Abstract
Bone condensation is thought to densify interfacial bone and thus improve implant primary stability, but scant data substantiate either claim. We developed a murine oral implant model to test these hypotheses. Osteotomies were created in healed maxillary extraction sites 1) by drilling or 2) by drilling followed by stepwise condensation with tapered osteotomes. Condensation increased interfacial bone density, as measured by a significant change in bone volume/total volume and trabecular spacing, but it simultaneously damaged the bone. On postimplant day 1, the condensed bone interface exhibited microfractures and osteoclast activity. Finite element modeling, mechanical testing, and immunohistochemical analyses at multiple time points throughout the osseointegration period demonstrated that condensation caused very high interfacial strains, marginal bone resorption, and no improvement in implant stability. Collectively, these multiscale analyses demonstrate that condensation does not positively contribute to implant stability.
Keywords: osseointegration, bone regeneration, bone mineral density, finite element analysis, Implantology, dental implants
Introduction
Condensation is often employed in clinical situations where bone density is considered insufficient to support a dental implant (Summers 1994). The procedure typically involves the creation of a pilot hole that is gradually enlarged by tapered osteotomes (Hahn 1999), and this process is assumed to increase the density of interfacial bone (Siddiqui and Sosovicka 2006; Koutouzis et al. 2011). Whether condensation increases bone density, however, has not been demonstrated (Fanuscu et al. 2007; Blanco et al. 2008; Trisi et al. 2016). In turn, bone with a higher density should be “stiffer” (Steiner et al. 2015) and allow an implant to better resist motion (e.g., superior primary stability). Whether condensation actually achieves this effect, however, is not clear. For example, resonance frequency analyses suggested that implants placed into condensed osteotomies had better primary stability (Marković et al. 2013); other clinical studies contradict this conclusion (Proff et al. 2008; Cehreli et al. 2009; Tabassum et al. 2014). Therefore, whether condensation actually improves initial implant stability remains a point of contention.
Here, we asked a series of questions about the effects of bone condensation, beginning with the hypothesis that condensation increases interfacial bone density and, in turn, implant stability. We employed micro–computed tomography (µCT) to assess bone volume/total volume (BV/TV) immediately after condensation, then experimentally measured the effect of condensation on primary stability. We used finite element (FE) modeling to estimate the magnitude of compressive strain created by condensation, and we evaluated how condensation affected bone microarchitecture and bone turnover. We concluded by testing the longer-term effects of condensation on secondary stability and bone remodeling. Collectively, our studies show that condensation increases interfacial bone density but simultaneously damages the bone, which triggers an immediate and protracted period of bone resorption. Our data do not support the conclusion that condensation positively contributes to either short- or longer-term implant stability.
Materials and Methods
Animal Surgery: Implants
Experimental protocols followed ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines and were approved by the Stanford Committee on Animal Research. Based on a power analysis, 58 mice were used in the study. After an adequate level of anesthesia was achieved, the mouth was rinsed with a povidone-iodine solution; then, small forceps were used to extract the maxillary first molars. In a separate series of experiments, healing of the extraction sockets was monitored. Based on histology, histomorphometric analyses, and µCT, the sockets were fully occupied by type III bone within 21 d of extraction.
After postextraction day 21, mice were again anesthetized, and a split-mouth experimental design was employed. In the control group, a 0.50-mm osteotomy was created in the healed extraction site with a dental engine (1,000 rpm) and a 0.50-mm diameter drill bit (Drill Bit City). The osteotomy was followed by insertion of a 0.62-mm titanium-6 aluminium-4 vanadium alloy implant (i.e., retopin; NTI Kahla GmbH). In the test (condensation) group, a 0.30-mm osteotomy was created in the healed extraction site with the same dental engine; bone was then condensed in a stepwise manner (Fig. 1A). Four self-developed osteotomes of increasing diameter were used to enlarge the prepared osteotomy to a diameter of 0.50-mm (Fig. 1; Appendix Table 1). Each osteotome remained in the osteotomy site for 1 min before the next osteotome was used. After a diameter of 0.50-mm was achieved, a 0.62-mm titanium implant was inserted. All implants were positioned at the height of the gingiva. All mice received subcutaneous injections of buprenorphine for analgesia 2 times per day for 3 d. No evidence of infection or prolonged inflammation was detected at any of the surgical sites.
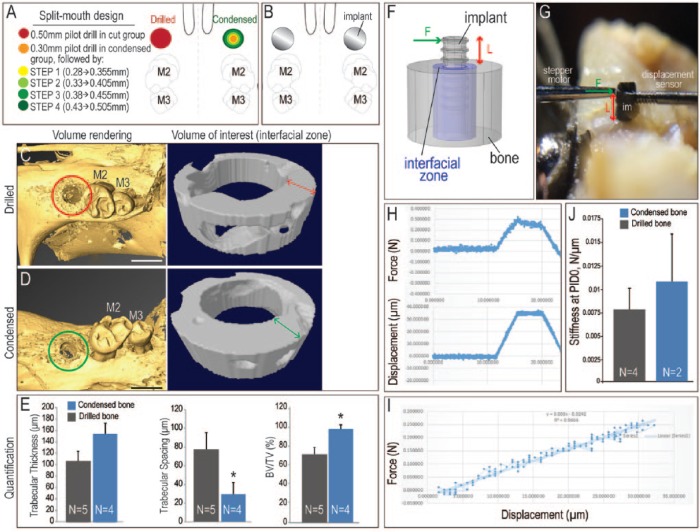
Figure 1.
Condensation-induced changes in bone microarchitecture. (A) Schematic of the experiment, where a split-mouth design was employed to test the effects of bone condensation prior to implant placement. In all animals, the maxillary first molar was extracted, and after 3 wk of healing, 0.50-mm osteotomies were created. In the drilled group, this was accomplished by drilling the bone (red circle). In the condensed bone group, 4 osteotomes were used in succession (green circles, see Methods). (B) A 0.62-mm titanium implant was then placed in the prepared osteotomies of both groups. (C) Immediately after osteotomy preparation, a subset of animals in the drilled bone group were sacrificed and examined by micro–computed tomography. The volume of interest (indicated with a red circle) was examined by volume rendering and by cross-sectional analysis, focusing on a 300-µm-wide annulus of peri-implant bone. (D) In the condensed bone group, the volume of interest (indicated with a green circle) was analyzed in the same manner. (E) Quantification of trabecular thickness, trabecular spacing, and bone volume/total volume (BV/TV). *P < 0.05. (F) Experimental system used to evaluate lateral stability of implants placed in osteotomies created by drilling or condensing. (G) A stepper motor delivered a defined displacement (Δx) at a specified height along the implant (L), and the resulting force (F) was recorded. (H) Representative recordings of force (measured in newtons) and resulting displacement (measured in microns) from a trial run. (I) Primary stability was equivalent regardless of whether the implant was placed into an osteotomy created by drilling (gray) or condensing (blue) the bone. (J) The slope of the F/Δx plot was calculated from the linear portion of the trace. PID, postimplant day. Scale bars: C, D = 1 mm.
For FE analysis, µCT, and lateral stability testing, as well as histology, histomorphometric analyses, and immunohistochemistry, see Appendix for details.
Statistical Analyses
Results were presented as mean ± SD. A 2-tailed Student’s t test was used to determine significant differences between data sets. A P value <0.05 was considered statistically significant, and all statistical analyses were performed with Microsoft Excel software (version 15.16).
Results
Condensation Increases Peri-implant Bone Density but Does Not Improve Implant Stability
A common justification for using condensation prior to implant placement is that condensation is thought to increase peri-implant bone density. We set out to analyze whether this reasoning was scientifically justified. To create a suitable oral implant bed, maxillary first molars were extracted; when the BV/TV of the extraction site was equivalent to adjacent pristine type III bone, the site was considered healed (Tanoue et al. 2015). Thereafter, a split-mouth model was employed, where a 0.50-mm diameter osteotomy was created on one side and a 0.30-mm pilot osteotomy was created on the other side, followed by 4 condensation steps (see Methods; Fig. 1A). The drilled and condensed bones then each received a 0.62-mm titanium implant (Fig. 1B). A total of 90 implants were placed.
Immediately thereafter, changes in peri-bone microarchitecture were evaluated by µCT (Fig. 1C, D). The condensation group showed a statistically significant reduction in trabecular spacing and a statistically significant increase in BV/TV (Fig. 1E). Thus, condensation caused an increase in the apparent density of peri-implant bone (Lan et al. 2013).
Biomechanics predicts that an increase in apparent density increases implant stability because elastic and strength properties of cancellous bone increase with apparent density (Hayes and Bouxsein 1997). We directly tested whether the increase in apparent density that we observed caused the implant to be more stable. A known lateral displacement was applied to the implant, and the force to cause that displacement was measured (Fig. 1F, G). Force and displacement were then plotted as a function of time (Fig. 1H), with interfacial lateral stiffness calculated by measuring the slope of the force versus the displacement plot (Fig. 1I). There was no significant difference in the lateral stiffness of implants in drilled versus condensed peri-implant bone (Fig. 1J). Thus, while condensation did increase the apparent density of interfacial bone, this “densification” did not significantly improve primary stability.
This finding was unexpected and suggested that the relationship between condensation and implant primary stability was more complicated.
Condensation Creates High Interfacial Strains and Extensive Interfacial Microdamage
Our next analyses focused on understanding how condensation affected peri-implant bone microarchitecture. FE models were generated through actual geometries (Fig. 2A, B; Appendix Table 3). The elastic modulus of bone was based on the BV/TV of bone in the healed extraction site. The modeled osteotome was inserted to a 5.0-mm depth (Fig. 2C), and the resulting strain distributions were assessed. The first osteotome created maximal compressive strains of ~20% around the top of the osteotome with lesser strain values toward the tapered tip (Fig. 2D). Each condensation step created ~10% additional strain (Fig. 2E). Combined with the strains created by implant placement (Fig. 2F, G), the total magnitude of accumulated strain in the crestal region was very high, on the order of 80% (red bar, Fig. 2E).
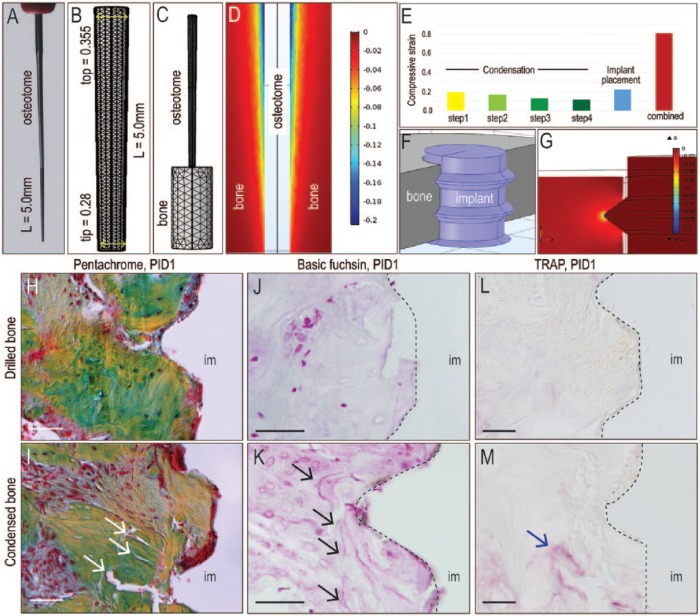
Figure 2.
Condensation creates high interfacial strains and damages peri-implant bone. (A) Representative osteotome used to condense the bone was (B) schematized in a finite element model, where the osteotome was positioned over the pilot drill hole in simulated bone, then inserted to a 5.0-mm depth. (C) Dimensions of the tapered osteotomes were represented in the finite element model. (D) A representative midlongitudinal image of the pattern and magnitude of the compressive strain created in response to insertion of the tapered osteotome. (E) Schematic of an implant placed into an osteotomy and (F) the resulting compressive strains created around the threads of the implant. (G) Magnitude of the compressive strain in bone created by each successive step of condensation and implant placement. The combined compressive strain is additive (red bar). Pentachrome staining of representative tissue sections from (H) a case where the implant was placed into drilled bone versus (I) a case where the implant was placed into condensed bone; arrows indicate microfractures. Basic fuchsin staining of representative tissue sections from (J) a case where the implant was placed into drilled bone versus (K) a case where the implant was placed into condensed bone; arrows indicate microdamage. Tartrate-resistant acid phosphatase (TRAP) activity on representative tissue sections from (L) a case where the implant was placed into drilled bone versus (M) a case where the implant was placed into condensed bone; arrow indicates bone remodeling around microfractures. im, implant; PID, postimplant day. Scale bars = 50 µm.
The immediate consequence of accumulated high strain was examined. In the control group, pentachrome staining showed a largely intact implant-bone interface on postimplant day 1 (PID1; Fig. 2H). In the condensation group, interfacial bone exhibited significant microfracturing (Fig. 2I). Basic fuchsin staining (Poundarik and Vashishth 2015) confirmed actual microdamage (Fig. 2J, K). Microdamage triggers bone resorption (Mori et al. 1993), and as anticipated, a concentration of resorptive TRAP+ve osteoclasts were localized around these microfractures (Fig. 2L, M). Thus, high interfacial strains created by condensation caused microdamage and triggered bone resorption.
Condensation Is Associated with Persistent Bone Resorption
The condensed bone underwent a prolonged period of bone resorption. On PID7, the pattern of osteoclastic activity was significantly broader in the condensed group (Fig. 3A–C). Molecular markers of osteogenic differentiation were used to analyze the onset of bone formation, and in samples where the bone had been condensed, significantly reduced levels of Runx2 (Fig. 3D–F) and Osterix (Fig. 3G–I) were observed.
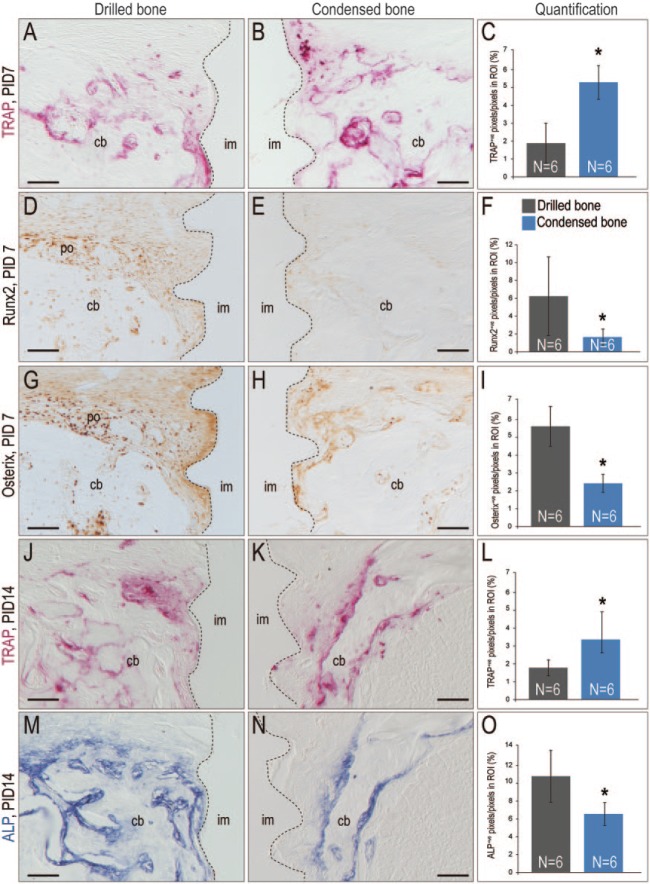
Figure 3.
Condensed bone exhibits diminished osteogenesis coupled with elevated and prolonged resorption. Tartrate-resistant acid phosphatase (TRAP) activity on representative tissue sections from (A) a case where the implant was placed into drilled bone versus (B) a case where the implant was placed into condensed bone; dotted line indicates bone-implant interface. (C) Quantification of TRAP+ve pixels in the region of interest (ROI; see Appendix). Runx2 expression on a representative tissue section from (D) a case where the implant was placed into drilled bone versus (E) condensed bone; (F) quantification of Runx2+ve pixels in the ROI. Similar expression analyses of Osterix in (G) drilled versus (H) condensed bone; (I) quantification of Osterix+ve pixels. TRAP activity in (J) drilled versus (K) condensed bone on PID14; (L) quantification of TRAP+ve pixels in ROI. Similar analyses of alkaline phosphatase (ALP) activity in (M) drilled versus (N) condensed bone; (O) quantification of ALP+ve pixels. cb, cancellous bone; im, implant; PID, postinsertion day; po, periosteum. Scale bars = 100 µm. *P < 0.05.
Enzymatic activity analyses on PID14 demonstrated that the bone resorptive state persisted. TRAP staining was significantly broader (Fig. 3J–L) and alkaline phosphatase (ALP) activity significantly more restricted (Fig. 3M–O) in the condensed group versus the drilled bone group (Appendix Fig.). Thus, in the condensed bone group, bone resorption exceeded bone formation.
Condensation Causes Crestal Region Bone Loss and Does Not Improve Secondary Stability
By PID21, murine oral implants typically osseointegrate (Mouraret, Hunter, Bardet, Brunski, et al. 2014a, Mouraret, Hunter, Bardet, Popelut, et al. 2014; Yin et al. 2016), and here, the same was observed: the control group showed uniform bone in contact with the implant surface, extending to the top thread (Fig. 4A). The interfacial bony matrix was mature, with no evidence of damage (Fig. 4B). Coupled with the absence of TRAP staining, these data demonstrated that osseointegration was largely complete by PID21 (Appendix Fig. 1E).
Figure 4.
Condensation induces a strain pattern predicting long-term resorption of peri-implant crestal bone. (A) Pentachrome staining after 21 d of implant insertion in drilled bone. (B) Aniline blue staining after 21 d of implant insertion in drilled bone; the dashed line indicates the edge of peri-implant bone. (C) Pentachrome staining after 21 d of implant insertion in condensed bone. (D) Aniline blue staining after 21 d of implant insertion in condensed bone; the dashed line indicates the edge of peri-implant bone. (E) The strain created by condensing in the crestal versus apical region of bone. (F) The strain created by condensing in the crestal region of bone. (G) Schematic of peri-implant bone volume in the crestal versus apical region. (H) Quantification of bone volume in the crestal, apical, and total area of peri-implant bone. (I) The change of implant stiffness after insertion. (J) The stiffness of implant for the drilled versus condensed groups on PID21. (K) Relationship between lateral stiffness and the elastic modulus of peri-implant bone. im, implant; PID, postinsertion day. Scale bar = 200 µm. *P < 0.05.
Implants placed into condensed bone osteotomies, however, showed a funnel-shaped deficit in crestal bone (dotted lines, Fig. 4C). The crestal space was occupied by a fibrous tissue collar (Fig. 4D). This pattern of interfacial bone loss mirrored the spatial distribution of predicted high strains induced by condensation (Fig. 4E, F).
Histomorphometric analyses were used to quantify interfacial bone volumes (Fig. 4G). In the crestal region, where condensation caused very high strains, condensed bone samples had significantly less bone (Fig. 4H). In the apical region, where condensation-induced strains were small, there was no difference in bone volumes (Fig. 4H). When summed over the total interface, no statistically significant differences in the volume of interfacial bone were observed (Fig. 4H). Experimental testing for lateral stiffness on PID21 confirmed that there was no significant difference in lateral stiffness between the drilled and condensed samples (Fig. 4I, J).
These data were quite surprising, given the number of claims that condensation increases implant stability (Marković et al. 2011; Nobrega et al. 2012; Shayesteh et al. 2013). We used a second FE model to revisit the question of how microdamage created by condensation affected secondary implant stability. We found that to match the measured value of interfacial bone stiffness at PID21, the modulus of the interfacial bone needed to be in the realm of 26 MPa for the drilled bone samples and 29 MPa for the condensed bone samples (Fig. 4K).
These modulus values at PID21 were moderately larger than at PID0, when the modulus of cut versus condensed samples was 17.2 and 23.5 MPa, respectively; this slight increase relative to PID0 was expected since interfacial bone at PID21 had undergone some healing and should have become stiffer (Marković et al. 2011).
Discussion
An In Vivo Model System for Assessing the Effects of Condensation
Clinically, condensation is applied when there is an insufficient amount of bone to support implant placement, for example, in type III bone (Summers 1994; Hahn 1999). To ensure that our model system mimicked this clinical scenario, we performed the condensation procedure on bone in healed extraction sites. A healed maxillary first molar extraction site constitutes type III bone (Lekholm and Zarb 1985; Shah et al. 2013; Virdi et al. 2015).
Because our experiments were performed in healthy animals, one could question whether the bone density of the healed extraction site was higher than what might be found in patients who are candidates for condensation. Unfortunately, there is a near-complete lack of documentation of BV/TV values—either initially or immediately after condensation—for human subjects; consequently, a direct comparison is impossible. However, Monje et al. (2015) did report typical BV/TV values of about 0.5 (range, 0.1759 to 0.73) for pristine bone in the human maxillae. This value compares favorably with the BV/TV value for our healed extraction sites, about 0.7 (Fig. 1).
We wondered if our conclusions would change if the bone that we used had a lower elastic modulus (e.g., represented a poorer-quality bone with a smaller BV/TV), but FE analyses explained why altering this variable would not have changed the outcomes. An implant’s lateral stiffness (k) is linearly dependent on the modulus (E) of interfacial bone (Fig. 4K), and we found that the modulus was linearly dependent on apparent density (Fig. 1). Thus, for the same osteotome procedure, similar interfacial strains are produced regardless of the starting modulus. As such, the results of our study would not be appreciably different if the bone that underwent condensation had a lower initial BV/TV.
A standard clinical condensation protocol involves the creation of an initial osteotomy, ~1.0- to 2.0-mm in diameter, which is enlarged to create a 3.5- to 3.8-mm implant bed (Nkenke et al. 2002; Donati et al. 2013). Based on these values, the computed average radial strains are between 43% to 200%. In our model, we opted to create strains on the lower end of this range (66%; Fig. 1A). By ensuring that the degree of radial enlargement was on the conservative end of the spectrum, we were able to draw realistic conclusions on how condensation affects interfacial bone.
Condensation Densifies and Damages Interfacial bone
Following stepwise condensation, µCT analyses demonstrated that condensation significantly increased BV/TV (Fig. 1E). These data are in agreement with the view that condensation “densifies” bone (Blanco et al. 2008). It is tempting to then assume that an increase in interfacial BV/TV (Fig. 1) should increase interfacial apparent density and, in turn, the interfacial elastic modulus. This assumption would be in keeping with common power-law relationships between elastic modulus and apparent density (O’Mahony et al. 2000).
This assumption is flawed. The power-law relationships relating E and apparent density were developed from the mechanical testing of pristine cancellous bone. It is therefore incorrect to assume that the same relationship is applicable to damaged, compacted bone. Using this improper line of reasoning leads to an overestimate of the elastic modulus of condensed bone.
Compared with intact bone (Fig. 5A), condensed bone is denser (Fig. 5B, C) in that it has a higher BV/TV (Fig. 5D); however, condensed bone is also damaged, and this loss in connectivity (Fig. 5E) significantly weakens the structure (Keaveny et al. 1994; Kabel et al. 1999; Szabó et al. 2011; Hardisty et al. 2013), meaning that the weakened bone is less able to support an implant. Other investigators reached similar conclusions regarding orthopedic implants (Giesen et al. 1999).
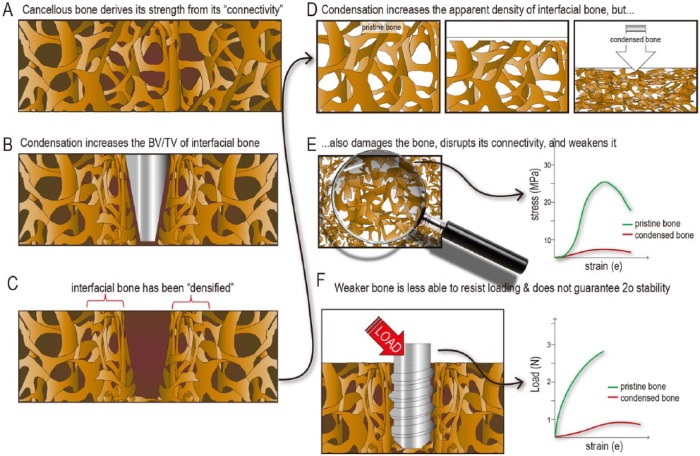
Figure 5.
Condensation provides questionable benefits to implant stability. (A) Compared with intact bone, (B) condensed bone has a higher bone volume/total volume (BV/TV). (C) This “densified” interfacial bone has an increased apparent density (D), but (E) condensation also disrupts the interconnected nature of the trabeculae, which leads to a decrease in elastic modulus. (F) Implants placed in bone with a low elastic modulus are less able to resist loading and thus more prone to failure.
Damaged bone remodels and becomes stiffer with time, but our experimental measurements demonstrated that condensed bone neither stiffened as quickly nor as much (Fig. 4I, J), as has been suggested. Collectively, the effect of condensation on implant primary and secondary stability appears to be minimal, at best (Fig. 5F).
Condensation and “Osseodensification”
Condensation is advertised as a means to densify bone and thus confer better primary stability to an implant (e.g., http://versah.com/versah-densah-bur-technology/). The concept seems plausible, so why is there a discrepancy between such claims and our actual experimental results?
The most straightforward answer is that few studies actually measured primary implant stability immediately after condensation. In a recent study of “osseodensification,” Osstell readings immediately after implant placement showed no difference between implants placed after standard drilling versus Versah counterclockwise drilling (Huwais and Meyer 2017). Nonetheless, the authors claimed that implants in Versah-drilled sites exhibited “better stability” based on indirect evidence from insertion torque data. Neither insertion torque nor bone-implant contact, however, is an accurate measure of implant stability (Cehreli et al. 2009; Degidi et al. 2009).
In other published work, investigators claimed that implants placed after Versah counterclockwise drilling exhibited a lower “value of actual micromotion” (VAM) as compared with implants placed after conventional drilling (Trisi et al. 2016). However, in this study, implant stability was not measured at the time of implant placement but 2 mo later, which is not an appropriate time for testing primary stability. It should also be pointed out that implants used in the Versah group had a larger diameter than those used in the control group (5.0-mm vs. 3.8- mm). Although the authors argue that different implant surfaces areas should not have too much of an effect on VAM, they neglected to calculate how this difference in diameter affects lateral stiffness or VAM. Using the values provided, we calculated a 58% difference in lateral stiffness due to diameter difference alone, with the larger diameter implant having greater lateral stability. Therefore, Trisi and colleagues’ (2016) conclusion that “osseodensification” increases an implant’s primary stability is open to question.
Conclusion
In this murine model, we provide evidence that condensation can increase the density of peri-implant bone; however, this did not ensure greater bone-implant contact, nor did it improve implant stability. Instead, even relatively conservative stepwise condensation created high interfacial strains that caused fractures and triggered a prolonged period of bone resorption. The resulting funnel-shaped bony deficits caused by condensation may help explain why implants placed into condensed osteotomies fail to show superior secondary stability.
Author Contributions
L. Wang, contributed to data analysis, acquisition, and interpretation, drafted and critically revised the manuscript; J.A. Helms, contributed to conception, data analysis, and interpretation, drafted and critically revised the manuscript; Y. Wu, contributed to data analysis, drafted the manuscript; K.C. Perez, U. Tulu, contributed to data acquisition and interpretation, critically revised the manuscript; S. Hyman, J. Brunski, B. Salmon, contributed to data analysis, critically revised the manuscript; C. Bao, contributed to conception and design, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Acknowledgments
We thank Jingtao Li and Xin Yin for their invaluable suggestions on establishing a mouse model; we thank Tao Chen and Bo Liu for invaluable aid in performing histology and data analysis.
Footnotes
This research project was supported by a grant from the National Institutes of Health (R01DE024000-11, J.A.H. and J.B.), from the National Key Research and Development Program of China (2016YFC1102702; L.W. and C.B.), and from the National Natural Science Foundation of China (81600910, 31400829; L.W.).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is available online.
References
- Blanco J, Suárez J, Novio S, Villaverde G, Ramos I, Segade LA. 2008. Histomorphometric assessment in human cadavers of the peri-implant bone density in maxillary tuberosity following implant placement using osteotome and conventional techniques. Clin Oral Implants Res. 19(5):505–510. [DOI] [PubMed] [Google Scholar]
- Cehreli MC, Kökat AM, Comert A, Akkocaoğlu M, Tekdemir I, Akça K. 2009. Implant stability and bone density: assessment of correlation in fresh cadavers using conventional and osteotome implant sockets. Clin Oral Implants Res. 20(10):1163–1169. [DOI] [PubMed] [Google Scholar]
- Degidi M, Perrotti V, Strocchi R, Piattelli A, Iezzi G. 2009. Is insertion torque correlated to bone-implant contact percentage in the early healing period? A histological and histomorphometrical evaluation of 17 human-retrieved dental implants. Clin Oral Implants Res. 20(8):778–781. [DOI] [PubMed] [Google Scholar]
- Donati M, Botticelli D, La Scala V, Tomasi C, Berglundh T. 2013. Effect of immediate functional loading on osseointegration of implants used for single tooth replacement: a human histological study. Clin Oral Implants Res. 24(7):738–745. [DOI] [PubMed] [Google Scholar]
- Fanuscu MI, Chang TL, Akça K. 2007. Effect of surgical techniques on primary implant stability and peri-implant bone. J Oral Maxillofac Surg. 65(12):2487–2491. [DOI] [PubMed] [Google Scholar]
- Giesen EB, Lamerigts NM, Verdonschot N, Buma P, Schreurs BW, Huiskes R. 1999. Mechanical characteristics of impacted morsellised bone grafts used in revision of total hip arthroplasty. J Bone Joint Surg Br. 81(6):1052–1057. [DOI] [PubMed] [Google Scholar]
- Hahn J. 1999. Clinical uses of osteotomes. J Oral Implantol. 25(1):23–29. [DOI] [PubMed] [Google Scholar]
- Hardisty MR, Zauel R, Stover SM, Fyhrie DP. 2013. The importance of intrinsic damage properties to bone fragility: a finite element study. J Biomech Eng. 135(1):011004. [DOI] [PubMed] [Google Scholar]
- Hayes WC, Bouxsein ML. 1997. Biomechanics of cortical and trabecular bone: implications for assessment of fracture risk. Philadelphia (PA): Lippincott-Raven. [Google Scholar]
- Huwais S, Meyer EG. 2017. A novel osseous densification approach in implant osteotomy preparation to increase biomechanical primary stability, bone mineral density, and bone-to-implant contact. Int J Oral Maxillofac Implants. 32(1):27-36. [DOI] [PubMed] [Google Scholar]
- Kabel J, Odgaard A, Van Rietbergen B, Huiskes R. 1999. Connectivity and the elastic properties of cancellous bone. Bone. 24(2):115–120. [DOI] [PubMed] [Google Scholar]
- Keaveny TM, Wachtel EF, Guo XE, Hayes WC. 1994. Mechanical behavior of damaged trabecular bone. J Biomech. 27(11):1309–1318. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG; National Centre for the Replacement, Refinement and Reduction of Amimals in Research. 2011. Animal research: reporting in vivo experiments—the ARRIVE guidelines. J Cereb Blood Flow Metab. 31(4):991–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutouzis T, Koutouzis G, Tomasi C, Lundgren T. 2011. Immediate loading of implants placed with the osteotome technique: one-year prospective case series. J Periodontol. 82(11):1556–1562. [DOI] [PubMed] [Google Scholar]
- Lan S, Luo S, Huh BK, Chandra A, Altman AR, Qin L, Liu XS. 2013. 3D image registration is critical to ensure accurate detection of longitudinal changes in trabecular bone density, microstructure, and stiffness measurements in rat tibiae by in vivo microcomputed tomography (μCT). Bone. 56(1):83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekholm U, Zarb G. 1985. Patient selection and preparation. Chicago (IL): Quintessence. [Google Scholar]
- Marković A, Calasan D, Colic S, Stojcev-Stajcic L, Janjic B, Misic T. 2011. Implant stability in posterior maxilla: bone-condensing versus bone-drilling: a clinical study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 112(5):557–563. [DOI] [PubMed] [Google Scholar]
- Marković A, Mišić T, Miličić B, Calvo-Guirado JL, Aleksić Z, Ðinić A. 2013. Heat generation during implant placement in low-density bone: effect of surgical technique, insertion torque and implant macro design. Clin Oral Implants Res. 24(7):798–805. [DOI] [PubMed] [Google Scholar]
- Monje A, González-Garcia R, Monje F, Chan HL, Galindo-Moreno P, Suarez F, Wang HL. 2015. Microarchitectural pattern of pristine maxillary bone. Int J Oral Maxillofac Implants. 30(1):125–132. [DOI] [PubMed] [Google Scholar]
- Mori S, Harruff R, Burr DB. 1993. Microcracks in articular calcified cartilage of human femoral heads. Arch Pathol Lab Med. 117(2):196–198. [PubMed] [Google Scholar]
- Mouraret S, Hunter DJ, Bardet C, Brunski JB, Bouchard P, Helms JA. 2014. A pre-clinical murine model of oral implant osseointegration. Bone. 58:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouraret S, Hunter DJ, Bardet C, Popelut A, Brunski JB, Chaussain C, Bouchard P, Helms JA. 2014. Improving oral implant osseointegration in a murine model via wnt signal amplification. J Clin Periodontol. 41(2):172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkenke E, Kloss F, Wiltfang J, Schultze-Mosgau S, Radespiel-Tröger M, Loos K, Neukam FW. 2002. Histomorphometric and fluorescence microscopic analysis of bone remodelling after installation of implants using an osteotome technique. Clin Oral Implants Res. 13(6):595–602. [DOI] [PubMed] [Google Scholar]
- Nobrega AR, Norton A, Silva JA, Silva JP, Branco FM, Anitua E. 2012. Osteotome versus conventional drilling technique for implant site preparation: a comparative study in the rabbit. Int J Periodontics Restorative Dent. 32(3):e109–e115. [PubMed] [Google Scholar]
- O’Mahony AM, Williams JL, Katz JO, Spencer P. 2000. Anisotropic elastic properties of cancellous bone from a human edentulous mandible. Clin Oral Implants Res. 11(5):415–421. [DOI] [PubMed] [Google Scholar]
- Poundarik AA, Vashishth D. 2015. Multiscale imaging of bone microdamage. Connect Tissue Res. 56(2):87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proff P, Bayerlein T, Rottner K, Mai R, Fanghänel J, Gedrange T. 2008. Effect of bone conditioning on primary stability of frialit-2 implants. Clin Oral Implants Res. 19(1):42–47. [DOI] [PubMed] [Google Scholar]
- Shah NJ, Hyder MN, Moskowitz JS, Quadir MA, Morton SW, Seeherman HJ, Padera RF, Spector M, Hammond PT. 2013. Surface-mediated bone tissue morphogenesis from tunable nanolayered implant coatings. Sci Transl Med. 5(191):191ra183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayesteh YS, Khojasteh A, Siadat H, Monzavi A, Bassir SH, Hossaini M, Alikhasi M. 2013. A comparative study of crestal bone loss and implant stability between osteotome and conventional implant insertion techniques: a randomized controlled clinical trial study. Clin Implant Dent Relat Res. 15(3):350–357. [DOI] [PubMed] [Google Scholar]
- Siddiqui AA, Sosovicka M. 2006. Lateral bone condensing and expansion for placement of endosseous dental implants: a new technique. J Oral Implantol. 32(2):87–94. [DOI] [PubMed] [Google Scholar]
- Steiner JA, Ferguson SJ, van Lenthe GH. 2015. Computational analysisof primary implant stability in trabecular bone. J Biomech. 48(5):807–815. [DOI] [PubMed] [Google Scholar]
- Summers RB. 1994. A new concept in maxillary implant surgery: the osteotome technique. Compendium. 15(2):152, 154,–156, 158. [PubMed] [Google Scholar]
- Szabó ME, Zekonyte J, Katsamenis OL, Taylor M, Thurner PJ. 2011. Similar damage initiation but different failure behavior in trabecular and cortical bone tissue. J Mech Behav Biomed Mater. 4(8):1787–1796. [DOI] [PubMed] [Google Scholar]
- Tabassum A, Meijer GJ, Walboomers XF, Jansen JA. 2014. Evaluation of primary and secondary stability of titanium implants using different surgical techniques. Clin Oral Implants Res. 25(4):487–492. [DOI] [PubMed] [Google Scholar]
- Tanoue R, Koi K, Yamashita J. 2015. Effect of alendronate on boneformation during tooth extraction wound healing. J Dent Res. 94(9):1251–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trisi P, Berardini M, Falco A, Podaliri Vulpiani M. 2016. New osseodensification implant site preparation method to increase bone density in low-density bone: in vivo evaluation in sheep. Implant Dent. 25(1):24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virdi AS, Irish J, Sena K, Liu M, Ke HZ, McNulty MA, Sumner DR. 2015. Sclerostin antibody treatment improves implant fixation in a model of severe osteoporosis. J Bone Joint Surg Am. 97(2):133–140. [DOI] [PubMed] [Google Scholar]
- Yin X, Li J, Chen T, Mouraret S, Dhamdhere G, Brunski JB, Zou S, Helms JA. 2016. Rescuing failed oral implants via wnt activation. J Clin Periodontol. 43(2):180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.