Abstract
Background
Coronary computed tomography angiography (CCTA) requires high spatial and temporal resolution, increased low contrast resolution for the assessment of coronary artery stenosis, plaque detection, and/or non-coronary pathology. Therefore, new reconstruction algorithms, particularly iterative reconstruction (IR) techniques, have been developed in an attempt to improve image quality with no cost in radiation exposure.
Purpose
To evaluate whether adaptive statistical iterative reconstruction (ASIR) enhances perceived image quality in CCTA compared to filtered back projection (FBP).
Material and Methods
Thirty patients underwent CCTA due to suspected coronary artery disease. Images were reconstructed using FBP, 30% ASIR, and 60% ASIR. Ninety image sets were evaluated by five observers using the subjective visual grading analysis (VGA) and assessed by proportional odds modeling. Objective quality assessment (contrast, noise, and the contrast-to-noise ratio [CNR]) was analyzed with linear mixed effects modeling on log-transformed data. The need for ethical approval was waived by the local ethics committee as the study only involved anonymously collected clinical data.
Results
VGA showed significant improvements in sharpness by comparing FBP with ASIR, resulting in odds ratios of 1.54 for 30% ASIR and 1.89 for 60% ASIR (P = 0.004). The objective measures showed significant differences between FBP and 60% ASIR (P < 0.0001) for noise, with an estimated ratio of 0.82, and for CNR, with an estimated ratio of 1.26.
Conclusion
ASIR improved the subjective image quality of parameter sharpness and, objectively, reduced noise and increased CNR.
Keywords: Computed tomography angiography (CTA), cardiac, image manipulation/reconstruction, perception image, vascular
Introduction
During the last decade, there has been a tremendous evolution in computed tomography (CT) technology (1), which has enabled its use with high diagnostic accuracy for the non-invasive assessment of the coronary arteries, called coronary computed tomography angiography (CCTA). CCTA requires high spatial and temporal resolution, increased low contrast resolution for the assessment of coronary artery stenosis, plaque detection, and/or non-coronary pathology (2). Therefore, iterative reconstruction (IR) techniques, have been developed in an attempt to possibly decrease the radiation dose or improve image quality (3–6). Diversity in reconstruction algorithms may result in images that do not look familiar to the observer and may thereby influencing the diagnostic performance (7).
Previous studies have shown that IR significantly reduces image noise without loss in the overall diagnostic information and thus holds the potential for substantial radiation dose reduction (1,8–12). However, previous studies only assessed the impact of IR on the overall image quality, either by objective or subjective assessments irrespective of the particular detailed anatomical image quality criteria and the intra-observer and inter-observer agreement among more than two observers. Key issues in CCTA interpretation are specifically the sharp visual reproduction of small anatomical structures that might be affected or disappear after the use of IR (13,14). This issue clarifies the need for a detailed assessment by more observers with respect to visual image quality perception (15,16) and for a correlation to objectively assess image quality, which has not yet been explored in CCTA to our knowledge.
The aim of this study was to evaluate whether adaptive statistical iterative reconstruction (ASIR) enhances the detailed perceived image quality in CCTA obtained by comparing the image quality for standard filtered back projection (FBP) with those for 30% ASIR and 60% ASIR reconstruction algorithms.
Material and Methods
Study population
Thirty consecutive patients with chest pain who were referred to CCTA for the assessment of suspected coronary artery disease (CAD) with a minimum of three clinical risk factors were included. The following exclusion criteria were used: age < 18 years, unable to hold the breath for 15 s, body mass index (BMI) > 40 kg/m2, atrial fibrillation, ectopic heart beats or other irregular heart rhythm, serum-creatinine ≥200 µmol/L, contrast media allergy, previous coronary artery bypass grafting, and inability to cooperate during the scan.
The need for written informed consent was waived by the local ethics committee due to the nature of the study, which only involved clinical data collection, which were collected anonymously.
CT acquisition
All CCTA examinations were performed on a CT750 HD scanner from GE Healthcare (Milwaukee, WI, USA) including 64 detector rows. Patients with a heart rate of > 60 bpm received IV β-blockers and all patients were given 0.4 mg of sublingual nitroglycerin immediately before the scan. A triple-phase contrast agent injection protocol was used for every patient: 55 mL of iodixanol (Visipaque 320 mgl/mL, GE Healthcare, Milwaukee, WI, USA), followed by 40 mL of a 60:40 mixture of iodixanol and saline, followed by a 50-mL saline flush. The iodinated contrast agent was injected through a venflon (18 G) in the brachial vein with a flow rate of 6.0 mL/s. The timing of the scan was time to peak in the aorta plus 10 s, giving an individual delay in the interval of 22–24 s from start of contrast injection.
The scanning parameters were: rotation time, 350 ms; collimation, 64 × 0.625 mm; tube voltage, 100 Kv; tube current, 650–750 mA depending on the patient chest size. Prospectively gated sequential scanning with 25 ms padding was used.
Each patient had one CCTA scan performed, yielding 30 image sets reconstructed with FBP in the 75% phase of the cardiac cycle. The raw data were retrospectively reprocessed using ASIR reconstruction to generate 30% ASIR and 60% ASIR image sets (given 90 image sets).
Subjective image quality assessment
The 108 image sets (90 image sets with 18 duplicated for intra-observer variability) were assessed for image quality using absolute visual grading analysis (VGA) (17–21). As no internationally recognized image criteria for CCTA are available, we chose to build five image criteria based on knowledge from specialized cardiologists, chest pain as clinical indication, and the criteria from the European Guidelines for chest CT (22) to secure the technical CT image quality criteria (14) (Table 1). The images were evaluated blindly in a random order by five experienced level 3-certified CCTA cardiologists from five different hospitals.
Table 1.
VGA image criteria connected to the referring technical image quality parameters for CT [14] and the VGA scoring scale used for assessing the quality of the visual reproduction of the anatomical structures [23-27]
| No. | Image criteria | Relation to technical image quality |
|---|---|---|
| 1 | Sharp/clear demarcation of the aortic wall | Sharpness of the edge in a large structure |
| 2 | Sharpness of the coronary artery contour | Sharpness of the edge in a relatively small structure |
| 3 | Sharp/clear reproduction of the anterior mitral valve | High contrast spatial resolution |
| 4 | Homogeneity in the left/right ventricle | Noise |
| 5 | Visualization of the myocardial septum between the right and left ventricle | Low contrast resolution |
| Score |
Definition |
Explanation |
| 1 | Not reproduced | The structure could not be discerned |
| 2 | Poorly reproduced | The structure was vaguely reproduced |
| 3 | Adequate reproduced | The structure was moderately reproduced |
| 4 | Well reproduced | The structure was clearly reproduced |
| 5 | Very well reproduced | The structure had a completely distinct shape |
Using Viewer for Digital Evaluation of X-ray images (ViewDex, Gothenburg, Sweden) (23,24), the images were presented to the observers in an individually randomized order and the scoring marks (Table 1) were noted directly on the monitor. Each observer’s individual quality ratings were stored and subsequently exported into Microsoft Excel. The images were displayed on a 10-bit grayscale 1600 × 1200 pixels 20.1” TFT Dicom-14 monitor with max luminance level of 250 cd/m2 (Olorin, MedicLine MC200D; Fineman, Birkerød, Denmark). The monitor was calibrated according to the Digital Imaging and Communications in Medicine (DICOM) part 14 (25).
To optimize the settings for the intra-/inter-observer agreement analysis and ensure that the full VGA rating scale was utilized, the evaluations were performed at the same location and with the same ambient surroundings, and a training session was conducted to illustrate the best and worst possible image qualities (16,19–21).
Objective image quality assessment
The following objective image criteria were evaluated:
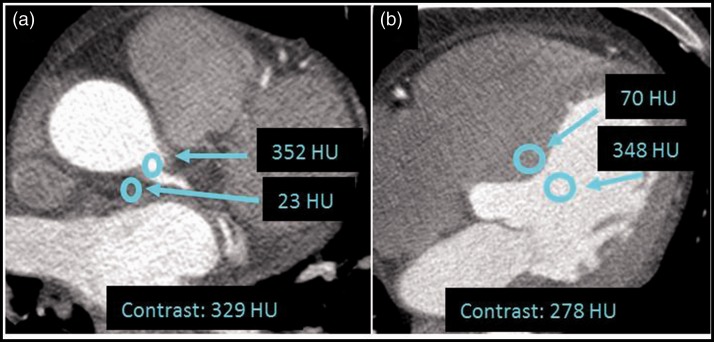
Contrast was determined as the difference in HU between two adjacent anatomical structures (13) as indicated in Fig. 1;
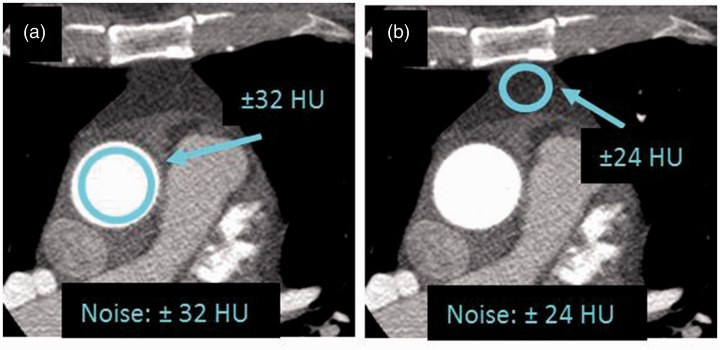
Noise was measured as the standard deviation (SD) of the pixel HU-value variations within each region of interest (ROI) of two anatomical areas (13). The positioning of the ROIs was chosen to be in expected anatomically homogenous areas where only the contribution to HU variability was from noise (26,27) (Fig. 2);
The contrast-to-noise ratio (CNR) was computed as the ratio between contrast, as measured in the myocardium at the ventricular septum (Fig. 1b) and noise as measured in the aorta.
Fig. 1.
Example of ROI locations for objective measures of contrast with a ROI diameter of 3 mm in measure A (a) and 5 mm in measure B (b).
Fig. 2.
The ROI locations for objective measures of noise with a diameter of 20 mm for measure A (a) and 10 mm for measure B (b).
Statistical analysis
The study was conducted in a paired design.
The analyses of the subjective image quality experiments were performed using an ordered logistic regression (proportional odds model), with the VGA scores explained by the reconstruction algorithms used (FBP, 30% ASIR, and 60% ASIR), and odds ratios (OR) were given. Intra-observer agreement was assessed using Cohen’ kappa (κ) for each of the five readers in combination with the image criteria (28). Cohen’s ĸ with Cicchetti-Allison (CA) weighting was applied to evaluate the scores for image criteria 1–5, and a respective 95% confidence interval (CI) was given using bootstrap techniques (29,30). Inter-observer agreement was assessed by Fleiss’ κ for five raters, and a respective 95% CI was given using bootstrap techniques similar to the intra-observer agreement.
For the objective image quality assessment analysis, a linear mixed effects model was used, with a random intercept varying for each patient; the independent explanatory variable was the reconstruction used (FBP, 30% ASIR, and 60% ASIR), and the response variables were the objective image quality parameters (contrast, noise, and CNR). As these response variables proved not to be normally distributed, the calculations were performed using log-transformed data. The log-transformed quality data for images reconstructed with ASIR (30% and 60%) were modeled as a linear response of the log-transformed quality data for the same images reconstructed with FBP, with no constraints on the intercept. The slope of the regressions was subsequently back-transformed to the original scale to yield the expected mean ratios of the outcome variable values for the 30% ASIR and 60% ASIR reconstructions compared with those for FBP. The inter- and intra-rater agreements were assessed by means of Bland–Altman plots. The agreements were analyzed by the limits of agreement. The importance of the scattering pattern for the clinical result of the objective image analysis was considered.
All statistical analyses were performed using © Stata/MP 12 (StataCorp LP, College Station, TX, USA).
Results
Patients and CT acquisition characteristics are shown in Table 2. The mA varied minimally based on the small variation in patient chest size affecting an almost equal signal-to-noise ratio (SNR) between the patient’s image sets.
Table 2.
Thirty patient and CT acquisition characteristics.
| Patient data | |
| Age (years), mean ± SD | 59 ± 10 |
| Sex, male/female | 20 (67%)/10 (33%) |
| Height (cm), mean ± SD | 176 ± 10 |
| Weight (kg), mean ± SD | 86 ± 19 |
| BMI (kg/m2): body mass index, mean ± SD (range) | 28 ± 5 (18.9–40.0) |
| Chest area at cardiac apex (ellipse in cm2), median (range) | 958 (572–1282) |
| Heart rate (bpm) | 59 ± 3 |
| Scanning data | |
| kVp | 100 |
| mA, median (range) | 650 (650–750) |
| Scan length (cm), mean ± SD | 13.7 ± 1.30 |
| DLP (mGy x cm), median (range) | 176 (138–176) |
| CTDIvol, mean ± SD (range) | 9.8 ± 0.53 (56.3–57.8) |
| Effective dose (mSv), mean ± SD | 2.5 ± 0.3 |
Subjective image quality assessment
For image criterion 1 (sharp demarcation of the aortic wall) and image criterion 2 (sharpness of the coronary artery contour), significant improvements were found for 30% ASIR and 60% ASIR compared with FBP. There was a trend favoring of 60% ASIR. For image criteria 3–5 (spatial resolution, noise, and low contrast resolution, see Table 1), no significant differences were found among the different reconstruction types (Table 3).
Table 3.
Results from the proportional odds model for the subjective analysis for five observers for each image criterion. FBP was the reference reconstruction method.
| Image criteria | Reconstruction method | Odds ratio | 95% CI | P value |
|---|---|---|---|---|
| 1: Sharp demarcation of aortic wall | FBP (reference) | |||
| 30% ASIR | 1.55 | 1.23–1.94 | <0.0001 | |
| 60% ASIR | 1.89 | 1.48–2.43 | <0.0001 | |
| 2: Sharpness of coronary artery contour | FBP (reference) | |||
| 30% ASIR | 1.54 | 1.15–2.06 | 0.004 | |
| 60% ASIR | 1.90 | 1.43–2.52 | <0.0001 | |
| 3: Sharp reproduction of anterior mitral valve | FBP (reference) | |||
| 30% ASIR | 1.05 | 0.64–1.72 | 0.85 | |
| 60% ASIR | 0.87 | 0.52–1.45 | 0.59 | |
| 4: Homogeneity in left/right ventricle | FBP (reference) | |||
| 30% ASIR | 1.08 | 0.70–1.67 | 0.72 | |
| 60% ASIR | 1.19 | 0.74–1.92 | 0.46 | |
| 5: Visualization of myocardial septum | FBP (reference) | |||
| 30% ASIR | 1.16 | 0.68–1.99 | 0.58 | |
| 60% ASIR | 1.28 | 0.74–2.19 | 0.38 |
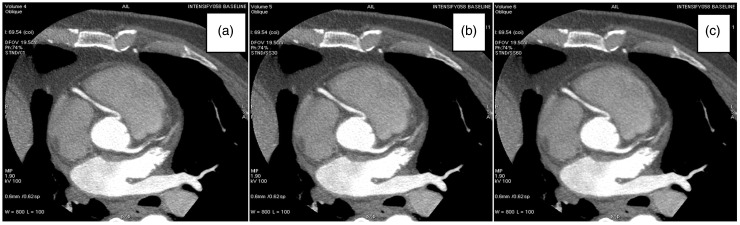
The visualized image quality is shown in Fig. 3, using one axial example.
Fig. 3.
CCTA images illustrating (a) FBP reconstructed image, (b) 30% ASIR image, and (c) image reconstructed with 60% ASIR.
Agreement study for the subjective image quality assessment
The intra-observer agreement varied from “poor” to “very good” depending on image criteria and observers, with κ values ranging from 0.16 (95% CI, –0.43 – 0.74) to 1 for the observer with the largest variability and κ values ranging from 0.54 (95% CI, 0.25–1.0) to 0.69 (95% CI, 0.24–0.84) for the observer with the smallest variability. In general, wide disagreement was found for most observers and, on average one-third of the repeated images deviated by only one VGA score, which is considered as clinical acceptable (see supplemental material). For all observers, the smallest κ value was found for criteria 1 and 2, and the largest κ-value was found for criteria 3 and 4.
The inter-observer κ values were calculated for each VGA criterion (Table 1), revealing a wide variation in the perceived image quality among the five observers. The κ values ranged from –0.005 (95% CI, –0.01 –0.002) for image criterion 5 to –0.10 (95% CI, –0.11 – –0.09) for image criterion 4, which indicate poor inter-observer agreement for all image criteria.
Objective image quality assessment
As shown in Table 4, significant differences between the reconstruction algorithms were found for noise and CNR, whereas no significant difference was found for contrast. For noise, the change in reconstruction showed a drop (30% ASIR: 0.94; 95% CI, 0.91–0.99; P = 0.009; 60% ASIR: 0.82; 95% CI, 0.79–0.86; P < 0.0001). The reverse relationship was observed for contrast (30% ASIR, 1.00; 95% CI, 0.95–1.05; P = 0.99; 60% ASIR: 1.01; 95% CI, 0.96–1.06; P = 0.76) and CNR (30% ASIR: 1.09; 95% CI, 1.02–1.15; P = 0.006; 60% ASIR: 1.26; 95% CI, 1.19–1.34; P < 0.0001).
Table 4.
Results of the linear mixed effects models for each image quality criterion after back-transformation to the original scale of HU: contrast, noise, and CNR. The reference reconstruction method was FBP, and the reference anatomical location was A, shown in Fig. 1 and explained for noise.
| Image quality | Reconstruction | Estimated ratio | 95% CI for estimated ratio | P value |
|---|---|---|---|---|
| Contrast | FBP (reference) | |||
| 30% ASIR | 1.00 | 0.95–1.05 | 0.99 | |
| 60% ASIR | 1.01 | 0.96–1.06 | 0.76 | |
| Anatomical location | 0.65 | 0.62–0.68 | <0.0001 | |
| Noise | FBP (reference) | |||
| 30% ASIR | 0.94 | 0.91–0.99 | 0.009 | |
| 60% ASIR | 0.82 | 0.79–0.86 | <0.0001 | |
| Anatomical location | 0.89 | 0.86–0.93 | <0.0001 | |
| CNR | FBP (reference) | |||
| 30% ASIR | 1.09 | 1.02–1.15 | 0.006 | |
| 60% ASIR | 1.26 | 1.19–1.34 | <0.0001 | |
| Anatomical location | 0.24 | 0.23–0.25 | <0.0001 | |
In the linear regression analysis, the anatomical ROI position was taken into consideration, showing a highly significant difference for contrast, noise, and CNR. All image quality parameters were associated with P values below 0.01, and the ratio of anatomical location B to A, as shown in Fig. 1 to be similar to noise, was estimated to be 0.89 (95% CI, 0.86–0.93), which meant a decrease in the standard deviation of HU, changing the reconstruction software. The same relationship was observed for contrast and CNR.
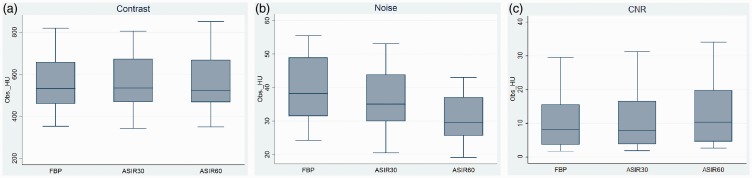
Box plots did not indicate any obvious difference in contrast among the reconstruction types (Fig. 4a), whereas noise decreased with an increasing percentage of ASIR (Fig. 4b). In contrast, CNR slightly increased with an increasing percentage of ASIR (Fig. 4c).
Fig. 4.
Objective image quality for FBP, 30% ASIR, and 60% ASIR illustrated as a box plot with the mean, median, 1st and 3rd quartiles and minimum and maximum HU for (a) contrast, (b) noise, and (c) CNR.
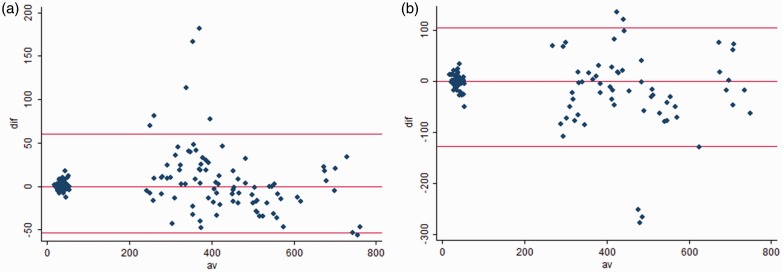
The variability of objective image quality assessment was performed and evaluated by Bland–Altman plots showing almost clinically sufficient intra- and inter-observer agreement for noise measures and small disagreements for contrast measures (Fig. 5).
Fig. 5.
Bland–Altman plot with indication a limits of agreement; (a) intra-observer variability, (b) inter-observer variability.
Discussion
This study demonstrated significant improvement in the observer’s subjective perceived sharpness of both the aortic wall and coronary artery contour when using ASIR compared with FBP. In addition, objective assessments showed a significant decrease of noise, and a corresponding significant increase in CNR using 60% ASIR compared with FBP.
Based on the subjective image quality assessment, significant improvements were found for the perceived sharpness of both the aortic wall and the coronary artery contour using 30% ASIR and 60% ASIR. These findings are quite surprising, given that ASIR primarily removes noise from the image. However, a potential disadvantage of ASIR is an artifactual oversmoothing in the image, which is visualized as a weak contrast demarcation among differences in attenuation between neighboring pixels (6). This oversmoothing was expected to negatively influence the human perception, as indicated by cognitive and perceptual mechanisms used to recognize anatomical contrast-enhanced structures with an edge-enhanced border (31). According to the significant findings of this study, the observers preferred the smoothest images best. This preference may be explained by the observations that the amount of ASIR processing applied to the image did not significantly affect image quality negatively (6,7) and that the observers were more disturbed by the minor noise represented in the image. Miéville et al. (12) found an increase in sharpness relying on a mean VGA score (VGAS) for a list of structures, including the left/right coronary artery and aorta for the use of 20% and 40% ASIR, followed by a decrease beyond 50% ASIR. They only focused on CCTA in children aged 0–7 years, which makes a clear difference, as pediatric structures are smaller and have a slimmer texture, which could explain the difference found to our study.
No significant differences were found for homogeneity or low contrast resolution. Based on the noise reduction by ASIR, the homogeneity and low contrast resolution were expected to increase, but this expected increase was not found to influence the VGA results. A possible explanation for this disconnect may be that the noise level was relatively low compared with the normal use of IR as the CT acquisitions were performed with an effective dose on 2.5 ± 0.3 mSv. Finally, there was no significant difference regarding sharpness in the reproduction of the anterior mitral valve related to the spatial resolution. This finding means that for ASIR to have an effect on the visibility of details in the image, these structures should either be of low contrast or be smaller than the anterior mitral valve. One should, however, bear in mind that the observer VGA scores were assessed collectively and that their disagreements, as indicated by the poor inter-observer agreement, could affect the non-statistical significance finding.
The majority of studies focusing on IR applied to CCTA evaluate the subjective image quality by only two observers based on an independently predefined scale for the overall image quality (1,9–11). Using this approach, it is problematic to compare outcome of the studies or to evaluate the IR influence on the individual image quality parameters. For example, Wang et al. (1) showed a significant improvement in both the subjective image quality and diagnostic accuracy using IR. Other studies (9–11) used a 4 - or 5-point scale to rate vessel blur, artifacts, and image noise individually defined for each of the four steps. Thus, they reported a better overall image quality than that of FBP.
The inter-observer agreement in VGA among the observers for the same image criterion was estimated to be in the range of pure random agreement. The rather poor observer agreement explains the complexity involved in evaluating image quality subjectively and the need to make the image assessment as detailed as possible and to include as many observers as possible. The intra-observer agreement was found to be clinical acceptable despite some very low κ values (see supplemental material).
Similar to previous studies, the objective image quality assessment demonstrated that the use of ASIR resulted in significant noise reduction and increased CNR. The largest difference was found for 60% ASIR compared with FBP, which was expected according to the processing algorithm (4–7). The noise measurements also showed the best intra- and inter-observer agreement. An increase in CNR means a larger ratio for the measured contrast signal to the background noise, which was improved based on the noise reduction (13). Hereby, the minor contrast differences were easier to visualize in HU. No differences between the reconstructions were found for the contrast measures, which could be explained by either the principle of IR and/or the largest variation in the assessment of intra- and inter-observer agreement. Dose reduction in CCTA using IR has been extensively investigated using objective image quality assessments (1,8–12,32,33). Our findings are in accordance with the results from those previous studies in which significant reductions in image noise and increases in CNR are reported even for 40–50% dose reductions. As our study did not involve dose reductions, the differences found between FBP and ASIR were more pronounced; however, our findings could contribute to the recommendation of the use of 60% ASIR.
An additional important finding was the influence of the heterogenic anatomical structures within the ROI measurements that were performed in two different axial slices. The difference between the two ROI placements showed high significance in all results. This finding highlights the importance of choosing the most appropriate axial slices for image assessment. It could be explained by the possibility that the two selected axial slice images emerged from different rotations on the 64 multislice CT scanner, resulting in a shift in the iodine contrast phase. As an identical iodine contrast concentration and protocol were used, even though the patients differed slightly in size and had a different cardiac output, the iodine contrast resolution and X-ray absorption varied between the different anatomical structures.
As found in the objective image quality assessment, the noise level significantly decreased using 30% ASIR and decreased further for 60% ASIR. This should then be expected to show up in the VGA score as a significant increase in perceived homogeneity in the ventricles. This was, however, not the case. Whereas the objective ROI measurements were taken in a uniform area, the subjective assessments related to the perception of the ventricles will always be influenced by other physical factors (e.g. scattered radiation, iodine contrast enhancement, and patient cardiac output) and the two sources of noise that affect our perception: (i) photonic noise; and (ii) the anatomical complexity in our area of interest (16,31). These effects could explain the differences between the observed objective and subjective assessments of noise. A study by Moore et al. (34) focusing on chest radiography in computed radiography systems is one of the few studies correlating subjective and objective image quality. The anatomical noise is known as the most limiting factor for diagnostic X-rays (35), and they show the importance of investigating detailed perceived image quality and not only focusing on the objective measures. The correlation between CNR and the average VGA score is good, and CNR is the only objective factor that can be used with clinical relevance.
The significant finding of the increase in objectively measured CNR using ASIR could affect the first three image criteria of the VGA. Enhanced contrast should make smaller structures more easily visible (anterior mitral valve), which was not found in these results, maybe because the smaller structures are extremely influenced by the heart moments (2). The lack of significance for the objective contrast measure may explain this lack instead.
This study has some limitations. First, this study consisted of a relatively small number of patients, which was considered to be applicable because of the paired design related to the three reconstructions. Second, only five specialized experienced cardiologists performed the subjective VGA. Due to the relatively large number of anatomical structures considered in the VGA and the time-consuming nature of the analysis, increasing the size of the observer cohort was difficult. The intra- and inter-observer agreement was assessed in both the objective and subjective assessments and showed quite a large variation, which is not unusual and is often observed especially related to subjective image quality assessments. Based on all these considerations, the results of this study are assessed as applicable, but more studies are needed to focus on possible dose reduction using IR or more studies that evaluate the actual influence on the identification of coronary plaques or stenosis.
In conclusion, the use of ASIR enhanced the perceived image quality compared with the FBP processing when the radiation dose was maintained, and these results suggest that the radiation dose could potentially be reduced. The subjective evaluation revealed a significant improvement in edge sharpening of the aortic wall and coronary artery contour using ASIR. The objective measurements revealed a significant difference for 60% ASIR with respect to noise reduction and increased CNR. The relationship between the subjective and objective image quality assessment was rather poor.
Supplementary Material
Acknowledgements
The authors give special thanks to IT Mark, Denmark, for support with the CCTA Dicom image preparation for the subjective analysis and the CT radiographers for scanning the patients and supporting the project at Odense University Hospital, Svendborg, Denmark. The investigators acknowledge the excellent assistance from each of the experienced CT Cardiologists: Bjarne Linde Nørgaard, Axel Diederichsen, Niels Peter Rønnow Sand, and Morten Böttcher for making the subjective image analysis and qualifying the paper in the writing process.
Declaration of conflicting interests
The author(s) declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The project was financially funded by research resources from the University College Lillebelt, University of Southern Denmark, Region of Southern Denmark and the Danish Council of Radiographers.
References
- 1.Wang R, Schoepf UJ, Wu R, et al. Diagnostic accuracy of coronary CT Angiography: comparison of filtered back projection and iterative reconstruction with different strengths. J Comput Assist Tomography 2014; 38: 179–184. [DOI] [PubMed] [Google Scholar]
- 2.Hassen A, Nazir SA, Alkadhi H. Technical challenges of coronary CT angiography: today and tomorrow. Eur J Radiol 2011; 79: 161–171. [DOI] [PubMed] [Google Scholar]
- 3.Willemink MJ, de Jong PA, Leiner T. Iterative reconstruction techniques for computed tomography Part 1: technical principles. Eur Radiol 2013; 6: 1623–1631. [DOI] [PubMed] [Google Scholar]
- 4.Beister M, Kolditz D, Kalender WA. Iterative reconstruction methods in X-ray CT. Physica Medica 2012; 28: 94–108. [DOI] [PubMed] [Google Scholar]
- 5.Silva AC, Lawder HJ, Hara A, et al. Innovations in CT dose reduction strategy: application of the adaptive statistical iterative reconstruction algorithm. Am J Roentgenol 2010; 194: 191–199. [DOI] [PubMed] [Google Scholar]
- 6.Nelson RC, Feuerlein S, Boll D. New iterative reconstruction technique for cardiovascular computed tomography: How do they work, and what are the advantages and disadvantages? J Cardiovasc Comput Tomography 2011; 5: 286–292. [DOI] [PubMed] [Google Scholar]
- 7.Kröpil P, Bigdeli AH, Nagel HD, et al. Impact of increasing levels of advanced iterative reconstruction on image quality in low dose cardiac CT angiography. RoFo 2014; 186: 567–575. [DOI] [PubMed] [Google Scholar]
- 8.Chen MY, Steigner ML, Leung SW, et al. Simulated 50% radiation dose reduction in coronary CT angiography using adaptive iterative dose reduction in three-dimensions (AIDR3D). Int J Cardiovasc Imaging 2013; 29: 1167–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kazakauskaite E, Husmann L, Stehli J, et al. Image quality in low-dose coronary computed tomography angiography with a new high-definition CT scanner. Int J Cardiovasc Imaging 2013; 29: 471–477. [DOI] [PubMed] [Google Scholar]
- 10.Utsunomiya D, Weigold G, Weissman G, et al. Effect of hybrid iterative reconstruction technique on quantitative and qualitative image analysis at 256-slice prospective gating cardiac CT. Eur Radiol 2012; 22: 1287–1294. [DOI] [PubMed] [Google Scholar]
- 11.Leipsic J, LaBounty TM, Heilbron B, et al. Adaptive statistical iterative reconstruction: assessment of image noise and image quality in coronary CT angiography. Am J Roentgenol 2010; 195: 649–654. [DOI] [PubMed] [Google Scholar]
- 12.Miéville FA, Gudinchet F, Rizzo E, et al. Paediatric cardiac CT examinations: impact of the iterative reconstruction method ASIR on image quality – preliminary findings. Pediatr Radiol 2011; 41: 1154–1164. [DOI] [PubMed] [Google Scholar]
- 13.Bushberg JT, Seibert JA, Leidholdt EM, et al. The essential physics of medical imaging. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2012:60–400.
- 14.Hsieh J. Computed tomography – principles, design, artifacts and recent advances. 2nd ed. Washington, DC: SPIE Press, 2009:37–141.
- 15.Wood BP. Decision making in radiology. Radiology 1999; 211: 601–603. [DOI] [PubMed] [Google Scholar]
- 16.Samei E, Krupinski E. The Handbook of: Medical image perception and techniques. 1st ed. New York, NY: Cambridge University Press, 2010.
- 17.Ledenius K, Svensson E, Stålhammar F, et al. A method to analyse observer disagreement in visual grading studies: example of assessed image quality in paediatric cerebral multidetector CT images. Br J Radiol 2010; 83: 604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ledenius K, Gustausson M, Johansson S, et al. Effect of tube current on diagnostic image quality in paediatric cerebral multidetector CT images. Br J Radiol 2009; 82: 313–320. [DOI] [PubMed] [Google Scholar]
- 19.Månsson LG. Methods for the evaluation of image quality: A review. Radiat Prot Dos 2000; 90: 89–99. [Google Scholar]
- 20.Båth M, Månsson LG. Visual grading characteristics (VGC) analysis: a non-parametric rank-invariant statistical method for image quality evaluation. Br J Radiol 2007; 80: 169–176. [DOI] [PubMed] [Google Scholar]
- 21.Sund P, Båth M, Kheddache S, et al. Comparison of visual grading analysis and determination of detective quantum efficiency for evaluating system performance in digital chest radiography. Eur Radiol 2004; 14: 143–150. [DOI] [PubMed] [Google Scholar]
- 22.European Commission. European guidelines on quality criteria for computed tomography. Available at: http://www.drs.dk/guidelines/ct/quality/htmlindex.htm (accessed 18 April 2014).
- 23.Börjesson S, Håkansson M, Båth M, et al. A software tool for increased efficiency in observer performance studies in radiology. Radiat Prot Dos 2005; 114: 45–52. [DOI] [PubMed] [Google Scholar]
- 24.Håkansson M, Svensson S, Zachrisson S, et al. VIEWDEX: an efficient and easy-to-use software for observer performance studies. Radiat Prot Dos 2010; 139: 42–51. [DOI] [PubMed] [Google Scholar]
- 25.National Electrical Manufacturers Association. Digital Imaging and Communications in Medicine (DICOM). Part 14: Grayscale Standard Display Function. PS 3. 14-2004. Available at: http://medical.nema.org/dicom/2004/04_14PU.pdf (accessed 14 April 2014).
- 26.Van Metter RL, Beutel J, Kundel HL. Handbook of Medical Imaging - Physics and Psychophysics. 2nd ed. Washington, DC: SPIE Press, 2004:79–222.
- 27.Bharkhada D, Yu H, Ge S, et al. Cardiac computed tomography radiation dose reduction using interior reconstruction algorithm with the aorta and vertebra as known information. J Comput Assist Tomogr 2009; 33: 338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleiss JL. Measuring nominal scale agreement among many raters. Psychol Bull 1971; 76: 378–382. [Google Scholar]
- 29.Efron B. Bootstrap methods: Another look at the jackknife. Ann Stat 1979; 7: 1–26. [Google Scholar]
- 30.Miller DP: Bootstrap 101: Obtain robust confidence intervals for any statistic. Proceedings of the Twenty-Ninth Annual SAS (R) Ovation Research Group International Conference, San Francisco, CA. Available at: http://www2.sas.com/proceedings/sugi29/193-29.pdf (accessed 22 November 2016).
- 31.Hendee WR, Wells PNT. The perception of visual information. New York, NY: Springer-Verlag, 1997.
- 32.Willemink MJ, Leiner T, de Jong PA, et al. Computed tomography: Iterative reconstruction techniques for computed tomography part 2: initial results in dose reduction and image quality. Eur Radiol 2013; 23: 1632–1342. [DOI] [PubMed] [Google Scholar]
- 33.Gosling O, Loader R, Venables P, et al. A comparison of radiation doses between state-of-the-art multislice CT coronary angiography with iterative reconstruction, multislice CT coronary angiography with standard filtered back-projection and invasive diagnostic coronary angiography. Heart 2010; 96: 922–926. [DOI] [PubMed] [Google Scholar]
- 34.Moore CS, Wood TJ, Beavis AW, et al. Correlation of the clinical and physical image quality in chest radiography for average adults with a computed radiography imaging system. Br J Radiol 2013; 86: 20130077–20130077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Båth M, Håkansson M, Börjesson S, et al. Nodule detection in digital chest radiography: effect of anatomical noise. Radiat Prot Dosimetry 2005; 114: 109–113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.