Abstract
The main reason cited for the replacement of dental composite restorations is the recurrence of caries. Numerous models—both in vitro, with acid gels or bacterial biofilms, and in situ, with dental appliances—have been used to study caries formation around dental composites. The literature shows that many factors may affect caries formation, including marginal gap formation, gap size, the local chemical environment, the durability of the bonded interface, the extent of bacterial penetration, and the presence of mechanical loading. Studies have also shown that what have been called wall lesions may form independent of surface lesions, though not likely due to microleakage through very small gap spaces in the clinical situation. Gap size and mechanical loading have been shown to be related to lesion severity within in vitro models, but these results do not correspond exactly with those obtained from in situ studies using restorations in dental appliances. Though not conclusive, some in vitro models have shown that certain materials possessing antimicrobial characteristics may reduce the severity of lesion formation, suggesting possible pathways for developing new composite and adhesive materials for restorations with potentially enhanced longevity.
Keywords: composite resins, dental caries, dental restorations, dental leakage, antimicrobial agents, mechanical stress
Introduction
Dental composites are polymer-based materials containing finely ground reinforcing glass or silica. Due to the fact that these materials can aesthetically mimic natural teeth, they have become the most popular dental material for replacing the lost or damaged tooth structure. While composites have been successfully used in dentistry for many years, their longevity is considered less than optimal. Results of clinical studies differ, with some showing average replacement times for dental composite restorations of approximately 6 y and others showing double or more service lifetimes. Regardless, the main reasons cited for the replacement of dental composite restorations is the presence of secondary caries and restoration fracture (Rasines Alcaraz et al. 2014; Astvaldsdottir et al. 2015), and this has not changed in well over 30 y.
Secondary caries has been defined as “lesions at the margins of existing restorations” (Mjör and Toffenetti 2000). There exists significant controversy over whether these lesions are a result of the presence of the dental restoration or simply a new primary lesion that forms in the same region as an initial lesion that has been restored. In any case, the presence or recurrence of these lesions is typically associated with the marginal areas of the restoration, and it has been stated that 80% to 90% of secondary caries will be found at the gingival margin (for class II to V restorations), irrespective of the type of restorative material (Mjör 1998, 2005). Furthermore, the occurrence of the initial lesion—and, most likely, the recurrence—is associated with patient caries risk factors. Presumably, this high incidence of failure at these sites is related to its hospitality to plaque formation, especially in susceptible individuals, and to the overall difficulty in cleansing it, especially when the margin is interproximal.
Aside from the most important aspect of the particular condition of the patients themselves, the primary characteristic that relates to this phenomenon of secondary caries is likely the presence of a bacterial biofilm, possibly in association with a discontinuity, or gap, at the margin between the composite and the tooth structure. This gap may be associated with the presence of an initially defective margin or may have resulted from a later degradation of the interface. In any case, the cause of the tooth demineralization has been attributed to leakage of either bacteria or their acid by-products within this gap, termed microleakage. Microleakage has been found to occur more readily on dentin than enamel margins, especially those undergoing mechanical loading (Campos et al. 2008). It has also been shown that larger gaps harbor a greater number of bacteria, even around dental amalgam (Kidd et al. 1995). When leakage is associated with these large gaps, it has been referred to as macroleakage (Mjör 2005). All of these observations imply that the key to eliminating or minimizing lesion recurrence is maintaining a biological and chemical seal of the interface. This seal should prevent the formation of a lesion along the wall of the tooth adjacent to the restorative (Kidd 1990; Kidd and O’Hara 1990), although it may not be expected to deter the formation of a lesion on the surface of the tooth near the marginal interface, a so-called outer lesion (Kidd 1990; Fig. 1).
Figure 1.
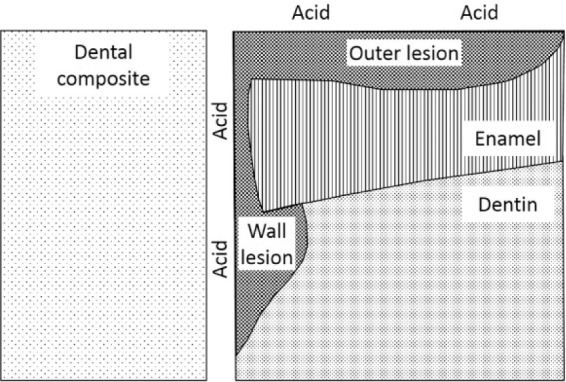
Schematic distinguishing 1) a lesion formed on the surface of the tooth near the margin of a restoration, a so-called outer lesion, from 2) a lesion forming within the interfacial gap, or wall lesion. Adapted from Kidd (1990 ).
The difficulty in achieving a seal of the tooth-composite interface relates to the demanding placement procedure for composites and to the polymerization shrinkage during curing that stresses the interfacial bond produced by the dental adhesive. The adhesion between the tooth and composite has been shown to degrade clinically with time (Tjäderhane 2015), presumably due to a hydrolytic or mechanical breakdown or enzymatic degradation of the resin material or the bonding layer (Li et al. 2014), and it has been associated with the loss of restorations during clinical service. Another important factor may be the extent of the composite cure, especially at the gingival margin of class II restorations, which is more difficult to access with the light curing unit. Insufficient curing of this material will allow it to “wash out” during aging in aqueous media, leaving an open space for plaque retention (Vandewalle et al. 2004). Perhaps many of the undocumented failures of posterior composites can be attributed to less-than-optimal curing of the material during placement.
What Needs to Be Studied?
The development of lesions adjacent to existing clinical restorations is a multifactorial problem that is difficult to study due to human variability and the time required for identifiable lesion formation. To better understand the cause of these lesions, it is important to study the resistance of the tooth and restorative materials, as well as their interface, to the chemical and bacterial components of the oral environment (Nedeljkovic et al. 2015). Saliva, food products, cleaning agents, and other abrasives are all likely important factors in lesion formation, as are the chemistry, geometry, and surface quality of the restorative materials themselves and the way that they interact with the oral microbiome. Controversy exists over many aspects of secondary caries formation around dental composite restorations, including simple terminology (Jokstad 2016), and much can be learned from additional studies focused on specific questions. For example, do wall lesions actually form independent from surface lesions? Is lesion formation or severity dependent on gap size? Does mechanical loading of the interface enhance or facilitate lesion formation? Can lesion occurrence or severity be affected by material formulation? These important issues are addressed in this article.
What Types of Models Exist?
Many models have been created to study the variables that influence the demineralization of tooth structure in the vicinity of a restoration (Fig. 2). These models involve both in vitro and in vivo methods, and they have largely been patterned after models for primary caries formation (Featherstone 1996; Lobo et al. 2005). The in vitro methods involve chemical or biological challenges and utilize static incubation periods at low pH or cycling between low pH and neutral remineralizing solutions that more closely approximate the conditions in the oral cavity. The biological challenges involve single-species, multispecies, or saliva-derived oral microcosm biofilms. The in vivo methods involve in situ models where dental composite-tooth interfaces are placed within dentures or removable appliances worn by volunteers or where restorations are directly placed into animals or humans. Some models have incorporated preformed composite-tooth gaps of controlled size, whereas others have subjected restoration interfaces to mechanical loading.
Figure 2.
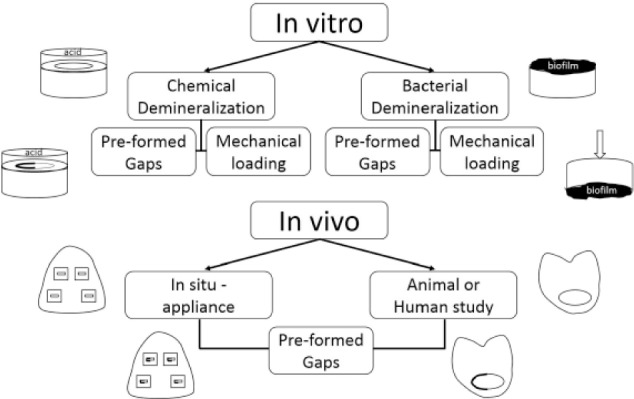
Comparison of the in vitro and in vivo models used to study caries formation around dental composite restorations. In vitro models are conducted with chemical or bacterial demineralization methods, in which the specimen 1) is submerged in an acidic solution or gel, for the former, or 2) has a biofilm grown upon it, for the latter. The in vivo models typically involve in situ models in which simulated restorations are placed within dental appliances worn by patients, but they can also involve restorations placed in animals or humans. Restorations with preformed gaps can be studied in either in vitro or in vivo models, and mechanical loading effects can be addressed specifically within in vitro models.
The following is a review of models that have been reported in the literature, predominantly within the past decade or so, to study the formation of caries in the vicinity of dental composite restorations. The different in vitro and in vivo models are described, along with their potential advantages and disadvantages, and the outcomes of many studies using the models are presented and compared.
Models for Studying Secondary Caries around Dental Composite Restorations
In Vitro: Chemical Demineralization—Static or Cycling
Perhaps the most straightforward method for studying secondary caries formation around dental composite restorations is to place a restored tooth or section of a restored tooth into an acidified solution or gel (pH <5) under static conditions and monitor passive demineralization of enamel and/or dentin over time (Dionysopoulos et al. 2003). While buffered lactic acid is the most common agent, there is evidence that lactic acid–sodium lactate with a small amount of hydroxyapatite in a methylcellulose gel slowly produces caries-like lesions similar to those formed in vivo (Kotsanos et al. 1989). These models may also be used with a demineralization-remineralization, or pH cycling, protocol to more closely mimic event in the oral cavity (Hara et al. 2002; Almeida Ayres et al. 2015; Dionysopoulos et al. 2016).
Storage times used to create lesions have ranged from 2 to 15 wk (Attar and Onen 2002; Hara et al. 2002; Dionysopoulos et al. 2003; Dionysopoulos et al. 2016). The methods for measuring demineralization include hardness testing (Hara et al. 2002; Almeida Ayres et al. 2015), polarized light microscopy (Attar and Onen 2002; Dionysopoulos et al. 2003; Dionysopoulos et al. 2016), microradiography (Kuper et al. 2013), laser confocal microscopy (Fontana et al. 1996), and optical coherence tomography (Turkistani et al. 2015).
In Vitro: Bacterial Demineralization
Because caries typically is caused by exposure to the acidic products of bacterial fermentation of carbohydrates, in vitro models often involve single- or multispecies biofilms grown on specimens incubated in simulated oral conditions (Fontana et al. 1996; Gama-Teixeira et al. 2007; Knight et al. 2007; Rudney et al. 2012; Li et al. 2014; Mayanagi et al. 2014; Schwendicke et al. 2014; Kramer et al. 2015). In some studies, gaps of specific size are created between the tooth and composite (Totiam et al. 2007; Cenci et al. 2009; Diercke et al. 2009; Hayati et al. 2011; Nassar and González-Cabezas 2011; Kuper, van de Sande, et al. 2015). In other studies, the effect of mechanical loading on marginal integrity and lesion formation has been assessed (Khvostenko et al. 2015; Khvostenko et al. 2016).
The majority of studies utilize Streptococcus mutans, but Streptococcus gordonii, Streptococcus sobrinus, and Lactobacillus have also been used. Some studies utilize a microcosm model derived from human saliva to produce a complex biofilm. Perhaps the microcosm model is the most clinically relevant, but it is also most difficult to control in terms of knowing specifically how individual bacterial species are involved in the process. For better control, mixed biofilms ≥2 species are desirable in that they establish a more realistic competitive environment for biofilm formation. However, determining the most appropriate species and their relative amounts is not inconsequential. In any case, nutrients may be supplied to the biofilm statically or in a flowing culture system. Studies typically utilize exposure times of 1 to 4 wk, and lesion progression is assessed as in the chemical demineralization studies.
In Vivo: In Situ
Many researchers have used models in which patients wear appliances containing specimens designed with tooth-composite interfaces, relying on the patient’s own bacteria to colonize the specimens (Sousa et al. 2009; Melo et al. 2013; van de Sande et al. 2014). Typically, patients are instructed to remove the appliance 8 to 10 times per day and submerge it in a high-concentration (i.e., 20%) sucrose solution for 10 min, thus mimicking an environment at high risk of caries (Kuper, van de Sande, et al. 2015). In many cases, specimens have been designed to have a preformed gap between the composite and the dentin or enamel (Thomas et al. 2007; Kuper et al. 2014; Kuper, van de Sande, et al. 2015; Montagner et al. 2015). Others have allowed a smaller gap to form by simply not applying an adhesive to the tooth structure (Cenci et al. 2008; Barata et al. 2012).
In Vivo: Human Clinical or Animal
The most clinically relevant model for studying secondary caries formation is within an animal or human, but these studies are seldom reported due to their enhanced complexity. Animal models for caries studies have been used for many years (Bowen 2013), but it is difficult to find reports of animal models used for secondary caries. The occurrence of these lesions is always assessed in clinical studies of composites, but few have focused efforts on directly assessing factors that influence them. There is a retrospective study in which restorations were evaluated after a minimum of 5 y to determine the potential influence of the location of the proximal gingival margin on secondary caries formation and restoration failure (Kuper et al. 2012). There is also a secondary caries study where restorations were place with preformed gaps in teeth scheduled for extraction (Papagiannoulis et al. 2002).
Advantages and Disadvantages of Models
The advantages and disadvantages of the different in vitro and in vivo models are summarized in Table 1. While in vitro models afford the opportunity to study many variables in a relatively simple, fast, and cost-effective manner, they likely lack overall clinical relevance and are best used for screening materials or systematically studying specific variables, such as gap size. In situ models, in contrast, require more time and expense, and interpretation of the results may be complicated by patient compliance issues, since most are removable appliances requiring adherence to specific protocols for wearing and care. Animal or human models have the greatest relevance but are also more time-consuming and expensive than in vitro studies and are seriously restricted in scope based on ethical issues. Perhaps the major point to emphasize is that in vitro models, as well as the in situ models that create artificial specimens, do not form true caries in the same time frame and under the same complex conditions as in the actual clinical case of a dental restoration in a real patient’s tooth.
Table 1.
Advantages and Disadvantages of Existing Models for Studying Secondary Caries Formation around Dental Composite Restorations.
| Model | Advantages | Disadvantages |
|---|---|---|
| Chemical: in vitro | Simple—requires straightforward protocols and equipment Fast—significant demineralization occurs in short time frames Low cost—consumables are inexpensive and time frames short Reproducible—straightforward protocols and easily controlled variables (e.g., gap or no gap, gap size, mechanical loading) Materials—can evaluate experimental or commercial materials pH cycling can be included—combination of demineralization/remineralization cycles better replicates real clinical situations Easily tests many variables—due to short time frame, low cost, and control of test parameters |
Clinical relevance—lesions do not form under the same conditions and in the same time frame as occurs clinically No biological component—does not replicate biofilm formation, which is likely key component of clinical lesion formation |
| Biological: in vitro | Same as Chemical—though typically does not include the possibility of pH cycling Clinical relevance better than Chemical—includes biofilm formation, which better replicates the clinical condition |
Clinical relevance—lesions do not form under the same conditions and in the same time frame as occurs clinically No pH cycling—because biofilms form on the surface and remain, demineralization is primarily present without the potential for surface remineralization, as occurs clinically in times when bacteria are inactive due to a lack of a food source |
| In situ | Clinical relevance better than In Vitro Relatively fast (vs. Clinical) Control—specific variables can be controlled (e.g., gap or no gap, gap size, multiple materials in the same patient) |
Time—requires longer times than In Vitro, though less than Clinical Expense—requires potentially costly appliances, clinical chair time, and possibly patient remuneration Variability—needs multiple patients, who have variable oral conditions Patient compliance—requires patients to wear and care for appliance as directed |
| Clinical | Most clinical relevance Compliance is not an issue (vs. In Situ) |
Time—requires longer times for caries to form Expense—requires chair time and possibly patient remuneration Variability—needs multiple patients, who have variable oral conditions Limited questions can be addressed—ethics |
Outcomes from Models for Assessing Caries Formation around Dental Composite Restorations
In Vitro: Chemical Demineralization—Static or Cycling
Most chemical demineralization studies of composite restorations have generally been comparing composite to glass ionomer or some other fluoride-releasing material. This is not surprising, since it is long known that composite does not protect the tooth from demineralization in a highly acidic environment, except potentially along the cavity wall when bonding is excellent. In general, these studies have provided evidence that the extent of lesions formed in dentin and enamel around restorations that leach fluoride is reduced as compared with nonfluoride releasing materials (Hicks et al. 2000; Attar and Onen 2002; Dionysopoulos et al. 2016). Similar results have been shown for restorations involving root dentin (Hicks et al. 2000; Hara et al. 2002; Dionysopoulos et al. 2003) in a pH cycling model (Hara et al. 2002; Almeida Ayres et al. 2015; Dionysopoulos et al. 2016).
Optical coherence tomography has been used to correlate lesion formation with gap length along the enamel and dentin walls in composite restorations exposed to an acidic gel for 5 wk (Turkistani et al. 2015). The gaps were formed by shrinkage of the flowable composite restorative placed in the absence of a bonding agent and further accentuated by thermal cycling. The study also compared flowable composite restorations bonded with 4 different dental adhesives and showed significant correlations between increased demineralization area and increased gap length on both surfaces (better for enamel), as well as evidence for reduced rate of lesion formation at margins associated with fluoride releasing adhesives. A similar beneficial effect of a fluoride-releasing composite reducing actual mineral loss from dentin located across a 200-µm gap was demonstrated by microradiography in another study, although the lesion depth was not reduced in the acidic gel (Dijkman et al. 1994).
One study investigated the effect of mechanical loading on lesion formation at bonded and nonbonded composite-tooth interfaces after exposure to acid for 14 d (Kuper et al. 2013). It found wall lesions in the nonbonded restorations when mechanically loaded, likely due to gap opening and enhanced interfacial fluid exchange. Furthermore, the extent of the lesion was increased with higher load levels (350 g vs. 200 g). Nonloaded samples did not form wall lesions, even in the absence of bonding, thus emphasizing the effect of loading on lesion formation. It is not clear what size gap existed in the nonbonding case, as it was not measured. Because it appears that the composite was allowed to shrink unconstrained, it is possible that there was little actual space between it and the tooth. However, several bonded specimens subjected to the highest load level actually developed wall lesions, with subsequent analysis showing that a gap had formed, possibly due to bond failure during loading.
In Vitro: Bacterial Demineralization
Models involving the formation of bacterial biofilms on composite restorations have tended to focus on identifying the potential beneficial effect of fluoride releasing materials. Similar to the acidic gel studies, these have provided evidence for reduced lesion formation for glass ionomers versus composites, suggesting that this could be due to a direct effect of fluoride or to a buffering effect from the glass ionomer as it is solubilized by the bacterial acids (Gama-Teixeira et al. 2007; Knight et al. 2007; Rudney et al. 2012; Li et al. 2014; Mayanagi et al. 2014; Schwendicke et al. 2014; Kramer et al. 2015). The effect of the biofilm on degradation of the interfacial adhesion between the composite and the tooth structure has also been demonstrated (Peris et al. 2007; Li et al. 2014).
The effect of preformed gaps on lesion formation around composite restorations exposed to bacterial biofilms has been studied with gap sizes of as little as 25 µm to as large as 1,000 µm. Single- or multispecies biofilms have been used for periods from 8 to 30 d, as summarized in Table 2. These in vitro studies all confirmed that lesion severity was increased with the size of the gap. Coating the surface of the tooth with an impermeable varnish to inhibit the formation of outer lesions, one group verified the independent formation of lesions along the enamel and dentin walls adjacent to marginal gaps of 50 to 250 µm, with the lesion size being somewhat influenced by gap size (Diercke et al. 2009). Although they did not confirm it in this study, the authors noted that in other work they could verify bacteria colonization of 50-µm gaps in vitro.
Table 2.
In Vitro Studies Using Biofilms to Form Secondary Caries around Dental Composite Restorations to Study the Effect of Gap Size.
| Study | Gap Size, µm | Biofilm | Time, d | Gap Effect? |
|---|---|---|---|---|
| Totiam et al. (2007) | 0, 25, 250, 1,000 | Streptococcus mutans | 8 | Yes |
| Cenci et al. (2009) | 0, 50, 100, 180, 250 | Microcosm | 18 | Yes |
| Diercke et al. (2009) | 50, 100, 250 | S. mutans | 21 | Yes |
| Hayati et al. (2011) | Bond–no bond | S. mutans, Streptococcus sobrinus, Streptococcus gordonii | 30 | Yes |
| Nassar and González-Cabezas (2011) | 30, 525, 1000 | S. mutans | 8 | Yes |
| Kuper, van de Sande, et al. (2015) | 200 to 400 | Microcosm | 20 | Yes |
In a recent study, researchers employed biofilms to create lesions around composite restorations while examining the influence of gap size and mechanical loading (Khvostenko et al. 2015). This study showed 1) that bacteria, in a static or constant flow environment, could penetrate shrinkage gaps as small as 20 to 25 µm and 2) that penetration was enhanced with mechanical loading, permitting bacteria to colonize the full depth of the interfacial gap at the axial wall. Surface lesions and lesions associated with the bacteria on the dentin cavity wall were evident, with the demineralization verified by confocal microscopy and microhardness measurements. Scanning electron microscopy of the dentin wall surface within the cavity preparation, exposed by breaking open the restoration, showed clear evidence for a bacteria biofilm within the shrinkage gap space and extending to the full depth of the margin, with associated dentin demineralization (Fig. 3). A follow-up study further showed a reduced penetration of bacteria into the gap when the dental composite contained a mildly antimicrobial bioactive glass additive (Khvostenko et al. 2016).
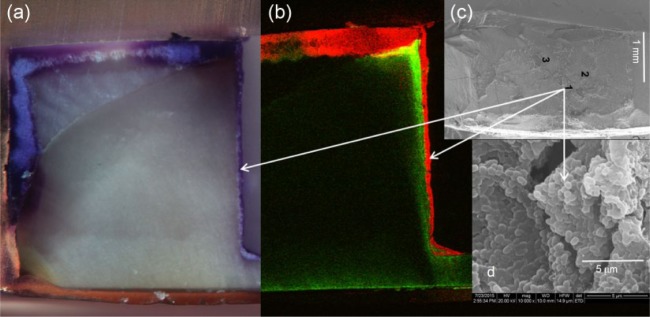
Figure 3.
Direct evidence of bacterial penetration into a shrinkage gap at the tooth-composite margin from an in vitro biofilm model of a restoration exposed to mechanical loading: (a) stereomicroscope image of the cross-section of the restoration showing, by Gram stain, the bacterial colonization of the surface demineralized enamel and along the interfacial gap between the composite and enamel/dentin (purple areas; total preparation depth, ~1.75 mm); (b) confocal microscopy image of the section in panel a, showing the location of the bacteria at the surface and penetrating the full length of the gap (red) and the presence of demineralized dentin (bright green areas); (c) scanning electron microscopy image of the dentin surface of the cavity wall after breaking open the specimen to show the presence of the bacterial biofilm (arrows point to the approximate location height of the image, although this was deeper into the restoration as compared with the cross-section surface); and (d) higher-magnification image of panel c showing clumps of Streptococcus mutans cells in the biofilm, indicative of a mature biofilm (14 d old).
In Vivo: In Situ
In situ studies, in which gap-free restorations have been placed within dental appliances worn by patients for weeks to months, have shown some evidence for reduced demineralization around specific fluoride-releasing materials (Sousa et al. 2009; de Moraes et al. 2016), as well as those containing antimicrobial (van de Sande et al. 2014) or remineralizing (Melo et al. 2013) compounds.
When examining the effect of the presence of marginal gaps, the majority of in situ studies have verified lesions on the cavity walls, most often when the gap is larger than a shrinkage gap (Thomas et al. 2007; Cenci et al. 2008; Lima et al. 2009; Barata et al. 2012; Kuper et al. 2014; Kuper, Montagner, et al. 2015; Montagner et al. 2015). However, wall lesions do not form in the absence of a gap, even in the presence of leakage for a nonbonded restoration (Thomas et al. 2007; Cenci et al. 2008; Barata et al. 2012; Kuper et al. 2014). Consistent with in vitro studies, in situ studies have confirmed that wall lesions can form independent of outer lesions. However, contrary to results from in vitro models, wall lesions did not form when gaps of limited width formed due to polymerization shrinkage only. Wall lesions required gaps of at least 50 µm, though no specific threshold size has been identified and a true dependence of lesion size on gap size could not be shown (Kuper et al. 2014). When composites were well bonded to the tooth, there was no evidence that the presence of the composite margin enhanced surface lesion progression (Thomas et al. 2007). Lesions form parallel to the surface, as a primary lesion would, with a slightly triangular shape at the composite interface but without progressing more apically. With a larger gap, lesions form the same triangular shape at the margin but then extend down the wall, producing an “L” shape, with evidence of a true wall lesion. Similar triangular lesions, or marginal widening, formed at the composite-dentin margin with a shrinkage gap within in vitro biofilm studies and in the absence of shrinkage gaps when mechanically loaded (Khvostenko et al. 2015; Fig. 4).
Figure 4.
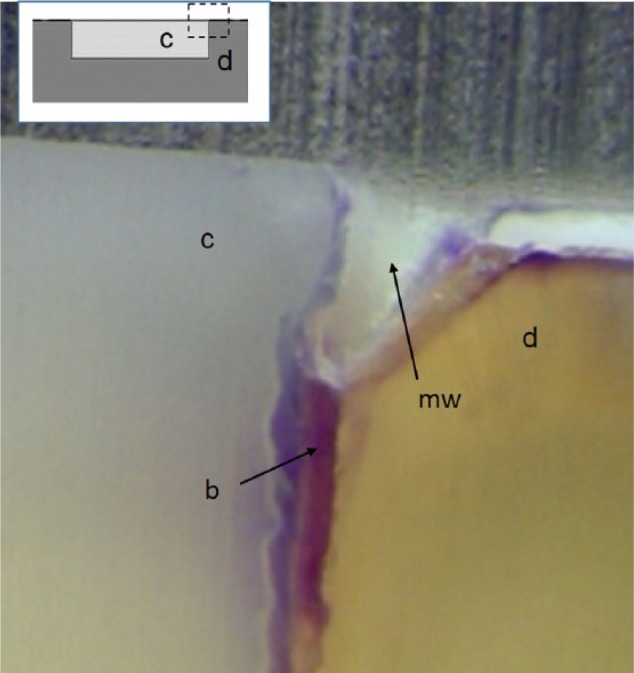
Light micrograph shows marginal widening (mw) extending approximately 20% of the depth of the cavity preparation. Such widening is due to enhanced demineralization at the interface of a composite (c)–dentin (d) restoration having a contraction gap and being colonized by bacteria (b) in an in vitro biofilm model.
Lesion progression in enamel and dentin within in situ models correlates well with lesion progression in clinical restorations, being about 1 to 2 µm per day in enamel and about 2.5 times that rate in dentin (Thomas et al. 2007). Others have shown greater mineral loss adjacent to composites as compared with glass ionomer, independent of leakage (i.e., bonded vs. nonbonded; Cenci et al. 2008) and consistent with in vitro studies. However, the difference in lesion extent between composite and glass ionomer was true only when a nonfluoridated toothpaste was used 3 times per day; the lesions were similar when a fluoridated tooth paste was used, similar to the results of a study with root dentin (Hara et al. 2006).
In further comparing the results of in situ and in vitro models, it seems that in vitro models may create a more aggressive environment where the tooth is more susceptible to lesion formation than in the oral cavity. Also, in situ studies appear to confirm that leakage alone does not promote wall lesions.
In Vivo: Human Clinical
Few clinical studies exist that are specifically designed to study secondary caries formation around composite restorations. Kuper et al. (2012) conducted a retrospective study of a database from a private dental practice in the Netherlands to determine if the gingival extension of a proximal amalgam or composite restoration influenced secondary caries formation. They assessed the radiographic depth of the cervical margins of 1,193 restorations after at least 5 y of service among 83 patients who had had at least 1 restoration replaced due to the diagnosis of secondary caries. They found dental composites to have a higher risk of secondary caries formation as compared with amalgam, but composites with gingival extensions apical to (vs. coronal to) the cementum-enamel junction did not fail more often due to secondary caries.
At least one clinical study prospectively assessed the influence of a marginal gap on secondary caries with dental composite restorations and included an in vitro caries model study for comparison (Papagiannoulis et al. 2002). Composite and glass ionomer restorations were placed with preformed gaps of 40 µm on the buccal surface of all 4 premolars of 4 volunteers at low risk for caries. The teeth were scheduled for extraction for orthodontic purposes after 6 mo. A second group of extracted premolars with similar restorations were exposed to a pH 4 gel for 4 wk. Caries was assessed on the extracted teeth with polarized light microscopy. The in vivo study showed no lesion development at the margins that were free of gaps, thereby emphasizing the importance of marginal sealing. Lesions were formed at most but not all margins with gaps, with no beneficial effect demonstrated with glass ionomer. Furthermore, the lesions were confined to the gap entrance and were not present down the entire wall of the gap. This study verified clinically that the presence of a relatively small gap alone is not sufficient criteria for secondary caries formation. The in vitro results differed from the in vivo in that lesions formed in vitro with and without gaps and were less extensive in dimension for glass ionomer versus composite. Thus, the outcomes of this clinical study were divergent from in vitro and in situ studies comparing fluoride-releasing and non-fluoride-releasing materials.
Summary
Despite the fact that in vitro models cannot reproduce the clinical situation, they are indispensable for evaluating the many variables involved with caries formation in association with dental restorations. The ability to conduct pH cycling is indeed an advantage of the chemical in vitro tests. However, because clinical caries is most likely dependent on biofilm formation, the biological tests seem to be the most relevant of the in vitro tests. In any case, these models are critical because it is simply not feasible to conduct mechanistic studies that include all of the many variables involved through in situ or clinical studies. While clinical studies are the most relevant, they are also the most variable due to patient factors and the time and costs involved. Therefore, perhaps the most effective testing model is the in situ one, which optimizes the balance between clinical relevance and control of key variables. Perhaps the greatest contribution to the current knowledge at this point might be more studies focusing on correlating the outcomes of specific models with real clinical secondary caries.
The following general conclusions can be drawn regarding secondary caries models:
Wall lesions can form and be distinct from outer lesions.
There is some evidence from both in vitro and in situ models of a protective effect on demineralization from fluoride and other potentially antibacterial compounds.
With in vitro studies, larger and deeper lesions form with larger gaps, but there does not appear to be a threshold gap size for lesion formation.
With in vivo studies, no wall lesions form if there is no gap or a small gap formed by composite shrinkage only, and larger gap size does not further influence lesion size.
Mechanical loading increases bacterial penetration and lesion depth in vitro.
Clinical outcomes may differ from in vitro outcomes. The lesion characteristics are similar, but the presence and extent based on the presence of gaps or leakage differ.
Author Contributions
J. Ferracane, contributed to conception, design, and data acquisition, drafted and critically revised the manuscript. The author gave final approval and agrees to be accountable for all aspects of the work.
Footnotes
A portion of this work was supported by a grant from the National Institutes of Health/National Institute of Dental and Craniofacial Research (RO1 DE021372).
The author declares no potential conflicts of interest with respect to authorship and/or publication of this article.
References
- Almeida Ayres AP, Tabchoury CP, Bittencourt Berger S, Yamauti M, Bovi Ambrosano GM, Giannini M. 2015. Effect of fluoride-containing restorative materials on dentin adhesion and demineralization of hard tissues adjacent to restorations. J Adhes Dent. 17(4):337–345. [DOI] [PubMed] [Google Scholar]
- Astvaldsdottir A, Dagerhamn J, van Dijken JW, Naimi-Akbar A, Sandborgh-Englund G, Tranaeus S, Nilsson M. 2015. Longevity of posterior resin composite restorations in adults: a systematic review. J Dent. 43(8):934–954. [DOI] [PubMed] [Google Scholar]
- Attar N, Onen A. 2002. Artificial formed caries-like lesions around esthetic restorative materials. J Clin Pediatr Dent. 26(3):289–296. [PubMed] [Google Scholar]
- Barata JS, Casagrande L, Pitoni CM, De Araujo FB, Garcia-Godoy F, Groismann S. 2012. Influence of gaps in adhesive restorations in the development of secondary caries lesions: an in situ evaluation. Am J Dent. 25(4):244–248. [PubMed] [Google Scholar]
- Bowen WH. 2013. Rodent model in caries research. Odontology. 101(1):9–14. [DOI] [PubMed] [Google Scholar]
- Campos PE, Barceleiro Mde O, Sampaio-Filho HR, Martins LR. 2008. Evaluation of the cervical integrity during occlusal loading of class II restorations. Oper Dent. 33(1):59–64. [DOI] [PubMed] [Google Scholar]
- Cenci MS, Pereira-Cenci T, Cury JA, Ten Cate JM. 2009. Relationship between gap size and dentine secondary caries formation assessed in a microcosm biofilm model. Caries Res. 43(2):97–102. [DOI] [PubMed] [Google Scholar]
- Cenci MS, Tenuta LM, Pereira-Cenci T, Del Bel Cury AA, ten Cate JM, Cury JA. 2008. Effect of microleakage and fluoride on enamel-dentine demineralization around restorations. Caries Res. 42(5):369–379. [DOI] [PubMed] [Google Scholar]
- de Moraes MD, de Melo MA, Bezerra Dda S, Costa LS, Saboia Vde P, Rodrigues LK. 2016. Clinical study of the caries-preventive effect of resin-modified glass ionomer restorations: aging versus the influence of fluoride dentifrice. J Investig Clin Dent. 7(2):180–186. [DOI] [PubMed] [Google Scholar]
- Diercke K, Lussi A, Kersten T, Seemann R. 2009. Isolated development of inner (wall) caries like lesions in a bacterial-based in vitro model. Clin Oral Investig. 13(4):439–444. [DOI] [PubMed] [Google Scholar]
- Dijkman GE, de Vries J, Arends J. 1994. Secondary caries in dentine around composites: a wavelength-independent microradiographical study. Caries Res. 28(2):87–93. [DOI] [PubMed] [Google Scholar]
- Dionysopoulos D, Koliniotou-Koumpia E, Helvatzoglou-Antoniades M, Kotsanos N. 2016. In vitro inhibition of enamel demineralisation by fluoride-releasing restorative materials and dental adhesives. Oral Health Prev Dent. 14(4):371–380. [DOI] [PubMed] [Google Scholar]
- Dionysopoulos P, Kotsanos N, Koliniotou-Koubia E, Tolidis K. 2003. Inhibition of demineralization in vitro around fluoride releasing materials. J Oral Rehabil. 30(12):1216–1222. [DOI] [PubMed] [Google Scholar]
- Featherstone JD. 1996. Modeling the caries-inhibitory effects of dental materials. Dent Mater. 12(3):194–197. [DOI] [PubMed] [Google Scholar]
- Fontana M, Dunipace AJ, Gregory RL, Noblitt TW, Li Y, Park KK, Stookey GK. 1996. An in vitro microbial model for studying secondary caries formation. Caries Res. 30(2):112–118. [DOI] [PubMed] [Google Scholar]
- Gama-Teixeira A, Simionato MR, Elian SN, Sobral MA, Luz MA. 2007. Streptococcus mutans-induced secondary caries adjacent to glass ionomer cement, composite resin and amalgam restorations in vitro. Braz Oral Res. 21(4):368–374. [DOI] [PubMed] [Google Scholar]
- Hara AT, Turssi CP, Ando M, Gonzalez-Cabezas C, Zero DT, Rodrigues AL, Jr, Serra MC, Cury JA. 2006. Influence of fluoride-releasing restorative material on root dentine secondary caries in situ. Caries Res. 40(5):435–439. [DOI] [PubMed] [Google Scholar]
- Hara AT, Turssi CP, Serra MC, Nogueira MC. 2002. Extent of the cariostatic effect on root dentin provided by fluoride-containing restorative materials. Oper Dent. 27(5):480–487. [PubMed] [Google Scholar]
- Hayati F, Okada A, Kitasako Y, Tagami J, Matin K. 2011. An artificial biofilm induced secondary caries model for in vitro studies. Aust Dent J. 56(1):40–47. [DOI] [PubMed] [Google Scholar]
- Hicks J, Garcia-Godoy F, Milano M, Flaitz C. 2000. Compomer materials and secondary caries formation. Am J Dent. 13(5):231–234. [PubMed] [Google Scholar]
- Jokstad A. 2016. Secondary caries and microleakage. Dent Mater. 32(1):11–25. [DOI] [PubMed] [Google Scholar]
- Khvostenko D, Hilton TJ, Ferracane JL, Mitchell JC, Kruzic JJ. 2016. Bioactive glass fillers reduce bacterial penetration into marginal gaps for composite restorations. Dent Mater. 32(1):73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khvostenko D, Salehi S, Naleway SE, Hilton TJ, Ferracane JL, Mitchell JC, Kruzic JJ. 2015. Cyclic mechanical loading promotes bacterial penetration along composite restoration marginal gaps. Dent Mater. 31(6):702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd EA. 1990. Caries diagnosis within restored teeth. Adv Dent Res. 4:10–13. [DOI] [PubMed] [Google Scholar]
- Kidd EA, Joyston-Bechal S, Beighton D. 1995. Marginal ditching and staining as a predictor of secondary caries around amalgam restorations: a clinical and microbiological study. J Dent Res. 74(5):1206–1211. [DOI] [PubMed] [Google Scholar]
- Kidd EA, O’Hara JW. 1990. The caries status of occlusal amalgam restorations with marginal defects. J Dent Res. 69(6):1275–1277. [DOI] [PubMed] [Google Scholar]
- Knight GM, McIntyre JM, Craig GG, Mulyani Zilm PS, Gully NJ. 2007. An in vitro investigation of marginal dentine caries abutting composite resin and glass ionomer cement restorations. Aust Dent J. 52(3):187–192. [DOI] [PubMed] [Google Scholar]
- Kotsanos N, Darling AI, Levers BG, Tyler JE. 1989. Simulation of natural enamel caries in vitro with methylcellulose acid gels: effect of addition of calcium and phosphate ions. J Biol Buccale. 17(3):159–165. [PubMed] [Google Scholar]
- Kramer N, Mohwald M, Lucker S, Domann E, Zorzin JI, Rosentritt M, Frankenberger R. 2015. Effect of microparticulate silver addition in dental adhesives on secondary caries in vitro. Clin Oral Investig. 19(7):1673–1681. [DOI] [PubMed] [Google Scholar]
- Kuper NK, Montagner AF, van de Sande FH, Bronkhorst EM, Opdam NJ, Huysmans MC. 2015. Secondary caries development in in situ gaps next to composite and amalgam. Caries Res. 49(5):557–563. [DOI] [PubMed] [Google Scholar]
- Kuper NK, Opdam NJ, Bronkhorst EM, Huysmans MC. 2012. The influence of approximal restoration extension on the development of secondary caries. J Dent. 40(3):241–247. [DOI] [PubMed] [Google Scholar]
- Kuper NK, Opdam NJ, Bronkhorst EM, Ruben JL, Huysmans MC. 2013. Hydrodynamic flow through loading and in vitro secondary caries development. J Dent Res. 92(4):383–387. [DOI] [PubMed] [Google Scholar]
- Kuper NK, Opdam NJ, Ruben JL, de Soet JJ, Cenci MS, Bronkhorst EM, Huysmans MC. 2014. Gap size and wall lesion development next to composite. J Dent Res. 93 Suppl 7:108S–113S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuper NK, van de Sande FH, Opdam NJ, Bronkhorst EM, de Soet JJ, Cenci MS, Huysmans MC. 2015. Restoration materials and secondary caries using an in vitro biofilm model. J Dent Res. 94(1):62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Carrera C, Chen R, Li J, Lenton P, Rudney JD, Jones RS, Aparicio C, Fok A. 2014. Degradation in the dentin-composite interface subjected to multi-species biofilm challenges. Acta Biomater. 10(1):375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima FG, Romano AR, Correa MB, Demarco FF. 2009. Influence of microleakage, surface roughness and biofilm control on secondary caries formation around composite resin restorations: an in situ evaluation. J Appl Oral Sci. 17(1):61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MM, Goncalves RB, Ambrosano GM, Pimenta LA. 2005. Chemical or microbiological models of secondary caries development around different dental restorative materials. J Biomed Mater Res B Appl Biomater. 74(2):725–731. [DOI] [PubMed] [Google Scholar]
- Mayanagi G, Igarashi K, Washio J, Domon-Tawaraya H, Takahashi N. 2014. Effect of fluoride-releasing restorative materials on bacteria-induced ph fall at the bacteria-material interface: an in vitro model study. J Dent. 42(1):15–20. [DOI] [PubMed] [Google Scholar]
- Melo MA, Weir MD, Rodrigues LK, Xu HH. 2013. Novel calcium phosphate nanocomposite with caries-inhibition in a human in situ model. Dent Mater. 29(2):231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjör IA. 1998. The location of clinically diagnosed secondary caries. Quintessence Int. 29(5):313–317. [PubMed] [Google Scholar]
- Mjör IA. 2005. Clinical diagnosis of recurrent caries. J Am Dent Assoc. 136(10):1426–1433. [DOI] [PubMed] [Google Scholar]
- Mjör IA, Toffenetti F. 2000. Secondary caries: a literature review with case reports. Quintessence Int. 31(3):165–179. [PubMed] [Google Scholar]
- Montagner AF, Kuper NK, Opdam NJ, Bronkhorst EM, Cenci MS, Huysmans MC. 2015. Wall-lesion development in gaps: the role of the adhesive bonding material. J Dent. 43(8):1007–1012. [DOI] [PubMed] [Google Scholar]
- Nassar HM, González-Cabezas C. 2011. Effect of gap geometry on secondary caries wall lesion development. Caries Res. 45(4):346–352. [DOI] [PubMed] [Google Scholar]
- Nedeljkovic I, Teughels W, De Munck J, Van Meerbeek B, Van Landuyt KL. 2015. Is secondary caries with composites a material-based problem? Dent Mater. 31(11):e247–e277. [DOI] [PubMed] [Google Scholar]
- Papagiannoulis L, Kakaboura A, Eliades G. 2002. In vivo vs in vitro anticariogenic behavior of glass-ionomer and resin composite restorative materials. Dent Mater. 18(8):561–569. [DOI] [PubMed] [Google Scholar]
- Peris AR, Mitsui FH, Lobo MM, Bedran-russo AK, Marchi GM. 2007. Adhesive systems and secondary caries formation: assessment of dentin bond strength, caries lesions depth and fluoride release. Dent Mater. 23(3):308–316. [DOI] [PubMed] [Google Scholar]
- Rasines Alcaraz MG, Veitz-Keenan A, Sahrmann P, Schmidlin PR, Davis D, Iheozor-Ejiofor Z. 2014. Direct composite resin fillings versus amalgam fillings for permanent or adult posterior teeth. Cochrane Database Syst Rev. 3:CD005620. [DOI] [PubMed] [Google Scholar]
- Rudney JD, Chen R, Lenton P, Li J, Li Y, Jones RS, Reilly C, Fok AS, Aparicio C. 2012. A reproducible oral microcosm biofilm model for testing dental materials. J Appl Microbiol. 113(6):1540–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendicke F, Kern M, Blunck U, Dorfer C, Drenck J, Paris S. 2014. Marginal integrity and secondary caries of selectively excavated teeth in vitro. J Dent. 42(10):1261–1268. [DOI] [PubMed] [Google Scholar]
- Sousa RP, Zanin IC, Lima JP, Vasconcelos SM, Melo MA, Beltrao HC, Rodrigues LK. 2009. In situ effects of restorative materials on dental biofilm and enamel demineralisation. J Dent. 37(1):44–51. [DOI] [PubMed] [Google Scholar]
- Thomas RZ, Ruben JL, ten Bosch JJ, Fidler V, Huysmans MC. 2007. Approximal secondary caries lesion progression: a 20-week in situ study. Caries Res. 41(5):399–405. [DOI] [PubMed] [Google Scholar]
- Tjäderhane L. 2015. Dentin bonding: can we make it last? Oper Dent. 40(1):4–18. [DOI] [PubMed] [Google Scholar]
- Totiam P, González-Cabezas C, Fontana MR, Zero DT. 2007. A new in vitro model to study the relationship of gap size and secondary caries. Caries Res. 41(6):467–473. [DOI] [PubMed] [Google Scholar]
- Turkistani A, Nakashima S, Shimada Y, Tagami J, Sadr A. 2015. Microgaps and demineralization progress around composite restorations. J Dent Res. 94(8):1070–1077. [DOI] [PubMed] [Google Scholar]
- van de Sande FH, Opdam NJ, Truin GJ, Bronkhorst EM, de Soet JJ, Cenci MS, Huysmans MC. 2014. The influence of different restorative materials on secondary caries development in situ. J Dent. 42(9):1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandewalle KS, Ferracane JL, Hilton TJ, Erickson RL, Sakaguchi RL. 2004. Effect of energy density on properties and marginal integrity of posterior resin composite restorations. Dent Mater. 20(1):96–106. [DOI] [PubMed] [Google Scholar]