Assessments: Apparent Modulus of Elasticity and Collagen Solubilization
Dentin beams (n = 10; dimensions, 0.5 × 1.7 × 6 mm) were prepared from midcoronal dentin of freshly extracted human molars (Institutional Review Board protocol no. 2011-0312) and fully demineralized with 10% phosphoric acid (Vidal, Aguiar, et al. 2014; Vidal, Leme, et al. 2014; Phansalkar et al. 2015). The treatment solution was prepared by dissolving e-GSE 6.5% weight per volume (w/v) in 20mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (HEPES) buffer at pH 7.2. Specimens were immersed in treatment solution for 1 h at room temperature. Control group specimens were immersed in HEPES for the same period. The apparent modulus of elasticity was evaluated at different time points through a customized 3-point bending device (EZ Graph, Shimadzu; Vidal, Aguiar, et al. 2014). The tests were performed at baseline, after 1-h treatment, and after 3, 6, and 12 mo of storage in simulated body fluid (SBF) at 37 °C. The SBF was changed every 2 wk and consisted of 5mM HEPES, 2.5mM CaCl2, 0.05mM ZnCl2, and 0.3mM NaN3 (Tezvergil-Mutluay et al. 2010).
Collagen solubilization by endogenous proteases was assessed by the quantification of hydroxyproline in the storage media (SBF) collected every 2 wk and pooled into 2 time points: first 6 mo and between 7 and 12 mo. The hydroxyproline assay followed a standard protocol (Reddy and Enwemeka 1996) with modifications (see Appendix). Data were statistically analyzed through 2-way analysis of variance (ANOVA) and Tukey’s post hoc test (α = 0.05).
Tissue Surface Assessment by Nanoindentation
Effect of Treatment Time and Concentration
Human third molars (n = 4) had their occlusal enamel removed, were cut into 4 fragments, and embedded in epoxy resin. Coronal dentin was gloss polished, and a layer of dentin matrix depleted of inter- and intrafibrillar dentin was exposed with 35% phosphoric acid conditioning for 1 min (Balooch et al. 2008). Three concentrations (6.5%, 15%, and 30% w/v) of e-GSE primer (prepared as described above) were investigated at baseline (etched surface) and after 1, 15, and 30 min of cumulative treatment. The reduced modulus of elasticity (Er) was determined by quasi-static indentations, and the storage (E′) and loss modulus (E″) used to calculate the complex modulus (E* = E′ + E″) and tan δ (see Appendix) were obtained by dynamic mechanical analysis (nano-DMA). Data were analyzed statistically through 2-way ANOVA, followed by Scheffe’s or Games-Howell post hoc tests (α = 0.05).
H-bond Destabilization Effect by Urea
Additional specimens (n = 4 teeth) were prepared in the same manner described above for the nanoindentation studies and subjected to a protein destabilization protocol with urea to accelerate aging of the cross-links (Zou et al. 1998; Usha and Ramasami 2002). Experimental groups included 1-min treatment of HEPES (control) or 30% w/v e-GSE. Specimens were incubated in 4M urea (MP Biomedicals) for 1 h. A series of indentations were carried out at baseline, after treatment with HEPES or e-GSE 30% and after urea challenge through the same settings described earlier (see Appendix).
Artificial-Natural Material Interfaces: Resin-Dentin Adhesive Properties
Adhesion studies were carried out with glycolic acid as a surface conditioner and experimental resin blends with varying 2-hydroxyethyl methacrylate (HEMA) concentrations (see Appendix for complete method details and supporting data). Human third molars with complete root formation (n = 7) had the occlusal enamel removed and dentin surface polished with silicon carbide abrasive papers (180, 320, and 600; Buehler). The dentin surface was etched with 35% glycolic acid solution (pH 1.5) for 15 s and rinsed. The surface was treated for 1 min with either 30% w/v e-GSE in HEPES or HEPES only (control). After rinsing, 2 layers of the respective H0, H6, and H18 experimental adhesives were applied (no HEMA, 6 % HEMA, and 18% HEMA); the excess solvent was evaporated; and the adhesive layer was light cured for 40 s (intensity of 700 mW/cm2; Optilux 501, Kerr). A resin composite block (A2 body, Filtek Supreme; 3M ESPE) was incrementally built on the surface. The specimens were immediately immersed in SBF at 37 °C, and 24 h later, they were sectioned into resin-dentin beams with a cross-sectional area of 0.8 × 0.8 mm2 to evaluate the interfacial bond strength and sealing ability.
Resin-Dentin Interfacial Bond Strength
The adhesive bond strength was assessed by tensile (n = 7 teeth; Castellan et al. 2013) at 24 h and after 1 y of storage in SBF at 37 °C, with the SBF replaced every 2 wk. Microtensile bond strength (TBS) was calculated by dividing the peak load by the cross-sectional area. The data were analyzed statistically through 3-way ANOVA and Tukey’s post hoc test (α = 0.05).
Sealing of the Adhesive Interface
The micropermeability of the adhesive interface was assessed with confocal laser scanning microscopy (CLSM; complete method description: Aydin et al. 2016). The resin-dentin beams were embedded in epoxy resin and polished with SiC abrasive papers 320, 600, 800, and 1,200. Specimens were kept immersed in 0.1M rhodamine-B solution (pH 7.2, RITC/Rhodamine-B; Sigma) for 1 h, rinsed, and immediately observed under CLSM (LSM 710; Zeiss). The interfacial micropermeability was calculated as the intensity of rhodamine-B at the adhesive interface, captured by a He-Ne laser of 514-nm wavelength (red fluorescence). The intensity of collagen cross-links was assessed at the adhesive interface by the quantification of collagen autofluorescence, with an Ar laser of 488-nm wavelength (green fluorescence). Three images were obtained from each resin-dentin beam (n = 14) and processed in expert mode, with the profile tool in the CLSM software. Data were obtained as fluorescence emission intensity and evaluated statistically with 3-way ANOVA and Games Howell tests (α = 0.05).
Results
Tissue Bulk Assessments: Apparent Modulus of Elasticity and Collagen Solubilization
The e-GSE-treated group exhibited significantly higher apparent modulus of elasticity for all time points (P < 0.001). Although a significant decrease in the modulus of elasticity of the e-GSE group occurred within the first 3 mo of storage (P < 0.001), there were no statistically significant differences among 3, 6, and 12 mo of storage (P > 0.05). The typical collagen solubilization by endogenous proteases observed in the control group was significantly reduced by e-GSE primer (P < 0.05; Fig. 1).
Figure 1.

Results of the long-term apparent modulus of elasticity (A) of the dentin matrix and collagen solubilization by endogenous proteases reported as cumulative hydroxyproline (HYP; B) released in the 0 to 6 mo (lighter gray) and between 7 and 12 mos (darker gray). Asterisks (*) depict statistically significant difference between groups (P < 0.05). e-GSE, enriched grape seed extract.
Tissue Surface Assessment by Nanoindentation
Effect of Treatment Time and Concentration
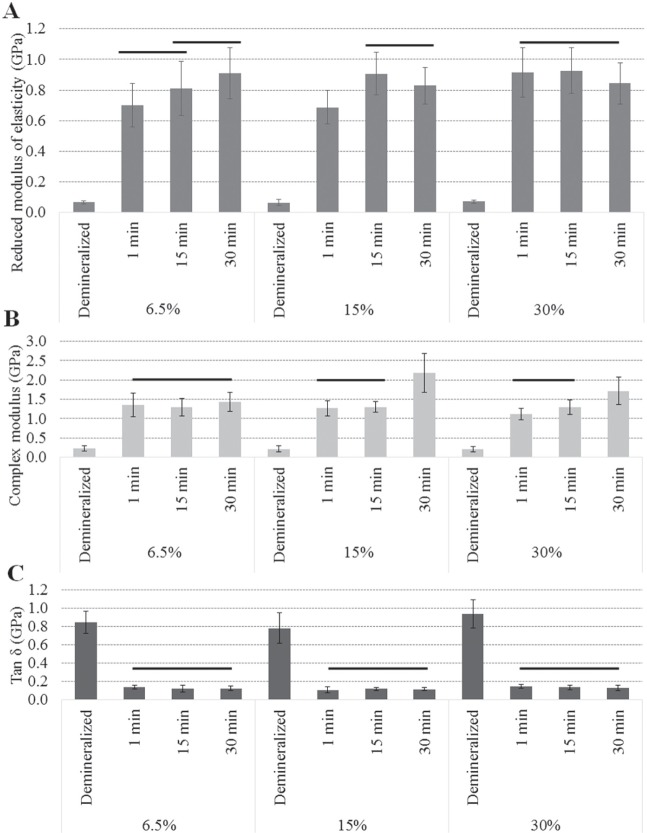
Within 1 min of application of e-GSE, the nanomechanical properties of the dentin matrix (Fig. 2) increased significantly for all tested concentrations (P < 0.001). The reduced modulus of elasticity (Er) of the dentin matrix was positively affected by the treatment time (P < 0.001) and concentration (P < 0.001). The use of lower e-GSE concentrations resulted in a gradual increase of the Er. In contrast, the application of 30% e-GSE resulted in a rapid increase in Er.
Figure 2.
Results of the nanomechanical properties of the dentin matrix surface before and after biomodification with enriched grape seed extract solutions at 6.5%, 15%, and 30% weight per volume (w/v): (A) the reduced modulus of elasticity (Er) after 1 min of surface treatment with enriched grape seed extract, (B) the complex modulus (E*), and (C) the tan δ values of the dentin matrix. Bars depicts statistically significant differences (p < 0.05) between the treatment times for each e-GSE concentration.
Moreover, a significant increase in the complex modulus was also observed after 1-min treatment with all e-GSE concentrations (P < 0.001). A significant decrease in tan δ indicated that the e-GSE-biomodified dentin matrices exhibited a more elastic-like behavior when compared with the native tissue. No differences were observed by applying cumulative treatment times and/or increasing the concentration of e-GSE (P > 0.05; Fig. 2).
H-bond Destabilization Effect by Urea
No significant effects of the urea protocol were observed on the dynamic nanomechanical properties of the native dentin matrix (P > 0.05; Fig. 3). When urea was applied to e-GSE-modified surfaces, a significant decrease in the complex modulus was observed (P < 0.001); however, the complex modulus still remained significantly higher as compared with baseline (P < 0.001). No change in tan δ was observed after urea challenge (P > 0.05).
Figure 3.

The complex modulus and viscoelastic behavior of enriched grape seed extract (e-GSE)–modified dentin and control dentin, as well as the effects of urea exposure. Different symbols depict statistically significant differences (P < 0.05) between time points: baseline, after e-GSE surface modification, and after urea exposure.
Artificial-Natural Materials Interfaces: Resin-Dentin Adhesive Properties
Resin-Dentin Interfacial Bond Strength
The adhesive properties were remarkably higher for the e-GSE primer groups (Fig. 4). Within the control groups, the TBS was significantly influenced by the hydrophilicity of the experimental adhesive resin (H18 > H6 > H0, P < 0.001) and aging (P < 0.001). In contrast, no such differences were observed among the experimental resins for the e-GSE-modified interfaces (P > 0.05). All groups treated with e-GSE primer remained stable after 1 y of aging (P > 0.05).
Figure 4.

Results of the microtensile bond strengths after 24 h and 1 y of storage in artificial saliva. Horizontal bars indicate statistically significant difference in control groups between 24 h and 1 y. Statistically significant differences (P < 0.05) among groups are depicted by different symbols (*, ϕ, α, and δ). H0, no HEMA; H6, 6% HEMA; H18, 18% HEMA. Primers: deionized water (control) and 30% enriched grape seed extract (e-GSE).
Sealing of the Adhesive Interface
Regardless of the experimental resin composition (H18, H6, and H0), the collagen fluorescence emission (Fig. 5A) of the e-GSE-treated groups was significantly higher than that of the control groups (P < 0.001). Similarly, the rhodamine-B fluorescence intensity observed for e-GSE groups was significantly lower than that of control groups (P < 0.001). Representative confocal images are shown in Figure 5B.
Figure 5.
Results of the micropermeability at the adhesive interface with the different experimental adhesives (H18, H6, and H0) and experimental primers (deionized water [control] and 30% enriched grape seed extract [e-GSE]). (A) The data are reported as mean ±SE. Different lowercase and uppercase letters depict statistically significant (P < 0.05) differences between collagen autofluorescence and interface micropermeability, respectively. Asterisk (*) depicts differences between control and e-GSE groups. (B) Representative confocal laser scanning microscopy images of the dentin-resin adhesive interfaces formed by experimental primers and resin blends. Red and green fluorescence observed at the adhesive interfaces depicts the permeability of the interface and collagen autofluorescence, respectively. No specimens were retrieved from control/H0. H0, no HEMA; H6, 6% HEMA; H18, 18% HEMA.
Discussion
The presence of OPAC-mediated collagen cross-links and coating of the dentin matrix exerted long-lasting enhanced mechanical properties and a protective effect against enzymatic biodegradation. For the first time, the dentin-resin adhesion mechanism was attributed to 2 main factors: 1) physicochemical interactions of the OPACs with the dentin matrix, inducing surface and bulk tissue modifications, and 2) adhesive properties of the catechol moieties in the OPACs, leading to bridging of the collagen molecules with the methacrylate resin. Thus, all 3 proposed study hypotheses were confirmed by the experimental outcomes. OPACs consist of flavan-3-ol units, connected by A-type (C4 → C6/C8 and C2 oxygen at C7) and B-type (C4 → C6/C8) linkages at various degrees of polymerization. The wide availability of OPACs in nature (He et al. 2008) and the possibility for further refinement and targeted biological effects have encouraged their application as bioactive compounds for tissue repair (Bedran-Russo et al. 2012; Vidal, Leme, et al. 2014; Phansalkar et al. 2015).
The collagen molecule is the fundamental building block of a highly organized extracellular matrix that constitutes the majority of the dentin matrix (Orgel et al. 2006). Type I collagen molecules are staggered, resulting in gaps and overlap zones (Bertassoni, Marshall, and Swain 2012), where areas within the collagen fibril corresponding to the overlap zones determine a more viscous-like behavior (Bertassoni, Marshall, and Swain 2012). Specific structural features of the OPAC molecules—such as the degree of polymerization, the presence or absence of galloyl moieties, and the amount and positioning of hydroxyl groups available to interact with collagen—determine the type and density of mediated cross-links within the dentin matrix (Vidal, Aguiar, et al. 2014; Vidal, Leme, et al. 2014). The OPAC catechol moieties cross-link within organic tissues via the formation of hydrogen and covalent bonds with -NH2, -COOH, -OH, -SH functional groups (Han et al. 2003; Lee et al. 2007; Ryu et al. 2011; Jenkins et al. 2013; Zhang et al. 2014). OPAC-collagen interactions are stable, as demonstrated by the modulus of elasticity of e-GSE-modified dentin matrix up to 1 y (Fig. 1). The initial 6-mo drop is attributed to the break of hydrogen bonds between collagen and OPACs and to potential leaching of weakly bound OPACs from the dentin matrix, rather than collagen solubilization. Collagen solubilization was significantly reduced in the e-GSE-treated dentin when compared with the control group, particularly in the first 6 mo (Fig. 1).
Considering the size of the OPACs present in e-GSE (degrees of polymerization primarily between 2 and 5, as well as catechins and the constituent phenolic monomers), cross-links can occur within the different structural hierarchy levels of collagen (Vidal, Leme, et al. 2014). It is possible that in the modified dentin matrix, previously existing spaces between the gap and overlap zones were occupied by the exogenous intermolecular cross-links tipping the viscoelastic properties of the dentin matrix to be more elastic-like (Fig. 2). The presence of e-GSE-mediated exogenous cross-links at the intermicrofibrillar structural level induced increased elastic properties of dentin (e.g., modulus of elasticity, Er, and complex modulus). The high density of cross-links mediated by OPACs reduces the surface hydrophilicity (He et al. 2011) and the intrinsic water dynamics (Fathima et al. 2010) of the tissue, resulting in decreased bound water within the extracellular matrix. Thus, as the moisture level and the presence of bound and unbound water of the tissue decreased, the viscoelastic properties of collagen changed from a more viscous to a more elastic-like (Fig. 2; Yamashita et al. 2001; Al-Ammar et al. 2009; Bertassoni and Swain 2012).
The decrease in complex modulus observed in the e-GSE-modified dentin matrix after exposure to urea indicates the break of hydrogen bonds between the OPACs and the collagen, not affecting the tan δ. However, the complex modulus of the e-GSE-treated dentin matrix remained higher than that of the control group after urea exposure, revealing permanent modifications of the dentin matrix. These permanent modifications accounted for nearly 50% of the total increase in the complex modulus and the viscoelasticity of e-GSE-biomodified dentin matrices, indicating presence of covalent bonds between OPACs and collagen (Han et al. 2003; He et al. 2011). The intermolecular bonds between the cross-linker and the collagen affect the viscoelastic properties of the tissue (Fathima et al. 2010). Thus, as covalent bonds are highly stable, they play significant roles in the viscoelastic properties of the e-GSE-modified dentin matrix and its long-term stability (Figs. 1, 2).
Depletion of mineral was carried out with preestablished demineralization protocols for mechanical tests of the dentin matrix (Balooch et al. 2008; Vidal, Aguiar, et al. 2014). The hybrid zone between dentin and resin is a vital mechanism of micromechanical adhesion, and the dentin demineralization depth may jeopardize adhesion (Hashimoto et al. 2000). Glycolic acid was herein used to demineralize dentin (~1 to 2 µm thick), yielding similar bond strength as standard phosphoric acid (~3 µm thick; see Appendix). Glycolic acid is a relatively mild alpha hydroxyl organic acid, potentially less aggressive (Kim et al. 1998; Kim and Won 1998) than phosphoric acid. Consistent with commercially available dental adhesives, porous adhesive interface was evident in the control group (high rhodamine-B fluorescence intensity) due to incorporation of water with the resin-dentin interface. In contrast, significantly reduced permeability and higher intensity of cross-link autofluorescence was detected at the adhesive interface of the e-GSE groups. Water is present in the dentin matrix as bound water, weakly bound water, and free/lattice water to collagen, and the hydrodynamics are known to change in the presence of a ligand (Fathima et al. 2010). Cross-links mediated by OPAC molecules changed the tissue water dynamics by displacing the bound water during collagen cross-linking, which facilitated further resin infiltration and reduced interfacial permeability. Proteoglycans are tightly bound to collagen fibrils and have a pivotal role in tissue hydration, where negatively charged glycosaminoglycans chains (GAGs) attract water to the interfibrillar spaces (Bertassoni, Orgel, et al. 2012; Rigozzi et al. 2013). A precursor source used to prepare e-GSE removes GAGs from the dentin matrix (Bedran-Russo et al. 2012), and the same phenomenon was observed by e-GSE (see Appendix). Enzymatic removal of proteoglycans in dentin matrix was shown to increase resin monomer infiltration (Mazzoni et al. 2008). Herein, the depletion of GAGs may have further favored resin diffusion within interfibrillar spaces as well as increased accessibility to the collagen fibrils for coating with OPACs.
Adhesion in the control group relied on HEMA monomers and exhibited severe degradation after 1 y (Fig. 4), highlighting the shortcomings of contemporary materials (Yiu et al. 2005). On the contrary, the adhesion of e-GSE-primed biointerfaces was not affected by the hydrophilicity of the experimental resin blends (Fig. 4). The stiffer e-GSE-treated dentin matrix appears to be structurally stable to the intrinsic moisture, thus maintaining the collagen interfibrillar spaces. As discussed above, substantially altered tissue water dynamics favor resin infiltration, reducing permeability and consequently resulting in highly stable adhesive joints.Interestingly, the presence of HEMA did not negatively affect the long-term outcomes in the e-GSE-treated biointerfaces, unraveling favorable adhesion by supplementary mechanisms. Catechol-containing compounds have been studied as biological adhesives on wet substrates (Lee et al. 2007; Jenkins et al. 2013; Zhang et al. 2014; Maier et al. 2015). Catechols chemically interact with acrylates in the dental resin chemistry (Cho et al. 2013; Zhang et al. 2014). Thus, the available catechol group in the OPACs chemically bridged methacrylate groups and collagen via e-GSE-coated collagen fibrils. Such chemical bridging has the potential to reduce the pitfalls associated with the unavoidable wet adhesion in dental restorative therapies.
Conclusion
Enriched plant-based OPACs provide a new mechanism of adhesion to type I collagen–rich tissue. This approach does not rely on hydrophilic monomers to promote strong and stable resin-dentin biointerfaces. The bioadhesion mechanism involves physicochemical modifications to the dentin matrix, reduced biodegradation, and chemical interactions with methacrylate resins.
Author Contributions
A.A. Leme-Kraus, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; B. Aydin, contributed to design, data acquisition, analysis, and interpretation, critically revised the manuscript; C.M.P. Vidal, contributed to data acquisition and analysis, critically revised the manuscript; R.M. Phansalkar, contributed to design, data acquisition, and interpretation, drafted and critically revised the manuscript; J.W. Nam, J. McAlpine, contributed to data interpretation, critically revised the manuscript; G.F. Pauli, A.K. Bedran-Russo, contributed to conception, design, data analysis, and interpretation, drafted and critically revised the manuscript; S.-N. Chen, contributed to design, data analysis, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Acknowledgments
The authors thank Bisco Inc. for providing the experimental resin formulations.
Footnotes
A supplemental appendix to this article is available online.
This study was supported by National Institutes of Health/National Institute of Dental and Craniofacial Research research grant DE021040.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Al-Ammar A, Drummond JL, Bedran-Russo AK. 2009. The use of collagen cross-linking agents to enhance dentin bond strength. J Biomed Mater Res B Appl Biomater. 91(1):419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Dental Association. 2007. 2005–06 survey of dental services rendered. Chicago (IL): American Dental Association. [Google Scholar]
- Astvaldsdottir A, Dagerhamn J, van Dijken JW, Naimi-Akbar A, Sandborgh-Englund G, Tranaeus S, Nilsson M. 2015. Longevity of posterior resin composite restorations in adults: a systematic review. J Dent. 43(8):934–954. [DOI] [PubMed] [Google Scholar]
- Aydin B, Saleh-Hassan L, Viana G, Bedran-Russo AK. 2016. Probing collagen and micro-permeability at the proanthocyanidins-treated dentin-resin interface. J Adhes Dent. In press. [DOI] [PubMed] [Google Scholar]
- Balooch M, Habelitz S, Kinney JH, Marshall SJ, Marshall GW. 2008. Mechanical properties of mineralized collagen fibrils as influenced by demineralization. J Struct Biol. 162(3):404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedran-Russo AK, Castellan CS, Shinohara MS, Hassan L, Antunes A. 2012. Characterization of biomodified dentin matrices for potential preventive and reparative therapies. Acta Biomater. 7(4):1735–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertassoni LE, Marshall GW, Swain MV. 2012. Mechanical heterogeneity of dentin at different length scales as determined by AFM phase contrast. Micron. 43(12):1364–1371. [DOI] [PubMed] [Google Scholar]
- Bertassoni LE, Orgel JP, Antipova O, Swain MV. 2012. The dentin organic matrix: limitations of restorative dentistry hidden on the nanometer scale. Acta Biomater. 8(7):2419–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertassoni LE, Swain MV. 2012. Influence of hydration on nanoindentation induced energy expenditure of dentin. J Biomech. 45(9):1679–1683. [DOI] [PubMed] [Google Scholar]
- Castellan CS, Bedran-Russo AK, Antunes A, Pereira PN. 2013. Effect of dentin biomodification using naturally derived collagen cross-linkers: one-year bond strength study. Int J Dent. 2013:918010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaraputt S, Mai S, Huffman BP, Kapur R, Agee KA, Yiu CK, Chan DC, Harnirattisai C, Arola DD, Rueggeberg FA, et al. 2008. Changes in resin-infiltrated dentin stiffness after water storage. J Dent Res. 87(7):655–660. [DOI] [PubMed] [Google Scholar]
- Cho JH, Shanmuganathan K, Ellison CJ. 2013. Bioinspired catecholic copolymers for antifouling surface coatings. ACS Appl Mater Interfaces. 5(9):3794–3802. [DOI] [PubMed] [Google Scholar]
- Fathima NN, Baias M, Blumich B, Ramasami T. 2010. Structure and dynamics of water in native and tanned collagen fibers: effect of crosslinking. Int J Biol Macromol. 47(5):590–596. [DOI] [PubMed] [Google Scholar]
- Han B, Jaurequi J, Tang BW, Nimni ME. 2003. Proanthocyanidin: a natural crosslinking reagent for stabilizing collagen matrices. J Biomed Mater Res A. 65(1):118–124. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Ohno H, Endo K, Kaga M, Sano H, Oguchi H. 2000. The effect of hybrid layer thickness on bond strength: demineralized dentin zone of the hybrid layer. Dent Mater. 16(6):406–411. [DOI] [PubMed] [Google Scholar]
- He F, Pan QH, Shi Y, Duan CQ. 2008. Biosynthesis and genetic regulation of proanthocyanidins in plants. Molecules. 13(10):2674–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Mu C, Shi J, Zhang Q, Shi B, Lin W. 2011. Modification of collagen with a natural cross-linker, procyanidin. Int J Biol Macromol. 48(2):354–359. [DOI] [PubMed] [Google Scholar]
- Jenkins CL, Meredith HJ, Wilker JJ. 2013. Molecular weight effects upon the adhesive bonding of a mussel mimetic polymer. ACS Appl Mater Interfaces. 5(11):5091–5096. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Park JH, Kim DH, Won YH, Maibach HI. 1998. Increased in vivo collagen synthesis and in vitro cell proliferative effect of glycolic acid. Dermatol Surg. 24(10):1054–1058. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Won YH. 1998. The effect of glycolic acid on cultured human skin fibroblasts: cell proliferative effect and increased collagen synthesis. J Dermatol. 25(2):85–89. [PubMed] [Google Scholar]
- Lee H, Dellatore SM, Miller WM, Messersmith PB. 2007. Mussel-inspired surface chemistry for multifunctional coatings. Science. 318(5849):426–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leme AA, Vidal CM, Hassan LS, Bedran-Russo AK. 2015. Potential role of surface wettability on the long-term stability of dentin bonds after surface biomodification. J Biomech. 48(10):2067–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier GP, Rapp MV, Waite JH, Israelachvili JN, Butler A. 2015. Biological adhesives: adaptive synergy between catechol and lysine promotes wet adhesion by surface salt displacement. Science. 349(6248):628–632. [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Nascimento FD, Carrilho M, Tersariol I, Papa V, Tjäderhane L, Di Lenarda R, Tay FR, Pashley DH, Breschi L. 2012. MMP activity in the hybrid layer detected with in situ zymography. J Dent Res. 91(5):467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni A, Pashley DH, Ruggeri A Jr, Vita F, Falconi M, Di Lenarda R, Breschi L. 2008. Adhesion to chondroitinase ABC treated dentin. J Biomed Mater Res B Appl Biomater. 86(1):228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedeljkovic I, Teughels W, De Munck J, Van Meerbeek B, Van Landuyt KL. 2015. Is secondary caries with composites a material-based problem? Dent Mater. 31(11):e247–e277. [DOI] [PubMed] [Google Scholar]
- Orgel JP, Irving TC, Miller A, Wess TJ. 2006. Microfibrillar structure of type I collagen in situ. Proc Natl Acad Sci U S A. 103(24):9001–9005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phansalkar RS, Nam JW, Chen SN, McAlpine JB, Napolitano JG, Leme A, Vidal CM, Aguiar T, Bedran-Russo AK, Pauli GF. 2015. A galloylated dimeric proanthocyanidin from grape seed exhibits dentin biomodification potential. Fitoterapia. 101:169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy GK, Enwemeka CS. 1996. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 29(3):225–229. [DOI] [PubMed] [Google Scholar]
- Rigozzi S, Muller R, Stemmer A, Snedeker JG. 2013. Tendon glycosaminoglycan proteoglycan sidechains promote collagen fibril sliding-AFM observations at the nanoscale. J Biomech. 46(4):813–818. [DOI] [PubMed] [Google Scholar]
- Ryu JH, Lee Y, Kong WH, Kim TG, Park TG, Lee H. 2011. Catechol-functionalized chitosan/pluronic hydrogels for tissue adhesives and hemostatic materials. Biomacromolecules. 12(7):2653–2659. [DOI] [PubMed] [Google Scholar]
- Tezvergil-Mutluay A, Agee KA, Hoshika T, Carrilho M, Breschi L, Tjäderhane L, Nishitani Y, Carvalho RM, Looney S, Tay FR, et al. 2010. The requirement of zinc and calcium ions for functional MMP activity in demineralized dentin matrices. Dent Mater. 26(11):1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usha R, Ramasami T. 2002. Effect of hydrogen-bond-breaking reagent (urea) on the dimensional stability of rat tail tendon (RTT) collagen fiber. J Appl Polym Sci. 84(5):975–982. [Google Scholar]
- Vidal CM, Aguiar TR, Phansalkar R, McAlpine JB, Napolitano JG, Chen SN, Araújo LS, Pauli GF, Bedran-Russo A. 2014. Galloyl moieties enhance the dentin biomodification potential of plant-derived catechins. Acta Biomater. 10(7):3288–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal CM, Leme AA, Aguiar TR, Phansalkar R, Nam JW, Bisson J, McAlpine JB, Chen SN, Pauli GF, Bedran-Russo A. 2014. Mimicking the hierarchical functions of dentin collagen cross-links with plant derived phenols and phenolic acids. Langmuir. 30(49):14887–14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita J, Furman BR, Rawls HR, Wang X, Agrawal CM. 2001. The use of dynamic mechanical analysis to assess the viscoelastic properties of human cortical bone. J Biomed Mater Res. 58(1):47–53. [DOI] [PubMed] [Google Scholar]
- Yiu CK, Pashley EL, Hiraishi N, King NM, Goracci C, Ferrari M, Carvalho RM, Pashley DH, Tay FR. 2005. Solvent and water retention in dental adhesive blends after evaporation. Biomaterials. 26(34):6863–6872. [DOI] [PubMed] [Google Scholar]
- Zhang H, Bré LP, Zhao T, Zheng Y, Newland B, Wang W. 2014. Mussel-inspired hyperbranched poly(amino ester) polymer as strong wet tissue adhesive. Biomaterials. 35(2):711–719. [DOI] [PubMed] [Google Scholar]
- Zou Q, Habermann-Rottinghaus SM, Murphy KP. 1998. Urea effects on protein stability: hydrogen bonding and the hydrophobic effect. Proteins. 31(2):107–115. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.