Abstract
Given the extensive application of carbon nanotubes (CNTs) in biomedical fields, there is increasing concern regarding unintentional health impacts. Research into safe usage is therefore increasingly necessary. This study investigated the responses of the mouse brain to single-walled CNTs (SWCNTs) delivered via intraperitoneal (IP) injection and compared these results with the previous study where SWCNTs were delivered via intravenous (IV) injection so as to explore which administration route is potentially better for SWCNTs application. This study suggests SWCNTs delivered via IP injection can have negative effects on the mouse brain through oxidative stress and inflammation at high concentration exposure, but these responses were not consistent and showed no dose-dependent effect. In a previous study, the results showed that IV-delivered SWCNTs induced a more consistent and dose-dependent effect. The comparison of the 2 studies suggested that using SWCNTs at a safe dosage delivered via IV injection may be a better administration route for SWCNTs in biomedical applications.
Keywords: single-walled carbon nanotubes (SWCNTs), intraperitoneal injection, intravenous injection, administration route
Introduction
Nanotechnology has been growing rapidly worldwide over the last decade. Carbon nanotubes (CNTs) are representative and significant nanomaterials and have been used in many fields since their discovery in the 1990s.1–2 CNTs have many special physical and chemical properties, and these characteristics offer great opportunities for their broad application.3–6
Owing to the wide use of CNTs, many researchers are becoming increasingly interested in their toxic effects. Murray et al demonstrated that single-walled CNTs (SWCNTs) could induce dermal toxicity via oxidative stress and inflammation.7 According to research by Urankar et al, without a significant pulmonary inflammatory response, oropharyngeal aspiration of Multi-walled CNTs (MWCNTs) promotes an increased susceptibility of cardiac tissue to ischemia/reperfusion injury.8 Yang et al reported that nanotubes accumulated in the brain over a 28-day experimental period, suggesting that the nanotubes could overcome the blood–brain barrier to enter the brain.9 However, this observation is still preliminary and further experimental verification and mechanistic elucidation are required. Among the toxicity studies of CNTs, pulmonary toxicity of CNTs has been the primary focus.10–12
Single-walled CNTs used as a drug delivery mechanism were shown to be able to carry acetylcholine into the brain.13 Carbon nanotubes therefore have potential application in the treatment of central nervous system diseases by delivering and enhancing the efficacy or bioavailability of targeted drugs. Further study is needed to determine how to ensure that the use of CNTs for drug delivery to the brain is safe.
In a previous study,14 mice were exposed to SWCNTs through intravenous (IV) injection to determine whether there were any adverse effects caused by SWCNTs on the mouse brain and also to find a safe application dosage if possible. In this study, intraperitoneal (IP) injection was used to mimic the human administration route. Histopathological alterations in the mouse brain were detected after SWCNTs exposure. In order to explore the possible mechanisms of these alterations, oxidative stress and inflammation levels in the mouse brain were examined. The possible protective effects of ascorbic acid (Vit C) on SWCNTs-induced brain damage were also investigated and then compared these results with the previous study. The aim of this work is to explore a possible appropriate application route for SWCNTs and to ensure that it is safe.
Materials and Methods
Ethics Statement
With a certificate of Application for the Use of Animals dated March 1, 2012 (approval ID: CCNU-IACUC-2012-011), all animal experiments were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Office of Scientific Research Management of Central China Normal University (Wuhan, People’s Republic of China).
Experimental Protocol
Fifty-four male Kunming mice were divided into 6 experimental groups of 9 mice each randomly. Each group was exposed to one of the following protocols: SWCNTs (0, 3.125, 6.25, or 12.5 mg/kg/d); SWCNTs (6.25 mg/kg/d) + Vit C (100 mg/kg/d); or Vit C (100 mg/kg/d). We exposed the mice to the SWCNTs or Vit C through a daily IP injection; the exposure cycle was 9 days. The SWCNTs were dispersed in 0.9% NaCl with 0.1% Tween 80, and the solution was sonicated for 10 minutes before each day’s use to promote dispersion of the suspension. Twenty-four hours after the last exposure, the brains of all the mice were collected for analysis.
Characterization of SWCNTs
Transmission electron microscopy (TEM), scanning electron microscopy, atomic force microscopy (AFM), X-ray photoelectron spectroscopy (XPS), and Raman spectroscopy were used to characterize the physicochemical properties of SWCNTs (Sigma-Aldrich, St Louis, Missouri).
Body Weight Increase and Brain Index
Before the first exposure, body weight of each mice was weighted (body weight1); then 24 hours after the ninth exposure, the body weight of each mice was weighted again (body weight2).
Brain index is organ coefficient of mouse brain; 24 hours after the ninth exposure, the mouse brains were collected and then weighted.
Histological Examination
Twenty-four hours after the ninth day’s IP injection, the mouse brains were collected for histological examination. The brains were soaked in a mixture (saturated in 2,4,6-trinitrophenol/formalin/glacial acetic acid [15:5:1 vol/vol/vol]) for 24 hours at least under normal conditions. Pieces were embedded in paraffin and then sectioned into 10-μm slices. Hematoxylin and eosin– and Nissl-stained slices were then prepared. All sections were examined and photographed at ×40 magnification through a microscope (DM 4000B; Leica, Berlin, Germany). The average optical density (OD) of each Nissl-stained slice was obtained using Image-Pro Plus 6.0 software (Media Cybernetics, Bethesda, Maryland). To determine the OD, nonstained regions of the slices were used as background.
Brain Tissue Homogenate
Mouse brains were collected and weighed and then put into a glass homogenizer. A 1× phosphate-buffered saline solution (pH = 7.5, 10 mL/g) was added and the mixture homogenized on ice. The homogenate was then centrifuged (10 000 rpm, 10 minutes, 4°C), and the supernatant was collected for assay. The protein concentration of the supernatant was determined using a Lowry assay.15
Oxidative Stress Level Detection
The reactive oxygen species (ROS), malondialdehyde (MDA), and glutathione (GSH) content in the mouse brain were measured using 2′,7′-dichlorofluorescin diacetate, 2-thiobarbituric acid, and 3-carboxy-4-nitrophenyl disulfide, respectively, as previously described.16
Analysis of Tumor Necrosis Factor α and Interleukin 1β Contents
The concentrations of tumor necrosis factor α (TNF-α) and interleukin 1β (IL-1β) were measured using enzyme-linked immunosorbent assay (ELISA) kits (eBioscience, San Diego, California), according to the manufacturers’ instructions. The sensitivities of the ELISA kits were 8 pg/mL (TNF-α) and 80 pg/mL (IL-1β).
Statistical Analyses
The findings were presented as mean ± standard deviation (SD). The significance of the differences between groups was determined by one-way analysis of variance combined with Tukey multiple comparison tests. Values of P < .05 were considered statistically significant. Data analyses were carried out using SPSS 13.0 (Chicago, Illinois). Statistical graphs were generated using GraphPad Prism 5.0 (San Diego, California).
Results
Characterization of SWCNTs
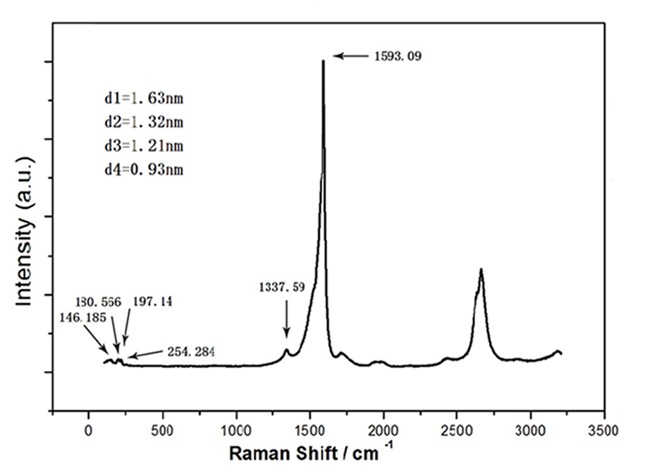
Scanning electron microscopy and TEM showed that the SWCNTs in our study comprised a smooth single-walled tube structure. The main metal catalysts in the SWCNTs samples were tested using XPS analysis (Figure S1). Raman spectral measurement (514-nm excitation wavelength) showed that G/D ratios were 15.64, and the Raman radial breathing mode peak analysis indicated that the diameter of the SWCNTs ranged from 0.88 to 1.56 nm (Figure S2). The AFM image shows that the diameter of the SWCNTs was around 1.0 nm and the length of the SWCNTs was around 1.38 μm (Figure S3). The biological effects of nanomaterials are related to their properties. The SWCNTs we used were the same as those used in our previous study. As shown by our data, the quality of these SWCNTs perfectly met the requirements of the experiments (Figure 1).
Figure 1.
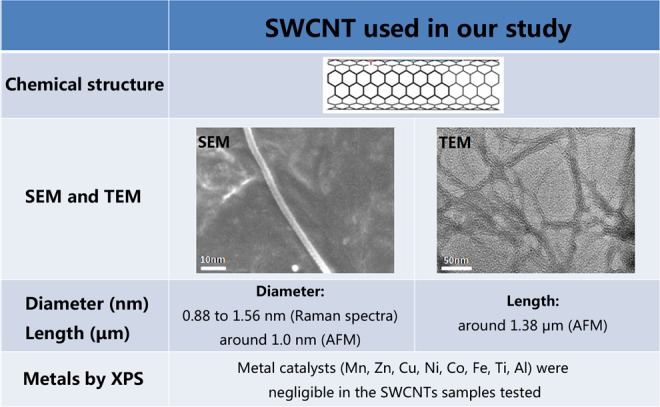
Characterization of SWCNTs. These characterization data indicated that the quality of these SWCNTs perfectly met the requirements of the experiments. SWCNTs indicate single-walled carbon nanotubes.
Mouse Body Weight and Brain Index After SWCNTs Exposure
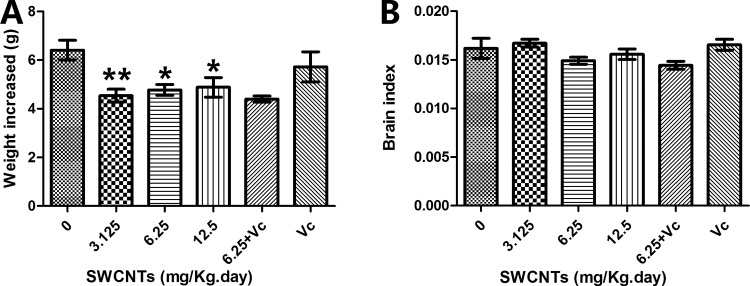
After 9 days exposure, all mice showed an increase in body weight. However, the degree of increase differed across groups. As shown in Figure 2A, the control group gained the most weight. The brain index (brain weight/body weight) of the mice did not show a significant change (Figure 2B).
Figure 2.
Body weight increase and brain index. A, The body weight increase. B, The brain index. *P < .05; **P < .01, compared with the control group (0 mg/kg/d SWCNTs). SWCNTs indicate single-walled carbon nanotubes.
Histological Observation of the Mouse Hippocampus After SWCNTs Exposure
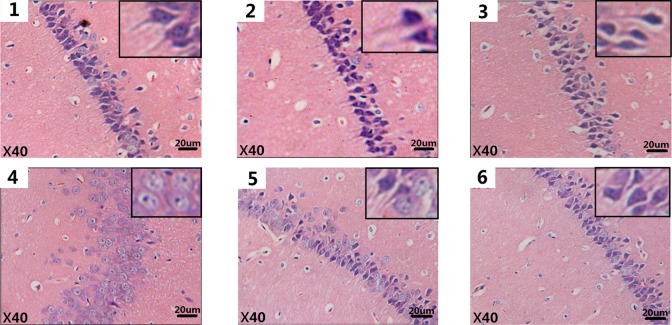
From the histopathological examination of the brain, histological changes were observed in the pyramidal cells of the CA1 region of the hippocampus. The cells in the hippocampus of the control group were arranged neatly together, with clear edges and polygonal shapes, and the radial apical dendrites stretched to the inner hippocampus. As the exposure concentrations increased, some histopathological lesions appeared. Cells became loosely arranged, disorderly, swelling deformations were observed in the shape of some cells, and the radial apical dendrites shortened or even disappeared (Figure 3).
Figure 3.
Histological alterations (×40) seen in mice brains after SWCNTs exposure (H&E staining). Each of the 6 images (1-6) correspond to 1 of the 6 different exposure groups: 0, 3. 125, 6.25, and 12.5 mg/kg/d SWCNTs; 6.25 mg/kg/d SWCNTs + 100 mg/kg/d ascorbic acid (block group); and 100 mg/kg/d ascorbic acid, respectively. H&E indicates hematoxylin and eosin; SWCNTs, single-walled carbon nanotube.
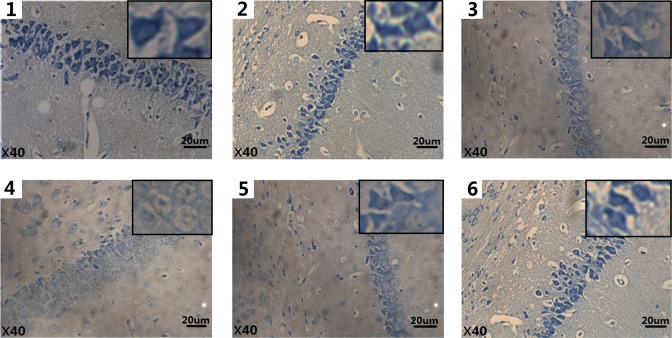
Nissl Substance Loss After SWCNTs Exposure
Nissl substance is a critical component in the synthesis of proteins, including many enzymes that are involved in neurotransmitter synthesis in the nerve cell, and it is very sensitive to pathological stimuli. For this reason, it is considered a good indicator of neuronal injury. Loss of Nissl substance was seen in the pyramidal cells of the hippocampus (Figure 4, Table 1).
Figure 4.
Histological alterations (×40) seen in mice brains after SWCNTs exposure (Nissl staining). Each of the 6 images (1-6) correspond to 1 of the 6 different exposure groups: 0, 3. 125, 6.25, and 12.5 mg/kg/d SWCNTs; 6.25 mg/kg/d SWCNTs + 100 mg/kg/d ascorbic acid (block group); and 100 mg/kg/d ascorbic acid, respectively. SWCNTs indicates single-walled carbon nanotube.
Table 1.
The Average Optical Density of the Nissl Staining.
| Group | Average Optical Density | Group | Average Optical Density |
|---|---|---|---|
| 0 mg/kg/d | 0.1385 ± 0.0027 | 12.5 mg/kg/d | 0.0482 ± 0.0031a |
| 3.125 mg/kg/d | 0.1288 ± 0.0039 | Block group | 0.0855 ± 0.0043 |
| 6.25 mg/kg/d | 0.0970 ± 0.0078b | 100 mg/kg/d Vit C | 0.1295 ± 0.0025 |
Abbreviation: Vit C, ascorbic acid.
a P < .01, compared with the control group (0 mg/kg/d SWCNTs).
b P < .05, compared with the control group (0 mg/kg/d SWCNTs).
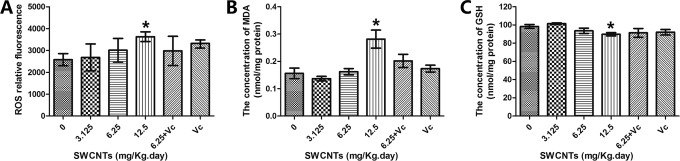
Oxidative Stress in the Brain After SWCNTs Exposure
After 9 days of exposure to SWCNTs, the ROS concentration increased in the brain tissue (Figure 5A). ROS, the key signaling molecules during cell signaling and homeostasis, are reactive species of molecular oxygen. Malondialdehyde is a typical indicator for evaluating lipid peroxidation. Figure 5B shows that the MDA content of the brain in the 12.50 mg/kg/d group increased significantly when compared with that of the mice in the control group. Figure 5C showed that after SWCNTs exposure, there was also a significant reduction in GSH, a major scavenger of ROS in tissues, thus reducing oxidation.
Figure 5.
Oxidative stress levels in mouse brains after SWCNTs exposure. A, The relative fluorescence of ROS. B, The concentration of MDA. C, The concentration of GSH. *P < .05, compared with the control group (0 mg/kg/d SWCNTs). GSH indicates glutathione; MDA, malondialdehyde; ROS, reactive oxygen species; SWCNTs, single-walled carbon nanotube.
The Pro-Inflammation Cytokines in the Brain After SWCNTs Exposure
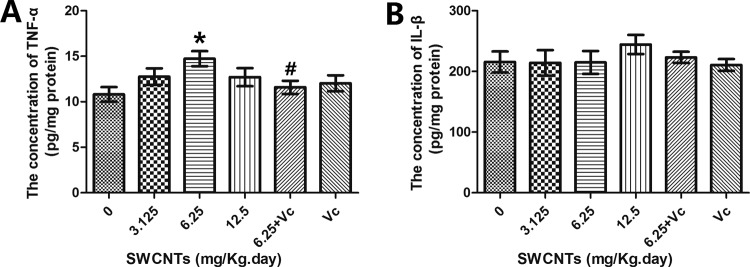
According to the hierarchical oxidative stress hypothesis, higher levels of oxidative stress will lead to pro-inflammatory effects. TNF-α and IL-1β are important cytokines that are involved in the regulation of immune and inflammatory responses. In this study, the TNF-α levels rose, and when compared to the 6.25 mg/kg/d SWCNT group, a TNF-α reduction could be observed in the 6.25 mg/kg/d SWCNT + Vit C group (Figure 6A); however, the concentration of IL-1β did not change (Figure 6B).
Figure 6.
Inflammation levels in mouse brains after SWCNTs exposure. A, The concentration of TNF-α. B, The concentration of IL-1β. *P < .05, compared with the control group (0 mg/kg/d SWCNTs), #P < .05, comparisons between the 6.25 mg/kg/d SWCNTs group and the block group (6.25 mg/kg/d SWCNTs + 100 mg/kg/d ascorbic acid). IL-1β indicates interleukin 1β; SWCNTs, single-walled carbon nanotube; TNF-α, tumor necrosis factor α.
Discussion
Although SWCNTs have broad potential applications in biomedical fields, especially in neuroscience, some studies have shown that there are adverse effects from SWCNTs exposure. Further study is needed to determine how to ensure the safe use of CNTs in the brain. To solve this problem, explorations into safe use dosages and suitable administration routes are crucial.
Previous study has already demonstrated that administering SWCNTs to mice through IV injection could induce brain damage, however, a 3.125 mg/kg/d SWCNTs dose had zero or only minor adverse effects in mice. However, whether the effects induced by SWCNTs are dependent not only on the administration route but also on the exposure dosage remains an open question. Since the IP route is also frequently used in treating humans,17 this simply repeated previous study on the dosage effects of SWCNTs but administered them via IP injection to determine which administration route is better.
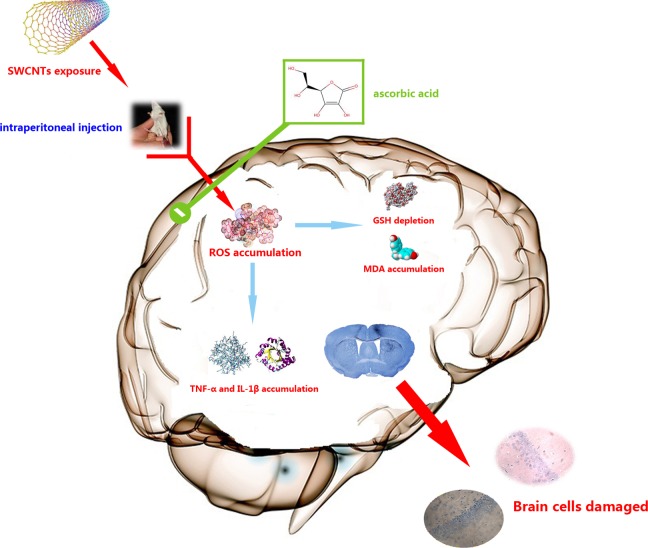
This study found that after exposing mice to SWCNTs via IP injection, the pyramidal neurons of the CA1 region were damaged and that Nissl substance loss occurred in pyramidal cells. Increased levels of oxidative stress and inflammation were also seen in the mouse brain (Figure 7).
Figure 7.
The possible molecular mechanism of SWCNTs-induced damage in the mouse brain. The SWCNTs-induced damage in the mouse brain occurs through enhancing ROS generation. Oxidative stress then induces cell damage in the brain, whereas treatment with ascorbic acid may protect cells by decreasing oxidative stress. This figure was produced by Xudong Liu. SWCNTs indicates single-walled carbon nanotube; ROS, reactive oxygen species.
At first glance, we found these results to be very similar to the previous study’s data. Both of the administration routes induced similar adverse effects at high exposure dosages. However, when comparing these data carefully, the data from the mice in the IP groups reflected inconsistent and no significant dose-dependent effects. In addition, Vit C did not show any protective effects in the IP group. We therefore reanalyzed the previous data and found that there was indeed a significant dose-dependent effect. The correlation coefficient (R 2) between the contents (ROS, MDA, GSH, TNF-α, IL-1β) and the exposure dosages of SWCNTs were 0.9874, 0.9038, 0.9590, 0.9438, and 0.9966, respectively.
Although IP injection is easier and faster to perform than an IV injection, it may result in unnoticed erroneous injections into the bowel or retroperitoneum. In addition, the first-pass effect of an IP injection can lead to an unknown amount of a drug in the circulatory system and a slow and decreasing cerebral uptake,18,19 the inconsistent data from our IP study may demonstrate this.
Nowadays, the main potential application of CNTs is as a drug carrier to deliver and enhance the efficacy or bioavailability of targeted drugs. Some studies have demonstrated that CNTs can cross the blood-brain barrier through different exposure routes,9,20 but the effects induced by different routes were limited. Our goal is to achieve the most effective treatment using a minimum number of CNTs. We have shown with the 2 studies that administering SWCNTs via IP injection may result in inconsistent biological effects, whereas IV administration does not. This may indicate that the number of SWCNTs transported to the brain is dependent on the exposure route. In other words, IV-administered SWCNTs transport drugs to the brain in a more consistent manner. For determining the dosage of a target drug and improving the drug’s efficacy, knowing which administration route is most consistent is very useful. This result may provide a reference for pharmaceutical and medical researchers who want to use SWCNTs in biomedical applications.
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Authors’ Note: Xudong Liu and Qing Guo have contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported financially by the National Natural Science Foundation of China (21577045).
Supplemental Material: The online supplemental figures are available at http://journals.sagepub.com/doi/suppl/10.1177/1559325816681320.
References
- 1. Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354(7):56–58. [Google Scholar]
- 2. Iijima S, Ichihashi T. Single-shell carbon nanotubes of 1-nm diameter. Nature. 1993;363(6430):603–615. [Google Scholar]
- 3. Liu Z, Chen K, Davis C, et al. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res. 2008;68(16):6652–6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Venkatesan J, Qian Z, Ryu B, et al. Preparation and characterization of carbon nanotube-grafted-chitosan-natural hydroxyapatite composite for bone tissue engineering. Carbohydr Polym. 2011;83(2):569–577. [Google Scholar]
- 5. Chen RJ, Bangsaruntip S, Drouvalakis KA, et al. Noncovalent functionalization of carbon nanotubes for highly specific electronic biosensors. Proc Natl Acad Sci U S A. 2003;100(9):4984–4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang Q, Huang JQ, Qian WZ, Zhang YY, Wei F. The road for nanomaterials industry: a review of carbon nanotube production, post-treatment, and bulk applications for composites and energy storage. Small. 2013;9(8):1237–1265. [DOI] [PubMed] [Google Scholar]
- 7. Murray AR, Kisina E, Leonarda SS, et al. Oxidative stress and inflammatory response in dermal toxicity of single-walled carbon nanotubes. Toxicology. 2009;257(3):161–171. [DOI] [PubMed] [Google Scholar]
- 8. Urankar RN, Lust RM, Mann E, et al. Expansion of cardiac ischemia/reperfusion injury after instillation of three forms of multi-walled carbon nanotubes. Part Fibre Toxicol. 2012;9:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang ST, Guo W, Lin Y, et al. Biodistribution of pristine single-walled carbon nanotubes in vivo. J Phys Chem C. 2007;111(48):17761–17764. [Google Scholar]
- 10. Shvedova AA, Kisin E, Murray AR, et al. Inhalation vs. aspiration of single-walled carbon nanotubes in C57BL/6 mice: inflammation, fibrosis, oxidative stress, and mutagenesis. Am J Physiol Lung Cell Mol Physiol. 2008;295(4):L552–L565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lam CW, James JT, McCluskey R, et al. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci. 2004;77(1):126–134. [DOI] [PubMed] [Google Scholar]
- 12. Chou CC, Hsiao HY, Honq QS, et al. Single-walled carbon nanotubes can induce pulmonary injury in mouse model. Nano Lett. 2008;8(2):437–445. [DOI] [PubMed] [Google Scholar]
- 13. Al-Jamal KT, Gherardini L, Bardi G, et al. Functional motor recovery from brain ischemic insult by carbon nanotube-mediated siRNA silencing. Proc Natl Acad Sci U S A. 2011;108(27):10952–10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu XD, Zhang YC, Li JQ, et al. Cognitive deficits and decreased locomotor activity induced by single-walled carbon nanotubes and neuroprotective effects of ascorbic acid. Int J Nanomedicine. 2014;9:823–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 16. Li JQ, Li L, Chen HQ, et al. Application of vitamin E to antagonize SWCNTs-induced exacerbation of allergic asthma. Sci Rep. 2014;4: 4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dou S, Smith M, Wang Y, et al. Intraperitoneal injection is not always a suitable alternative to intravenous injection for radiotherapy. Cancer Biother Radiopharm. 2013;28(4):335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arioli V, Rossi E. Errors related to different techniques of intraperitoneal injection in mice. Appl Microbiol. 1970;19(4):704–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schiffer WK, Mirrione MM, Dewey SL. Optimizing experimental protocols for quantitative behavioral imaging with 18F-FDG in rodents. J Nucl Med. 2007;48(2):277–287. [PubMed] [Google Scholar]
- 20. Wang HF, Wang J, Deng XY, et al. Biodistribution of carbon single-wall carbon nanotubes in mice. J Nanosci Nanotechnol. 2004;4(8):1019–1024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials